Introduction
Lung cancer is the leading cause of
cancer-associated mortalities worldwide, with an estimated 1.61
million new cases and 1.38 million mortailies in 2008 (1,2).
Approximately 85% of primary lung cancer patients have
non-small-cell lung cancer (NSCLC) (3). Surgery is the standard treatment for
early-stage NSCLC. However, the majority of patients diagnosed at
an advanced stage are unsuitable for surgical resection or
extensive mediastinal lymphadenopathy (4,5).
Chemo- and radiotherapy are the current standard of care for
patients with unresectable advanced NSCLC (6,7).
However, there are several limiting factors of chemo- and
radiotherapy, including dose tolerance limitation of normal tissue
and tumor radioresistance (8).
Accordingly, identifying effective agents for enhancing tumor
sensitivity to radiation and reducing adverse effects on normal
tissues are crucial.
Acting as radiosensitizers, drugs can accelerate the
killing of cancer cells by increasing the effectiveness of
radiation with little effect on normal cells (9). The PI3K/Akt and ERK pathways play an
essential role in confirming sensitivity or resistance to radiation
by inhibiting a survival pathway that induces cell apoptosis
(10,11). Previous studies also showed that
treatment with the ERK inhibitor can reduce radioresistance and
suppressed activation of PI3K/Akt can promote radiosentization
(12,13). Inhibition of growth of cancer cells
and the induction of apoptosis are important determinants of the
response to anticancer therapy (14).
Bioflavonoids, which are the phytochemicals that are
abundant in a variety of plants have a vital role in cancer
prevention as they can scavenge free radicals (15,16).
Kaempferol is a flavonol that is present in tea, broccoli,
grapefruit, Brussels sprouts and apples. It is claimed to have an
anti-proliferative effect on colon cancer cell lines (17,18).
The anti-angiogenic properties of kaempferol have also been well
documented (19). Among the
flavonols, kaempferol is absorbed particularly well when
administered orally, even in low doses, with minimal
inter-individual variation (20).
Kaempferol is reportedly effective against pancreatic cancer, human
lung non-small carcinoma and glioma cells (21–23).
It has been reported to act synergistically with quercetin to cause
a considerable anti-proliferative effect in human gut cells and
breast cancer cells (24). However,
the combination of kaempferol with radiation against cancer remains
to be evaluated. In the present study, the anticancer capacity of
kaempferol and its ability to sensitize tumors radiation were
assessed in in vitro and in vivo studies. Its effects
on signal transduction in lung cancer cells were also
investigated.
Materials and methods
Materials and chemicals
Kaempferol was purchased from Sigma-Aldrich (St.
Louis, MO, USA). All other chemicals and reagents were of
analytical grade. Chemicals were obtained from Sigma-Aldrich,
except where otherwise indicated. The antibodies for α-tubulin,
p-ERK (E-4) and PI3K p85α(Z-8) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The antibodies for
phospho-Akt (Ser473) and caspase-7 were purchased from Cell
Signaling Technology (Danvers, MA, USA). The antibody for caspase-3
was purchased from Millipore (Billerica, MA, USA). MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was
purchased from USB Corp. (Cleveland, OH, USA). The Annexin V
conjugates and PI (propidium iodide) solid were all purchased from
Invitrogen Corp. (Carlsbad, CA, USA).
Cell culture
The human A-549 lung carcinoma and the human HFL1
normal lung fibroblast cell lines were obtained from BCRC
(Bioresource Collection and Research Center) in Taiwan. A-549 cells
were cultured in Ham's F12K medium supplemented with 10% fetal
bovine serum (FBS), 2 mM L-glutamine and 1.5 g/l sodium
bicarbonate. HFL1 cells were also cultured in Ham's F12K medium
supplemented with 10% FBS. The cells were maintained under standard
cell culture conditions at 37°C and 5% CO2 in a humid
environment. Adherent cells were harvested using trypsin and
re-suspended in a serum-containing medium before use in the assays,
as described below.
MTT assay
MTT assay was performed to determine the effect of
kaempferol on cell growth. This assay is based on the cleavage of
the yellow tetrazolium salt MTT to the purple formazan crystal by
mitochondrial succinate dehydrogenase from living cells. This
reduction occurs only when mitochondrial reductase enzymes are
active, thus the extent of the reduction is directly associated
with the number of viable cells.
Briefly, A-549 cells were seeded at a density of
1×104 cells/well in 24-well plates and pre-incubated for
24 h. The medium was then replaced with serum-free medium, to which
indicated doses of kaempferol were added for 48 h. Following
treatment with kaempferol, the medium was replaced with 0.5 mg/ml
MTT medium and incubated for 4 h. The MTT solution was removed from
the wells and the formazan crystals were dissolved in DMSO. The
concentration was then determined using a microplate reader at 570
nm.
Western blot analysis
After the cells were treated with kaempferol, they
were rinsed three times with PBS. The cells were then directly
solubilized on ice in a lysis buffer (0.5 M Tris-HCl, pH 7.4, 1.5 M
NaCl, 2.5% deoxycholic acid, 10% NP-40, 10 mM EDTA) that contained
a protease inhibitor cocktail. After 5 min, the cells were scraped
and the lysate was collected in an Eppendorf tube. The lysate was
cleared by centrifugation at 12,000 x g for 30 min at 4°C, and the
protein concentration in the supernatant was determined by the
Bradford method (Bio-Rad protein assay; Hercules, CA, USA).
For western blotting, equal amounts of proteins were
resolved over 10–12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene
fluoride (PVDF) membrane. The non-specific sites on the blots were
blocked by incubating them in a blocking buffer (5% non-fat dry
milk/TBS, pH 7.4) for 1 h at room temperature. Incubation was
performed using an appropriate monoclonal primary antibody in TBS
overnight at 4°C, and then incubated with a horseradish
peroxidase-conjugated secondary antibody for 1 h at room
temperature. Immunoreactive bands were visualized using an enhanced
chemiluminescence system.
Cell cycle analysis
To determine the effect of kaempferol on the cell
cycle, A-549 cells (1×106/10-cm dish) were treated with
56 µM kaempferol for the indicated time points (0, 3, 6, 12,
24 and 48 h). Each time-point was evaluated with at least three
plates. Briefly, the cells were treated with kaempferol at the
specified time points. The cell pellet was washed with cold
phosphate-buffered saline (PBS), fixed with 95% ethanol overnight,
and stained with 1.0 ml PI/Triton X-100 for 30 min in the dark. The
stained cells were analyzed using a flow cytometer (BD FACSCanto;
BD Biosciences, San Diego, CA, USA). The data were analyzed using
ModFit LT 3.0 software (Verity Software House, Inc., Topsham, ME,
USA).
Radiation exposure
The A-549 cells in dishes with diameters of 10 cm
were exposed to radiation by photons from a linear accelerator. The
dishes were placed within the exposure area on an acrylic sheet
with an area of 25×25 cm and covered by a bolus with an area of
1.5×1.5 cm. The cells were exposed to the indicated doses (2, 4, 6,
8, 10 and 12 Gy). The summarized radiation dosage on the cells and
exposure period was well calculated and monitored from the control
room. Following exposure, each dish was immediately transferred to
a cell culture incubator.
Clonogenic assay
Cell survival via radiation alone or the combination
of kaempferol with radiation was analyzed using a clonogenic assay.
A-549 cells were seeded in 10-cm dishes with an appropriate amount
of radiation or kaempferol with radiation. The cells were exposed
to 0, 2, 4, 6, 8, 10 and 12 Gy radiation and treated with 56
µM kaempferol for 48 h, followed by different doses of
radiation. The cell medium was replaced with a new medium and
cultured in an incubator for two weeks. After fixation and staining
with 5% crystal violet, colonies containing ≥50 cells were counted
under a microscope. The plating efficiency was defined as the
average number of cell colonies counted divided by the number of
initial cells for the control group which were not exposed to
kaempferol or radiation. The surviving fraction was determined as
the average number of cell colonies counted divided by the number
of seeding cells multiplied for the plating efficiency.
Tumor xenograft studies
A-549 cells (2×106 in 200 µl PBS)
were implanted subcutaneously in BALB/c nude mice near the left
hind leg. The mice were obtained from BioLasco Taiwan Co., Ltd.
(Taipei, Taiwan). The tumors were allowed to reach a volume of 350
mm3 prior to initiation of treatment (3 weeks after
tumor implantation). The mice (3 mice per group) were treated
(intraperitoneally) with radiation at 4 Gy alone or radiation plus
kaempferol (4 h before radiation). The tumor volume and size were
recorded every 5 days.
Histological stains
The mice were sacrificed and tissues were harvested
and placed in tissue wells filled with Tissue-Tek OCT. The tissue
wells were rapidly frozen and then dried. The frozen specimens were
sectioned using a cryostat microtome. hematoxylin and eosin
(H&E)-stained specimens were used to determine histological
morphology and nuclear structures. An anti-caspase-3
immunohistochemical (IHC) stain was used to label the apoptotic
cells. The cryosections were fixed with 4% formaldehyde overnight
and washed with PBS. Non-specific binding sites were blocked with
2% (w/v) BSA solution in PBS prior to labeling with antibody. The
specimens were washed several times with PBS, and incubated
overnight at 4°C with a 1:200 dilution of polyclonal anti-caspase-3
antibody. The sections were then counterstained with hematoxylin
and mounted on a cover slide for optical microscopic
examination.
Statistical analysis
Numerical data were presented as mean ± standard
deviation from at least three experiments. Statistical comparisons
were made using the Student's t-test or one-way analysis of
variance (ANOVA) followed by post hoc Fisher's LSD multiple
comparison test, as indicated. Any difference was regarded as
significant at a probability of P<0.05. Data were analyzed using
the SPSS software version 10.0 (SPSS, Inc., Chicago, IL, USA).
Results
Kaempferol selectively suppresses the
growth of A-549 cells and induces cell apoptosis through inhibition
of PI3K/AKT and ERK pathways
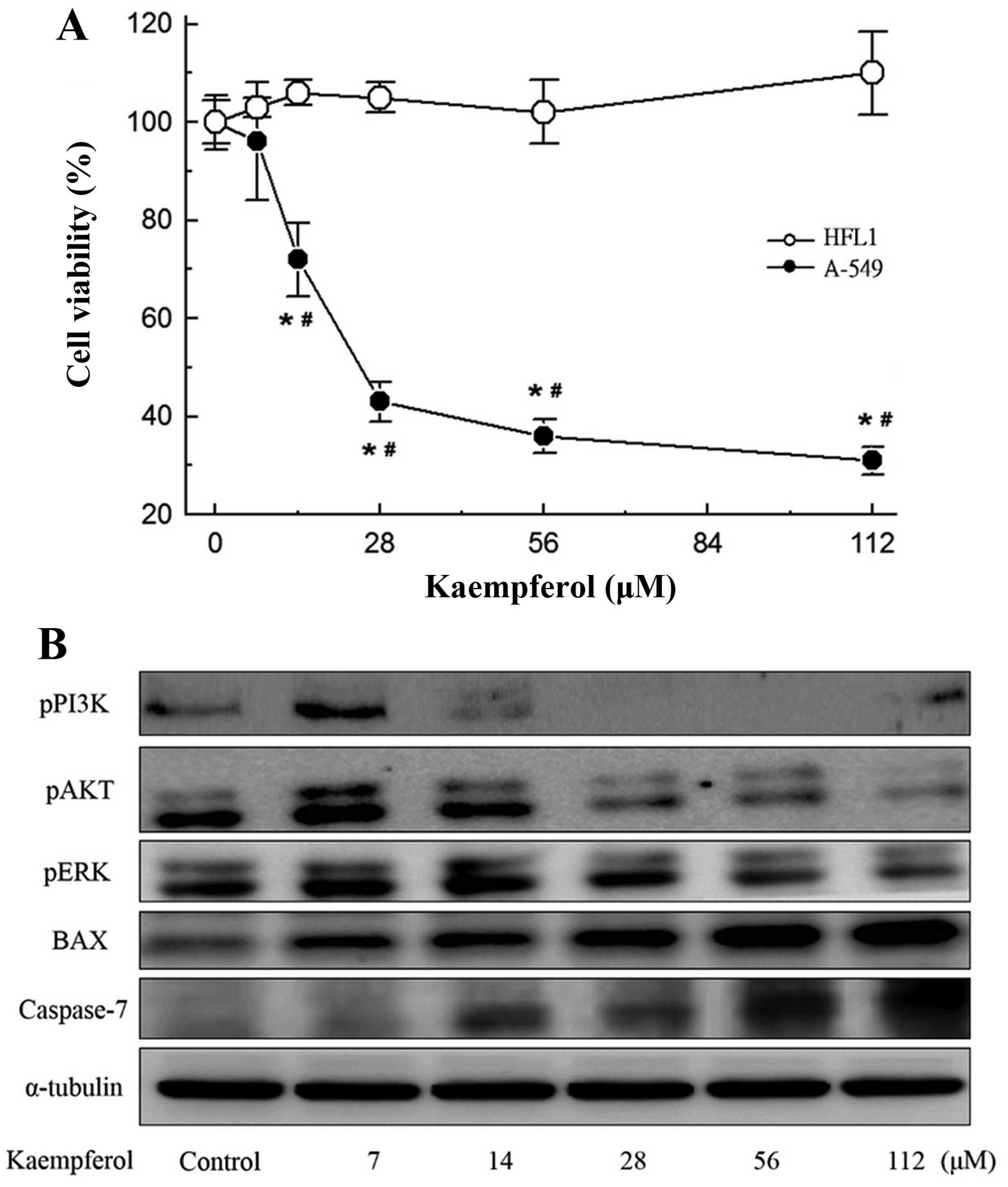
The cell viability of A-549 lung cancer cells and
HFL1 normal lung cells when treated with kaempferol was assessed
using an MTT assay. As shown in Fig.
1A, the treatment of A-549 cells with kaempferol at doses of
14-112 µM inhibited growth by 28, 57, 65 and 68%. The same
doses did not inhibit the growth of HFL1 normal lung cells
(Fig. 1A). The inhibitory effects
on the two cell lines differed significantly (P<0.05).
To elucidate the mechanism that underlies the
kaempferol-induced death of A-549 cells, the roles of caspases were
investigated by examining their activation. As shown in Fig. 1B, the expression of caspase-7
increased significantly with the dose of kaempferol. Since members
of the Bcl-2 family are the main regulators of apoptosis, the
effect of kaempferol on the levels of such proteins in A-549 cells
was determined. Western blot analysis revealed a dose-dependent
increase in the expression of Bax, verifying the induction of the
apoptotic process. Inhibition of the PI3K/AKT and ERK pathways also
increased the level of Bax, thus, we also detected the
phosphorylation of AKT, PI3K and ERK. Additionally, kaempferol
suppressed the phosphorylation of AKT, PI3K and ERK.
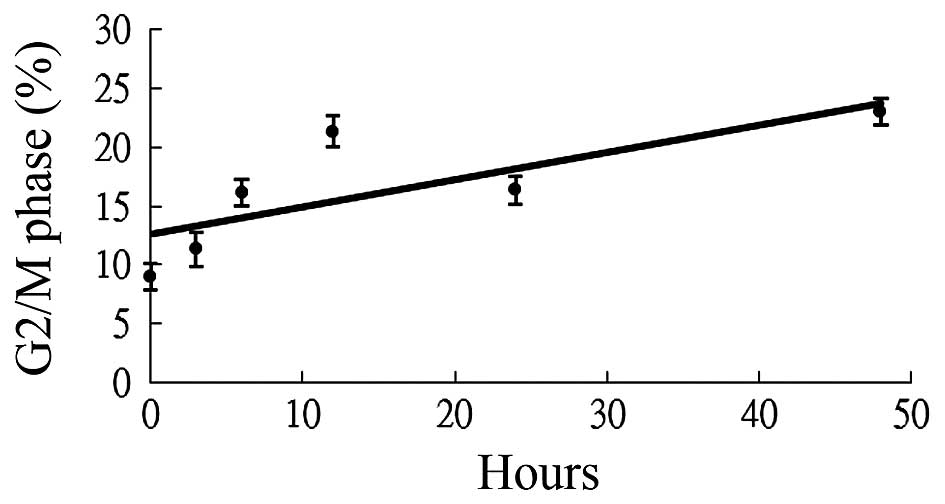
Kaempferol induces G2/M cell cycle
arrest
To determine whether kaempferol was a
radiosensitizer, cell cycle analysis was first performed. The
effects of kaempferol on the cell cycle progression of A-549 cells
are shown in Fig. 2. There was an
increase in the cell population in the G2/M phase at 48 h compared
with the other groups (0, 3, 6, 12 and 24 h) following treatment
with 56 µM of kaempferol. The trend line of the G2/M phase
showed that the cell population exhibited a time-dependent increase
following treatment.
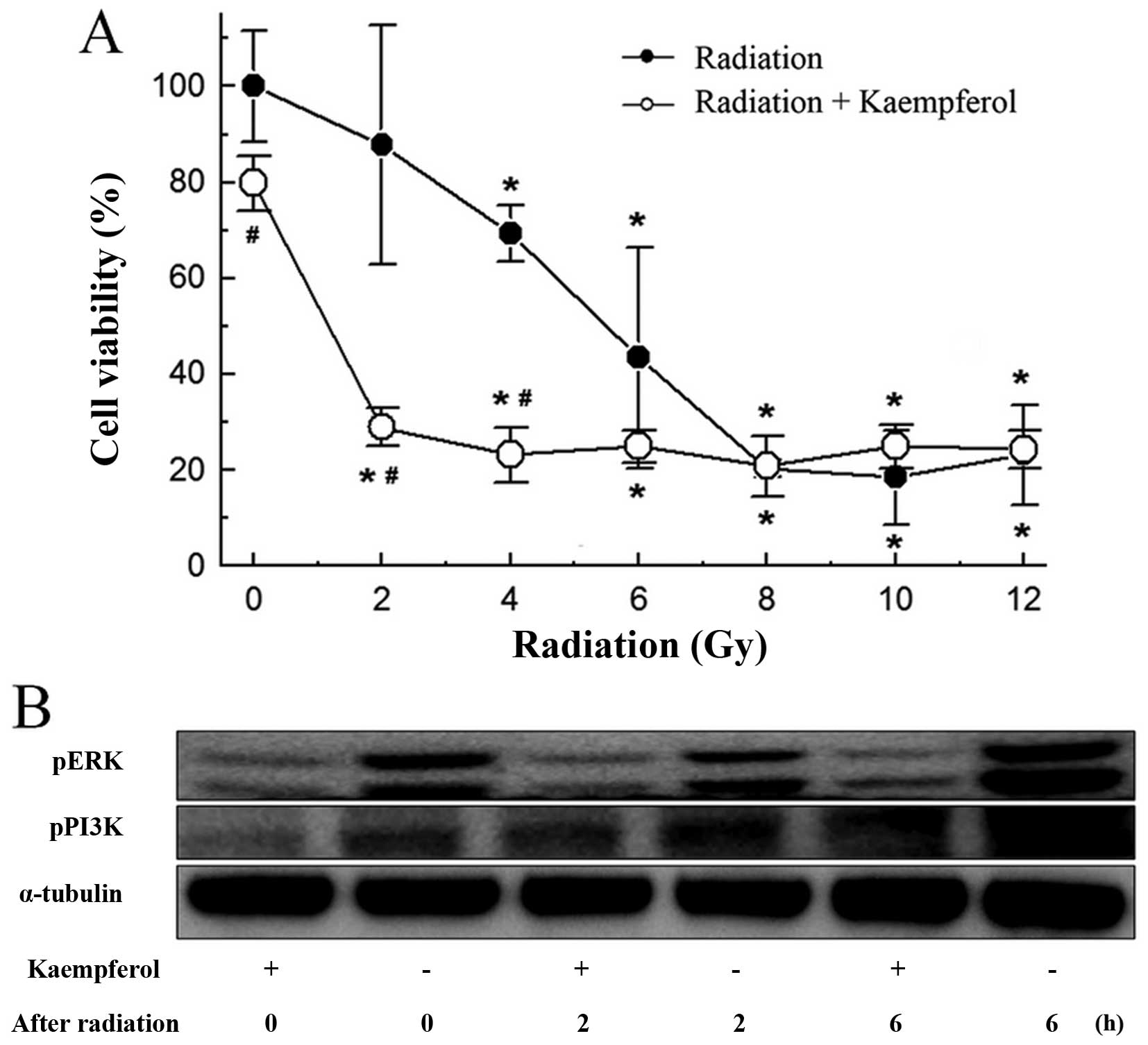
Kaempferol induces enhancement of
radiation-induced death and retards phosphorylation of PI3K and
ERK
Kaempferol at a dose of 14 µM, which
inhibited cell growth by 28% (Fig.
1A), was used to examine the radiosensitizing effects of
kaempferol on A-549 cells. As shown in Fig. 3A, radiation exposure alone inhibited
cell growth dose-dependently from 2 to 12 Gy. Kaempferol treatment
at 48 h prior to radiation exposure enhanced inhibition of cancer
cell growth from 2 to 6 Gy. The application of kaempferol prior to
radiation exposure enhanced A-549 cell radiosensitivity and
strengthened the inhibitory effects.
To achieve a good prognosis, inhibition of
radioresistant protein PI3K or ERK activated after radiation
exposure was crucial. We found treatment with kaempferol before
radiation exposure inhibited PI3K and ERK, and reduced
phosphorylation of ERK sustainability after radiation exposure of 6
h (Fig. 3B).
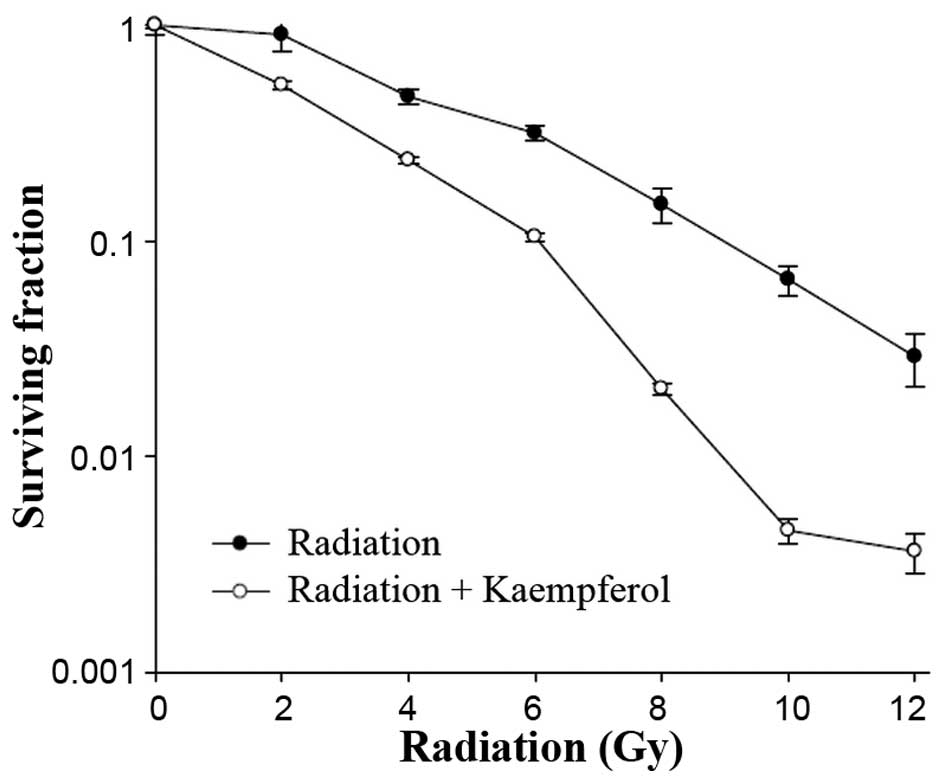
Kaempferol decreases the clonogenic
survival
To determine the effects of kaempferol on the
radiosensitivity of A-549 cells, clonogenic assays were performed
after exposure to 0–12 Gy of radiation with and without 48-h
pretreatment with 56 µM of kaempferol. We found that
treatment of the cells with radiation alone led to a minimal effect
on clonogenic survival. However, when pretreated with kaempferol
before radiation exposure, the surviving fraction decreased
significantly (Fig. 4). The dose
enhancement factor (DEF) was 2, which was the ratio of the dose
reducing the surviving fraction to 0.5 in the absence vs. presence
of kaempferol.
Kaempferol increases tumor cell killing
of radiation in vivo
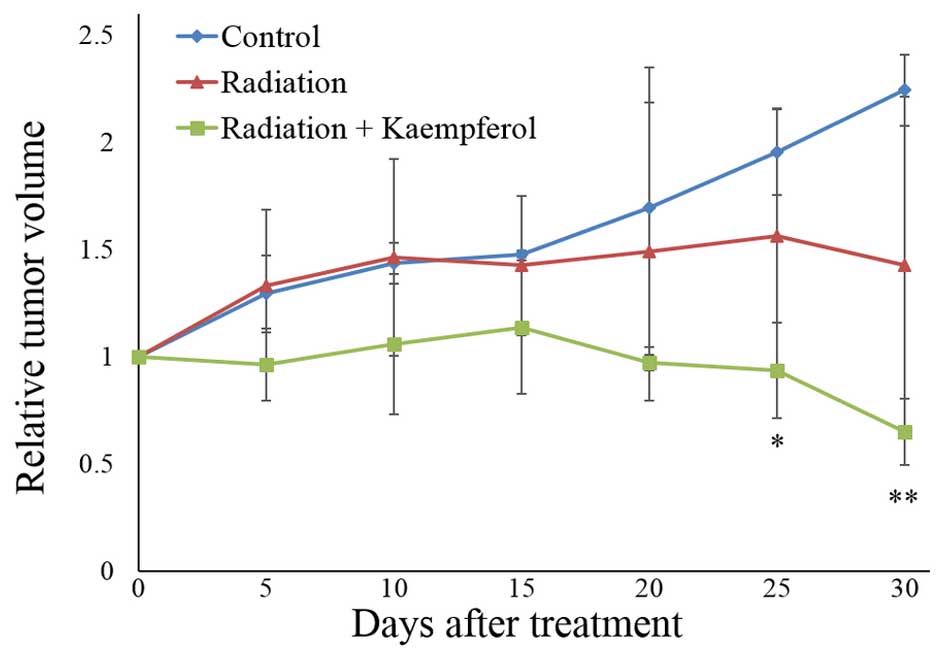
To determine the in vivo radiosensitivity of
kaempferol, we used BALB/c nude mice with A-549 tumor xenografts.
As shown in Fig. 5, treatment with
radiation alone showed no significant difference in tumor growth
compared to the control group (saline-treated). However, treatment
with radiation plus kaempferol (4 h before radiation) resulted in a
significant suppression of tumor growth 25 days after treatment. In
addition, the tumor volume of the radiation plus kaempferol group
decreased ~35% of the starting volume 30 days after treatment.
Kaempferol enhances tumor cell apoptosis
of radiation in vivo
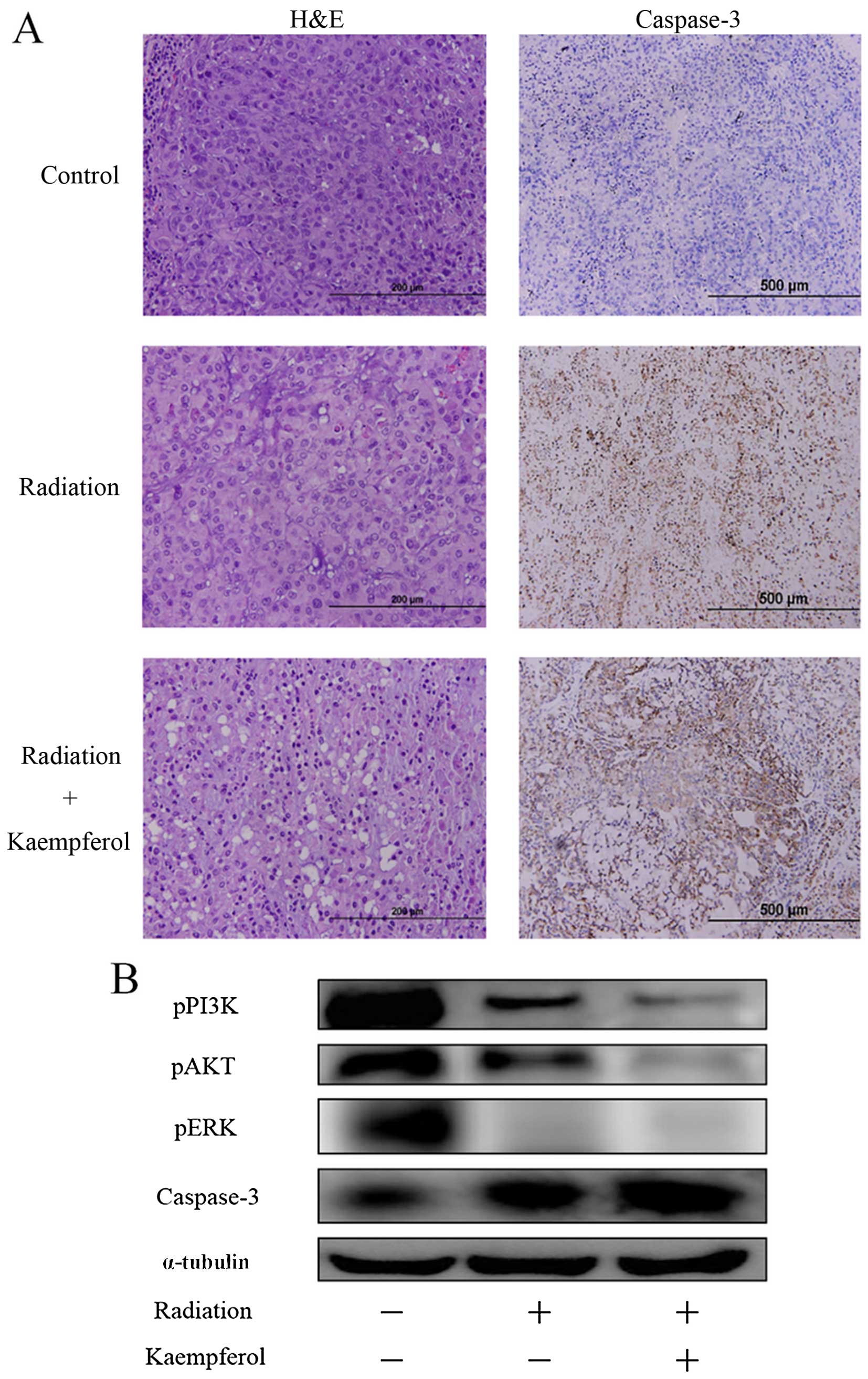
To investigate histological differences between
treated and control tumors, we performed tumor sections by H&E
staining and IHC of caspase-3 (Fig.
6A). Although control tumors exhibited a homogeneous
distribution of viable cells, the sections of the radiation plus
kaempferol-treated tumors showed apoptotic evidence of cell
shrinkage, nuclear condensation and fragmentation via H&E
staining. IHC staining of caspase-3 was used to verify cell
apoptosis (dark-brown cells). There was considerably more
dark-brown staining of the radiation plus kaempferol group,
compared with radiation only group.
Tumor tissue sections were extracted and processed
for western blot analysis. As shown in Fig. 6B, treatment kaempferol plus
radiation, not only inhibited the phosphorylation of PI3K, AKT and
ERK, but also enhanced the expression of caspase-3, inducing tumor
cell apoptosis.
Discussion
An important issue is to develop anticancer agents
such as tumor-specific radiosensitizers that promote tumor
radiosensitization to ultimately improve the therapeutic ratio and
overcome tumor radioresistance. Kaempferol is a common flavonoid
that is found in vegetables, fruits and tea (25,26).
Although kaempferol reportedly has anti-proliferative and cytotoxic
effects on human lung cancer cells, to the best of our knowledge,
no study has previously demonstrated that it can enhance
radiosensitivity (27,28). The present study provides evidence
that kaempferol has radiosensitization potential for lung cancer
in vitro and in vivo.
Kaempferol dose-dependently reduced the viability of
A-549 cells, but had no significant effect on the normal HFL1 cell
line. Such properties are relevant to the development of an
anticancer drug that is not cytotoxic towards normal cells, unlike
most currently used clinical drugs. Inhibition of the PI3K/AKT and
ERK pathway may promote apoptosis proteins of Bcl-2 family members
such as Bax, and then trigger caspase to induce cell apoptosis
(29,30). In the present stuyd, kaempferol
suppressed the phosphorylation of AKT, PI3K and ERK, and then
increased the expression of Bax, and subsequently stimulated
caspase-7. The data demonstrate that kaempferol inhibited A-549
cells through activation of the mitochondria apoptosis pathway,
which is consistent with the findings of a previous study (31).
Prior to investigating whether kaempferol was
capable of enhancing the inhibitory effects of radiation on the
growth of cancer cells, cell cycle regulation is important in
mediating radiosensitivity. Cells have varying radiosensitivity in
different phases of the cell cycle. The G2/M phase is most
sensitive to radiation (32). In
the current study, the cell population of G2/M phase exhibited a
time-dependent increase following treatment with kaempferol.
Additionally, treatment with kaempferol prior to radiation exposure
inhibited the growth of A-549 lung cancer cells more effectively.
Kaempferol also inhibited the phosphorylation of PI3K and ERK
sustainability after radiation exposure for 6 h. We then verified
the radiosentization effect of kaempferol using a clonogenic assay,
which is also used to determine cell reproductive death after
treatment with ionizing radiation (33). It was found that following
pretreatment with kaempferol prior to radiation exposure, the
surviving fraction decreased significantly. These data demonstrate
that kaempferol has a radiosensitization potential for A-549
cells.
An animal model was used to determine the
radiosensitization of kaempferol in vivo. Treatment with
kaempferol prior to radiation (4 h) resulted in significant
suppression of tumor growth and decreased tumor volume.
Additionally, treatment with kaempferol prior to radiation enhanced
the induction of cell apoptosis in tumor tissue by staining the
histological sections. The protein level of the phosphorylation of
AKT, PI3K and ERK from tumor tissue was also more inhibited
following treatment with kaempferol plus radiation, and
significantly activated caspase-3 to induce tumor apoptosis. The
results demonstrate that kaempferol was able to increase in
vivo tumor cell killing by radiation, indicating that
kaempferol functions as a powerful radiosensitizer.
In conclusion, the results showed that, kaempferol
increased tumor cell killing by radiation in vitro and in
vivo through inhibition of the AKT/PI3K and ERK pathways and
activation of the mitochondria apoptosis pathway. Thus, this study
provided solid evidence that kaempferol is a safe and potential
radiosensitizer for NSCLC.
Acknowledgments
The authors would like to thank the National Science
Council of the Republic of China, Taiwan (contract no.
NSC100-2628-E-039-002-MY3) and the China Medical University
(contract no. CMU100-S-36) for financially supporting this
research.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pisters KM, Evans WK, Azzoli CG, Kris MG,
Smith CA, Desch CE, Somerfield MR, Brouwers MC, Darling G, Ellis
PM, et al Cancer Care Ontario; American Society of Clinical
Oncology: Cancer Care Ontario and American Society of Clinical
Oncology adjuvant chemotherapy and adjuvant radiation therapy for
stages I-IIIA resectable non small-cell lung cancer guideline. J
Clin Oncol. 25:5506–5518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crinò L, Weder W, van Meerbeeck J and
Felip E; ESMO Guidelines Working Group: Early stage and locally
advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21(Suppl 5): v103–v115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Furuse K, Fukuoka M, Kawahara M, Nishikawa
H, Takada Y, Kudoh S, Katagami N and Ariyoshi Y: Phase III study of
concurrent versus sequential thoracic radiotherapy in combination
with mitomycin, vindesine, and cisplatin in unresectable stage III
non-small-cell lung cancer. J Clin Oncol. 17:2692–2699.
1999.PubMed/NCBI
|
|
7
|
Pfister DG, Johnson DH, Azzoli CG, Sause
W, Smith TJ, Baker S Jr, Olak J, Stover D, Strawn JR, Turrisi AT,
et al American Society of Clinical Oncology: American Society of
Clinical Oncology treatment of unresectable non-small-cell lung
cancer guideline: Update 2003. J Clin Oncol. 22:330–353. 2004.
View Article : Google Scholar
|
|
8
|
Eberhardt W, Pöttgen C and Stuschke M:
Chemoradiation paradigm for the treatment of lung cancer. Nat Clin
Pract Oncol. 3:188–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wardman P: Chemical radiosensitizers for
use in radiotherapy. Clin Oncol (R Coll Radiol). 19:397–417. 2007.
View Article : Google Scholar
|
|
10
|
Kim IA, Bae SS, Fernandes A, Wu J, Muschel
RJ, McKenna WG, Birnbaum MJ and Bernhard EJ: Selective inhibition
of Ras, phosphoinositide 3 kinase, and Akt isoforms increases the
radiosensitivity of human carcinoma cell lines. Cancer Res.
65:7902–7910. 2005.PubMed/NCBI
|
|
11
|
Reardon DB, Contessa JN, Mikkelsen RB,
Valerie K, Amir C, Dent P and Schmidt-Ullrich RK: Dominant negative
EGFR-CD533 and inhibition of MAPK modify JNK1 activation and
enhance radiation toxicity of human mammary carcinoma cells.
Oncogene. 18:4756–4766. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fokas E, Im JH, Hill S, Yameen S,
Stratford M, Beech J, Hackl W, Maira SM, Bernhard EJ, McKenna WG,
et al: Dual inhibition of the PI3K/mTOR pathway increases tumor
radiosensitivity by normalizing tumor vasculature. Cancer Res.
72:239–248. 2012. View Article : Google Scholar
|
|
13
|
Wang T, Hu YC, Dong S, Fan M, Tamae D,
Ozeki M, Gao Q, Gius D and Li JJ: Co-activation of ERK, NF-kappaB,
and GADD45beta in response to ionizing radiation. J Biol Chem.
280:12593–12601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuo WT, Ho YJ, Kuo SM, Lin FH, Tsai FJ,
Chen YS, Dong GC and Yao CH: Induction of the mitochondria
apoptosis pathway by phytohemagglutinin erythroagglutinating in
human lung cancer cells. Ann Surg Oncol. 18:848–856. 2011.
View Article : Google Scholar
|
|
15
|
Miean KH and Mohamed S: Flavonoid
(myricetin, quercetin, kaempferol, luteolin, and apigenin) content
of edible tropical plants. J Agric Food Chem. 49:3106–3112. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Agarwal OP and Nagaratnam A:
Radioprotective property of flavonoids in mice. Toxicon.
19:201–204. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JS, Rho HS, Kim DH and Chang IS:
Enzymatic preparation of kaempferol from green tea seed and its
antioxidant activity. J Agric Food Chem. 54:2951–2956. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Du B, Wang T, Wang S and Zhang J:
Kaempferol induces apoptosis in human HCT116 colon cancer cells via
the Ataxia-Telangiectasia Mutated-p53 pathway with the involvement
of p53 upregulated modulator of apoptosis. Chem Biol Interact.
177:121–127. 2009. View Article : Google Scholar
|
|
19
|
Luo H, Rankin GO, Liu L, Daddysman MK,
Jiang BH and Chen YC: Kaempferol inhibits angiogenesis and VEGF
expression through both HIF dependent and independent pathways in
human ovarian cancer cells. Nutr Cancer. 61:554–563. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
DuPont MS, Day AJ, Bennett RN, Mellon FA
and Kroon PA: Absorption of kaempferol from endive, a source of
kaempferol-3-glucuronide, in humans. Eur J Clin Nutr. 58:947–954.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nöthlings U, Murphy SP, Wilkens LR,
Henderson BE and Kolonel LN: Flavonols and pancreatic cancer risk:
The multiethnic cohort study. Am J Epidemiol. 166:924–931. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leung HW, Lin CJ, Hour MJ, Yang WH, Wang
MY and Lee HZ: Kaempferol induces apoptosis in human lung non-small
carcinoma cells accompanied by an induction of antioxidant enzymes.
Food Chem Toxicol. 45:2005–2013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeong JC, Kim MS, Kim TH and Kim YK:
Kaempferol induces cell death through ERK and Akt-dependent
down-regulation of XIAP and survivin in human glioma cells.
Neurochem Res. 34:991–1001. 2009. View Article : Google Scholar
|
|
24
|
Ackland ML, van de Waarsenburg S and Jones
R: Synergistic antiproliferative action of the flavonols quercetin
and kaempferol in cultured human cancer cell lines. In Vivo.
19:69–76. 2005.PubMed/NCBI
|
|
25
|
Kowalski J, Samojedny A, Paul M, Pietsz G
and Wilczok T: Effect of kaempferol on the production and gene
expression of monocyte chemoattractant protein-1 in J774.2
macrophages. Pharmacol Rep. 57:107–112. 2005.PubMed/NCBI
|
|
26
|
Sotibrán AN, Ordaz-Téllez MG and
Rodríguez-Arnaiz R: Flavonoids and oxidative stress in Drosophila
melanogaster. Mutat Res. 726:60–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moon SS, Rahman MA, Manir MM and Jamal
Ahamed VS: Kaempferol glycosides and cardenolide glycosides,
cytotoxic constituents from the seeds of Draba nemorosa
(Brassicaceae). Arch Pharm Res. 33:1169–1173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu X, Xie H, Hao J, Jiang Y and Wei X:
Flavonoid glycosides from the seeds of Litchi chinensis. J Agric
Food Chem. 59:1205–1209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fletcher JI, Meusburger S, Hawkins CJ,
Riglar DT, Lee EF, Fairlie WD, Huang DC and Adams JM: Apoptosis is
triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc
Natl Acad Sci USA. 105:18081–18087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nguyen TT, Tran E, Ong CK, Lee SK, Do PT,
Huynh TT, Nguyen TH, Lee JJ, Tan Y, Ong CS, et al:
Kaempferol-induced growth inhibition and apoptosis in A549 lung
cancer cells is mediated by activation of MEK-MAPK. J Cell Physiol.
197:110–121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar
|