Introduction
HOXB13 is one of the 39 HOX homeodomain proteins in
humans and manifests tissue-specific expression in prostate and
rectum (1). Overexpression of
HOXB13 has been reported in various cancers of the prostate, breast
and ovary (2–4). However, the biological role of HOXB13
is not clearly understood, mainly due to the lack of phenotypes of
genetically altered HOXB13 knockout mouse models (5,6). In
our previous reports, HOXB13 was predominantly overexpressed in
castration resistant prostate cancers (CRPC) to provide positive
signals for cancer cell growth under harsh androgen-deprived
conditions (7). The growth
promoting function of HOXB13 seems to be mediated through the
regulation of E2F1/RB pathways and intracellular zinc concentration
(7,8). At the same time, we demonstrated that
HOXB13 inhibits expression of p21WAF1/CIP1 protein (p21)
in prostate cancer cells grown under androgen-deprived conditions
without gaining a clear mechanical understanding.
p21 is involved in the control of cell proliferation
and differentiation. p21 is an effector of the p53 protein and it
negatively regulates the cell cycle by inhibiting cyclin-dependent
kinases (CDK) that directly inhibits the activity of cyclin-CDK2
and cyclin-CDK4 complexes. Thus, p21 is considered as a regulator
of cell cycle progression at the G1/S phase checkpoint (9). The p21 gene has p53 transcriptional
regulatory motifs, and the cells lacking functional p53 express
very a low level of p21, suggesting that p53 directly regulates p21
expression. On the contrary, there are a number of agents that
activate p21 transcription independent of the p53 pathway (10).
To explore HOXB13-mediated growth promotion of
prostate cancer cells under androgen-deprived conditions, we
studied the effect of p21 regulated by HOXB13. In the present
study, HOXB13 inhibited both p21 expression and JNK signals. While
it is known that c-Jun activates p21 expression, this study also
demonstrated that HOXB13-mediated p21 inhibition affects JNK/c-Jun
activity, suggesting that there seems to be reciprocal
communication between p21 and JNK/c-Jun-mediated signals.
Materials and methods
Patient cohort and
immunohistochemistry
Twenty-eight tumor specimens from patients were
included in the present study, including 8 specimens of
castration-resistant prostate cancers (CRPC). These
paraffin-embedded human primary PCa specimens were acquired from
patients who provided informed consent at Chonnam National
University Hospital between 1997 and 2005. The tumors used in the
present study were acquired by transurethral resection of the
prostate. All cases had clinical follow-up of at least 10 years
(Table I). Immunohistochemical
studies were performed on both paraffin-embedded tissue sections of
human hormone dependent prostate cancers (HDPC, 20 specimens) and
CRPC (8 specimens) as previously described (7). The antibodies used were anti-p21
monoclonal antibody (DCS60; Cell Signaling Technology, Danvers, MA,
USA) and anti-HOXB13 antibodies. The stained slides were evaluated
by two different investigators, including a pathologist, who were
blinded to the patient's clinical features. The intensity of the
staining was classified into one of the two grades (+, positive; −,
negative).
 | Table IProstate cancer patient cohort
(N=28). |
Table I
Prostate cancer patient cohort
(N=28).
| Characteristics | Data |
|---|
| Age median | 68 (56–82) |
| Preoperative PSA
(ng/ml) | |
| <10 | 12 |
| 10–20 | 6 |
| >20 | 10 |
| Pathological
stage | |
| Stage 2 | 22 |
| Stage 3 | 6 |
| Invasion | |
| Extraprostatic
invasion | 8 |
| Seminal vesicle
invasion | 2 |
| Angiolymphatic
invasion | 8 |
| Perineural
invasion | 13 |
| Unknown | 8 |
| Castration resistant
disease | 8 |
| Gleason score | |
| ≤6 | 6 |
| 7 | 8 |
| ≥8 | 14 |
Plasmids and reagents
The synthetic testosterone R1881, was purchased from
NEN Life Science (Boston, MA, USA) and used at a final
concentration of 10 nM (nmol/l). Both fetal bovine serum (FBS) and
charcoal dextran-treated (CDT) FBS were purchased from Invitrogen
(Carlsbad, CA, USA). The pFLAG-p21 and pCDNA-FLAG-p21 were
generated by PCR cloning. The pFLAG-HOXB13 was previously described
(11). Antibodies to JNK,
phosphorylated JNK (Thr183/185), ERK, AKT, STAT3 and p53 were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Anti-FLAG M5, c-Fos, c-Jun, phosphorylated c-Jun (Ser73) and
β-actin antibodies were purchased from Sigma-Aldrich (St. Louis,
MO, USA). Antibodies against p21WAF1/CIP1 were purchased
from Upstate Biotechnology (Lake Placid, NY, USA). The dominant
negative c-Jun mutant was kindly provided by Dr Kyung Keun Kim
(Chonnam National University, Gwangju, Korea). An inhibitor of
c-Jun N-terminal kinase (SP600125) was obtained from Sigma-Aldrich.
Reporter vectors, including AP1-luc and c-Jun-luc, were purchased
from Stratagene (Santa Clara, CA, USA).
Cell culture
Human prostate cell lines, including LNCaP and PC3,
were routinely cultured in RPMI media (Invitrogen, Carlsbad, CA,
USA) supplemented with 5% FBS at 37°C in an atmosphere containing
5% CO2. HOXB13-suppressed LNCaP and HOXB13-overexpressed
PC3 cells have been previously described (7). Wild-type HCT116-p21(+/+) and
HCT116-p21(−/−) were kindly provided by Dr Bert Vogelstein. All
cultures were fed with fresh medium every 3–4 days.
MTT in vitro cell proliferation
assay
To determine the hormone effect, the cells were
grown under 5% CDT-FBS for two days. The next day, cells were
treated with either R1881 or ethanol and were grown for up to 7
days. After incubation for 24 h,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide in
vitro proliferation assay was performed as previously described
(11). Briefly, the cells were
stained with 100 µl of 5 mg/ml MTT (Sigma-Aldrich) solution
and incubated for 4 h at 37°C. The reaction was stopped and then
the absorbance at 570 nm was measured using a microplate reader
with SoftMax PRO software (Molecular Devices, Sunnyvale, CA, USA).
Densitometric values were analyzed with the Student's t-test, using
GraphPad Prism software (San Diego, CA, USA).
Western blotting
The cells were grown to 80% confluence and then
lysed in protein extraction RIPA buffer (25 mM Tris-HCl pH 7.6, 150
mM NaCl, 1% NP-40, 1% sodium deoxycholate and 0.1% SDS) containing
a cocktail of protease inhibitors and phosphatase inhibitors.
Twenty micrograms of total cell lysates were loaded onto a 10%
Bis-Tris gel and separated using the electroporation system
(Bio-Rad Laboratories, Hercules, CA, USA). After the proteins were
transferred to a PVDF membrane, primary antibodies were applied,
followed by incubation with horse peroxidase-conjugated secondary
antibodies. The blots were developed by the ECL detection system
(Thermo Fisher Scientific Inc., Rockford, IL, USA).
Luciferase assay
Approximately 1×105 cells were plated in
a 24-well plate 16 h before transfection. Each transfection was
carried out using Lipofectamine 2000 (Invitrogen) with 0.1
µg of reporter and 2 ng of Renilla luciferase
reporter as described in the manufacturer's protocol. Six hours
later, the cells were washed and fed with medium containing 5% FBS.
Addition of SP600125 was performed 18 h post-transfection.
Subsequently, the cells were washed with PBS, harvested for 24 h,
lysed with 100 µl of passive lysis buffer. Then luciferase
activities were assayed as relative light units (RLU) using the
Dual-Luciferase reporter assay system (Promega, Madison, WI, USA)
as per the instructions. The transfection experiments were
performed in triplicate and the results are reported as the mean ±
SD. For each transfection, Renilla luciferase reporter was
used to normalize for differences in transfection efficiency. The
RLU from the experimental group were normalized to the control
group and the values were represented as fold change.
Chromatin immunoprecipitation (ChIP)
Chromatin immuno-precipitation was performed using
antibodies against c-Jun, HOXB13 or IgG as previously described
(12). The immuno-precipitated DNA
was amplified by using specific primers as follows: p21 (forward,
ctcacatcctccttcttcag and reverse, cacacacagaatctgactccc).
Statistical analysis
The data were expressed as mean ± standard deviation
and processed using the SPSS software 17.0 (SPSS, Inc., Chicago,
IL, USA). The Pearson's correlation coefficient test was used to
estimate the correlation between tumors and HOXB13 and p21.
Statistical significance was set at P-value <0.05.
Results
Expression of HOXB13 and p21 in
castration-resistant prostate cancer
Our previous studies demonstrated that HOXB13
inhibits p21 expression without clearly indicating the underlying
mechanism (7,8). Since HOXB13 is predominantly
overexpressed in CRPC, we screened the expression of both HOXB13
and p21 in selected prostate cancers including CRPC in order to
demonstrate correlated expression of these two proteins.
Immunohistochemistry was performed on formalin-fixed and
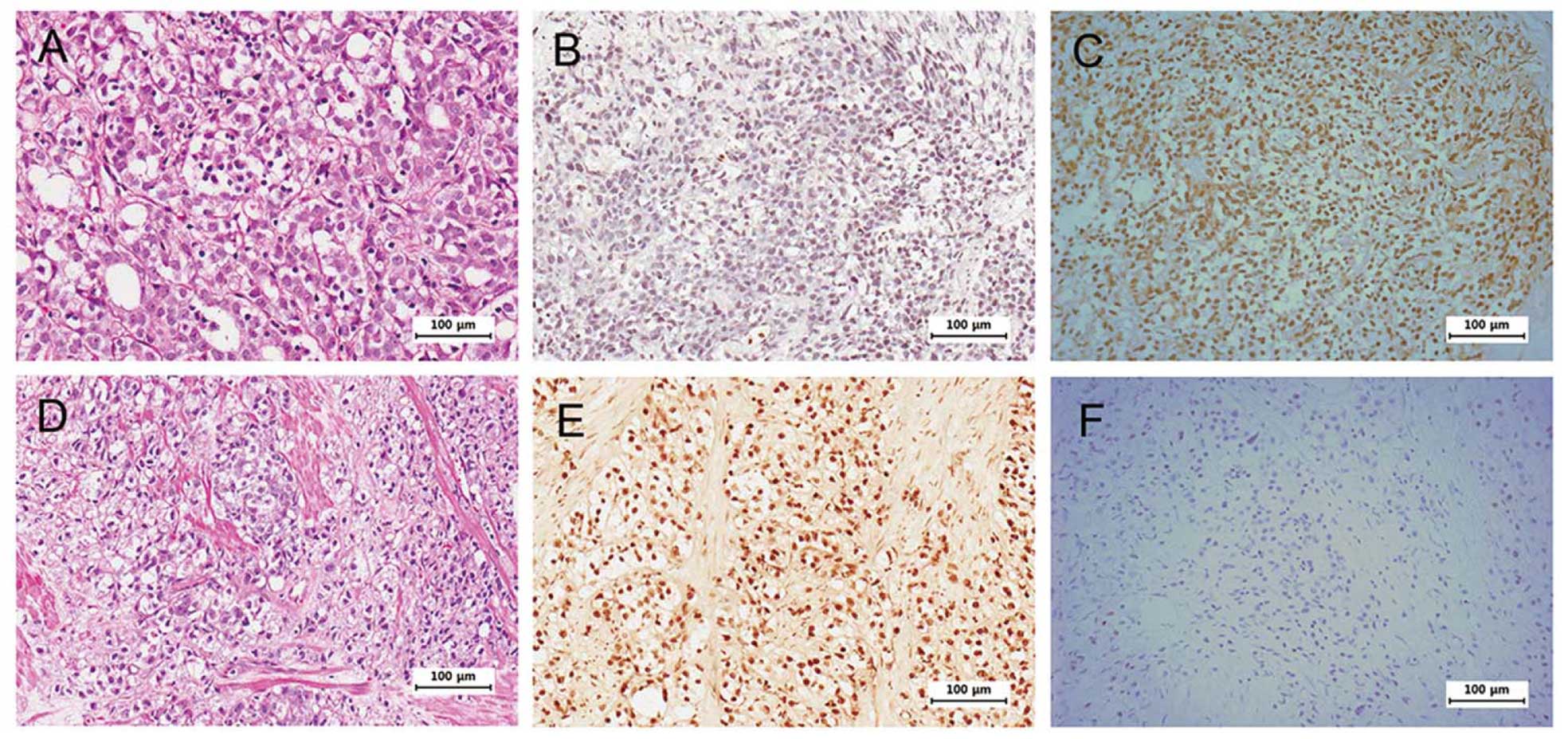
paraffin-embedded tumor specimens. Fig.
1 shows negatively correlated expression of HOXB13 and p21 in
some selected tumors. Intensity of HOXB13 staining was classified
into one of the four grades (0, absent; 1, weak; 2, intermediate;
and 3, strong) to statistically correlate the expression of HOXB13
and p21. We further classified the grades as follows: 0–1 as
negative and 2–3 as positive. Due to the heterogeneous nature of
prostate cancer and small sample size, there were no statistically
significant correlations between HOXB13 and p21 expression
(Table II). However,
HOXB13-deficient tumors had higher odds for expressing p21 than
HOXB13-positive tumors with negative p21 expression. Although the
sample size was small resulting in lack of statistical
significance, CRPC showed a more negative correlation between
HOXB13 and p21 than HDPC.
 | Table IIExpression of HOXB13 and p21 in
prostate cancer samples. |
Table II
Expression of HOXB13 and p21 in
prostate cancer samples.
Tumor type
Total | HOXB13 | p21
|
|---|
| Positive | Negative | |
|---|
| ADPC | Positive | 2 | 4 | 6 |
| Negative | 6 | 8 | 14 |
| CRPC | Positive | 2 | 3 | 5 |
| Negative | 2 | 1 | 3 |
The negative effect of p21 on cell growth
is minimal in the absence of androgen
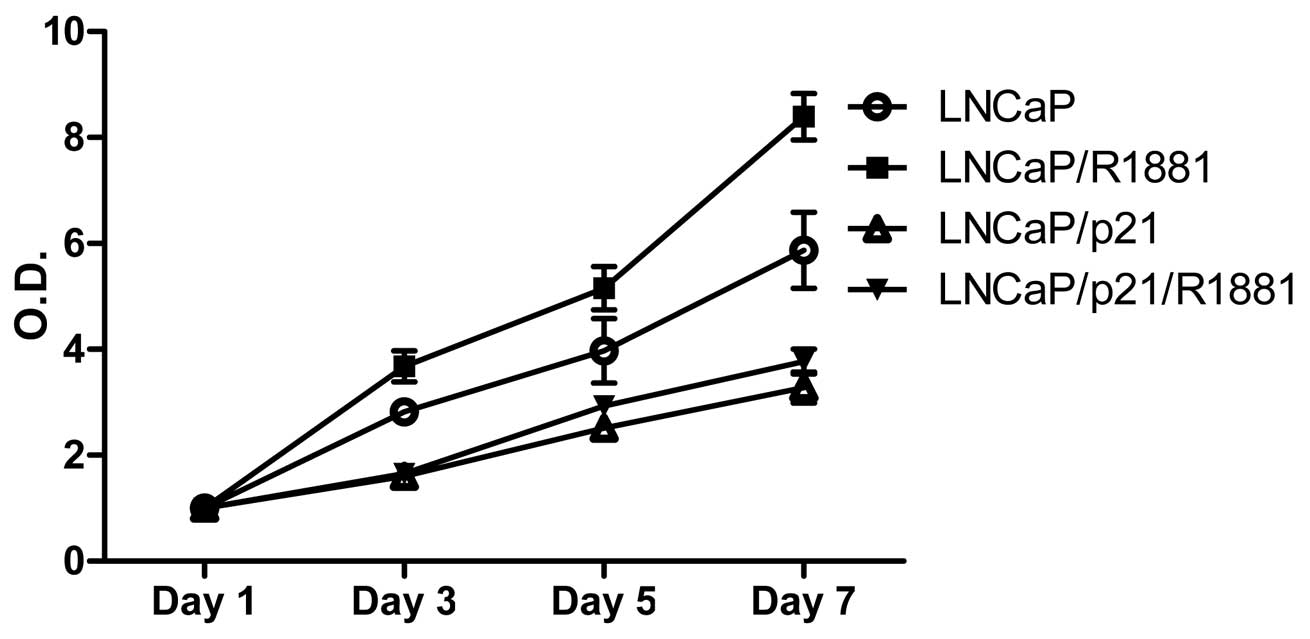
To demonstrate the growth-regulating role of p21 in
the absence of androgen, androgen-responsive LNCaP PCa cells were
transfected with either pCDNA or pCDNA-p21. Cells were grown up to
7 days in the presence of androgen (final concentration of 1 nM
R1881) followed by MTT in vitro proliferation assays. As
shown in Fig. 2, addition of R1881
promoted proliferation of LNCaP cells, while p21 generally
suppressed cell proliferation. Noticeably, androgen did not
stimulate cell proliferation in the presence of p21, suggesting
that p21 suppression is an important step in survival of PCa cells
under androgen-deprived conditions.
HOXB13 suppresses p21 expression and
JNK-mediated signaling
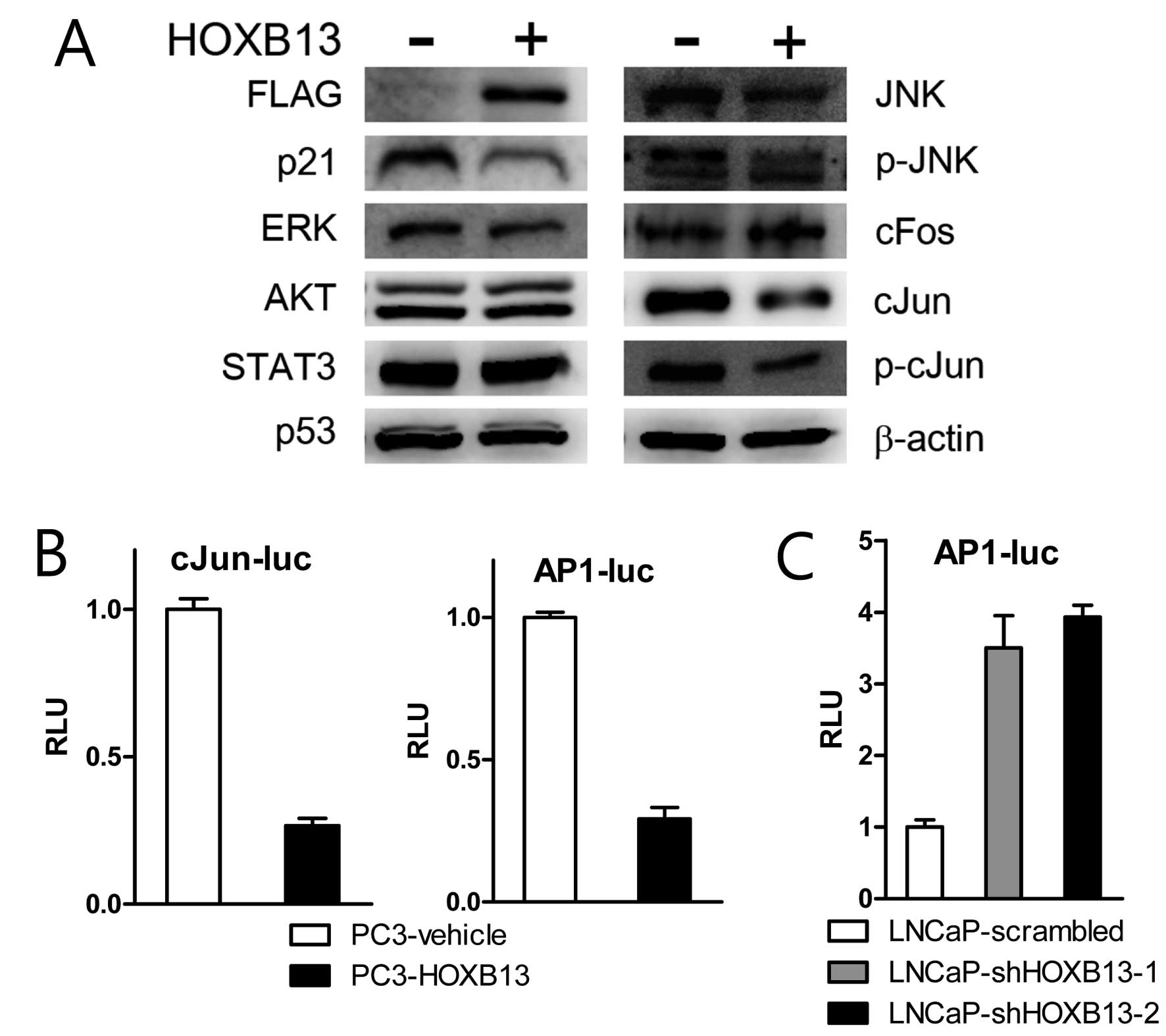
To explore the detailed mechanism by which HOXB13
suppresses p21 expression, PC3 cells stably transfected with HOXB13
were used for western blot analysis. HOXB13 suppressed not only p21
expression but also c-Jun N-terminal kinase (JNK)-related proteins
(Fig. 3A). There were no
significant alterations in extracellular signal-regulated kinase
(ERK), AKT, STAT3 and p53 proteins. While HOXB13 inhibited
expression of total forms of JNK and c-Jun and phosphorylated forms
of JNK and c-Jun, there was no significant difference in c-fos
proteins. JNK is known to bind and phosphorylate the DNA binding
protein c-Jun, which is a central component of the AP-1 family of
transcription factors. AP-1 complex consists of homodimers or
heterodimers of c-Jun and other transcription factors such as
c-fos. Consequently, the reporter transcription assay demonstrated
that HOXB13 suppressed c-Jun and AP-1 mediated transcription
(Fig. 3B). Suppression of HOXB13 in
LNCaP cells also indicated stimulation of AP-1 activity (Fig. 3C).
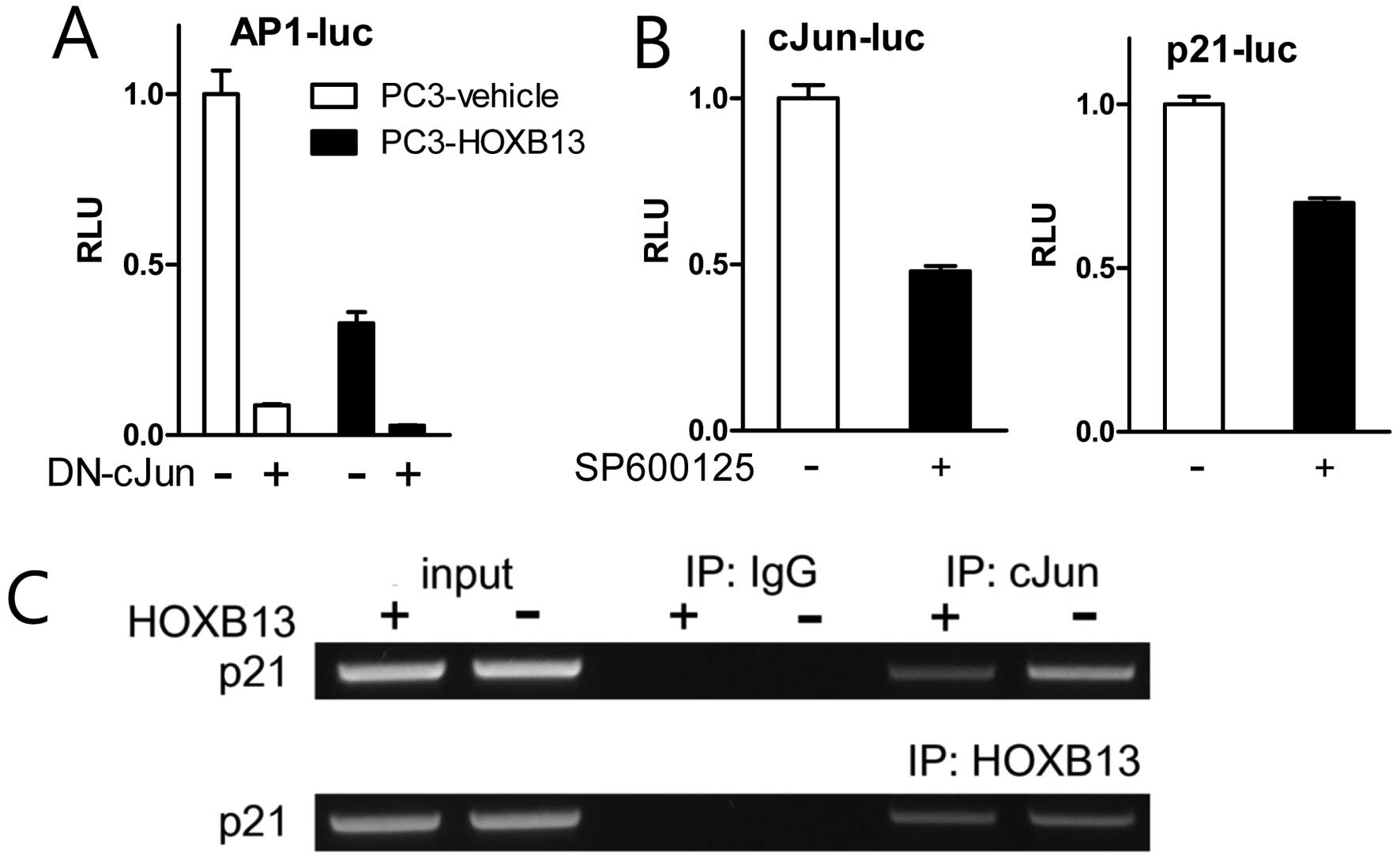
To confirm the effect of HOXB13 on c-Jun and AP-1
activity, TAM67 (dominant-negative c-Jun) and SP600125
(pharmacological inhibitor of JNK) were used in the reporter
transcription assay. HOXB13 further downregulated AP-1 activity
suppressed by TAM67 (Fig. 4A).
Treatment with SP600125 blocked not only the activation of c-Jun
but also the activation of p21 (Fig.
4B). Chromatin immunoprecipitation assay demonstrated that
HOXB13 accommodated less amount of c-Jun on the p21 promoter region
while HOXB13 association did not change (Fig. 4C). These results suggest that HOXB13
affects both AP-1 activity and p21 activity and there might be a
mutual communication between these two proteins.
HOXB13-mediated suppression of p21
stimulates c-Jun activity
c-Jun negatively regulates transcription of p53 by
binding to multiple AP-1 sites in the p53 promoter and represses
its target p21 expression (13). As
shown in Fig. 3A, however, HOXB13
suppressed the expression of both p21 and c-Jun, implying that
there is no negative correlation between c-Jun and p21. To assess
if there is c-Jun mediated regulation of p21 activity,
p21-deficient HCT116 cells were tested for c-Jun activity. Compared
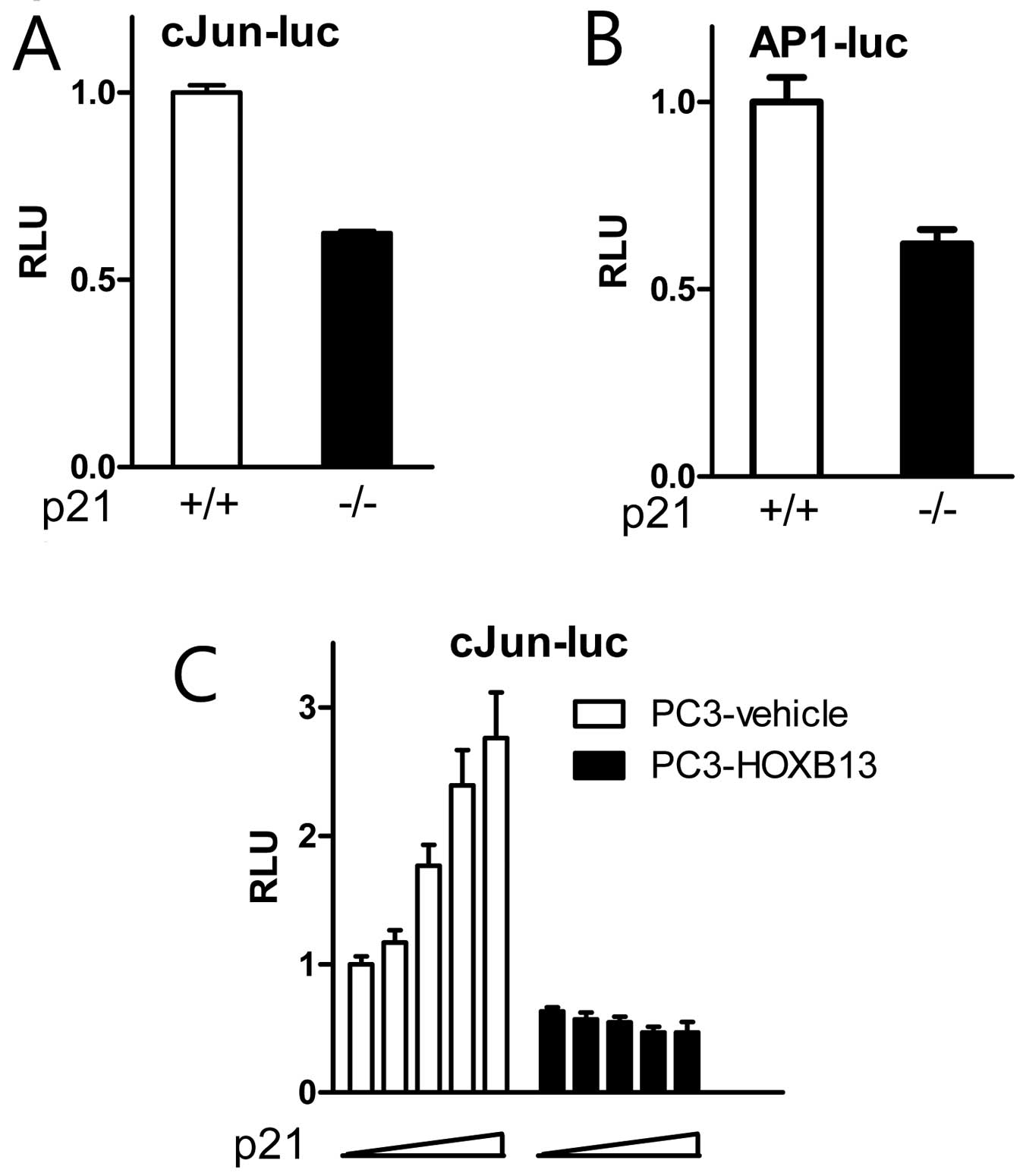
to wild-type cells, p21(−/−) cells showed inhibited activity of
c-Jun (Fig. 5A) and AP-1 (Fig. 5B). A further study demonstrated that
exogenous p21 stimulated c-Jun activity in a dose-responsive manner
in HOXB13-deficient PC3 cells, while the effect of p21 on c-Jun
activity was minimal in HOXB13-overexpressed PC3 cells (Fig. 5C). These results suggest that the
cell cycle inhibitor p21 may have a novel role in the regulation of
c-Jun signaling in the presence of HOXB13.
Discussion
Androgens are the most dominant growth stimulatory
factors for both normal and malignant prostate cells. Therefore,
various androgen deprivation therapies are the most effective and
final treatment for patients with recurrent PCa. The clinical
response is, however, transient due to the presence of
androgen-independent PCa cells. As a long-term goal with the
involvement of HOXB13 homeodomain protein, we explored the
mechanism by which PCa becomes androgen-independent and survives in
an androgen-deprived environment. We have previously demonstrated
that HOXB13 is overexpressed in most CRPC and it inhibits the
expression of p21 as a result of which E2F1 signaling is activated
to provide positive effects on prostate cancer cells under
androgen-deprived conditions (7).
More precisely, inhibition of p21 promotes the activity of CDK2 and
consequently phosphorylates the RB protein to release E2F1
transcription factors. These oncogenic factors help prostate cancer
cells to survive under harsh androgen-deprived conditions. This
report demonstrates for the first time that HOXB13-mediated
inhibition of p21 expression is also involved in JNK/c-Jun
signaling. HOXB13 inhibited both c-Jun-mediated AP-1 activity and
p21 promoter activity. While activation of c-Jun has been shown to
promote p21 expression, we demonstrated that HOXB13-mediated p21
inhibition also reciprocally regulates c-Jun-mediated AP-1
signaling. We also showed that there is tendency for negative
correlation between HOXB13 and p21 expression in PCa including CRPC
and HDPC.
The p21 protein and JNK are two well-characterized
cellular modulators that play important roles in regulating cell
growth, differentiation and apoptosis. The p21 inhibits the cell
cycle through its interaction with cyclin-CDK complexes (14). The expression of p21 is regulated
via p53-dependent and p53-independent mechanisms (15,16).
In response to stimuli, JNK binds and phosphorylates the c-Jun
transcription factor and increases its transcriptional activity.
c-Jun is a central component of the AP-1 family of transcription
factors, which consist of homodimers or heterodimers of either
c-Fos or other transcription factors (17). Activation of JNK leads to both cell
cycle arrest and cell cycle progression (18). Overexpression of c-Jun represses p53
and p21 expression and accelerates cell proliferation (13). c-Jun negatively regulates
transcription of p53 by direct binding to a variant AP-1 site in
the p53 promoter. On the other hand, JNK1 can also stabilize p21
protein by phosphorylation (19,20)
and c-Jun can transactivate the p21 promoter by activation of Sp1
(21). Another study has also shown
that androgen via p21 inhibits tumor necrosis factor α-induced JNK
activation and apoptosis, suggesting that p21 may mediate crosstalk
between androgen-androgen receptor signaling and JNK in prostate
cancer cells (22). This study
demonstrates that HOXB13 transcription factor inhibits the
expression of p21 and JNK signaling. We have previously shown that
HOXB13 suppresses the expression of p21 at the transcriptional
level (7). Althogh HOXB13 inhibits
AP-1 activity through the downregulation of JNK/c-Jun expression,
there seems to be a sequential effect of JNK/c-Jun mediated
suppression of p21 expression. In addition, our result also implies
that p21 suppression by HOXB13 conversely affects JNK signaling. In
conclusion, our findings demonstrate that HOXB13 inhibits both p21
expression and JNK signaling in prostate cancer cells and its
regulation seems to be mainly accomplished through the mutual
regulatory mechanism between p21 and JNK signaling. The present
study provides insights suggesting that HOXB13 plays an important
role in prostate tumorigenesis and malignant progression via the
regulation of both p21 and JNK signaling and suggests that HOXB13
might be a new therapeutic target.
Acknowledgments
This research was supported by the National Research
Foundation of Korea (MRC, 2011-0030132) funded by the Korea
government and by the Basic Science Research Program through the
National Research Foundation of Korea funded by the Ministry of
Science, ICT and future Planning (2014R1A2A2A01005160).
References
|
1
|
Jung C, Kim RS, Zhang HJ, Lee SJ and Jeng
MH: HOXB13 induces growth suppression of prostate cancer cells as a
repressor of hormone-activated androgen receptor signaling. Cancer
Res. 64:9185–9192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edwards S, Campbell C, Flohr P, Shipley J,
Giddings I, Te-Poele R, Dodson A, Foster C, Clark J, Jhavar S, et
al: Expression analysis onto microarrays of randomly selected cDNA
clones highlights HOXB13 as a marker of human prostate cancer. Br J
Cancer. 92:376–381. 2005. View Article : Google Scholar
|
|
3
|
Ma XJ, Wang Z, Ryan PD, Isakoff SJ,
Barmettler A, Fuller A, Muir B, Mohapatra G, Salunga R, Tuggle JT,
et al: A two-gene expression ratio predicts clinical outcome in
breast cancer patients treated with tamoxifen. Cancer Cell.
5:607–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miao J, Wang Z, Provencher H, Muir B,
Dahiya S, Carney E, Leong CO, Sgroi DC and Orsulic S: HOXB13
promotes ovarian cancer progression. Proc Natl Acad Sci USA.
104:17093–17098. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Economides KD and Capecchi MR: Hoxb13 is
required for normal differentiation and secretory function of the
ventral prostate. Development. 130:2061–2069. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Economides KD, Zeltser L and Capecchi MR:
Hoxb13 mutations cause overgrowth of caudal spinal cord and tail
vertebrae. Dev Biol. 256:317–330. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim YR, Oh KJ, Park RY, Xuan NT, Kang TW,
Kwon DD, Choi C, Kim MS, Nam KI, Ahn KY, et al: HOXB13 promotes
androgen independent growth of LNCaP prostate cancer cells by the
activation of E2F signaling. Mol Cancer. 9:1242010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim YR, Kim IJ, Kang TW, Choi C, Kim KK,
Kim MS, Nam KI and Jung C: HOXB13 downregulates intracellular zinc
and increases NF-κB signaling to promote prostate cancer
metastasis. Oncogene. 33:4558–4567. 2014. View Article : Google Scholar
|
|
9
|
Dzau VJ, Braun-Dullaeus RC and Sedding DG:
Vascular proliferation and atherosclerosis: New perspectives and
therapeutic strategies. Nat Med. 8:1249–1256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kennett SB, Udvadia AJ and Horowitz JM:
Sp3 encodes multiple proteins that differ in their capacity to
stimulate or repress transcription. Nucleic Acids Res.
25:3110–3117. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung C, Kim RS, Lee SJ, Wang C and Jeng
MH: HOXB13 homeodomain protein suppresses the growth of prostate
cancer cells by the negative regulation of T-cell factor 4. Cancer
Res. 64:3046–3051. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee SJ, Lee K, Yang X, Jung C, Gardner T,
Kim HS, Jeng MH and Kao C: NFATc1 with AP-3 site binding
specificity mediates gene expression of
prostate-specific-membrane-antigen. J Mol Biol. 330:749–760. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schreiber M, Kolbus A, Piu F, Szabowski A,
Möhle-Steinlein U, Tian J, Karin M, Angel P and Wagner EF: Control
of cell cycle progression by c-Jun is p53 dependent. Genes Dev.
13:607–619. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
el-Deiry WS, Tokino T, Velculescu VE, Levy
DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and
Vogelstein B: WAF1, a potential mediator of p53 tumor suppression.
Cell. 75:817–825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parker SB, Eichele G, Zhang P, Rawls A,
Sands AT, Bradley A, Olson EN, Harper JW and Elledge SJ:
p53-independent expression of p21Cip1 in muscle and other
terminally differentiating cells. Science. 267:1024–1027. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Angel P and Karin M: The role of Jun, Fos
and the AP-1 complex in cell-proliferation and transformation.
Biochim Biophys Acta. 1072:129–157. 1991.PubMed/NCBI
|
|
18
|
Behrens A, Sibilia M and Wagner EF:
Amino-terminal phosphorylation of c-Jun regulates stress-induced
apoptosis and cellular proliferation. Nat Genet. 21:326–329. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan Y, Chen H, Qiao B, Liu Z, Luo L, Wu Y
and Yin Z: c-Jun NH2-terminal kinase decreases ubiquitination and
promotes stabilization of p21(WAF1/CIP1) in K562 cell. Biochem
Biophys Res Commun. 355:263–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K
and Friedman E: The stress-activated protein kinases p38 alpha and
JNK1 stabilize p21(Cip1) by phosphorylation. J Biol Chem.
277:29792–29802. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kardassis D, Papakosta P, Pardali K and
Moustakas A: c-Jun transactivates the promoter of the human
p21WAF1/Cip1 gene by acting as a superactivator of the
ubiquitous transcription factor Sp1. J Biol Chem. 274:29572–29581.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang F, Kokontis J, Lin Y, Liao S, Lin A
and Xiang J: Androgen via p21 inhibits tumor necrosis factor
alpha-induced JNK activation and apoptosis. J Biol Chem.
284:32353–32358. 2009. View Article : Google Scholar : PubMed/NCBI
|