Introduction
Lung cancer is one of the leading causes of
mortality among all malignant diseases worldwide. More than 80% of
lung cancers belong to the histopathological type termed non-small
cell lung cancer (NSCLC), with squamous cell carcinomas,
adenocarcinomas and large cell carcinoma as the most noticeable
forms (1,2). The remaining 20% belong to the small
cell lung cancer (SCLC) type. Risk factors include smoking as well
as genetic risk factors (3) which
include activating point mutations in the KRAS oncogene, frequent
loss of heterozygosity and frequent mutations in the p53 gene among
others. Increased risk of lung cancer has also been connected with
several polymorphisms of cytochrome P450 genes and with deficits in
DNA restoration ability (4–6). Treatment strategies for SCLC primary
involve chemotherapy while as for NSCLC, surgical intervention
constitutes the first choice in early stages. For locally advanced
NSCLC, a combination of chemotherapy and radiotherapy is the
treatment of choice while as for metastatic patients, chemotherapy
alone is the best treatment regimen supplemented with adjuvant
chemotherapy (7).
Over the last few decades, chemotherapy has improved
the outcome for patients with late stage NSCLC, but only
marginally. Therefore, there is an urgent need to discover and
develop more effective antitumor agents which can improve the
outcome for patients with late stage NSCLC. Among them, natural
products have been shown to be an important source of anticancer
drugs (8).
Previously published studies on bergamottin have
revealed that this molecule suppressed the
phorbol-12-myristate-13-acetate-induced tumor cell invasion through
inhibition of protein kinase Cδ/p38 mitogen-activated protein
kinase and JNK/nuclear factor-κB-dependent matrix
metalloproteinase-9 expression (9).
Bergamottin as well as bergamot essential oil have been shown to
inhibit cancer cell proliferation in human neuroblastoma cells
(SH-SY5Y) (10). Bergamottin
obtained from grapefruit juice has also been reported to induce
apoptosis and chemosensitization through inhibition of the STAT3
signaling pathway in U266 (human multiple myeloma), DU145 (human
prostate cancer), RWPE-1 (normal human prostate epithelial cells),
MDA-MB-231 (human breast cancer) and Hep3B (human hepatocellular
carcinoma) tumor cells (11).
Although various studies on the anticancer effects
of bergamottin have been reported in the literature, the anticancer
activity of this compound against human NSCLC (A549 cells) has not
been reported to date. Moreover, the mechanistic details of the
present study have not yet been reported. Not only the in
vitro antitumor effects but also in vivo efficacy in a
nude mouse model of this furacoumarin were investigated in detail
making our research findings quite novel and promising.
In the present study, we report the anticancer
properties of bergamottin, a natural furanocoumarin isolated from
Citrus bergamia Risso et Poiteau fruit (Rutaceae), against
A549 lung cancer cells in vitro and in vivo. We also
studied the underlying mechanism of its action by demonstrating the
role of this compound on apoptosis, cell cycle arrest, cancer cell
migration and invasion and mitochondrial membrane potential. To the
best of our knowledge, the effect of bergamottin on the A549 cell
line along with its anticancer mechanism of action have not been
previously studied.
Materials and methods
Materials and reagents
Bergamottin was dissolved in dimethyl sulfoxide
(DMSO) at a stock solution of 100 mM and stored at −20°C.
Dulbecco's modified Eagle's medium (DMEM), RPMI-1640 medium,
propidium iodide (PI), Triton X-100,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
penicillin/streptomycin solution and Hoechst 33342 were obtained
from Sigma Chemical Co. (St. Louis, MO, USA). Fetal bovine serum
(FBS) was obtained from Gibco-BRL (Grand Island, NY, USA). Annexin
V-FITC/PI apoptosis detection kit was purchased from Beyotime
Institute of Biotechnology (Shanghai, China).
Plant material, extraction and
isolation
The fruit of C. bergamia was collected from
July to August 2014 from Zhengzhou City, China and identified by
Professor Ying Lin. A voucher specimen (18-097-018-14) was
deposited in the Herbarium of Southeast University, Nanjing, China.
A hot extraction procedure was used to prepare the extract. The
vacuum dried, finely powdered peel of the fruit (4 kg) was
extracted for 48 h with chloroform in a soxhlet apparatus to yield
the extract, which was concentrated under reduced pressure. The
chloroform extract (50 g) was loaded onto a silica gel (60–120
mesh, 200 g) column and eluted with an increasing gradient of
hexane and dichloromethane. Fractions of 100 ml volume each were
collected and pooled according to TLC analysis. Five major
fractions were collected (90:10, 80:20, 70:30, 60:40, 50:50 and
40:60). The fraction (hexane, dichloromethane; 70:30) yielded
bergamottin as a colorless crystalline solid which was identified
by 1H and 13CNMR in comparison with previous
studies (12).
Cell line and culture conditions
Human lung adenocarcinoma cancer A549 cells were
obtained from the Shanghai Institute of Cell Resource Center of
Life Science (Shanghai, China). Cells were grown in DMEM
supplemented with 10% FBS and 100 U/ml penicillin and 100
µg/ml streptomycin. Cells were cultured in a CO2
incubator (New Brunswick Galaxy 170R; Eppendroff) with an internal
atmosphere of 95% air and 5% CO2 gas and the cell lines
were maintained at 37°C. The media were stored at low temperature
(2–8°C) and the medium for cryopreservation contained 20%
phosphate-buffered saline (PBS) and 10% DMSO in growth medium.
Cell viability assay
Inhibition of cell proliferation by bergamottin was
measured by the MTT assay. Briefly, the cells were plated in
96-well culture plates (1×105 cells/well). After 24 h of
incubation, the cells were treated with bergamottin (0, 5, 10, 25,
50, 75 and 100 µM) for 24 and 48 h, MTT solution (10 mg/ml)
was then added to each well. After a 4-h incubation, the formazan
precipitate was dissolved in 100 µl DMSO, and then the
absorbance was measured in an automated micro-plated reader
(Bio-Tek, Winooski, VT, USA) at 570 nm. The cell viability ratio
was calculated by the following formula: Inhibitory ratio (%) =
(ODcontrol − ODtreated)/ODcontrol
× 100%. Cytotoxicity was expressed as the concentration of
bergamottin needed to inhibit cell growth by 50% (IC50
value).
Colony formation assay
Cells were suspended in 1 ml of DMEM containing 0.5%
agarose (Amresco, Solon, OH, USA) and 10% FBS, and plated on a
bottom layer containing 0.8% agarose and 10% FBS in 6-well plate in
triplicate. The cells were treated with 0, 5, 10, 25 and 50
µM of bergamottin for 48 h. After 1 week, the plates were
stained with 0.3% gentian violet, and the colonies were counted
under a light microscope (13). In
each plate ~500 cells were chosen and observed under the
microscope.
Invasion assay
The invasion assay was carried out in a 24-well
plate. Matrigel (BD) coating was carried out on a
polyvinylpyrrolidone-free polycarbonate filter (6-mm pore size).
The lower chamber was filled with medium containing 10% FBS. A549
cells (1×105 cells/well) were pre-incubated with
different bergamottin concentrations (0, 5, 10, 25 and 50
µM) for 20 min at room temperature and the cell medium
containing bergamottin was seeded onto the upper chamber wells.
Following incubation for 48 h, the filter was fixed and stained
with 3% ethanol containing 0.3% crystal violet for 20 min. The
stained cells were counted under a light microscope.
In vitro wound healing assay
The wound healing assay was performed using a
standard method (14). Cells
(1×106 cells/ml) were seeded in a 6-well plate and
incubated at 37°C until a 95–100% full confluent monolayer was
obtained. Subsequent to 12 h of starvation, a 100-ml pipette tip
was used to create a straight cell-free wound. Each well was washed
twice with PBS to remove any debris and then exposed to various
concentrations of bergamottin (0, 10, 25 and 50 µM) in a
medium. After 48 h of incubation, the cells were fixed and stained
with 3% ethanol containing 0.5% crystal violet powder for 20 min,
and randomly chosen fields were photographed under a light
microscope. The number of cells that migrated into the scratched
area were counted.
Morphological study by phase contrast
microscopy
A549 cells were seeded into 6-well plates at a
density of 1×106 cells/well in 1 ml medium. The cells
were treated with the different concentrations (0, 10, 25 and 50
µM) of bergamottin for 48 h. The morphological changes were
observed and the images were captured under an inverted light
microscope (Olympus; Olympus Optical Co., Ltd., Tokyo, Japan) after
48 h. The same spot of cells was marked and captured. The images
were captured at a magnification of ×200.
Morphological study of apoptosis using
fluorescence microscopy
A549 cells were seeded into 12-well plates at a
density of 1×106 cells/well in 1 ml culture medium.
After treatment with the different concentrations (0, 10, 25 and 50
µM) of bergamottin for 48 h, cell apoptosis was determined
by the Hoechst staining kit according to the manufacturer's
instructions. After the treatment, cells were fixed with 4%
polyoxymethylene and then incubated in Hoechst solution for 10–15
min in the dark. The staining images were recorded using a UV
fluorescence microscope (Olympus) using a UV filter at a
magnification of ×200 to detect morphological evidence of
apoptosis.
Quantification of cell apoptosis by
Annexin V-FITC/PI assay
Apoptotic cells were quantified using an Annexin
V-fluorescein isothiocyanate (FITC)/PI kit (BD Biosciences, San
Jose, CA, USA) and detected using flow cytometry using a
FACSCalibur flow cytometer (Becton-Dickinson and Company, Franklin
Lakes, NJ, USA) and analyzed using ModFit and CellQuest™ software.
A549 cells were plated at a density of 1×106 cells/well
into 12-well plates and incubated overnight. The cells were then
treated with bergamottin at varying doses (0, 10, 25 and 50
µM) for 48 h. Cells grown in media containing an equivalent
amount of 0.11% DMSO without any drug served as the control. Cells
were then collected and resuspended in binding buffer. Cells were
incubated with Annexin V-FITC and PI for 30 min in the dark,
previous to flow cytometric analysis. Annexin V-positive cells were
considered to be in the early stage of apoptosis, whereas Annexin V
and PI-positive cells were considered to be in the late stage of
apoptosis.
Effect of bergamottin on cell cycle phase
distribution
Briefly, A549 cells (1×106 cells/ml) were
seeded into each well of 6-well plates and incubated for 24 h for
cell attachment and recovery. The cells were treated with different
concentrations (0, 10, 25 and 50 µM) of bergamottin for 48
h. After incubation for 24 h, the cells were harvested and fixed
with ice-cold 70% ethanol (2 ml) at −20°C for 1 h. Prior to
analysis, the cells were washed with cold PBS and re-suspended in
450 µl of PBS, 25 µl PI and 25 µl RNase A. The
DNA contents were recorded by a FACSCalibur flow cytometer equipped
with Cell Quest software.
Effect of bergamottin on the
mitochondrial membrane potential (ΔΨm) loss
The effect of bergamottin on mitochondrial membrane
potential in human non-small cell lung cancer cells (A549) was
detected using the Rhodamine 123 (5 mM) fluorescent probe. A549
cells (2×105 cells/dish) were treated with different
concentrations of bergamottin (0, 10, 25 and 50 µM) for 48
h. Rhodamine 123 (2 mM) was added 2 h before the termination of the
experiment. Mitochondrial membrane potential was measured by flow
cytometry, a BD FACSCalibur flow cytometer and Cell Quest software
3.0 (Becton-Dickinson).
In vivo antitumor activity of bergamottin
in a mouse xenograft model
The effects of bergamottin on tumor progression were
also observed using a nude mouse model. Female BALB/c nude mice
(six weeks old) were purchased from the Shanghai Laboratory Animal
Center (SLAC; Shanghai, China). All mice (a total of 20 were
obtained) were maintained with water and food ad libitum in
a pathogen-free environment with a 12 h light and 12 h dark cycle
in an animal care facility and according to animal welfare
regulations and protocols approved by the Affiliated Tumor Hospital
of Zhengzhou University/Tumor Hospital of Henan Province,
Zhengzhou, China. Human non-small cell lung carcinoma A549 cells
(2×106 cells/mouse) were injected into the right axilla
of the nude mice (5 mice/group) to create tumors in the mice.
Subsequent to tumor development, the mice were divided into 4
groups and treated with bergamottin injected intraperitoneally. The
control group in the study was treated with an equal amount of PBS
while the other three groups were treated with 25, 50 and 100 mg/kg
of bergamottin. Afterwards, the mice were sacrificed after 18 days,
and the tumor weight and volume of each mouse were evaluated. Tumor
length and width were measured using a Vernier caliper (Fisher,
Pittsburgh, PA, USA) and the tumor volume (TV) was calculated using
the formula: TV = length × width × 0.5 width.
Statistical analysis
All data were derived from at least three
independent experiments. The results are expressed as the mean ±
SD. Differences between groups were analyzed using the Student's
t-test. P<0.05 was considered to indicate a statistically
significant result.
Results
Effects of bergamottin on proliferation
and colony formation in non-small cell lung cancer A549 cells
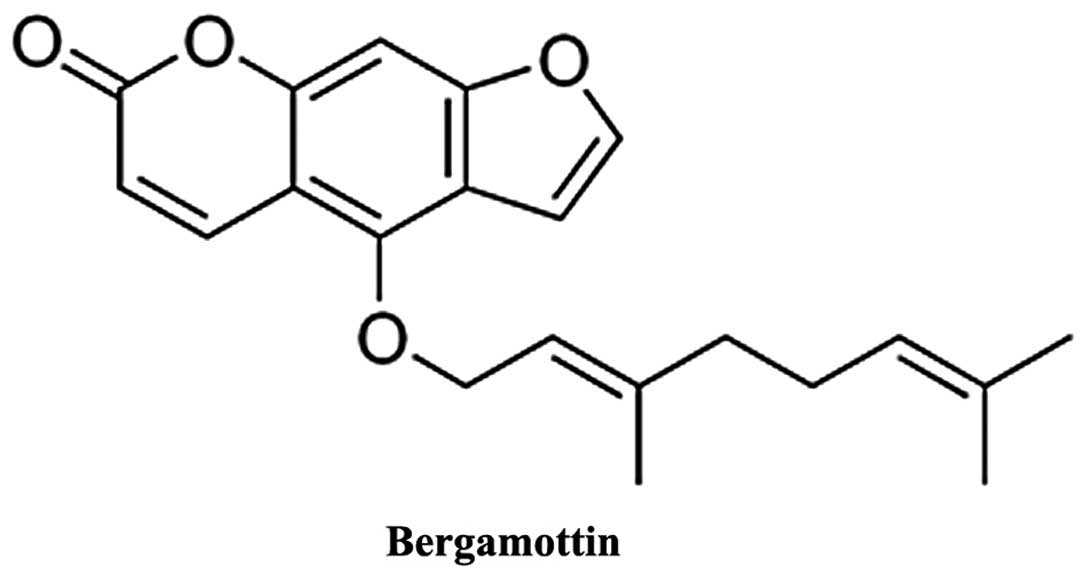
The chemical structure of bergamottin is shown in
Fig. 1. Initially we demonstrated
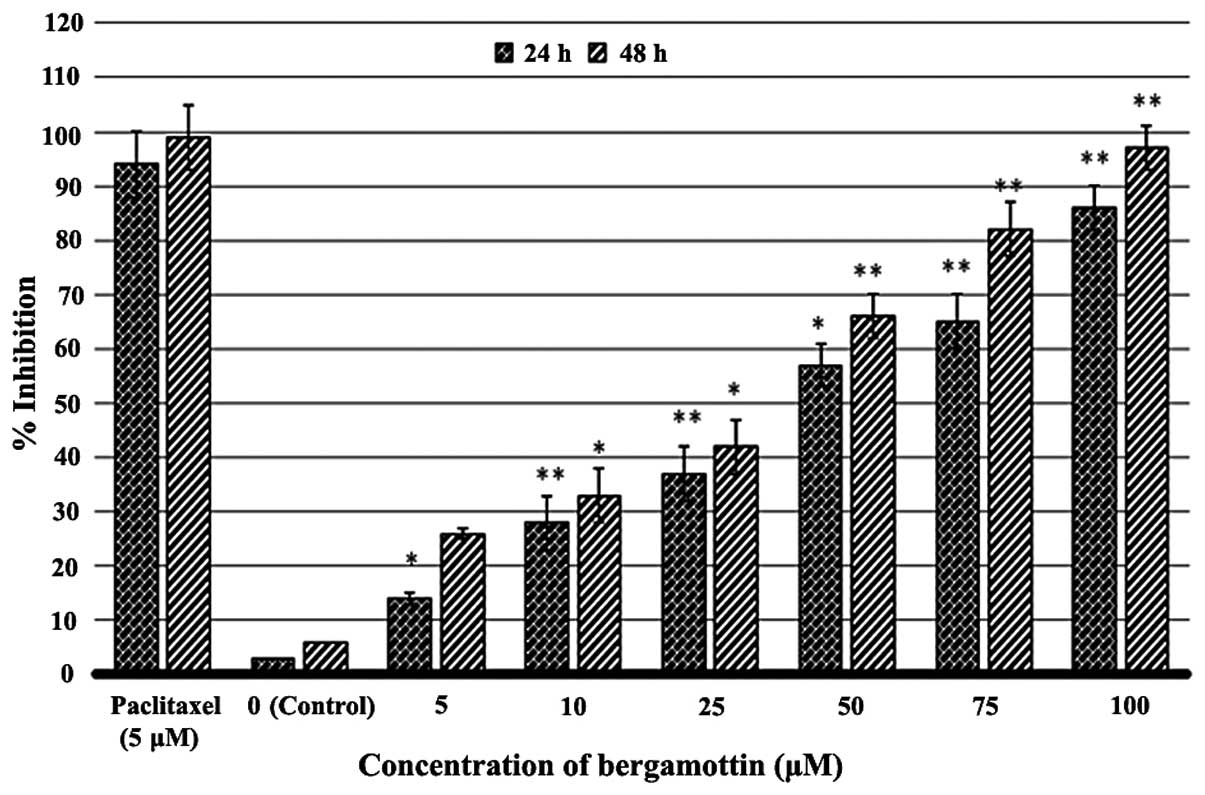
the antiproliferative activity of bergamottin on A549 cells using
MTT assay. The results revealed that bergamottin had potent
antiproliferative effects on the A549 cells. Bergamottin showed
both concentration-dependent as well as time-dependent growth
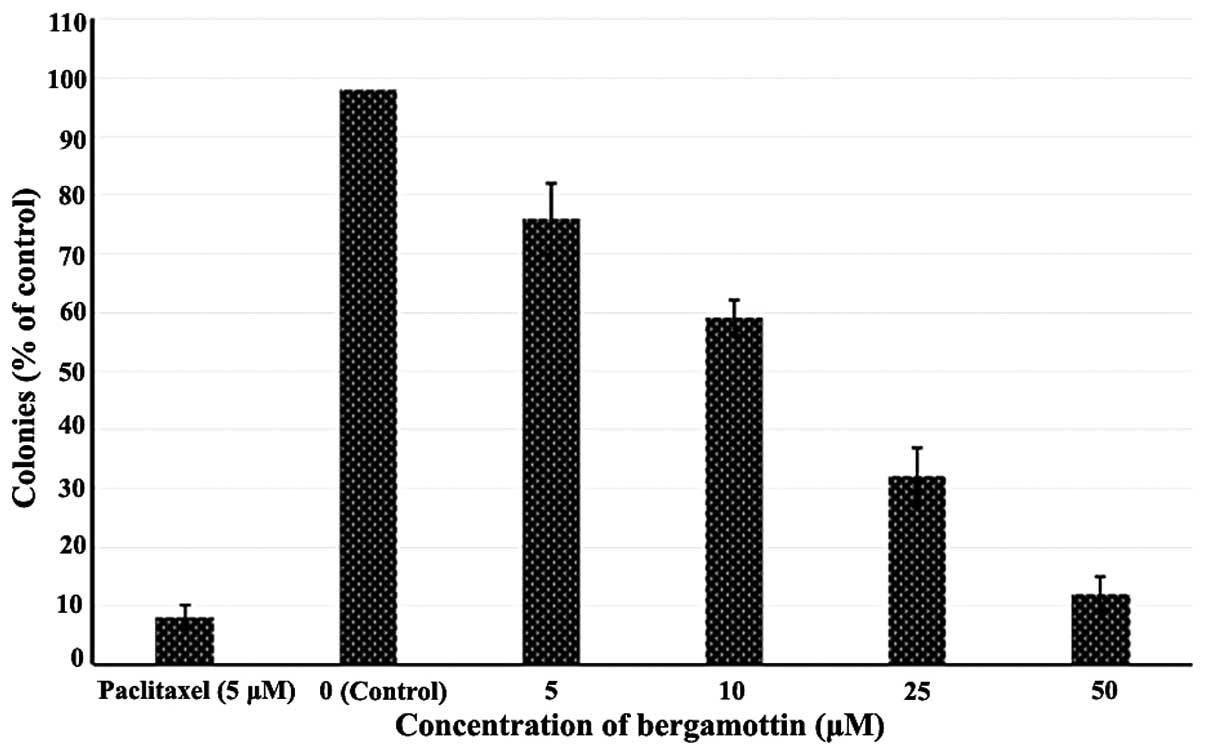
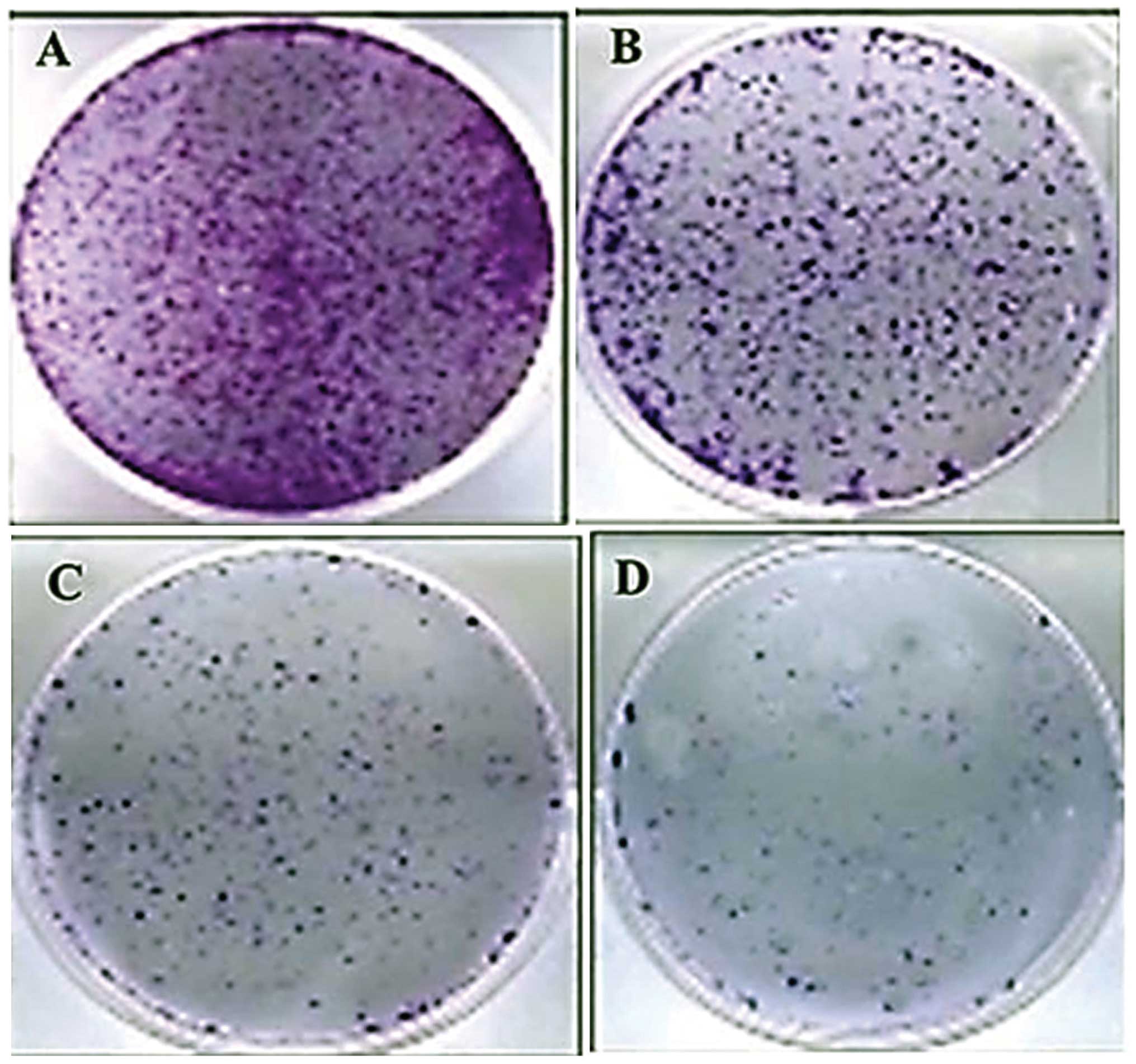
inhibitory effects against these cells (Fig. 2). Bergamottin also inhibited the
clonogenic activity of the A549 cancer cells by reducing the number
of cancer colony forming cells. A reduction in clonogenicity also
followed the concentration dependence on bergamottin (Figs. 3 and 4). The results indicate that bergamottin
has the tendency to inhibit both cell proliferation
(anchorage-dependent) and colony formation (anchorage-independent)
growth of NSCLC cells in vitro. Paclitaxel at 5 µM
was used as a positive control.
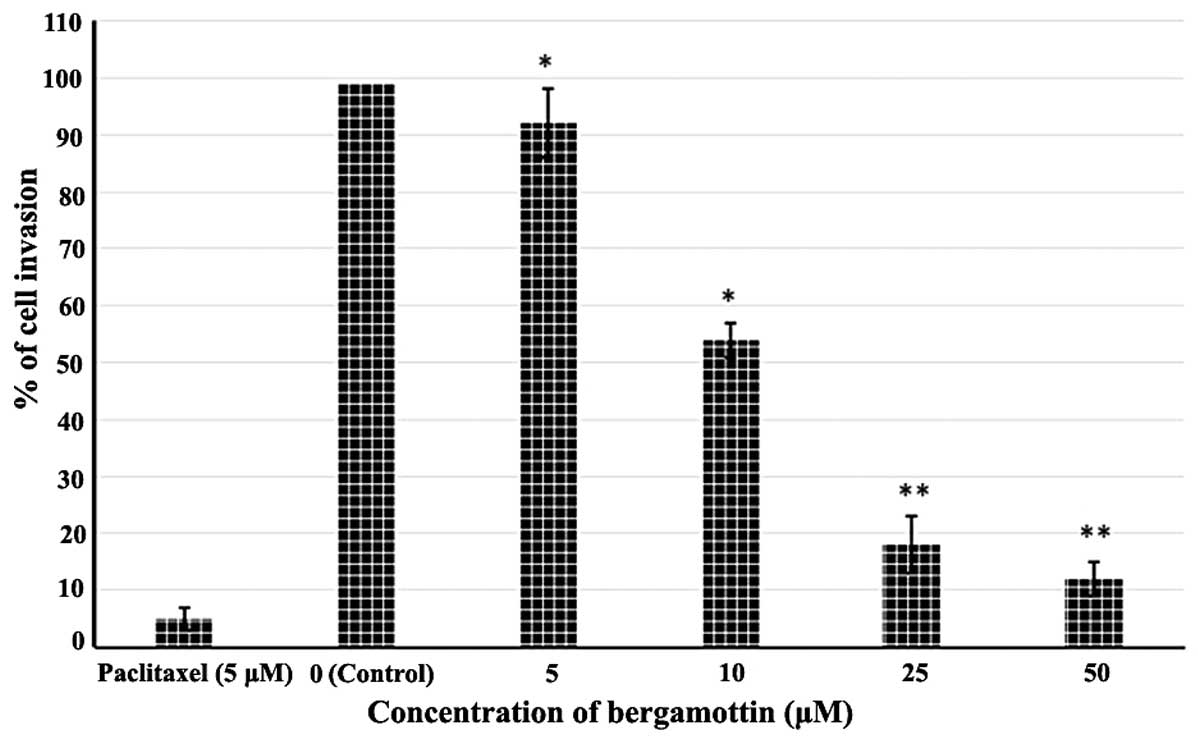
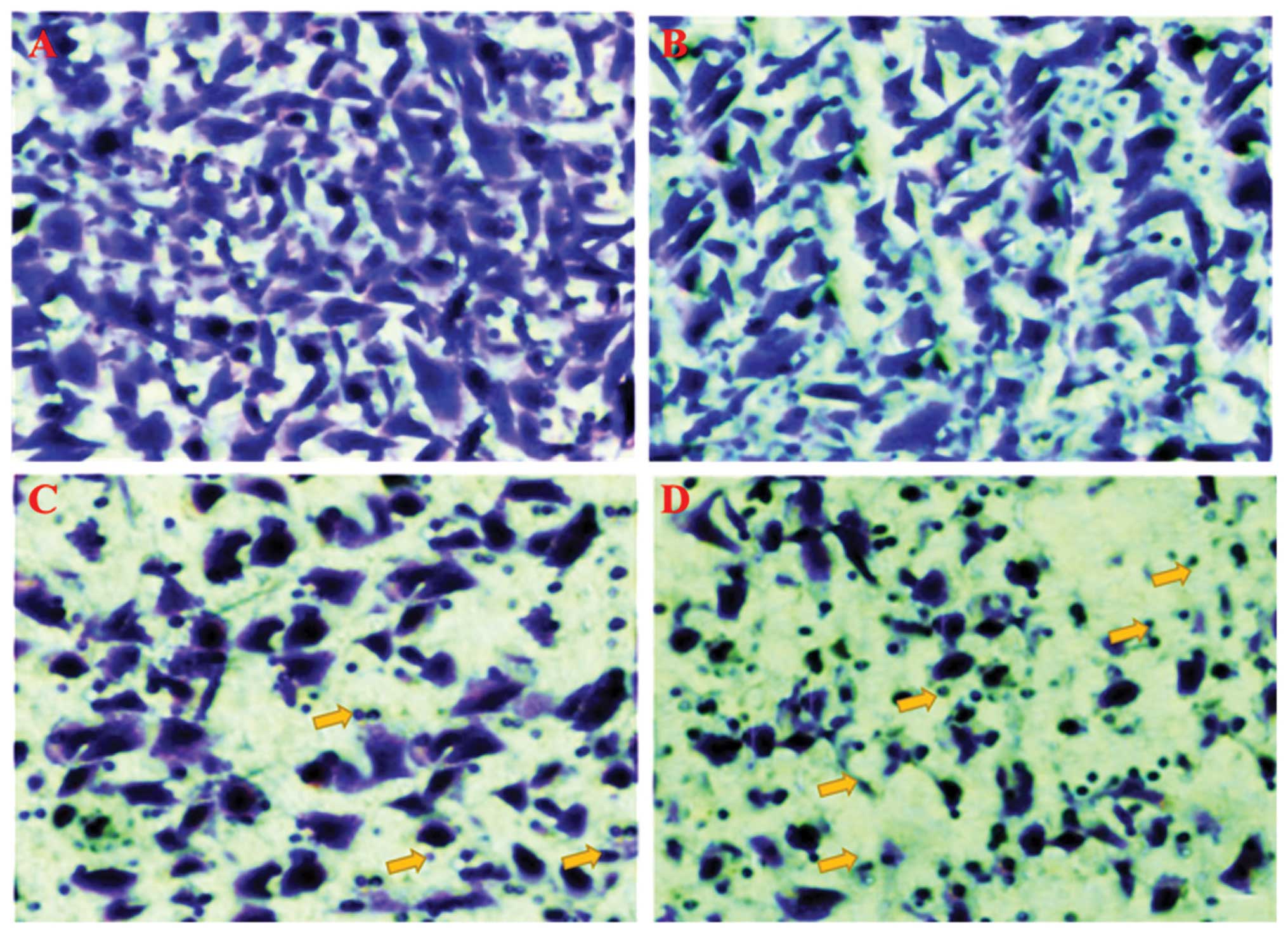
Effects of bergamottin on the invasion of
A549 cells
Lung cancer is very lethal since it is highly
invasive particularly in later stages. In this part of the study,
we demonstrated whether bergamottin could inhibit the invasive
behavior of the NSCLC A549 cells. The invasive assay was designed
using A549 cells using Matrigel-coated 24-well microchemotaxis
chambers in the presence of bergamottin at various concentrations.
As shown in Figs. 5 and 6, bergamottin at various concentrations
(0, 5, 10, 25 and 50 µM) considerably inhibited the invasion
of the A549 cells in a dose-dependent manner. Paclitaxel was used
as a positive control and it was observed that the inhibition of
cell invasion induced by bergamottin at higher doses was comparable
to paclitaxel.
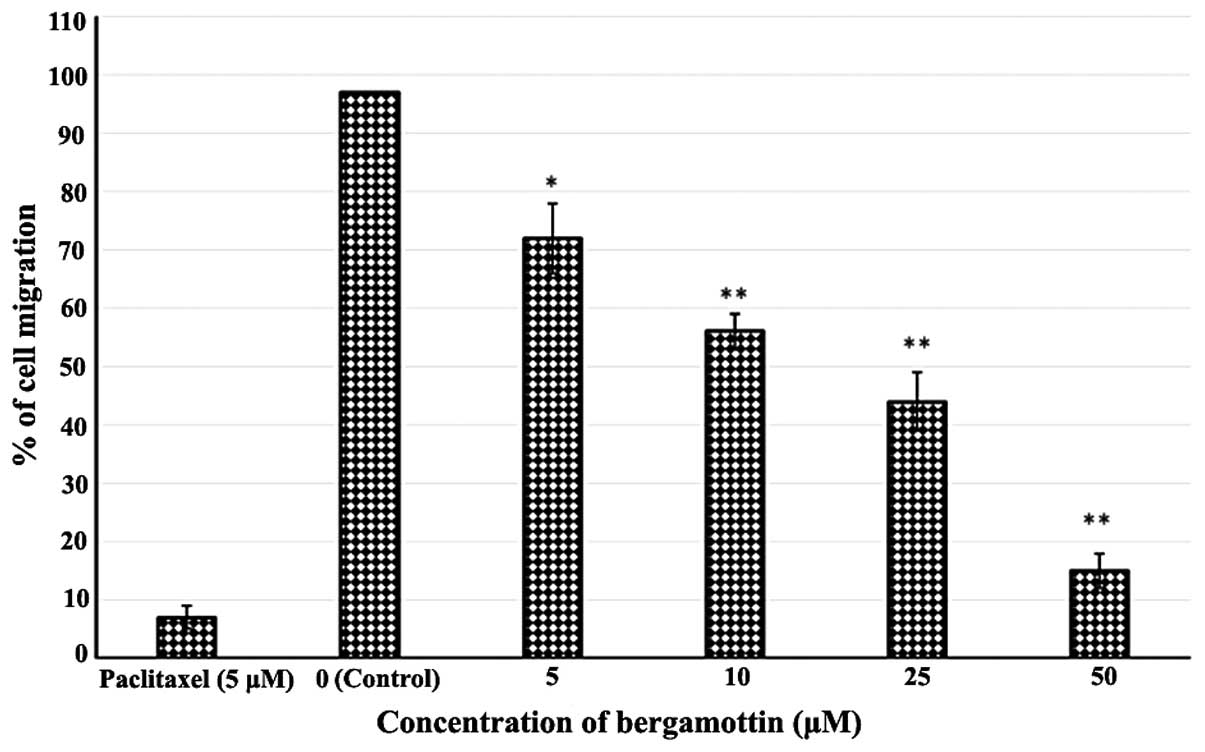
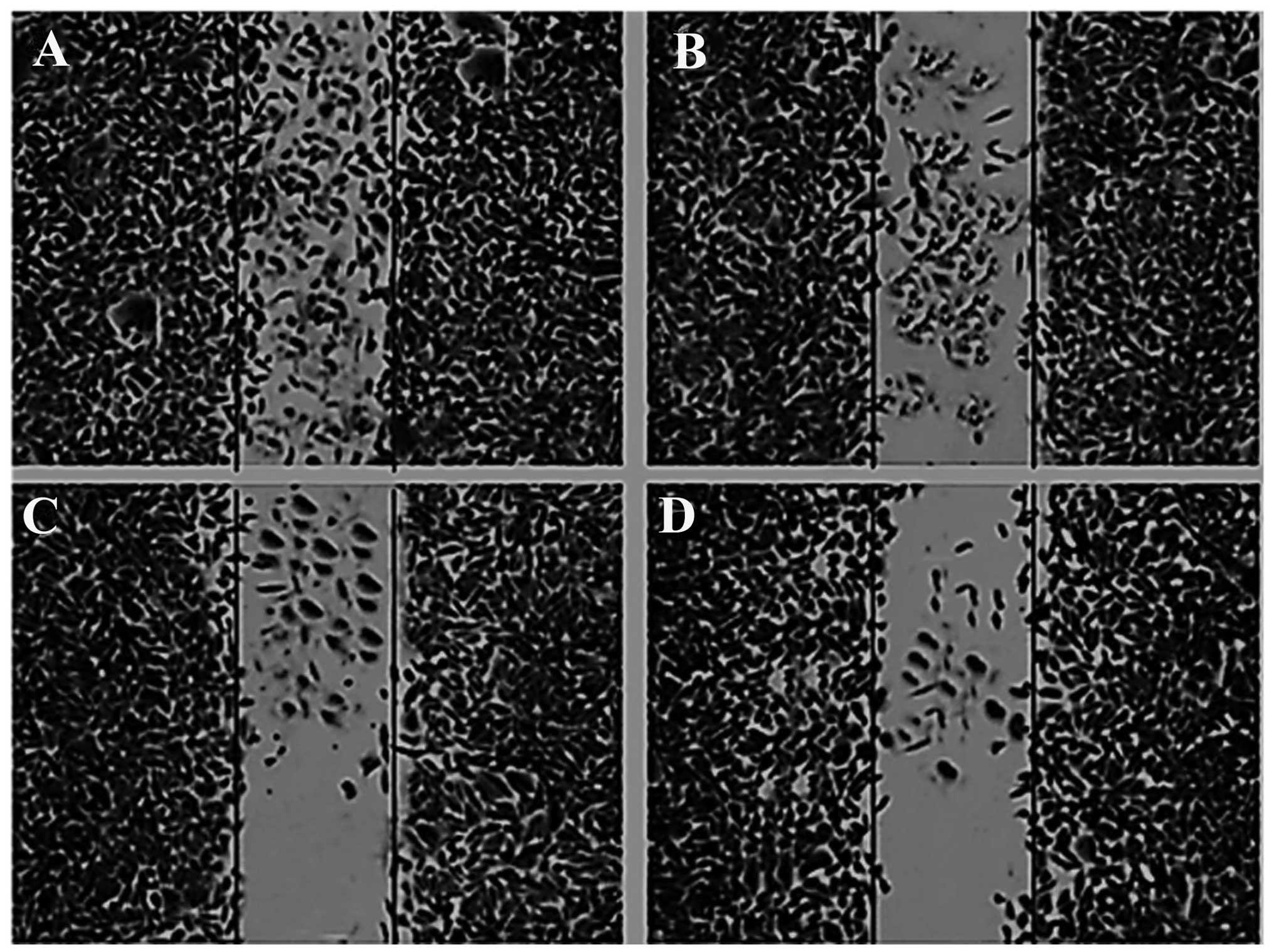
Effects of bergamottin on the migration
of A549 cells
In this experiment, we evaluated the effect of
bergamottin on the cell migration in NSCLC A549 cells. Confluent
cells were scratched and then treated with bergamottin in a
complete medium for 48 h. The number of cells that migrated into
the scratched area was photographed (magnification, ×40) and
calculated as a percentage (%) of migration. As shown in Figs. 7 and 8, bergamottin markedly reduced A549 cell
migration in a concentration-dependent manner. Fig. 8A represents untreated (0 µM)
control cells while Fig. 8B–D
represent the effect of a 10, 25 and 50 µM dose of
bergamottin, respectively. Paclitaxel at a dose of 5 µM was
used as a positive control.
Morphological study of apoptosis using
phase contrast and fluorescence microscopy
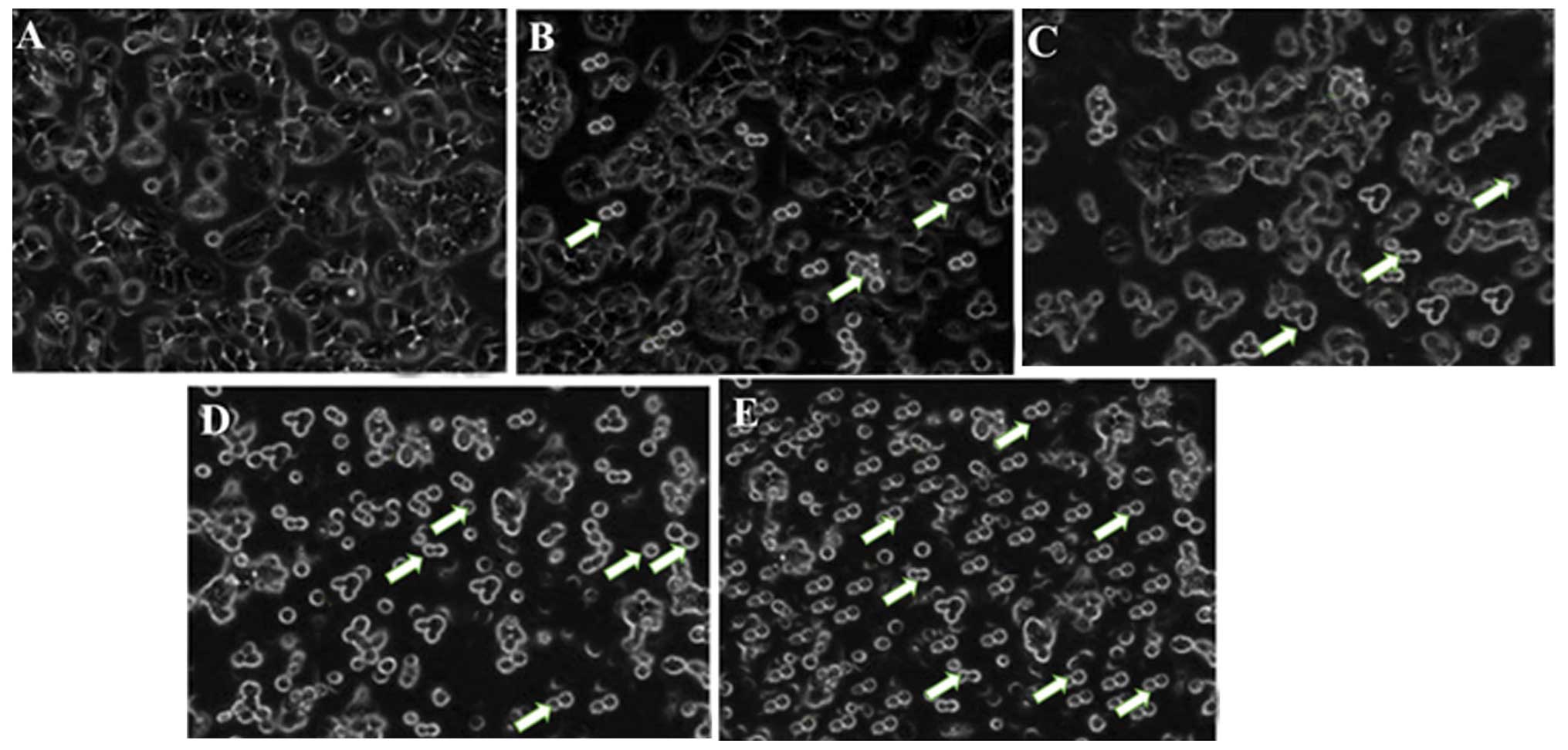
In the present study, the morphological alterations
in the human NSCLC A549 cells untreated and treated with
bergamottin were observed under an inverted light microscope. The
most conspicuous changes characteristic of apoptosis were observed
in the treated cells that included the detachment of the cells from
the substratum and cell shrinkage. As revealed by inverted light
microscopy, the untreated control cells were evenly distributed on
the substratum. A decrease in the cell population was noted with
the increase in the bergamottin concentration. As shown in Fig. 9A–E, the untreated A549 cells
appeared as densely packed and disorganized multilayers, whereas
after incubation with various concentrations of bergamottin for 48
h several of the cells became rounded and shrunken, and detached
from each other or floated in the medium. Paclitaxel was used as a
positive control and its effect is shown in Fig. 9E.
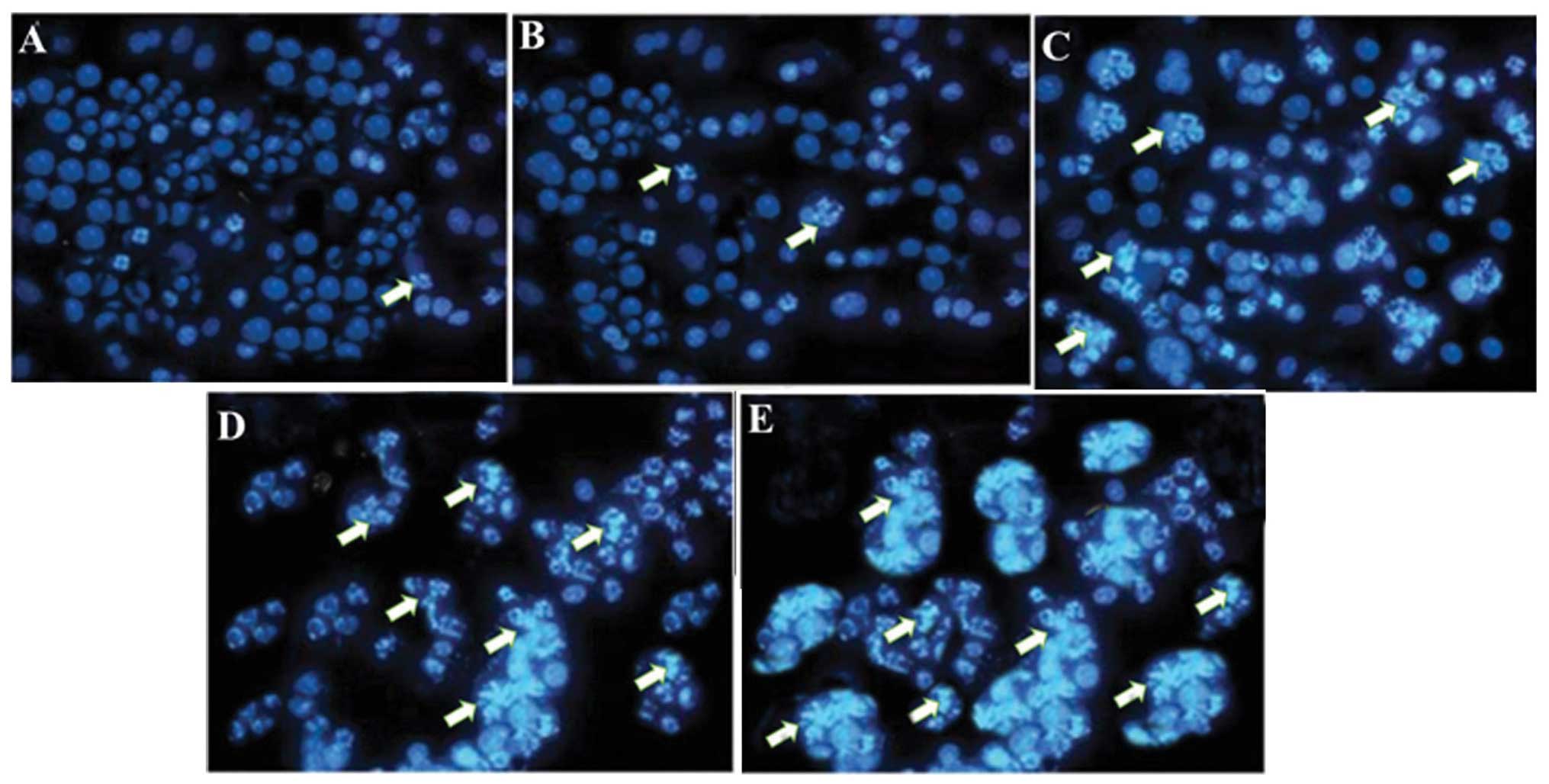
The apoptosis-inducing effect of bergamottin was
further assessed by Hoechst 33258 staining using fluorescence
microscopy. Following the treatment with different doses of
bergamottin (0, 10, 25 and 50 µM) for 48 h, the cells were
analyzed by fluorescence microscopy. Bergamottin-treated cells
stained with Hoechst 33258 revealed chromatin condensation,
fragmented nuclei and nuclear shrinkage which increased with the
increasing dose of bergamottin (Fig.
10A–E). The number of apoptotic cells increased to 43.2 and
66.4%, respectively, at 25 and 50 µM bergamottin dose for 48
h. At a lower dose of 10 µM, no significant increase in
apoptotic cells was noted. Paclitaxel was used as a positive
control and its effect is shown in Fig. 10E.
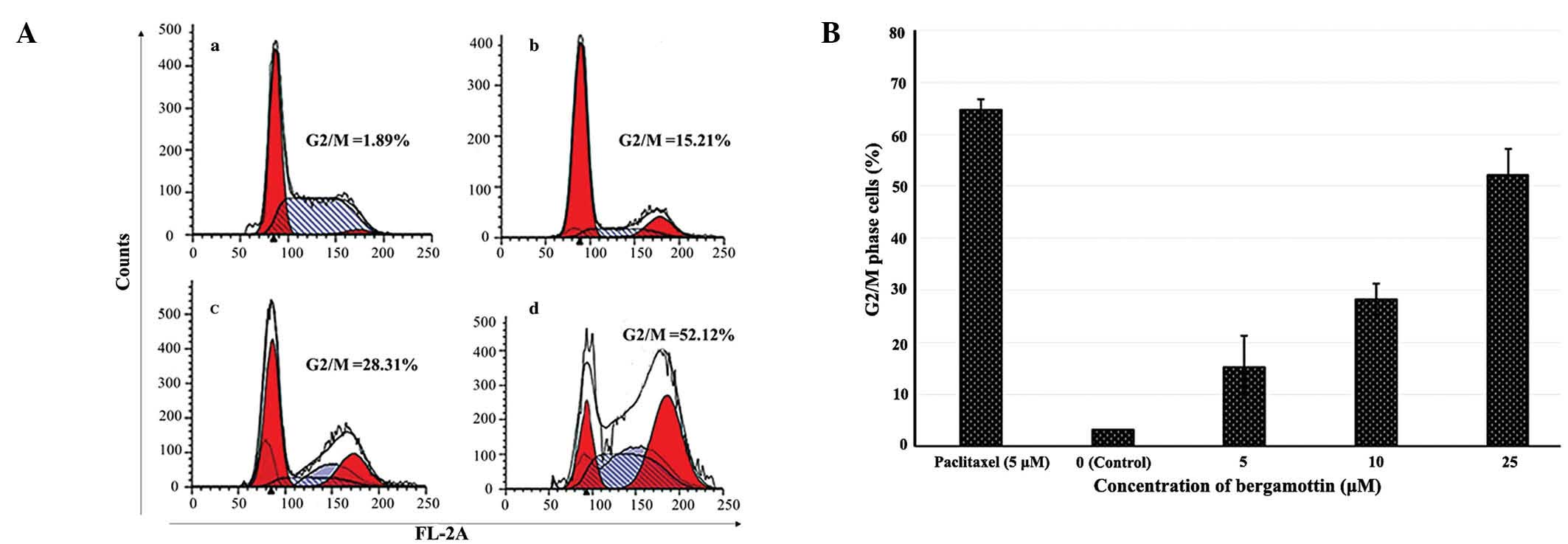
Bergamottin induces G2/M phase cell cycle
arrest in lung cancer A549 cells
Flow cytometry was employed to demonstrate the
effect of bergamottin on cell cycle phase distribution in the lung
cancer A549 cells. The results showed that the population of cells
in the G2/M phase of the cell cycle increased from 1.89% in the
control (untreated cells) (Fig.
11Aa) to 15.21, 28.31 and 52.12% in the A549 lung cancer cells
treated with 10, 25 and 50 µM of bergamottin, respectively
(Fig. 11Ab–d). Cell cycle arrest
and apoptosis may result from extensive DNA damage caused by
bergamottin in the A549 cancer cells. A higher number of A549 cells
accumulated in the G2/M phase after treatment with increasing
concentrations of bergamottin. Fig.
11B shows the graphical representation of the G2/M phase cell
population at different bergamottin concentrations.
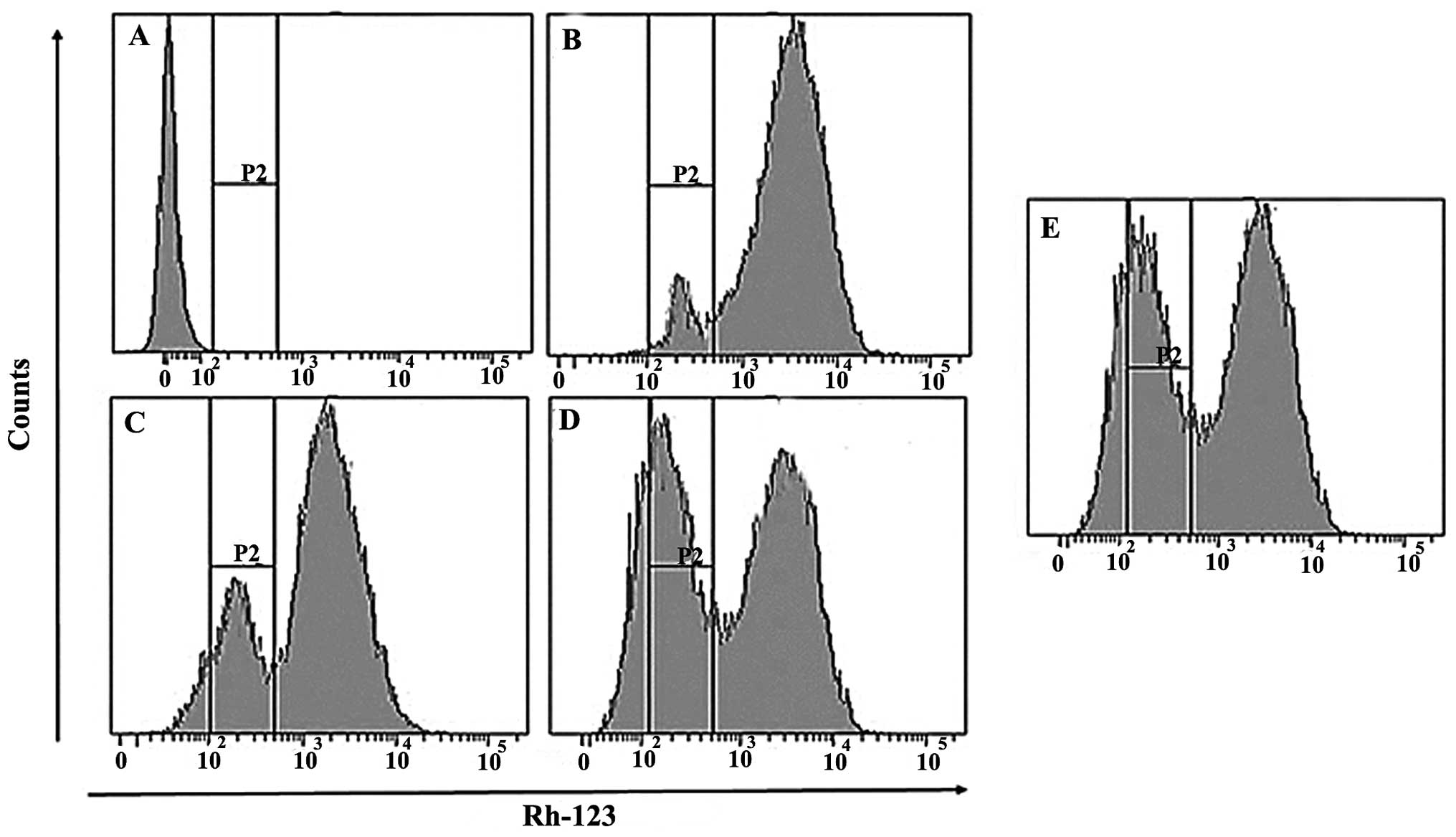
Bergamottin induces mitochondrial
membrane potential loss in lung cancer A549 cells
Rhodamine 123 is one of the most commonly used dyes
for measuring mitochondrial membrane potential (MMP) loss in cells.
In the present study, we evaluated the effect of bergamottin on MMP
loss in NSCLC cancer cells using flow cytometry. The results of the
effect of bergamottin on mitochondrial membrane potential are shown
in Fig. 12A–E, which revealed that
bergamottin induced a dose-dependent loss of MMP in the A549 cells.
The number of cells with intact mitochondrial membrane potential
decreased by 32.8 and 52.3%, respectively, at a 25 and 50 µM
dose, respectively. Fig. 12A
represents untreated cells, while Fig.
12B–E represent cells treated with 10, 25 and 50 µM and
paclitaxel (5 µM), respectively.
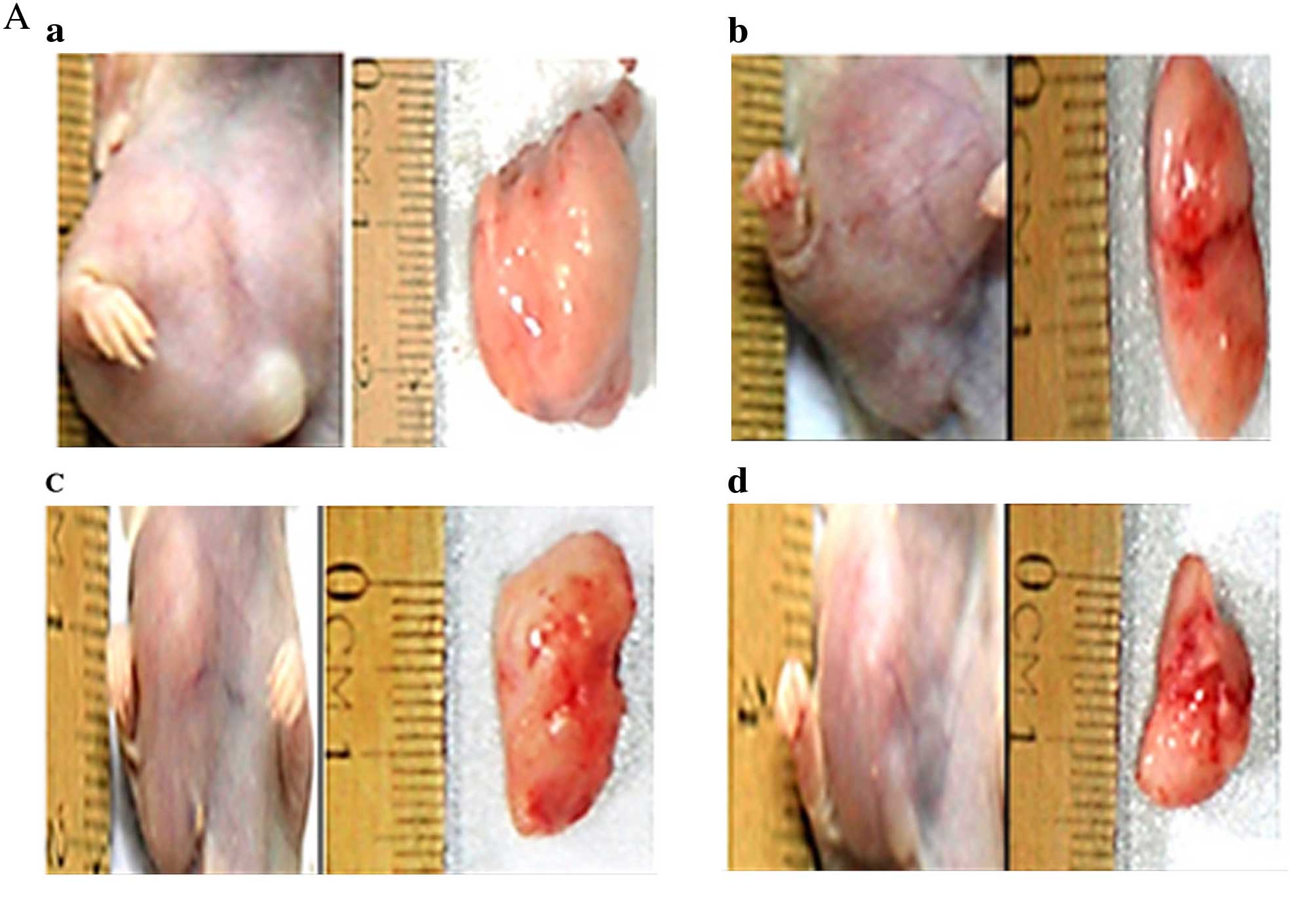
Bergamottin reduces tumor volume and
weight in female BALB/c nude mice
From the in vitro experiments, it was
confirmed that bergamottin exerts potent cytotoxic effects
inhibiting cell proliferation and inducing apoptosis. However,
there are many instances where a drug shows bioactivity in in
vitro experiments but fails when used for in vivo
experiments. Therefore, we also determined the anticancer efficacy
of this compound under in vivo conditions using female
BALB/c nude mice (a total of 20 mice were used). Tumors were
induced in the mice by injecting non-small cell lung cancer A549
cells (1×106 cells/mouse). After tumor formation, the
mice were sacrificed and tumors were removed and their weights and
volumes were calculated (Fig.
13A). The results showed that 25, 50 and 100 mg/kg bergamottin
injection reduced the tumor weight from 1.61 g in the PBS-treated
group (control) to 1.21, 0.42 and 0.15 g, respectively (Fig. 13B). Tumor weight in the nude mice
was reduced much more significantly in the highest-concentration
bergamottin group (100 mg/kg body weight) compared with the vehicle
group (P<0.05). Likewise, 25, 50 and 100 mg/kg bergamottin
injection reduced the tumor volume from 2.2 cm3 in the
PBS-treated group (control) to 1.71, 1.1 and 0.51 cm3,
respectively (Fig. 13C). The
periodic measurement of the tumor xenograft volume indicated that
the tumor volume in the nude mice was reduced considerably in the
highest-concentration bergamottin group (100 mg/kg body weight)
compared with the vehicle group (P<0.05).
Discussion
New chemotherapeutic or chemopreventive agents from
plants are potent alternative sources of anticancer drugs with the
potential to kill cancer cells. Various bioactive natural
product-based compounds inhibit cancer cell growth by upsetting the
cell cycle, which is in turn regulated and controlled by a series
of cell cycle regulators and check points. Cell cycle arrest,
encouraged by the failure of cell cycle progression, affords time
for the preservation of genomic integrity in response to DNA damage
(15). The present study revealed
that a higher percentage of A549 cells were accumulated in the G2/M
phase after treatment with increasing concentrations of
bergamottin. The population of cells in the G2/M phase of the cell
cycle increased from 1.89% in control (untreated cells) to 15.21,
28.31 and 52.12% in the A549 lung cancer cells treated with 10, 25
and 50 µM of bergamottin, respectively. Bergamottin is a
furanocoumarin compound found naturally in members of the
Citrus genus. It is most commonly associated with the
Citrus paradisi species, otherwise known as grapefruit, but
this compound was originally identified in the Citrus
bergamia or bergamot fruit. Bergamottin is a linear
furanocoumarin functionalized with side-chains derived from
geraniol.
Numerous anticancer drugs function predominantly to
induce apoptosis in cancer cells and prevent tumor development. The
morphological changes of apoptosis detected in most cell types
initially start with a decrease in cell volume and condensation of
the nucleus (16–18). Upon treatment with different doses
of bergamottin, it was observed that potent nuclear fragmentation
and chromatin condensation occurred. Tumor invasion is a collective
feature of many malignant and deadly tumors resulting in high
morbidity and death due to their high growth rate, invasive
potential and their resistance towards drug treatment. Migration
and invasion are the key features of cancer progression and
metastasis. Cell migration has previously been shown to be
regulated by numerous molecules, including PI3K, p38MAPK, pJNK and
FAK (19–21). As a result, therapeutic approaches
for preventing or suppressing cancer invasion, migration and
metastasis can significantly improve the survival of patients.
After migration, cancer cells need to disrupt and
intrude the cellular membrane to launch the metastasis process
successfully at a remote site. This cancer cell invasion is
accompanied by the degradation of the extracellular matrix, and
this damage has been credited to the activity of proteolytic
enzymes (22). Our data revealed
that bergamottin has the potential to inhibit both cancer cell
invasion as well as cancer cell migration. These cell invasion and
cell migration-inhibitory effects of bergamottin were
dose-dependent.
Apoptosis is a highly specialized biochemical
process which eliminates redundant cells from the body and as such
is the key for maintaining tissue homeostasis. Any disruption in
this process ultimately results in numerous diseases including
cancer. Both intrinsic as well as extrinsic stimuli can trigger the
process of apoptosis which finally leads to the activation of
proteases (caspases) and nucleases, resulting in destruction of the
cell (23–25). Bergamottin induced potent apoptosis
in the A549 cancer cells as revealed by inverted phase microscopy
as well as fluorescence microscopy.
In conclusion, bergamottin exhibited dose-dependent
anticancer effects by inhibiting colony formation, cell invasion
and cell migration in lung cancer A549 cells. It also induced
apoptotic effects through cell shrinkage and chromatin
condensation. Bergamottin also induced a potent cell cycle arrest
at G2/M phase of the cell cycle as well as a significant loss of
mitochondrial membrane potential in these cancer cells.
References
|
1
|
Travis WD: Pathology of lung cancer. Clin
Chest Med. 23:65–81. viii2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Butnor KJ and Beasley MB: Resolving
dilemmas in lung cancer staging and histologic typing. Arch Pathol
Lab Med. 131:1014–1015. 2007.PubMed/NCBI
|
|
3
|
Mulshine JL and Henschke CI: Prospects for
lung-cancer screening. Lancet. 355:592–593. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wright GS and Gruidl ME: Early detection
and prevention of lung cancer. Curr Opin Oncol. 12:143–148. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X, Miao X, Liang G, Hao B, Wang Y,
Tan W, Li Y, Guo Y, He F, Wei Q, et al: Polymorphisms in DNA base
excision repair genes ADPRT and XRCC1 and risk of lung cancer.
Cancer Res. 65:722–726. 2005.PubMed/NCBI
|
|
6
|
Shao M, Ma H, Wang Y, Xu L, Yuan J, Wang
Y, Hu Z, Yang L, Wang F, Liu H, et al: Polymorphisms in excision
repair cross-complementing group 4 (ERCC4) and susceptibility to
primary lung cancer in a Chinese Han population. Lung Cancer.
60:332–339. 2008. View Article : Google Scholar
|
|
7
|
Gridelli C, Perrone F, Nelli F, Ramponi S
and De Marinis F: Quality of life in lung cancer patients. Ann
Oncol. 12(Suppl 3): S21–S25. 2001. View Article : Google Scholar
|
|
8
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hwang YP, Yun HJ, Choi JH, Kang KW and
Jeong HG: Suppression of phorbol-12-myristate-13-acetate-induced
tumor cell invasion by bergamottin via the inhibition of protein
kinase Cdelta/p38 mitogen-activated protein kinase and JNK/nuclear
factor-kappaB-dependent matrix metalloproteinase-9 expression. Mol
Nutr Food Res. 54:977–990. 2010. View Article : Google Scholar
|
|
10
|
Navarra M, Ferlazzo N, Cirmi S, Trapasso
E, Bramanti P, Lombardo GE, Minciullo PL, Calapai G and Gangemi S:
Effects of bergamot essential oil and its extractive fractions on
SH-SY5Y human neuroblastoma cell growth. J Pharm Pharmacol.
67:1042–1053. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SM, Lee JH, Sethi G, Kim C, Baek SH,
Nam D, Chung WS, Kim SH, Shim BS and Ahn KS: Bergamottin, a natural
furanocoumarin obtained from grapefruit juice induces
chemosensitization and apoptosis through the inhibition of STAT3
signaling pathway in tumor cells. Cancer Lett. 354:153–163. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Girennavar B, Poulose SM, Jayaprakasha GK,
Bhat NG and Patil BS: Furocoumarins from grapefruit juice and their
effect on human CYP 3A4 and CYP 1B1 isoenzymes. Bioorg Med Chem.
14:2606–2612. 2006. View Article : Google Scholar
|
|
13
|
Ma L, Wen ZS, Liu Z, Hu Z, Ma J, Chen XQ,
Liu YQ, Pu JX, Xiao WL, Sun HD, et al: Overexpression and small
molecule-triggered downregulation of CIP2A in lung cancer. PLoS
One. 6:e201592011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pietenpol JA and Stewart ZA: Cell cycle
checkpoint signaling: Cell cycle arrest versus apoptosis.
Toxicology. 181–182:475–481. 2002. View Article : Google Scholar
|
|
16
|
Li W, Wang J, Jiang HR, Xu XL, Zhang J,
Liu ML and Zhai LY: Combined effects of cyclooxygenase-1 and
cyclooxygenase-2 selective inhibitors on ovarian carcinoma in vivo.
Int J Mol Sci. 12:668–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khoo BY, Chua SL and Balaram P: Apoptotic
effects of chrysin in human cancer cell lines. Int J Mol Sci.
11:2188–2199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moongkarndi P, Kosem N, Kaslungka S,
Luanratana O, Pongpan N and Neungton N: Antiproliferation,
antioxidation and induction of apoptosis by Garcinia mangostana
(mangosteen) on SKBR3 human breast cancer cell line. J
Ethnopharmacol. 90:161–166. 2004. View Article : Google Scholar
|
|
19
|
Lee WJ, Chen WK, Wang CJ, Lin WL and Tseng
TH: Apigenin inhibits HGF-promoted invasive growth and metastasis
involving blocking PI3K/Akt pathway and beta 4 integrin function in
MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol.
226:178–191. 2008. View Article : Google Scholar
|
|
20
|
Neudauer CL and McCarthy JB: Insulin-like
growth factor I-stimulated melanoma cell migration requires
phosphoinositide 3-kinase but not extracellular-regulated kinase
activation. Exp Cell Res. 286:128–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goncharova EA, Ammit AJ, Irani C, Carroll
RG, Eszterhas AJ, Panettieri RA and Krymskaya VP: PI3K is required
for proliferation and migration of human pulmonary vascular smooth
muscle cells. Am J Physiol Lung Cell Mol Physiol. 283:L354–L363.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goldfarb RH and Liotta LA: Proteolytic
enzymes in cancer invasion and metastasis. Semin Thromb Hemost.
12:294–307. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reed JC: Mechanisms of apoptosis. Am J
Pathol. 157:1415–1430. 2000. View Article : Google Scholar : PubMed/NCBI
|