Introduction
Epithelial-mesenchymal transition (EMT) is an
important process in tumor progression that causes epithelial cells
to acquire a migratory mesenchymal phenotype (1,2). EMT
is thought to be a critical step in the induction of cell invasion
and tumor metastasis (1).
Furthermore, it has been shown that cells with an EMT phenotype are
more resistant to chemoradiotherapy in regards to head and neck
squamous cell carcinoma (HNSCC) (3–6).
It has been indicated in recent studies that EMT
induces cancer stem cell (CSC)-like properties in many different
types of malignant tumors and that both EMT and a CSC-like
phenotype are associated with treatment resistance (7–9).
Prince et al demonstrated that the purified CD44+
population of HNSCC cells possesses the self-renewing properties of
CSCs (10). Aldehyde dehydrogenase
1 (ALDH1) has also been shown to be a putative marker of CSCs in
HNSCC (11). Furthermore, Chen
et al showed that CD44+/ALDH1+ cells
resist radiotherapy and may serve as a reservoir for developing
tumors and metastasis (6).
Additionally, the CD44high/EGFRlow
subpopulation exhibited the EMT phenotype and resistance to
treatment in HNSCC (12). Moreover,
a high level of expression of variant isoforms of CD44 (CD44v)
could suppress the clustering of EGFR at the surface of HNSCC cells
and thereby negatively regulate EGFR signaling in the absence of a
differentiation stimulus, suggesting that CD44v-negative HNSCC
cells rely on EGFR activity for survival (13).
However, the source and the mechanism of development
in regards to CSCs have not yet been fully elucidated. Moreover, it
has been shown that it is difficult to extract pure CSC populations
with only CSC markers such as CD44 and ALDH1. The same result is
shown with side populations (14).
Therefore, it is necessary not only to identify novel CSC markers
but also to clarify the mechanism of CSC development.
Snail, a member of the zinc-finger transcription
factor family, plays an important role in EMT by directly
repressing epithelial markers such as E-cadherin and by
upregulating mesenchymal markers (7,15–19).
Several studies have shown that Snail-related transcription factors
play a transcriptional and regulatory role in the invasion,
metastasis and poor outcome in different type of malignancies,
including HNSCC (20,21). These findings suggest that Snail
expression may regulate CSC-like properties via EMT in HNSCC.
We previously demonstrated that Snail overexpression
induced EMT, including cancer cell migration and invasion, and
promotes CSC-like phenotype such as
CD44+/ALDH1+ in head and neck cancer cells
(22). However, the key role of
Snail on the stemness of CSC in HNSCC has not been fully
elucidated. In the present study, we first demonstrated that
Snail-induced EMT gains CSC-like phenotype such as upregulation of
stem cell markers, including CD44 and ALDH1, and enhanced CSC-like
properties such as sphere formation capability, chemoresistance and
in vivo cancer invasion and metastasis in HNSCC.
Materials and methods
Cell lines and culture
Human HNSCC cells, SAS and HSC-4, were employed in
the present study. SAS and HSC-4 cells, obtained from the Japanese
Cancer Research Resource Bank (Tokyo, Japan), were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
heat-inactivated fetal bovine serum (FBS) (both from Invitrogen,
Carlsbad, CA, USA), 100 U/ml penicillin and 100 mg/ml streptomycin
(Gibco, Grand Island, NY, USA) at 37°C in 5% CO2.
Transient transfection
The cDNA fragment encoding human Snail (NM_005985.2)
was inserted into the pCR 3.1 mammalian expression vector
(Invitrogen). For transient transfection, SAS and HSC-4 cells
(1.5×105 cells) were plated into 6-well culture plates
and allowed to adhere for 12 h. Then, the cells were transfected
with 2 µg of either pCR 3.1-Snail or pCR 3.1-vector (without
insert DNA) with Lipofectamine 2000 reagent (invitrogen) according
to the manufacturer's instructions. We established SAS-Snail and
HSC-4-Snail as Snail-expressing cell lines and their respective
control cell lines.
Immunoblot analysis and antibodies
Total protein extracts were prepared according to
the freeze-thawing lysis method, and protein concentrations were
measured with a bovine serum albumin (BSA) protein assay. Samples
of extract containing 20 µg of protein were then separated
by SDS-PAGE and transferred to polyvinylidene difluoride membranes.
After washing with phosphate-buffered saline with Tween-20 (PBST),
the membranes were incubated first with rabbit anti-Nanog, rabbit
anti-Oct4, rabbit anti-ABCG2, rabbit anti-EGFR and rabbit
anti-pEGFR (Cell Signaling Technology, Danvers, MA, USA; diluted
1:1,000), rabbit anti-Sox2 and rabbit anti-Bmi1 (Abcam, Cambridge,
MA, USA; diluted 1:1,000) antibodies at 4°C overnight and then with
peroxidase-conjugated secondary anti-rabbit antibody or goat
immunoglobulin G (IgG) (diluted 1:1,000; Cell Signaling Technology)
for 1 h. After rinsing in PBST, immunodetection was accomplished
using an ECL western blot analysis detection reagent and analysis
system. The membranes were subsequently exposed to X-ray film as
previously described (23).
Real-time RT-PCR
Total RNA was isolated from each cell line using the
RNeasy Mini kit (Qiagen, Hilden, Germany). cDNA was then reverse
transcribed using ReverTra Ace® qPCR rT master mix with
gDNA remover (Toyobo Life Science, Tokyo, Japan) according to the
manufacturer's instructions. Real-time RT-PCR was then carried out
using the primers shown in Table I
and EXPRESS SYBR®-GreenER™ qPCR SuperMix with premixed
ROX (Invitrogen). PCR was performed with an initial step of 3 min
at 95°C followed by 40 cycles of 3 sec at 95°C and 20 sec at 60°C.
The level of target mRNA was normalized to the mRNA level of GAPDH
as an internal standard.
 | Table IPrimers for real-time RT-PCR. |
Table I
Primers for real-time RT-PCR.
| Gene | Primer
sequence |
|---|
| ABCG2 | S
5′-CATGTACTGGCGAAGAATATTTGGT-3′ |
| (NM_004827) | A
5′-CACGTGATTCTTCCACAAGCC-3′ |
| Bmi1 | S
5′-AAATGCTGGAGAACTGGAAAG-3′ |
| (NM_005180) | A
5′-CTGTGGATGAGGAGACTGC-3′ |
| Nanog | S
5′-ATTCAGGACAGCCCTGATTCTTC-3′ |
| (NM_024865) | A
5′-TTTTTGCGACACTCTTCTCTGC-3′ |
| Oct4 | S
5′-GTGGAGAGCAACTCCGATG-3′ |
| (NM_002701) | A
5′-TGCTCCAGCTTCTCCTTCTC-3′ |
| Sox2 | S
5′-CGAGTGGAAACTTTTGTCGGA-3′ |
| (NM_003106) | A
5′-TGTGCAGCGCTCGCAG-3′ |
| GAPDH | S
5′-CATCATCCCTGCCTCTACTG-3′ |
| (NM_002046) | A
5′-GCCTGCTTCACCACCTTC-3′ |
Sphere formation assay
The capability of self-renewal was assessed using
96-well NanoCulture plates (Scivax, Tokyo, Japan). Cells
(1×104) were seeded and cultured for 1 week in DMEM
supplemented with 10% FBS or serum-free medium, and phase-contrast
images were obtained.
Chemotherapy of the cultured cells
Chemosensitivity was assessed using the Cell
Counting Kit-8 (WST-8 cleavage; Dojindo, Mashikimachi, Japan) as
previously described (23). The
cells were seeded into 96-well plates at an initial density of
4×103 cells/well and incubated for 24 h. For
chemotherapy, cisplatin (Nihon Kayaku Co., Tokyo, Japan) at a
concentration of 1.0 µM was added to each well. Following
incubation for an additional 48 h, 10 µl of WST-8 solution
was added to each well, and the plate was incubated for a further 2
h. The absorbance of each well at 450 nm (reference wavelength at
620 nm) was measured by a Multiscan FC microplate photometer
(Thermo Scientific). The measurement was repeated at least three
times for each cell line.
Chick chorioallantoic membrane (CAM) in
vivo invasion assay
The CAM in vivo invasion assay was conducted
using 11-day-old chick embryos wherein HSC-4 or the transfected
cells (105 cells labeled with green Fluoresbrite
carboxylated polystyrene nanospheres of 45-nm diameter;
Polysciences) were seeded atop the CAM and incubated for three days
as previously described (24,25).
The CAM was dropped without damaging the epithelial basement
membrane (BM) by applying gentle negative pressure at the air sac,
and an opening of ~1 cm2 was cut in the shell above the
CAM with an electric drill.
Statistical analysis
Data are presented as the mean ± standard error
(SE). Experimental differences between groups were assessed with a
t-test. The differences were considered to indicate a statistically
significant result at P<0.05.
Results
Snail expression induces a CSC-like
phenotype
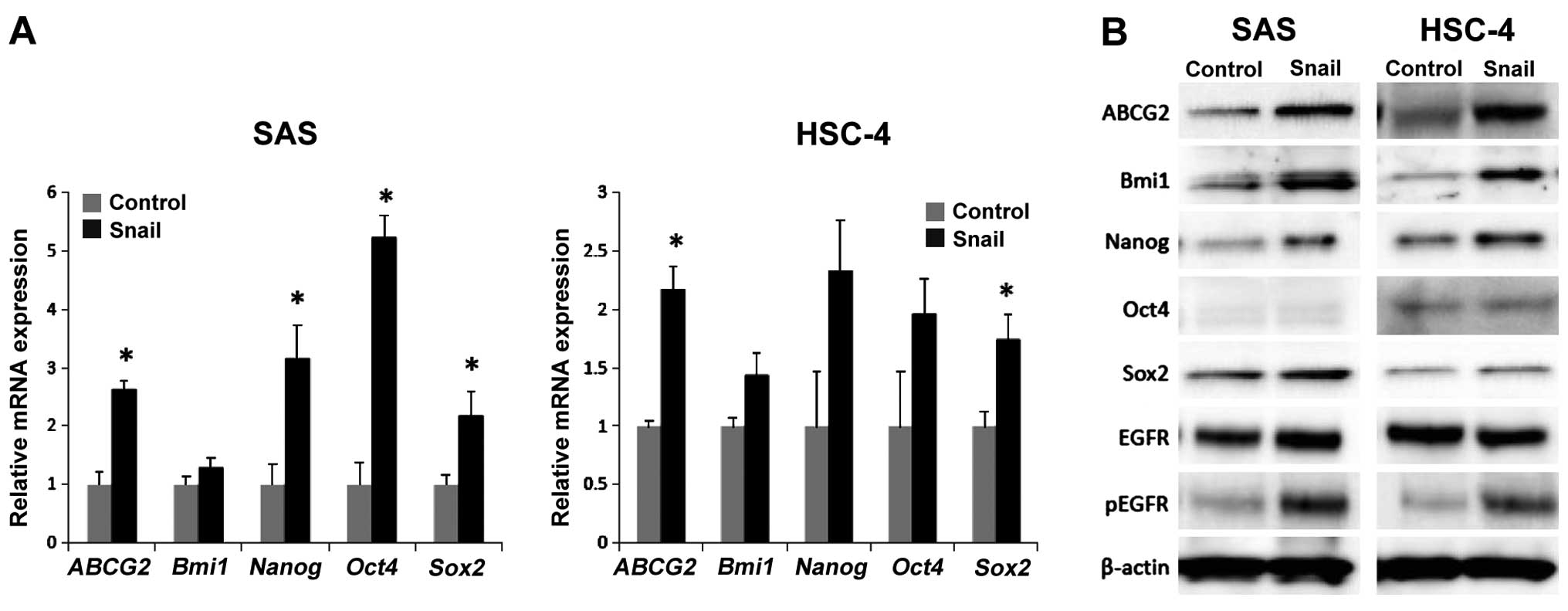
To elucidate that EMT by Snail expression induces a
stem cell-like phenotype in HNSCC cells, we investigated the
expression of CSC surface markers in HNSCC cells. We previously
demonstrated that expression of both CD44 and ALDH1 increased in
SAS-Snail and HSC-4-Snail cells compared with their control cells
(22). RT-PCR analysis revealed
that mRNA levels of several stem cell markers, Bmi1, Nanog, Oct4,
Sox2 and ABCG2, which are drug-resistant proteins, were high in the
Snail-transfected cells compared with the levels in the controls
(Fig. 1A). Moreover, at the protein
level, Nanog, Bmi1 and ABCG2 were also upregulated in the
Snail-transfected cells, whereas the expression of Oct4 and Sox2
showed no difference between the Snail-expressing cells and the
controls (Fig. 1B). These data
revealed that Snail-induced EMT elicits a CSC-like phenotypic
change as CD44+/ALDH+, and directly regulates
the expression of Nanog, Bmi1 and ABCG2. In addition, there were no
differences in the level of EGFR protein between the
Snail-transfected cells and the controls, whereas, the levels of
phospho-EGFR protein increased in the Snail-transfected cells
compared with that in the control (Fig.
1B).
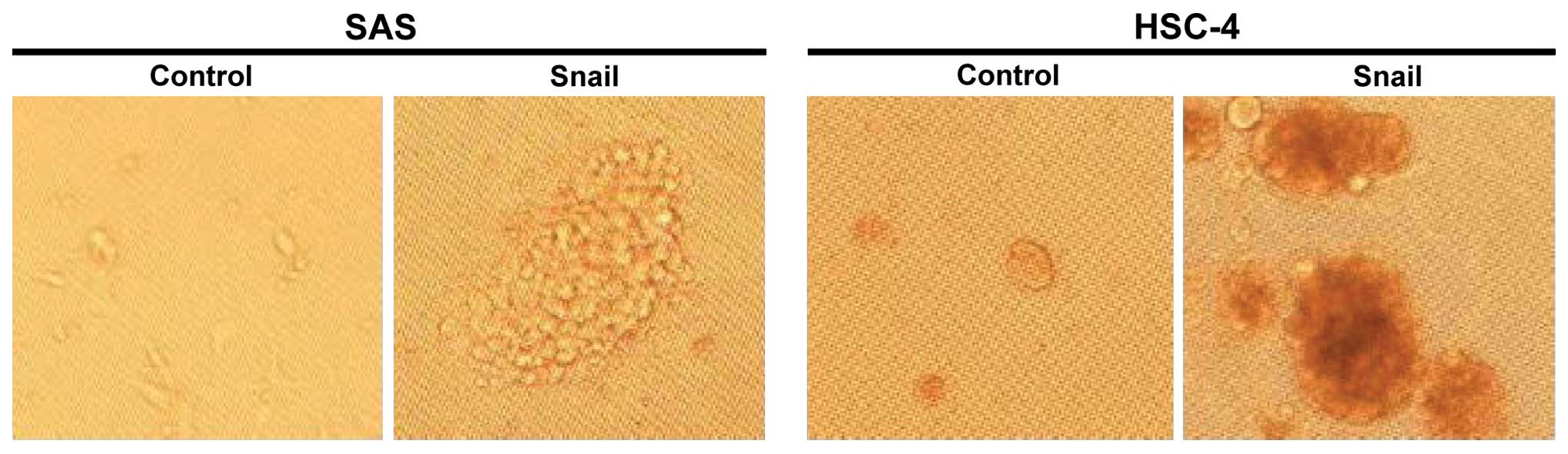
Snail induces CSC properties
The transfection of Snail induced tumor
sphere-forming capability in the SAS and HSC-4 cells, but not in
the control cells (Fig. 2).
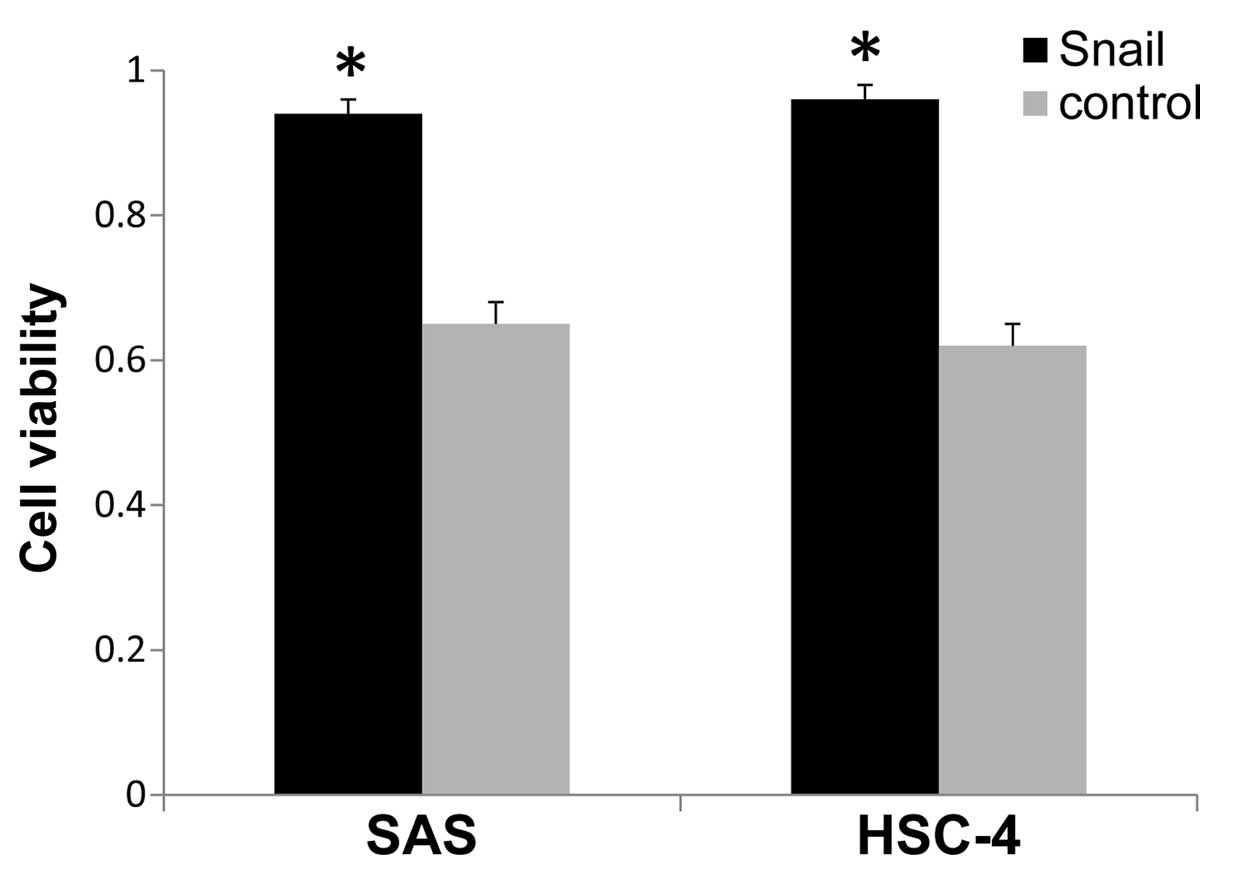
Furthermore, the cells transfected with Snail showed significantly
low chemosensitivity at 1.0 µM of cisplatin, as compared
with the control cells (Fig. 3).
Thus, these data revealed that the acquisition of a CSC-like
phenotype caused by Snail-induced EMT results in enhancement of the
ability of sphere formation and chemoresistance in the HNSCC
cells.
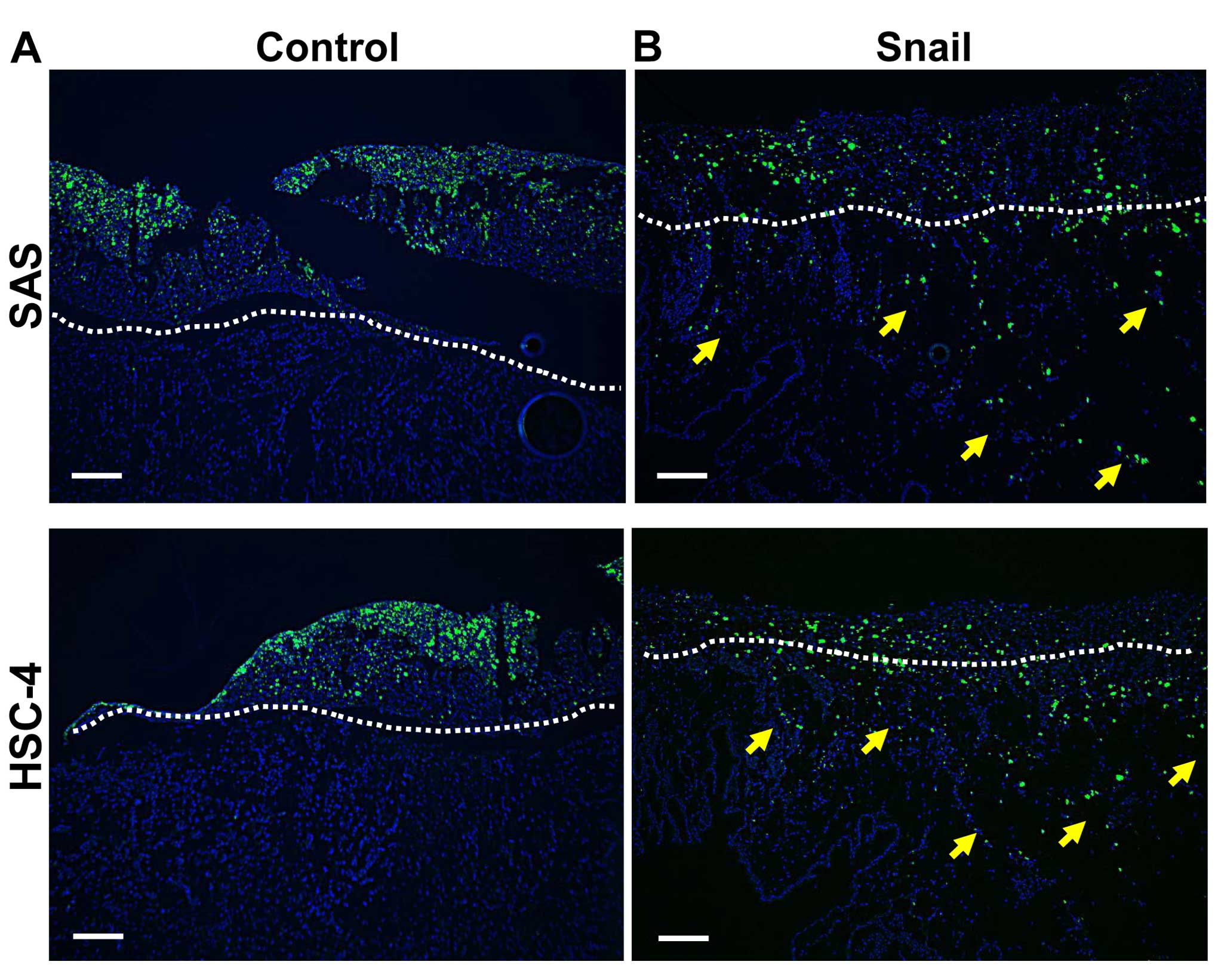
Snail induces EMT as a CSC property in
vivo
We previously demonstrated that Snail-regulated EMT
promotes CSC properties, including cell migration and invasion as
well as E-cadherin suppression in HNSCC cells in vitro
(22). Snail was also functional in
inducing in vivo cancer invasive progression of non-invasive
SAS and HSC-4 cells, as noted in the crossing of cells into the BM
in the CAM assay (Fig. 4).
Discussion
The identification of cell surface-specific markers
of CSCs is critical for the possible establishment of
target-specific cancer therapies. CD44 (10) and ALDH1 (11) have been reported to represent
candidate markers of CSCs in HNSCC. Recently, CD271 (26) and CD10 (27) have been identified as additional
markers of CSCs in HNSCC. However, it is difficult to extract CSCs
selectively with such markers only. Several studies have indicated
that there could be key markers of CSCs that regulate stemness
genes, such as Bmi1, Nanog, Oct4, Sox2 and ABCG2 as follows.
Bmi1 has been demonstrated to play a role in the
tumorigenesis of HNSCC (28,29).
Expression of Bmi1, ALDH1 and Snail could be associated with the
maintenance of stemness in CSCs and correlate with poor overall
survival in HNSCC patients (30).
Additionally, Bmi1 can regulate Snail and ALDH1 in inducing EMT and
CSC properties (30). In present
study, we obtained results consistent with those in our previous
study (22).
Nanog maintains the pluripotency of embryonic stem
cells and functionally blocks differentiation (31,32).
Recent studies have demonstrated that Nanog promotes CSC
properties, and the downregulation of Nanog inhibits sphere
formation and tumor development (33–36).
Another study suggested that Nanog was upregulated by TGF-β through
Smad signaling and that Snail directly regulates Nanog promoter
activity (37). Nanog expression
was also upregulated in the present study, whereas there were no
significantly differences in the expression of Oct4 and Sox2
between Snail-transfected cells and the controls. A previous study
showed that Oct4 and Sox2 regulate Nanog expression (38). Therefore, direct regulation against
the Nanog promoter by Snail may result in the suppression of Oct4
and Sox2.
Yoshikawa et al showed that the expression of
EGFR was low in CSCs, but high in non-CSCs. They suggested that a
high level of expression of variant isoforms of CD44 (CD44v) could
suppress the clustering of EGFR at the surface of HNSCC cells and
thereby negatively regulate EGFR signaling in the absence of a
differentiation stimulus, and that CD44v-negative HNSCC cells rely
on EGFR activity for survival (13). It has been suggested that the
suppression of differentiation and treatment resistance in CSCs
could be attributed to CD44v-positive and EGFR-negative expression.
In the present study, Snail overexpression induced CSC-like
properties, while there was no significantly difference in the
expression of EGFR between the Snail-transfected cells and the
controls. Furthermore, the expression of phospho-EGFR was enhanced
in the Snail-transfected cells compared with the controls. However,
this result is considered to be compatible with the findings in the
previous study. It was predicted from the present study that the
CSC-like properties acquired by Snail occurred not in all cells,
but in a portion of them. This raises the possibility that
upregulation of phospho-EGFR occurred in non-CSCs around CSCs. Wang
et al showed that reduction in E-cadherin resulted in
upregulation of EGFR transcriptionally and activation of EGFR
resulted in overexpression of Snail (39). It was suggested that Snail could not
only induce CSC-like properties, but also phosphorylates EGFR in
non-CSCs, and it contributed to the maintenance of the
microenvironment by interaction between CSCs and non-CSCs. Although
the precise involvement of CD44 and EGFR expression by Snail in the
regulation of CSCs remains to be elucidated, they may be involved
in a latent effect. The critical mechanisms remain to be further
investigated. The majority of studies on CSCs have been performed
in vitro to evaluate clinical cancer therapies. Therefore,
it should be noted that the results of in vitro studies on
CSCs cannot be translated to the same cells in vivo.
Although it is still too early to discuss its clinical efficiency,
these data could support the hypothesis that the interaction
between CSCs and non-CSCs contributes to cell proliferation and
treatment resistance.
In addition, the upregulation of ABCG2 expression
was also observed in the Snail-transfected cells. However, higher
expression of ABCG2 was observed not in ALDH1+ cells,
but in ALDH cells around CSCs (40). This suggests that ABCG2 itself is
not a CSC marker, but the interaction between CSCs and the cells
around CSCs results in upregulation of ABCG2. The upregulation of
ABCG2 in the present study could similarly support the possibility
that Snail contributes to the interaction between CSCs and
non-CSCs.
Furthermore, we first showed that Snail regulates
cancer cell invasion through EMT as a CSC-like property in HNSCC
cells in vivo as well as in vitro (22). These data suggest that Snail can
promote cancer invasion and metastasis as well as maintain the
stemness similar to CSCs.
In summary, we demonstrated the possibility that
Snail induces CSC-like properties through EMT and maintains
stemness by upregulating various CSC markers. However, other
transcription factors of EMT as well as Snail could be involved in
the maintenance of CSCs and the microenvironment. These
interactions between various CSC- and EMT-relating genes make it
difficult to select a pure CSC population. Snail-induced EMT is
also considered to play an essential role in tumor progression,
such as cancer invasion and metastasis in vivo. Although the
critical mechanisms remain to be further investigated, Snail could
prove to be one of the valid targets in the treatment for
HNSCC.
Acknowledgments
The present study was supported in part by Grants-in
Aids for Scientific Research from the Ministry of Education,
Culture, Sports, Science and Technology of Japan.
References
|
1
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hay ED: The mesenchymal cell, its role in
the embryo, and the remarkable signaling mechanisms that create it.
Dev Dyn. 233:706–720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsu DS, Lan HY, Huang CH, Tai SK, Chang
SY, Tsai TL, Chang CC, Tzeng CH, Wu KJ, Kao JY, et al: Regulation
of excision repair cross-complementation group 1 by Snail
contributes to cisplatin resistance in head and neck cancer. Clin
Cancer Res. 16:4561–4571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chiou SH, Kao CL, Chen YW, Chien CS, Hung
SC, Lo JF, Chen YJ, Ku HH, Hsu MT and Wong TT: Identification of
CD133-positive radioresistant cells in atypical teratoid/rhabdoid
tumor. PLoS One. 3:e20902008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen YC, Chang CJ, Hsu HS, Chen YW, Tai
LK, Tseng LM, Chiou GY, Chang SC, Kao SY, Chiou SH, et al:
Inhibition of tumorigenicity and enhancement of
radiochemosensitivity in head and neck squamous cell cancer-derived
ALDH1-positive cells by knockdown of Bmi-1. Oral Oncol. 46:158–165.
2010. View Article : Google Scholar
|
|
7
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang AD, Fan F, Camp ER, van Buren G, Liu
W, Somcio R, Gray MJ, Cheng H, Hoff PM and Ellis LM: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prince ME, Sivanandan R, Kaczorowski A,
Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF and Ailles
LE: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen YC, Chen YW, Hsu HS, Tseng LM, Huang
PI, Lu KH, Chen DT, Tai LK, Yung MC, Chang SC, et al: Aldehyde
dehydrogenase 1 is a putative marker for cancer stem cells in head
and neck squamous cancer. Biochem Biophys Res Commun. 385:307–313.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
La Fleur L, Johansson AC and Roberg K: A
CD44high/EGFRlow subpopulation within head
and neck cancer cell lines shows an epithelial-mesenchymal
transition phenotype and resistance to treatment. PLoS One.
7:e440712012. View Article : Google Scholar
|
|
13
|
Yoshikawa M, Tsuchihashi K, Ishimoto T,
Yae T, Motohara T, Sugihara E, Onishi N, Masuko T, Yoshizawa K,
Kawashiri S, et al: xCT inhibition depletes CD44v-expressing tumor
cells that are resistant to EGFR-targeted therapy in head and neck
squamous cell carcinoma. Cancer Res. 73:1855–1866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burkert J, Otto WR and Wright NA: Side
populations of gastrointestinal cancers are not enriched in stem
cells. J Pathol. 214:564–573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moody SE, Perez D, Pan TC, Sarkisian CJ,
Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD and
Chodosh LA: The transcriptional repressor Snail promotes mammary
tumor recurrence. Cancer Cell. 8:197–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Debies MT, Gestl SA, Mathers JL, Mikse OR,
Leonard TL, Moody SE, Chodosh LA, Cardiff RD and Gunther EJ: Tumor
escape in a Wnt1-dependent mouse breast cancer model is enabled by
p19Arf/p53 pathway lesions but not p16Ink4a
loss. J Clin Invest. 118:51–63. 2008. View
Article : Google Scholar
|
|
18
|
Kudo-Saito C, Shirako H, Takeuchi T and
Kawakami Y: Cancer metastasis is accelerated through
immunosuppression during Snail-induced EMT of cancer cells. Cancer
Cell. 15:195–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vincent T, Neve EP, Johnson JR, Kukalev A,
Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL,
et al: A SNAIL1-SMAD3/4 transcriptional repressor complex promotes
TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol.
11:943–950. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yokoyama K, Kamata N, Hayashi E, Hoteiya
T, Ueda N, Fujimoto R and Nagayama M: Reverse correlation of
E-cadherin and snail expression in oral squamous cell carcinoma
cells in svitro. Oral Oncol. 37:65–71. 2001. View Article : Google Scholar
|
|
21
|
Peinado H, Ballestar E, Esteller M and
Cano A: Snail mediates E-cadherin repression by the recruitment of
the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell
Biol. 24:306–319. 2004. View Article : Google Scholar :
|
|
22
|
Masui T, Ota I, Yook JI, Mikami S, Yane K,
Yamanaka T and Hosoi H: Snail-induced epithelial-mesenchymal
transition promotes cancer stem cell-like phenotype in head and
neck cancer cells. Int J Oncol. 44:693–699. 2014.
|
|
23
|
Hayashi K, Motoyama S, Sugiyama T, Izumi
J, Anbai A, Nanjo H, Watanabe H, Maruyama K, Minamiya Y, Koyota S,
et al: REG Ialpha is a reliable marker of chemoradiosensitivity in
squamous cell esophageal cancer patients. Ann Surg Oncol.
15:1224–1231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim
NH, Cha SY, Ryu JK, Choi YJ, Kim J, et al: A Wnt-Axin2-GSK3beta
cascade regulates Snail1 activity in breast cancer cells. Nat Cell
Biol. 8:1398–1406. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ota I, Li XY, Hu Y and Weiss SJ: Induction
of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration
program in cancer cells by Snail1. Proc Natl Acad Sci USA.
106:20318–20323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Imai T, Tamai K, Oizumi S, Oyama K,
Yamaguchi K, Sato I, Satoh K, Matsuura K, Saijo S, Sugamura K, et
al: CD271 defines a stem cell-like population in hypopharyngeal
cancer. PLoS One. 8:e620022013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fukusumi T, Ishii H, Konno M, Yasui T,
Nakahara S, Takenaka Y, Yamamoto Y, Nishikawa S, Kano Y, Ogawa H,
et al: CD10 as a novel marker of therapeutic resistance and cancer
stem cells in head and neck squamous cell carcinoma. Br J Cancer.
111:506–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brunner M, Thurnher D, Pammer J, Geleff S,
Heiduschka G, Reinisch CM, Petzelbauer P and Erovic BM: Expression
of VEGF-A/C, VEGF-r2, PDGF-alpha/beta, c-kit, EGFR, Her-2/Neu,
Mcl-1 and Bmi-1 in Merkel cell carcinoma. Mod Pathol. 21:876–884.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang P, Zhang Y, Mao L, Zhang Z and Chen
W: Side population in oral squamous cell carcinoma possesses tumor
stem cell phenotypes. Cancer Lett. 277:227–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu CC, Lo WL, Chen YW, Huang PI, Hsu HS,
Tseng LM, Hung SC, Kao SY, Chang CJ and Chiou SH: Bmi-1 regulates
Snail expression and promotes metastasis ability in head and neck
squamous cancer-derived ALDH1 positive cells. J Oncol. 2011:pii:
609259. 2011. View Article : Google Scholar
|
|
31
|
Chambers I, Colby D, Robertson M, Nichols
J, Lee S, Tweedie S and Smith A: Functional expression cloning of
Nanog, a pluripotency sustaining factor in embryonic stem cells.
Cell. 113:643–655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abelev GI and Lazarevich NL: Control of
differentiation in progression of epithelial tumors. Adv Cancer
Res. 95:61–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jeter Cr, Badeaux M, Choy G, Chandra D,
Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ and Tang
DG: Functional evidence that the self-renewal gene NANOG regulates
human tumor development. Stem Cells. 27:993–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jeter CR, Liu B, Liu X, Chen X, Liu C,
Calhoun-Davis T, Repass J, Zaehres H, Shen JJ and Tang DG: NANOG
promotes cancer stem cell characteristics and prostate cancer
resistance to androgen deprivation. Oncogene. 30:3833–3845. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong
CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, et al:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen C, Wei Y, Hummel M, Hoffmann TK,
Gross M, Kaufmann AM and Albers AE: Evidence for
epithelial-mesenchymal transition in cancer stem cells of head and
neck squamous cell carcinoma. PLoS One. 6:e164662011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dang H, Ding W, Emerson D and Rountree CB:
Snail1 induces epithelial-to-mesenchymal transition and tumor
initiating stem cell characteristics. BMC Cancer. 11:3962011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Niwa H: Molecular mechanism to maintain
stem cell renewal of ES cells. Cell Struct Funct. 26:137–148. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang D, Su L, Huang D, Zhang H, Shin DM
and Chen ZG: Downregulation of E-Cadherin enhances proliferation of
head and neck cancer through transcriptional regulation of EGFR.
Mol Cancer. 10:1162011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Emmink BL, Van Houdt WJ, Vries RG,
Hoogwater FJ, Govaert KM, Verheem A, Nijkamp MW, Steller EJ,
Jimenez CR, Clevers H, et al: Differentiated human colorectal
cancer cells protect tumor-initiating cells from irinotecan.
Gastroenterology. 141:269–278. 2011. View Article : Google Scholar : PubMed/NCBI
|