Introduction
Psoriasis is a common chronic skin disease that is
characterized by hyperproliferation and disrupted differentiation
of keratinocytes (1–3). Psoriasis leads to a decrease in
apoptotic cell death in keratinocytes and an increased resistance
of intralesional keratinocytes to apoptosis (4). We studied the inhibition of
keratinocyte proliferation and the regulation of keratinocyte
differentiation by induced apoptosis for the treatment of
psoriasis.
Resveratrol
(3,5,4′-trihydroxy-trans-stilbene) is a natural polyphenol,
mainly found in grapes, berries and red wine (5). There are several studies on the
bioactivities of resveratrol including anti-inflammatory,
antioxidant, antimicrobial and neuroprotective effects (6).
Additionally, resveratrol has strong chemopreventive
effects against skin, breast, prostate and lung tumors (7). The cancer preventive effects of
resveratrol were demonstrated in the prevention of tumor growth in
an animal model (8). Resveratrol is
reportedly an activator of Sirt1 both in vivo and in
vitro (9,10).
Silent information regulator 2 (Sir2) homolog 1
(Sirt1) is a member of the conserved family of NAD-dependent
deacetylases, the sirtuin family, that function in enzymatic
cleavage of NAD to the deacetylation of protein substrates. Sirt1
plays a critical role in the regulation of numerous cellular
processes, including cell cycle progression, nutrient metabolism,
cellular ageing (11) and cell
apoptosis (12). Sirt1 has nuclear
substrates such as p53, and the DNA repair proteins APE/Ref1 and
PARP1 (13–15). Resveratrol was found to inhibit cell
proliferation and differentiation by increasing the expression of
Sirt1 mRNA (16).
The serine/threonine kinase Akt, also known as
protein kinase B (PKB), is a downstream effector of
phosphatidylinositol 3-kinase (PI3K), activated by several stimuli,
such as growth factor stimulation, stress or protein phosphatase
inhibitors, in a PI3K-dependent manner (17). AKT is involved in multiple cellular
signaling pathways and functions as a transducer of many actions
initiated by growth factors and other receptors that activate PI3K
(18–20). One of the major roles of Akt is to
regulate cell survival (18,21),
including apoptosis and tumorigenesis as an oncogene (22). Recent studies have shown that Sirt1
inactivates Akt and suppresses survivin expression and the
subsequently activated mitochondrion-mediated pathway, including
MMP activity, cytochrome c release and caspase activation
(23).
The novel finding of the present study was that
resveratrol induced HaCaT keratinocyte cell death. We investigated
the role of resveratrol on the apoptosis of human HaCaT
keratinocytes and clarified the mechanisms. Resveratrol was
associated with increased expression and activation of Sirt1, by
enhancing the deacetylation of p53, which downregulated Akt
phosphorylation. The results indicated that resveratrol-induced
HaCaT keratinocyte cell death may be regulated by a
Sirt1/phospho-Akt-dependent signaling pathway.
Materials and methods
Cell culture
HaCaT, an immortalized, non-tumorigenic human
keratinocyte cell line was maintained in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS) and 1% antibiotics under standard conditions (37°C, 5%
CO2, in a humidified incubator). HaCaT cells were seeded
in 60-mm culture dishes in standard medium, or in the presence of
different concentrations of resveratrol (25 and 100 µM) for
the proliferation studies. Triplicate dishes were trypsinized at
appropriate intervals, and the cell number was determined by
counting cell suspension in a Neubauer hemacytometer. The values
reported represent the mean ± SEM of 3 independent samples per each
experimental point.
Crystal violet assay
Cell viability was evaluated by crystal violet
staining. Briefly, the cells were stained with staining solution
(0.5% crystal violet in 30% ethanol and 3% formaldehyde) for 10 min
at room temperature (RT) and washed 4 times with water. The stained
cells were lysed with 1% sodium dodecyl sulfate (SDS), and the
absorbance was measured at 550 nm. Cell viability was calculated
based on the relative dye intensity compared with the controls.
Lactate dehydrogenase (LDH) assay
Cytotoxicity was assessed in the supernatants using
the LDH cytotoxicity detection kit (Takara Bio, Tokyo, Japan)
according to the manufacturer's protocol. LDH activity was
determined by measuring the absorbance at 490 nm using a SpectraMax
M2 microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Annexin V assay
Apoptosis was assessed in detached cells with an
Annexin V assay kit (Santa Cruz Biotechnology, Santa Cruz, CA,
USA), according to the manufacturer's protocol. Annexin V levels
were determined by measuring fluorescence at a 488-nm excitation
and a 525/30 emission using the guava easyCyte HT System
(Millipore, Bedford, MA, USA).
Western blot analysis
HaCaT cells were lysed in buffer [25 mM HEPES, 100
mM NaCl, 1 mM EDTA, 5 mM MgCl2, 0.1 mM DTT
(dithiothreitol) and a protease inhibitor mixture at pH 7.4].
Proteins were electrophoretically resolved by 10–15% sodium dodecyl
sulfate polyacrylamide gel electrophoresis and transferred to a
nitrocellulose membrane. Immunoreactivity was detected through
sequential incubations with horseradish peroxidase-conjugated
secondary antibodies and enhanced chemiluminescence reagents. The
antibodies used for immunoblotting were Sirt1 (Santa Cruz
Biotechnology), phospho-Akt, acetyl-p53 (both from Epitomics,
Burlingame, CA, USA) and β-actin (Sigma-Aldrich, St. Louis, MO,
USA). Images were examined using a Fusion-FX7 imaging system
(Vilber Lourmat, Marne-la-Vallée, France).
Construction of recombinant
adenoviruses
The Sirt1-overexpressing adenovirus (Ad-Sirt1) was
provided by Professor Byung-Hyun Park of Chonbuk National
University (Jeonju, Jeonbuk, Korea). The lacZ-bearing adenovirus
(Ad-lacZ) was used as the control. Recombinant adenoviruses were
amplified in human embryonic kidney (HEK)-293 cells and purified
using the Vivapure AdenoPACK kit (Sartorius AG, Göttingen, Germany)
according to the manufacturer's instructions.
Statistical evaluation
All data are expressed as mean ± standard deviation
and were compared by Student's t-test, analysis of variance and
Duncan's test using SAS statistical software version 9.1 (SAS
Institute, Cary, NC, USA). Results were considered significant at
p<0.05, p<0.001 or p<0.01, as appropriate.
Results
Resveratrol induces cell damage in HaCaT
keratinocytes
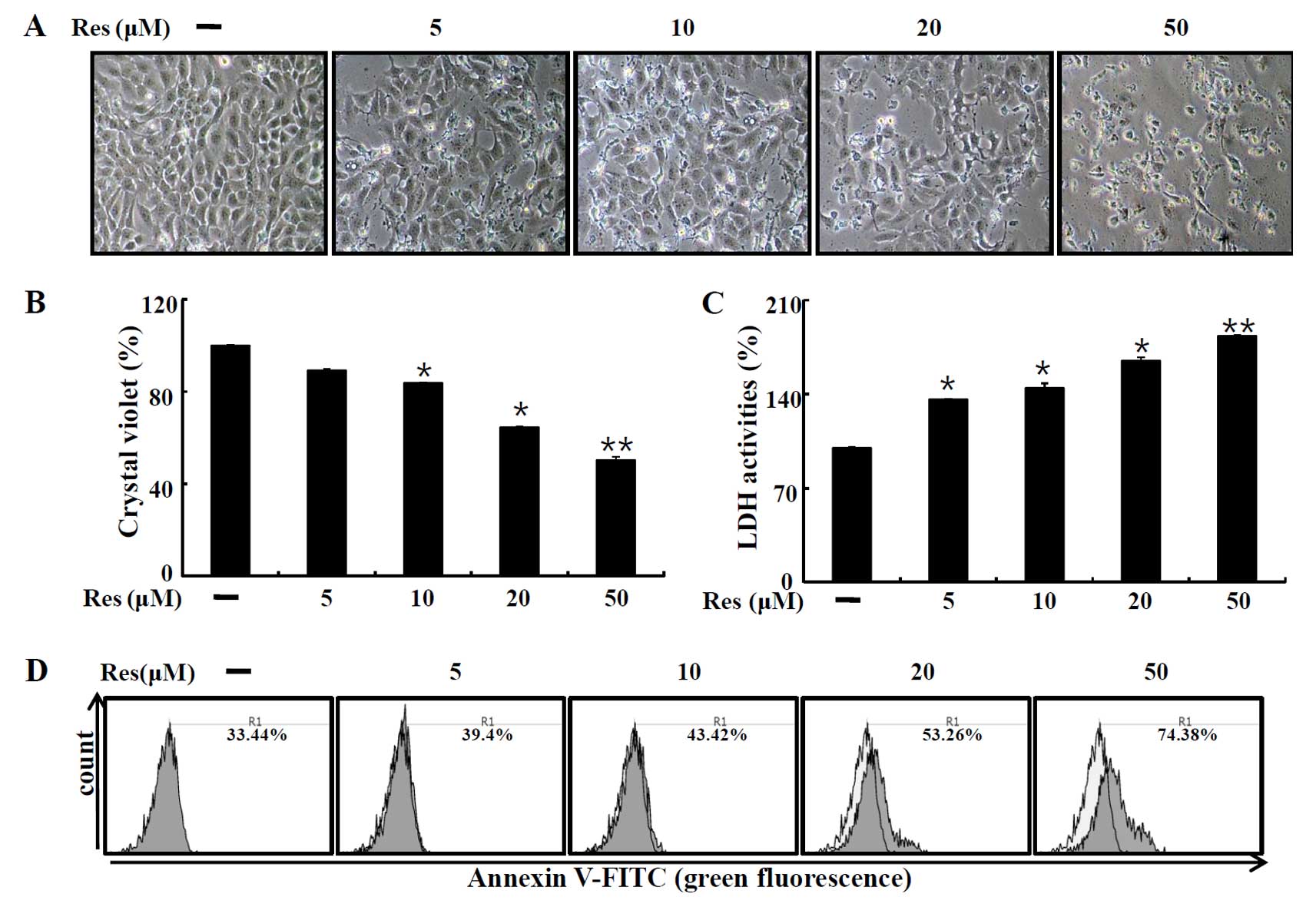
We first examined the influence of resveratrol on
the cell viability of human HaCaT cells to determine whether
resveratrol induces cell death or promotes cell proliferation and
cell survival. Cells were exposed to different concentrations (5,
10, 20 and 50 µM) of resveratrol for 12 h. After resveratrol
treatment, based on the cell morphology and the number of cells by
microscopy and crystal violet assay, we determined that resveratrol
induced cell death (Fig. 1A and B).
We next performed the LDH assay for detecting cell death. As shown
in Fig. 1C, resveratrol treatment
significantly increased LDH release. The Annexin V-positive cell
population was also increased following resveratrol treatment,
compared to that of the control (Fig.
1D). These data indicated that resveratrol induced HaCaT cell
death.
Resveratrol induces cell death through
the Sirt1 pathway
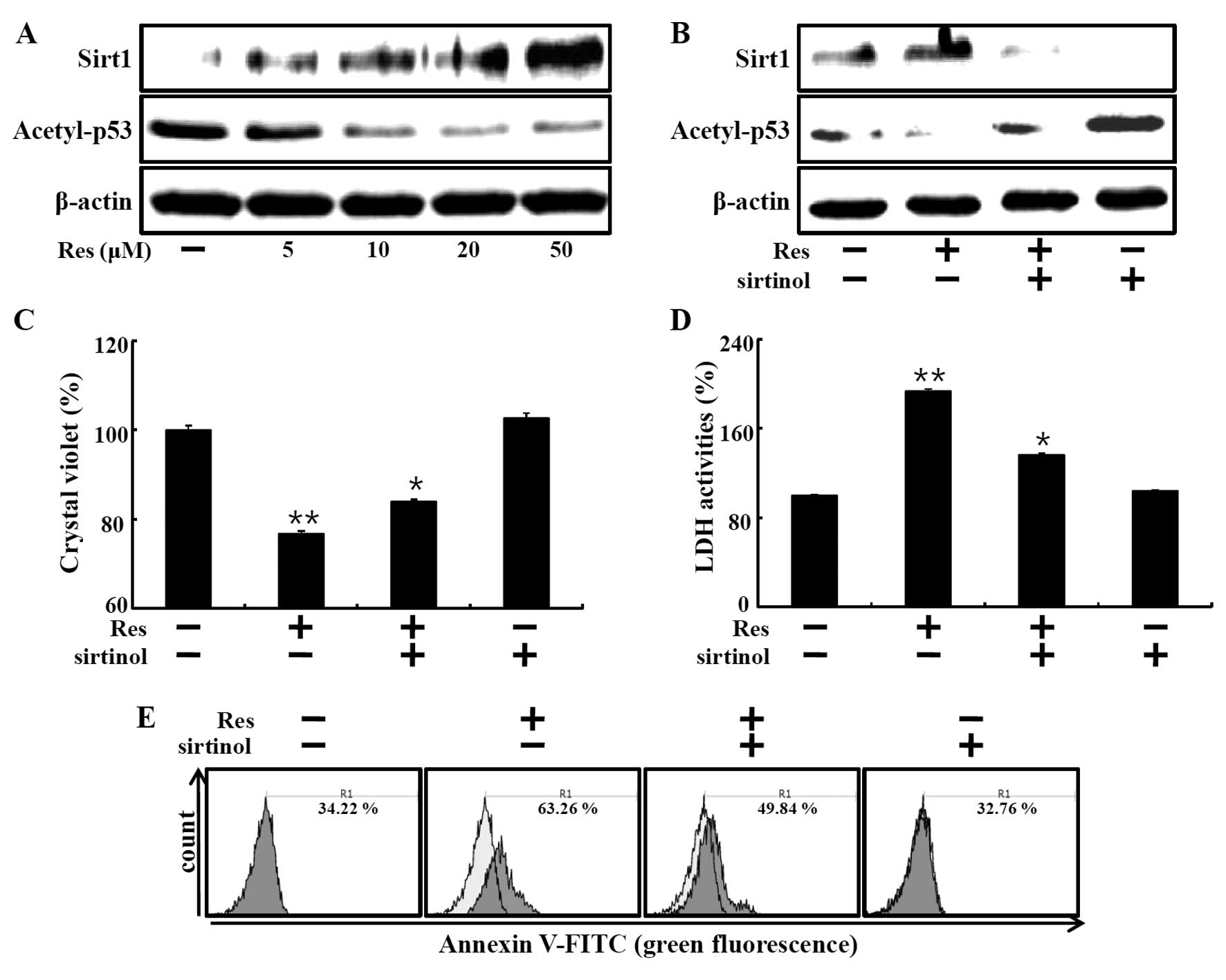
We examined the Sirt1 protein levels in the HaCaT
keratinocytes treated with resveratrol to understand the molecular
mechanism of resveratrol in human keratinocytes. Resveratrol
increases the deacetylase activities of Sirt1. Hence, we
investigated whether resveratrol induced cell death by increasing
Sirt1. First, we evaluated Sirt1 expression following resveratrol
treatment at concentrations of 5, 10, 20 and 50 µM. Sirt1
protein levels dose-dependently increased in the
resveratrol-treated group as compared to the control group
(Fig. 2A). We next determined
whether resveratrol-induced Sirt1 had deacetylase activity by
measuring the acetylated p53 protein levels. Acetyl-p53 protein
levels were gradually decreased (Fig.
2A). Moreover, sirtinol, Sirt1 inhibitor, inhibited the
resveratrol-induced increase in Sirt1 protein and decrease in
acetyl-p53 protein (Fig. 2B).
Next, to determine whether resveratrol-induced Sirt1
activation is related to cell death, HaCaT keratinocytes were
treated with resveratrol with or without sirtinol. Crystal violet
assay showed that resveratrol induced cell death and sirtinol
restored cell viability (Fig. 2C).
Consistent with these results, resveratrol-treated cells exhibited
increased LDH release, while treatment with sirtinol blocked the
effects of resveratrol (Fig. 2D).
The influence of sirtinol on resveratrol-induced apoptosis in HaCaT
keratinocytes was determined by the Annexin V assay (Fig. 2E). The resveratrol-mediated increase
in Annexin V-positive cells was decreased by treatment with
sirtinol. These results demonstrated that resveratrol induced cell
death via Sirt1.
Resveratrol-mediated cell death is
associated with Akt phosphorylation
The effect of resveratrol on apoptotic proteins and
survival signals was investigated by measuring the expression of
Akt phosphorylation by western blot analysis. The results showed
that resveratrol inhibited Akt activation dose-dependently
(Fig. 3A and B). Akt inhibition by
exposure to different concentrations of wortmannin (0.5, 1, 2 and 4
µM) also decreased the phospho-Akt protein levels (Fig. 3C and D). We evaluated the released
LDH activity in the media of the HaCaT keratinocytes treated with
wortmannin alone. The data showed that wortmannin dose-dependently
increased LDH release (Fig.
3E).
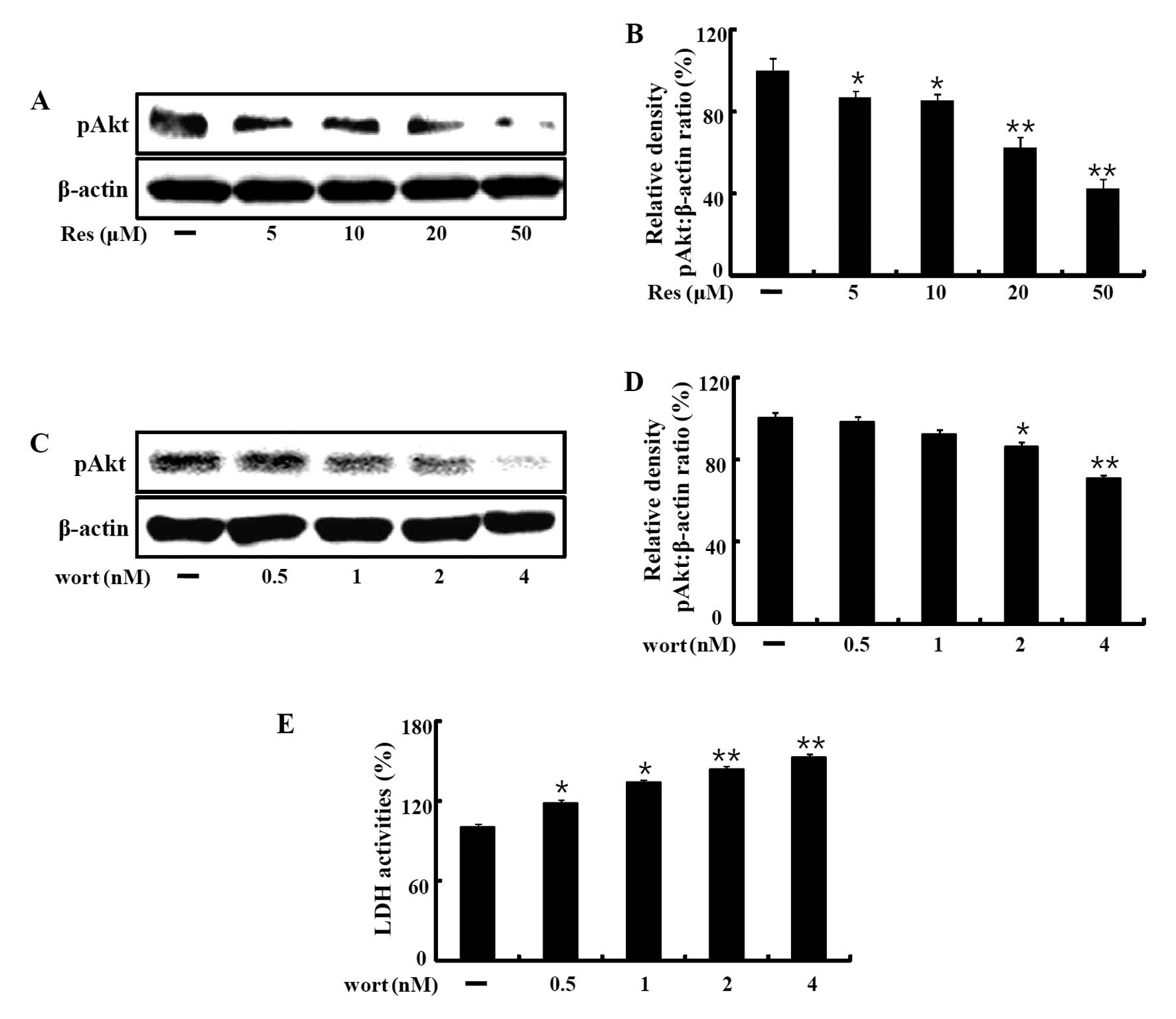 | Figure 3Resveratrol induces cell death through
inhibition of Akt phosphorylation. (A and B) HaCaT keratinocytes
were incubated with the indicated concentrations of resveratrol (5,
10, 20 and 50 µM) for 12 h. The treated cells were assessed
for phospho-Akt by western blot analysis. (C and D) HaCaT
keratinocytes were incubated with wortmannin, an Akt inhibitor, for
12 h at increasing concentrations (5, 10, 20 and 50 µM).
Western blot analyses for phospho-Akt were performed on HaCaT
keratinocytes. β-actin was used as the loading control. (E) LDH
assay was used to quantify LDH release into the medium. Bar graphs
indicates the mean ± SEM (n=3). *p<0.05,
**p<0.01, significant differences between the control
and each treatment group. Res, resveratrol; Wort, wortmannin. |
Resveratrol-induced Sirt1 increase leads
to cell death through Akt phosphorylation
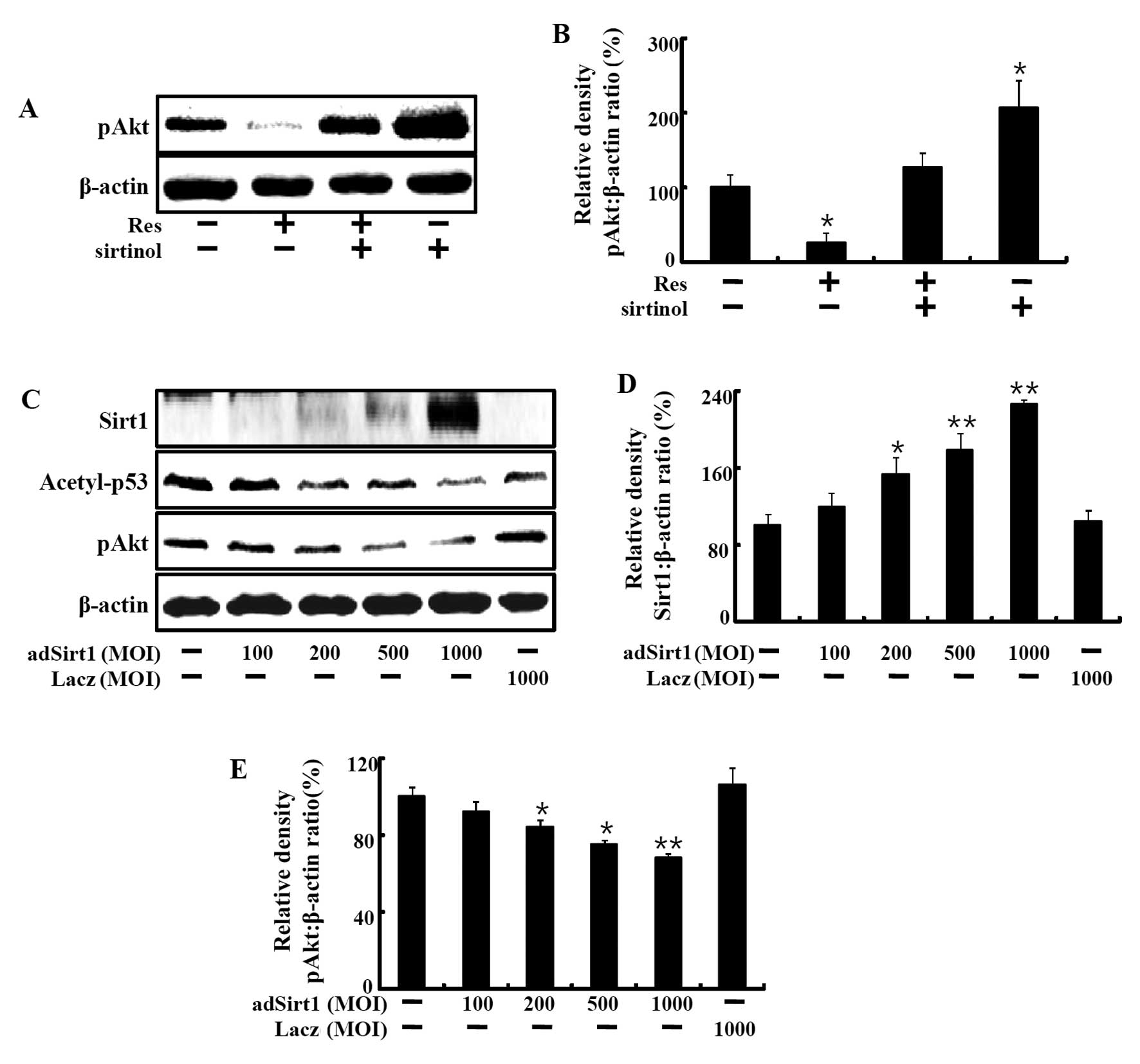
Next, we investigated whether resveratrol-induced
Sirt1 activation is involved in Akt phosphorylation. HaCaT
keratinocytes were treated with resveratrol with or without
sirtinol. Resveratrol decreased phospho-Akt protein expression at
50 µM, and this effect was restored by the Sirt1 inhibitor,
sirtinol (Fig. 4A and B). We next
treated the cells with an adenovirus of Sirt1 and found that AKT
phosphorylation was decreased dose-dependently. Cells were
transfected with adenoviruses expressing Sirt1 at a multiplicity of
infection (MOI) of 100, 200, 500 and 1,000 pfu/cell to directly
evaluate the role of Sirt1 in HaCaT keratinocytes.
Sirt1-overexpressing cells exhibited increased Sirt1 protein levels
and a markedly decrease in acetyl-p53 protein. Overexpression of
Sirt1 led to a decrease in phospho-Akt protein levels (Fig. 4C–E). Sirt1, acetyl-p53 and
phospho-Akt expression in Ad-LacZ-infected cells was not changed
when compared to the levels in the control cells. These results
showed that resveratrol-induced Sirt1 upregulation led to cell
death of HaCaT keratinocytes through phospho-Akt.
Discussion
Psoriasis is a chronic inflammatory skin disease
involving hyperproliferation and abnormal differentiation of
epidermal keratinocytes. Several therapies for mild psoriasis are
currently available (24). However,
due to the complex nature of psoriasis, most therapies have adverse
side-effects. Apoptosis is a physiological process in the skin to
regulate keratinocyte proliferation and epidermal growth. Lack of
apoptosis leads to increase in anti-apoptotic proteins in the skin
that cause psoriasis (24). We
investigated an effective therapy to inhibit hyperproliferation and
induce apoptosis in keratinocytes for psoriasis treatment.
Resveratrol
(3,5,4′-trihydroxy-trans-stilbene), a natural phenol known
as a stilbenoid (25), was found to
prevent inflammation and apoptosis of human and murine primary
keratinocytes against UVB irradiation or H2O2
(26–28). However, resveratrol exhibited
differential responses in HaCaT and primary human keratinocytes.
Resveratrol sensitized UV-induced apoptosis in HaCaT keratinocytes
(29). Cellular apoptosis is a main
mechanism for blocking proliferation of cultured cells, hence we
investigated whether resveratrol alone promoted apoptosis in HaCaT
keratinocytes.
Resveratrol has attracted much attention for its
ability to enhance the deacetylase activity of Sirt1 (30). Sirt1 is an NAD-dependent histone
deacetylase that is a major regulator of multiple biological
processes, including the stress response, apoptosis, the regulation
of gene transcription and the cell cycle (12,31).
Although Sirt1 mainly has protective effects against apoptosis in
many cells, some cells undergo cell death by Sirt1 expression and
activation (23,32). We showed that the
resveratrol-mediated increase in Sirt1 led to cell death in HaCaT
keratinocytes.
Numerous studies suggest that Akt plays a critical
role in tumorigenesis and cell survival. Resveratrol reportedly
inhibits skin tumorigenesis through PI3K and Akt, proteins that are
implicated in cancer development and progression (33). Akt phosphorylation involved in the
apoptotic process raised the possibility that Akt regulates cell
survival by directly phosphorylating components of the cell death
pathway (21). We found that
resveratrol inhibited phospho-Akt and the inhibition of PI3K by
wortmannin-mediated resveratrol-induced apoptosis in HaCaT
keratinocytes. Our data indicated that resveratrol inhibited cell
proliferation and induced apoptosis through the inactivation of Akt
signaling. Collectively, the present study demonstrated that
resveratrol regulated cell death in HaCaT keratinocytes via
Sirt1/phospho-Akt-mediated signaling pathways. Additionally, these
findings suggested that resveratrol may be used as a potential
therapeutic agent for psoriasis.
Acknowledgments
The present study was supported by the global Ph.D.
Fellowship Program through the National Research Foundation of
Korea (NRF) funded by the Ministry of Education (2014H1A2A1021117
and 2013R1A1A2063931).
References
|
1
|
Wrone-Smith T, Mitra RS, Thompson CB,
Jasty R, Castle VP and Nickoloff BJ: Keratinocytes derived from
psoriatic plaques are resistant to apoptosis compared with normal
skin. Am J Pathol. 151:1321–1329. 1997.PubMed/NCBI
|
|
2
|
Lebwohl M: Psoriasis. Lancet.
361:1197–1204. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nickoloff BJ and Nestle FO: Recent
insights into the immunopathogenesis of psoriasis provide new
therapeutic opportunities. J Clin Invest. 113:1664–1675. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ortonne N, Ram-Wolff C, Giustiniani J,
Marie-Cardine A, Bagot M, Mecheri S and Bensussan A: Human and
mouse mast cells express and secrete the GPI-anchored isoform of
CD160. J Invest Dermatol. 131:916–924. 2011. View Article : Google Scholar
|
|
5
|
Dolinsky VW and Dyck JR: Calorie
restriction and resveratrol in cardiovascular health and disease.
Biochim Biophys Acta. 1812:1477–1489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frémont L: Biological effects of
resveratrol. Life Sci. 66:663–673. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: Preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.PubMed/NCBI
|
|
8
|
Corpet DE and Pierre F: Point: From animal
models to prevention of colon cancer. Systematic review of
chemoprevention in min mice and choice of the model system. Cancer
Epidemiol Biomarkers Prev. 12:391–400. 2003.PubMed/NCBI
|
|
9
|
Borra MT, Smith BC and Denu JM: Mechanism
of human SIRT1 activation by resveratrol. J Biol Chem.
280:17187–17195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Howitz KT, Bitterman KJ, Cohen HY, Lamming
DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL,
et al: Small molecule activators of sirtuins extend Saccharomyces
cerevisiae lifespan. Nature. 425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blander G and Guarente L: The Sir2 family
of protein deacetylases. Annu Rev Biochem. 73:417–435. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo J, Nikolaev AY, Imai S, Chen D, Su F,
Shiloh A, Guarente L and Gu W: Negative control of p53 by Sir2alpha
promotes cell survival under stress. Cell. 107:137–148. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rajamohan SB, Pillai VB, Gupta M,
Sundaresan NR, Birukov KG, Samant S, Hottiger MO and Gupta MP:
SIRT1 promotes cell survival under stress by
deacetylation-dependent deactivation of poly(ADP-ribose) polymerase
1. Mol Cell Biol. 29:4116–4129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamori T, DeRicco J, Naqvi A, Hoffman
TA, Mattagajasingh I, Kasuno K, Jung SB, Kim CS and Irani K: SIRT1
deacetylates APE1 and regulates cellular base excision repair.
Nucleic Acids Res. 38:832–845. 2010. View Article : Google Scholar :
|
|
16
|
Bai L, Pang WJ, Yang YJ and Yang GS:
Modulation of Sirt1 by resveratrol and nicotinamide alters
proliferation and differentiation of pig preadipocytes. Mol Cell
Biochem. 307:129–140. 2008. View Article : Google Scholar
|
|
17
|
Testa JR and Bellacosa A: AKT plays a
central role in tumorigenesis. Proc Natl Acad Sci USA.
98:10983–10985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kandel ES and Hay N: The regulation and
activities of the multifunctional serine/threonine kinase Akt/PKB.
Exp Cell Res. 253:210–229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brazil DP and Hemmings BA: Ten years of
protein kinase B signalling: A hard Akt to follow. Trends Biochem
Sci. 26:657–664. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lawlor MA and Alessi DR: PKB/Akt: A key
mediator of cell proliferation, survival and insulin responses? J
Cell Sci. 114:2903–2910. 2001.PubMed/NCBI
|
|
21
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: A play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arboleda MJ, Lyons JF, Kabbinavar FF, Bray
MR, Snow BE, Ayala R, Danino M, Karlan BY and Slamon DJ:
Overexpression of AKT2/protein kinase Bbeta leads to up-regulation
of beta1 integrins, increased invasion, and metastasis of human
breast and ovarian cancer cells. Cancer Res. 63:196–206.
2003.PubMed/NCBI
|
|
23
|
Chen S, Xiao X, Feng X, Li W, Zhou N,
Zheng L, Sun Y, Zhang Z and Zhu W: Resveratrol induces
Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating
AMPK and suppressing AKT activity and survivin expression. J Nutr
Biochem. 23:1100–1112. 2012. View Article : Google Scholar
|
|
24
|
Zhou LL, Lin ZX, Fung KP, Cheng CH, Che
CT, Zhao M, Wu SH and Zuo Z: Celastrol-induced apoptosis in human
HaCaT keratinocytes involves the inhibition of NF-κB activity. Eur
J Pharmacol. 670:399–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soleas GJ, Diamandis EP and Goldberg DM:
Wine as a biological fluid: History, production, and role in
disease prevention. J Clin Lab Anal. 11:287–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Afaq F, Adhami VM and Ahmad N: Prevention
of short-term ultraviolet B radiation-mediated damages by
resveratrol in SKH-1 hairless mice. Toxicol Appl Pharmacol.
186:28–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aziz MH, Afaq F and Ahmad N: Prevention of
ultraviolet-B radiation damage by resveratrol in mouse skin is
mediated via modulation in survivin. Photochem Photobiol. 81:25–31.
2005. View Article : Google Scholar
|
|
28
|
Potapovich AI, Kostyuk VA, Kostyuk TV, de
Luca C and Korkina LG: Effects of pre- and post-treatment with
plant poly-phenols on human keratinocyte responses to solar UV.
Inflamm Res. 62:773–780. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vitale N, Kisslinger A, Paladino S,
Procaccini C, Matarese G, Pierantoni GM, Mancini FP and Tramontano
D: Resveratrol couples apoptosis with autophagy in UVB-irradiated
HaCaT cells. PLoS One. 8:e807282013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu W, Fu YC, Zhou XH, Chen CJ, Wang X, Lin
RB and Wang W: Effects of resveratrol on H2O2
induced apoptosis and expression of SIRTs in H9c2 cells. J Cell
Biochem. 107:741–747. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Michan S and Sinclair D: Sirtuins in
mammals: Insights into their biological function. Biochem J.
404:1–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Q, Ganapathy S, Singh KP, Shankar S
and Srivastava RK: Resveratrol induces growth arrest and apoptosis
through activation of FOXO transcription factors in prostate cancer
cells. PLoS One. 5:e152882010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roy P, Kalra N, Prasad S, George J and
Shukla Y: Chemopreventive potential of resveratrol in mouse skin
tumors through regulation of mitochondrial and PI3K/AKT signaling
pathways. Pharm Res. 26:211–217. 2009. View Article : Google Scholar
|