Introduction
Glycosylation is one of the most common forms of
post-translational modifications and is essential for many cellular
functions. Changes in the composition of glycans added to
glycoproteins and glycolipids are common events in malignancy
(1,2), and these changes can affect the course
of the disease (3–5). Poly-N-acetyllactosamine
(poly-LacNAc) linkage on glycoconjugates is a unique glycan
comprised of N-acetyllactosamine (LacNAc) repeats
(Galb1-4GlcNAcb1-3) n, and is associated with cancer progression
(6). For example, it has been
reported that β1,6-branched N-glycans containing poly-LacNAc
correlate with a variety of malignant phenotypes of tumor cells,
and affect cell proliferation (7)
and metastatic potential (8–10). In
addition, poly-LacNAc may be modified to carry important
carbohydrate structures such as Lewis-related antigens (11–14)
and human natural killer-1 (HNK-1) antigen (15). Poly-LacNAc and its related
structures may alter structural and functional characteristics of
proteins that carry them and play important roles in cell-cell
interaction, cell-extracellular matrix (ECM) interaction (16) and metastatic capacity (17). Notably, highly metastatic colon cell
lines were found to synthesize more N-glycans that contain
poly-LacNAc than poorly metastatic colon cell lines (18).
The presence and elongation of poly-LacNAc have been
attributed to the overexpression of a number of
β1,3-N-acetylglucosaminyltransferases (β3Gn-Ts). These
enzymes have different tissue distribution and different receptor
substrate specificity (19), but
all utilize UDP-N-acetylglucosamine (UDP-GlcNAc) as the
donor to transfer LacNAc to the non-reducing terminus of Gal to
form β1,3 linkage. Among these enzymes, β3Gn-T8 was first cloned by
our laboratory and was responsible for the synthesis of poly-LacNAc
chains on β1,6 branched N-glycans (20,21).
Our previous studies confirmed that β3Gn-T8 is abnormally expressed
in a variety of tumor cells, and influences cancer invasion and
metastasis ability by regulating matrix metalloprotein 2 (MMP2)
expression (22,23). We also found that another
glycoprotein CD147 was modified by β3Gn-T8 and was associated with
cancer metastatic potential in colon cancer cells (24). CD147 has been shown to be a
glycoprotein that carries a large amount of poly-LacNAc chains on
its N-glycans by LC/MS techniques in a variety of cancer
cell lines (25). However, how
β3Gn-T8 expression is regulated has not yet been reported.
Bioinformatic studies revealed that most glycosyltransferase genes
have TATA-less, CpG-associated promoters (26) and we found oncogenic transcription
factor Ets-1 and c-jun binding sites within the β3Gn-T8 promoter
region. In our preliminary experiments, no definite evidence was
obtained that β3Gn-T8 was regulated by the transcription factor
Ets-1 in gastric cancer cells (data not shown). Therefore, we
investigated whether β3Gn-T8 expression was mainly regulated by the
transcription factor c-jun in the SGC-7901 gastric cancer cell
line.
In the present study, three bioinformatics online
software tools (AliBaba 2.1, TESS and PATCH, data not shown) were
employed to predict the binding sites of transcription factors to
the β3Gn-T8 promoter. One transcription factor, c-jun, emerged as a
potential regulator of β3Gn-T8 expression. Luciferase reporter
assay, chromatin immunoprecipitation (ChIP) assay and point
mutation analysis were used to confirm the binding of c-jun on the
β3Gn-T8 promoter. Meanwhile, we also found that c-jun could
regulate the expression and enzymic activity of β3Gn-T8 and
N-glycans of HG-CD147 in the SGC-7901 cells. In addition, we
further demonstrated that c-jun is positively correlated with
β3Gn-T8 in human cancer tissues.
Materials and methods
Materials
Gastric cancer cell line SGC-7901 was preserved in
our laboratory. Plasmid pCI-neo, pGL3-basic-luc (pGL3) pRL-SV40 and
Dual-Luciferase Reporter Gene Assay kit were purchased from Promega
(Madison, WI, USA). RPMI-1640 medium was obtained from Gibco-BRL
(USA), and transfection reagent Lipofectamine 2000 and primers were
procured from Invitrogen. ChIP assay kit was purchased from
Beyotime Institute of Biotechnology (China) and c-jun antibody from
Abcam (Hong Kong).
Patients and samples
A total of 97 patient specimens were obtained from
the First and Second Affiliated Hospitals of Soochow University
between January 1, 2008 and March 31, 2010. In all cases, the
specimens obtained were inspected independently by two pathologists
according to the classification of gastric cancer by Lauren's
system. The clinical and pathological data collected included
gender, age, clinical stage (AJCC, American Joint Committee on
Cancer), histological grade, histological type (Lauren), depth of
invasion, and presence of lymph node metastasis. Ethical approval
was obtained from the First and Second Affiliated Hospitals and the
study was approved by the Soochow University Research Ethics
Committee.
Cell culture
The SGC-7901 cell line was cultured in RPMI-1640
medium containing 10% fetal calf serum, 100 µg/ml penicillin
and 100 µg/ml streptomycin in a water-saturated, 5%
CO2 atmosphere at 37°C.
Cloning and plasmid construction
The putative promoter region of the human β3Gn-T8
gene was amplified by PCR and cloned into a pGL3 vector, to
construct the recombinant vectors pGL3-luc (−1449/+107), pGL3-luc
(−947/+107), pGL3-luc (−760/+107), pGL3-luc (−561/+107), pGL3-luc
(−503/+107), pGL3-luc (−393/+107), pGL3-luc (−248/+107) and a
mutant plasmid pGL3-luc (−561/+8). Point mutations were generated
with the QuickChange II XL Site-Directed Mutagenesis kit
(Stratagene) using pGL3-561/+8-luc as a template. All plasmids were
confirmed by DNA sequencing.
Transient transfection and
dual-luciferase activity assay
The cells were cultured in 24-well plates at
1×105 cells/well on the day prior to transfection.
Plasmids were extracted, measured for concentration, and
transfected into SGC-7901 cells using Lipofectamine 2000. The cells
were co-transfected with 500 ng pCI-neo-jun vector, 500 ng
pGL3-β3Gn-T8-promoter vectors and 50 ng pRL-SV40 vector. After
transfection (48 h), the cells were lysed with 500 µl of
lysis buffer. Dual-luciferase activity assays were performed
according to the Dual-Luciferase Reporter Assay System technical
manual. The relative luciferase activity (firefly
luciferase/Renilla luciferase) of the transfected cells in
each group was determined with the Thermo Scientific Fluoroskan
Ascent FL.
Chromatin immunoprecipitation assays
The SGC-7901 cells were used for the ChIP assays. We
used the Beyotime Chip Assay kit and followed the manufacturer's
instructions. The ChIP analysis was conducted using antibodies
against c-jun and IgG. After the ChIP assessment, the samples were
purified using the PCR/DNA purification kit and products were
subjected to PCR amplification with the following primer sequences:
sense, 5′-TGTACGCGTGAGGCACATGGCAAAGG-3′ and anti-sense,
5′-GTTCTCGAGAGTGGGGAGGAAGTGGT-3′. The PCR products were subjected
to 1.5% agarose gel electrophoresis, and a gel imaging system was
used to analyze the bands.
RT-PCR
Total RNA from each experimental group of cells was
extracted using TRIzol (Gibco-BRL) according to the manufacturer's
instructions. cDNA was generated from total RNA using M-MLV Reverse
Transcriptase (Fermentas, USA). Amplification was performed for
>28 cycles. PCR products were separated by electrophoresis on a
1.5% agarose gel and stained with ethidium bromide to visualize the
bands. Primer sequences and expected product sizes are listed in
Table I.
 | Table IPrimer sequences for RT-PCR
analysis. |
Table I
Primer sequences for RT-PCR
analysis.
| Gene | Primer
sequences | Size (bp) |
|---|
| c-jun | Sense:
5′-GCCTCAGACAGTGCCCGAGAT-3′ | 245 |
| Antisense:
5′-GTTTAAGCTGTGCCACCTGTTCC-3′ | |
| β3Gn-T8 | Sense:
5′-CCCTGACTTCGCCTCCTAC-3′ | 362 |
| Antisense:
5′-GGTCTTTGAGCGTCTGGTTGA-3′ | |
| GAPDH | Sense:
5′-TGAACGGGAAGCTCACTGG-3′ | 307 |
| Antisense:
5′-TCCACCACCCTGTTGCTGTA-3′ | |
Western blot analysis
Western blot analysis was performed as previously
described (23,24). In brief, the cells were lysed with
lysis buffer and 40 µg of protein from each sample was
resolved by 10% SDS-PAGE gel and transferred to a nitrocellulose
membrane (Millipore, Bedford, MA, USA). The membrane was blotted
with antibodies β3Gn-T8, GAPDH, c-jun and CD147 (all purchased from
Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Flow cytometric analysis
For poly-LacNAc chain analysis, biotin-labeled LEA
(Sigma, USA), which is specific for poly-LacNAc residues, was used.
Cells were harvested and stained with 10 µg/ml LEA PBS
(containing 0.5% BSA and 0.05% sodium azide) at 37°C for 1 h. After
being washed three times with PBST (PBS + 0.05% Tween-20), the
cells were then stained with 10 µg/ml phycoerythrin
(PE)-conjugated streptavidin (Sigma) at 37°C for 1 h and washed
another three times with PBST. The fluorescence intensity of the
stained cells was measured with a flow cytometer and analyzed with
CellQuest (BD Biosciences, USA).
Immunofluorescence staining
Biotin-labeled LEA was used in this experiment to
examine poly-LacNAc chains on the cell surface. In brief, cells
were incubated with biotinylated Lycopersicon esculentum
(tomato) lectin (20 µg/ml) for 2 h at room temperature and
then incubated with streptavidin-R-phycoerythrin (0.4 µg/ml,
Sigma) for 1 h at room temperature. Images were obtained using an
inverted fluorescence microscope combined with a digital
camera.
Lectin blot analysis
The levels of poly-LacNAc were analyzed by lectin
blot analysis. In brief, the cells were lysed and cell extracts
were separated using 10% SDS-PAGE gel electrophoresis, and
transferred onto nitrocellulose membranes. The membranes were
incubated with biotinylated LEA (1:400 dilution) for 1 h and then
incubated with streptavidin-HRP (1:1,000 dilution) for 1 h. The
protein bands on the membranes were visualized using an ECL kit (GE
Healthcare).
Tissue microarrays and
immunohistochemistry
Tissue microarrays were constructed using 97 gastric
adenocarcinoma specimens paired with 89 adjacent non-tumor gastric
mucosa 5 cm away from the adenocarcinoma (eight samples of adjacent
non-tumor gastric tissues were lost). Immunohistochemical staining
was performed on 4-µm sections of paraffin-embedded tissue
samples to detect the expression levels of β3Gn-T8, c-jun, MMP2,
and tissue inhibitors of matrix metalloproteinases 2 (TIMP2)
protein. In brief, the slides were incubated in β3Gn-T8, c-jun
MMP2, and TIMP2 antibodies diluted to 1:100–300 at 4°C overnight.
The subsequent steps were performed using the EnVision™ FLEX High
pH 9.0 visualization system (Dako, Demark).
Statistical analysis
The results are presented as means ± SD. P<0.05
was considered to indicate statistically significant differences.
SPSS 13.0 was used for statistical analysis. The intensities of the
protein expression levels in gastric cancer and adjacent non-tumor
gastric tissue were compared with the chi-square test (McNemar's
test). The relationship between the intensity of protein expression
and clinical pathological parameters was analyzed with the
chi-square test.
Results
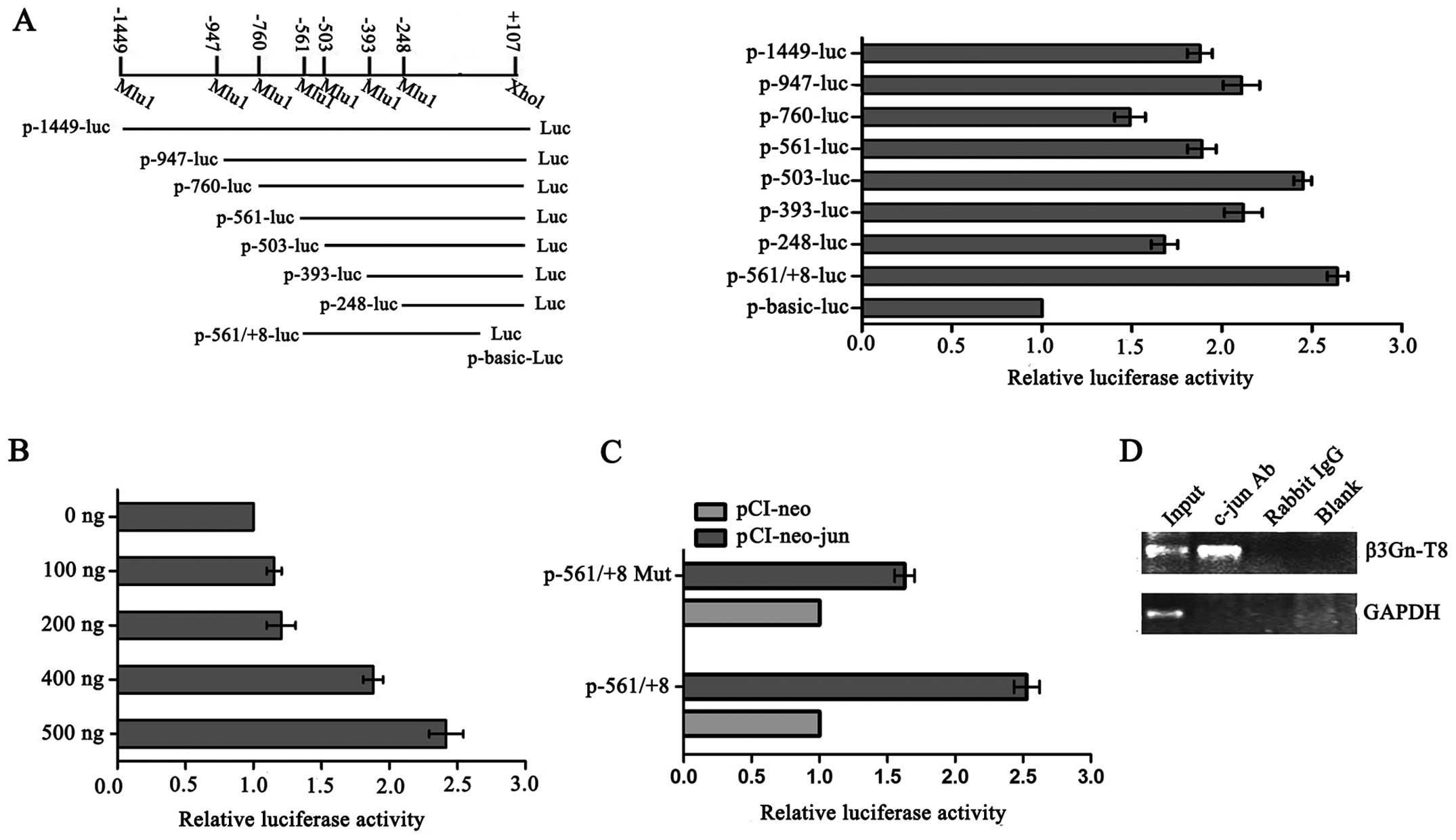
c-jun regulates β3Gn-T8 promoter activity
by directly binding to the β3Gn-T8 promoter
To map the c-jun binding site on the β3Gn-T8
promoter, a series of β3Gn-T8 promoter segments were generated and
analyzed by co-transfection with c-jun. Promoter fragments ranging
from −1449, −947, −760, −561, −503, −393, −248, −561 to +107
(related to the transcription start site) were cloned into a pGL3
vector upstream of a luciferase reporter gene and assessed for
their transcriptional activity in the SGC-7901 cell line. As shown
in Fig. 1A, compared with other
promoter segments, −561/+8 caused the biggest changes in the
reporter activity, and with the increasing amount of exogenous
c-jun, the reporter activity of the −561/+8 deletion mutant was
also gradually increased (Fig. 1B).
Furthermore, point mutations within this element were constructed
and luciferase assays were performed again (Fig. 1C). When we mutated the
TGAGTCA/TTAATCA conservative sequence (−160~−154), which is
critical for the binding of c-jun transcription factors, the
promoter activity was markedly reduced in the SGC-7901 cells. These
results suggest that −561/+8 is a potential c-jun binding sequence
on the β3Gn-T8 promoter. We next carried out a ChIP assay to
examine the in vivo relevance of c-jun's binding to the
β3Gn-T8 promoter. With an anti-c-jun antibody, immunoprecipitated
chromosomal DNA was subjected to RT-PCR. The results showed that
c-jun indeed interacted with the β3Gn-T8 promoter region in the
SGC-7901 cell line (Fig. 1D).
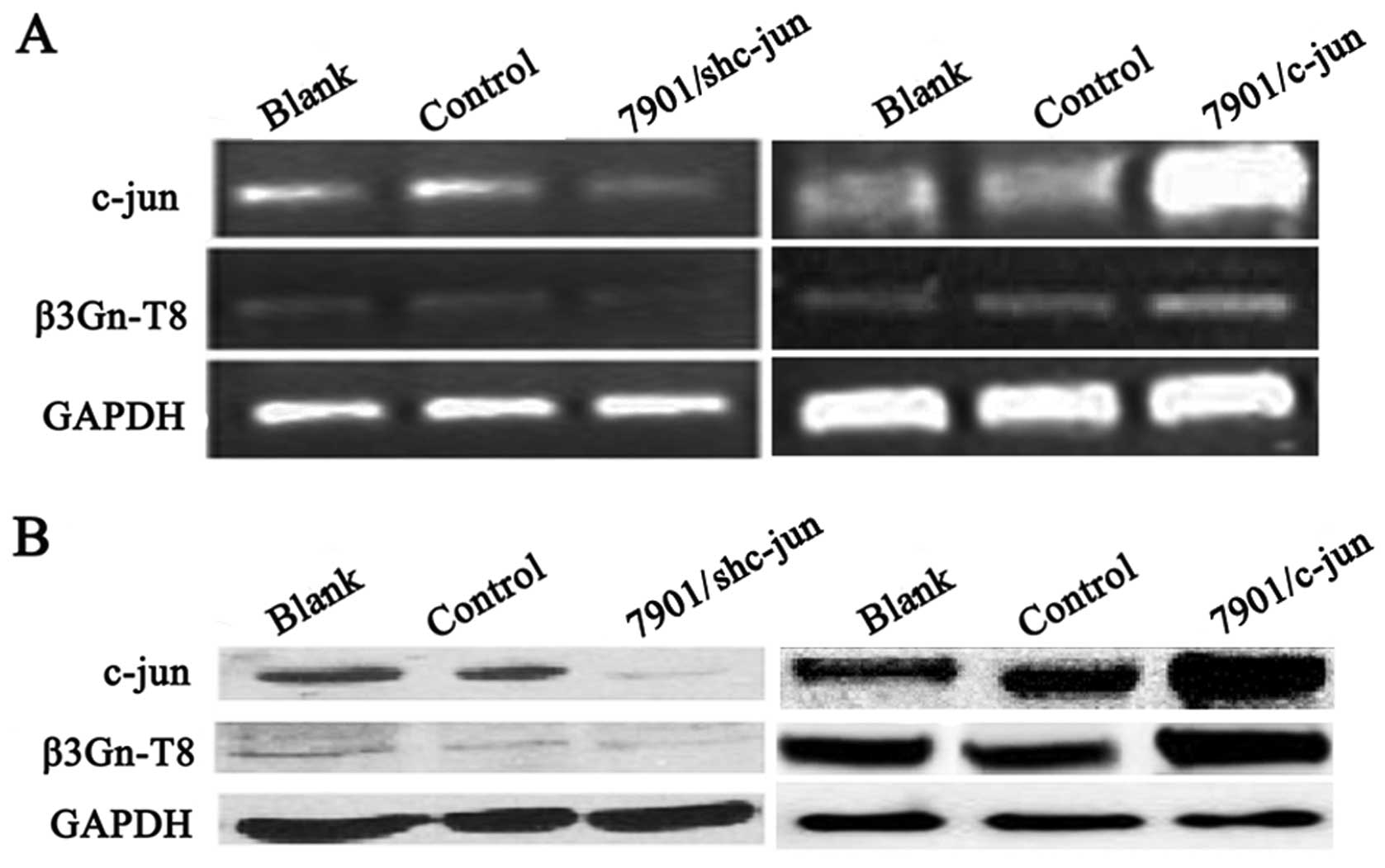
Correlation between c-jun and β3Gn-T8
expression in SGC-7901 cells
Next we explored whether c-jun could regulate
β3Gn-T8 transcription and protein expression in the SGC-7901 cells.
Firstly, stable cell lines with overexpression and
interference-expression of c-jun were established in the SGC-7901
cell line. As shown in Fig. 2, the
c-jun mRNA and protein levels in the SGC-7901 cells were measured
by RT-PCR and western blot analysis, respectively. When compared
with control groups, c-jun expression was significantly decreased
in the interference vector-transfected cells (7901/c-junSi) and
increased in the pCI-neo-c-jun expression vector-transfected cells
(7901/c-jun). Notably, c-jun overexpression upregulated β3Gn-T8
expression, and silencing downregulated β3Gn-T8 expression. These
results indicated that β3Gn-T8 expression was at least partially
regulated by c-jun.
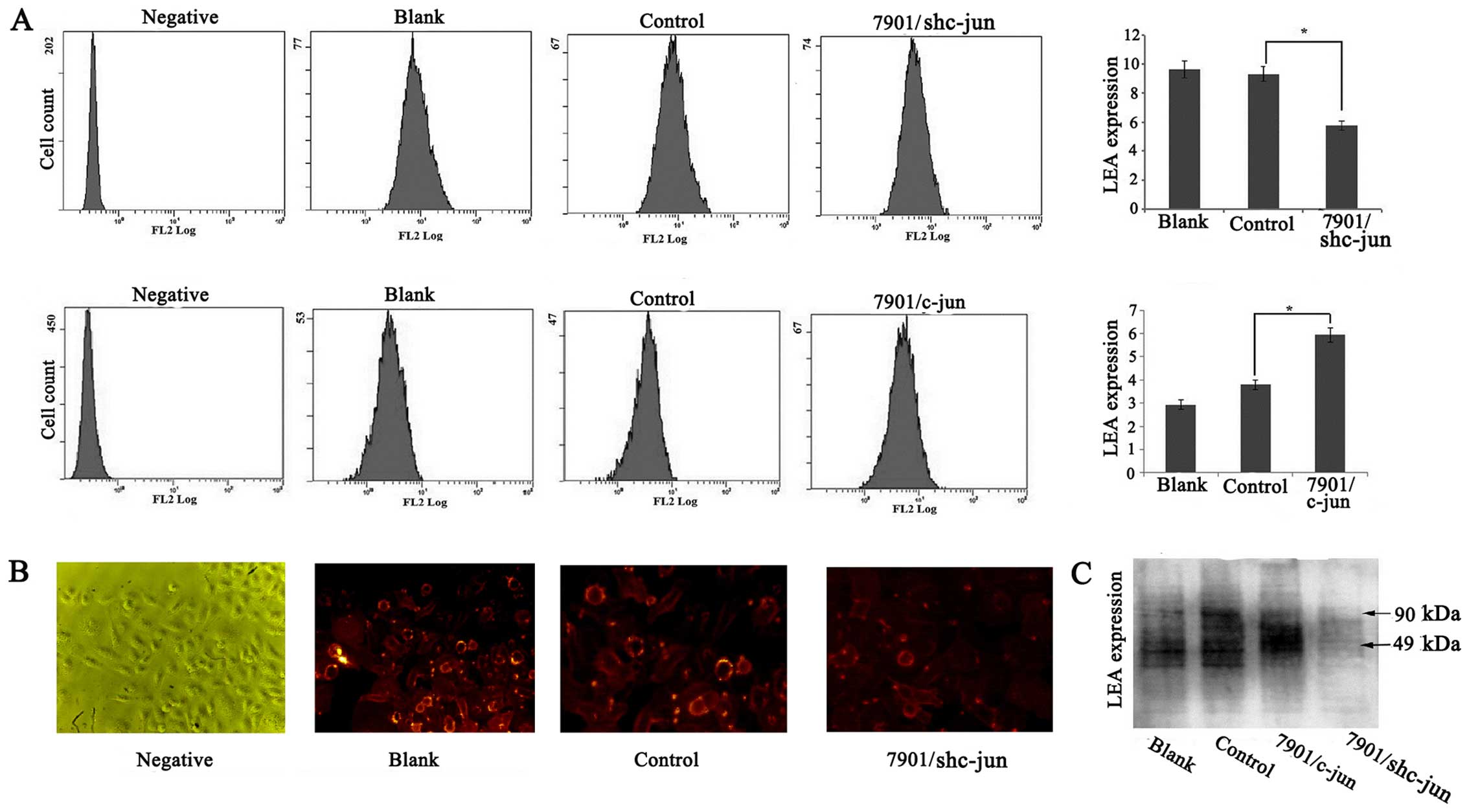
Effect of c-jun on the poly-LacNAc
expression in the SGC-7901 cells
β3Gn-T8 is involved in the synthesis of poly-LacNAc
chains, and hence, we investigated whether c-jun expression could
influence poly-LacNAc chain formation. The level of poly-LacNAc on
the cell membrane was detected by flow cytometric analysis. As
shown in Fig. 3A, the level of
poly-LacNAc in the SGC-7901/c-junSi cells was significantly
decreased compared with the level noted in the control groups but
was increased in the SGC-7901/c-jun cells (p<0.05). In order to
confirm the results, immunofluorescence staining was also performed
to examine the alteration of poly-LacNAc chains in the
SGC-7901/c-junSi cells and we obtained similar results (Fig. 3B). To further confirm the
relationship of c-jun and poly-LacNAc, lectin blot analysis was
used to detect whole poly-LacNAc expression in the SGC-7901 cells.
As shown in Fig. 3C, compared to
the control group, c-jun overexpression upregulated the
glycoprotein modified by poly-LacNAc, and c-jun silencing
downregulated the glycoprotein modified by poly-LacNAc expression.
In addition, the molecular size of glycoproteins regulated by c-jun
ranged from 49 to 90 kDa. These results indicated that c-jun may
affect poly-LacNAc expression and glycoprotein modified by
poly-LacNAc ranged from 49 to 90 kDa through regulation of β3Gn-T8
expression and enzymatic ability.
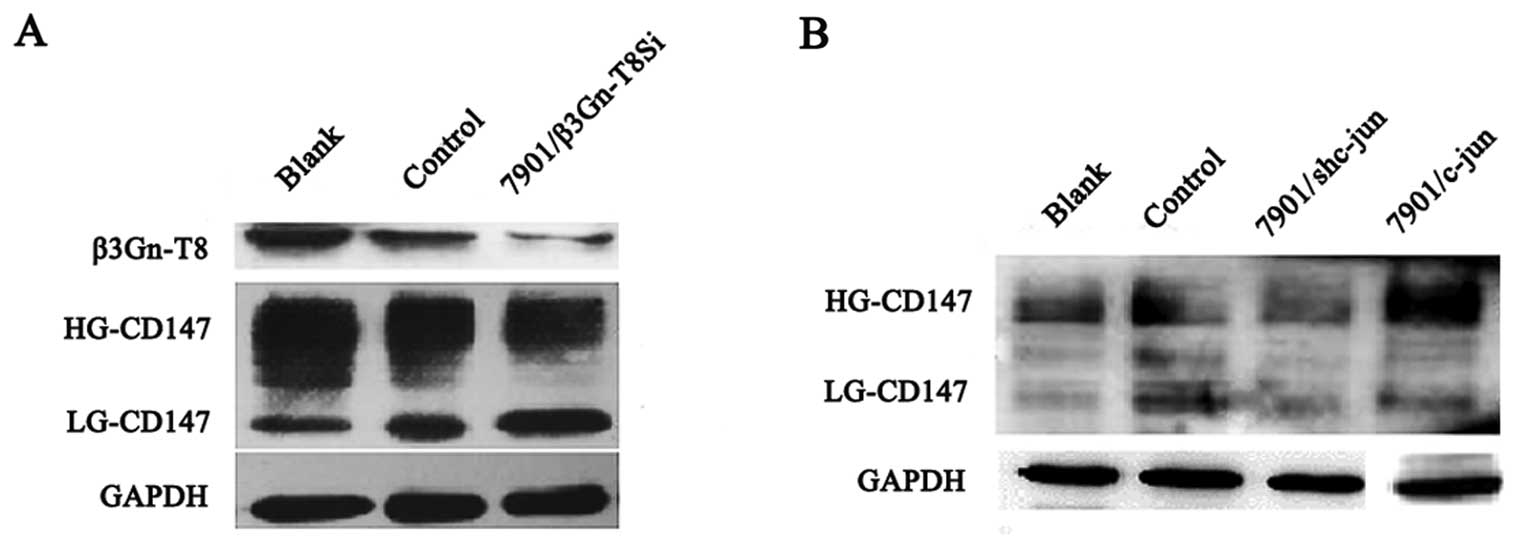
Correlation between c-jun and CD147
expression in SGC-7901 cells
To study the co-expression relationship between
β3Gn-T8 and CD147, western blot analysis was used to assess β3Gn-T8
and CD147 expression. As shown in Fig.
4A, the level of glycosylation of HG-CD147 was reduced
apparently with silenced β3Gn-T8 expression when compared to the
control group. The changes in the N-glycans of HG-CD147
indicated that β3Gn-T8 may be involved in the synthesis of
poly-LacNAc on N-glycans of HG-CD147 in the SGC-7901 cells.
To further study the correlation between c-jun and CD147
expression, c-jun was upregulated or downregulated in the SGC-7901
cell line. As shown in Fig. 4B, the
level of glycosylation of HG-CD147 was decreased with the silencing
of c-jun expression compared with the control cells but increased
in the c-jun-upregulated cells. The molecular size of HG-CD147 (55
kDa) was also in the range (49–90 kDa) of the glycoproteins
modified by poly-LacNAc by the aforementioned lectin blot analysis.
We speculated that c-jun affects N-glycans of HG-CD147
through regulation of β3Gn-T8 expression in the SGC-7901 cell
line.
c-jun and β3Gn-T8 expression and
clinicopathological features of gastric cancer
To investigate the clinical importance of c-jun and
β3Gn-T8 in gastric cancer tissues, we performed immunohistochemical
analysis in 97 human gastric cancer tissues and 89 matched adjacent
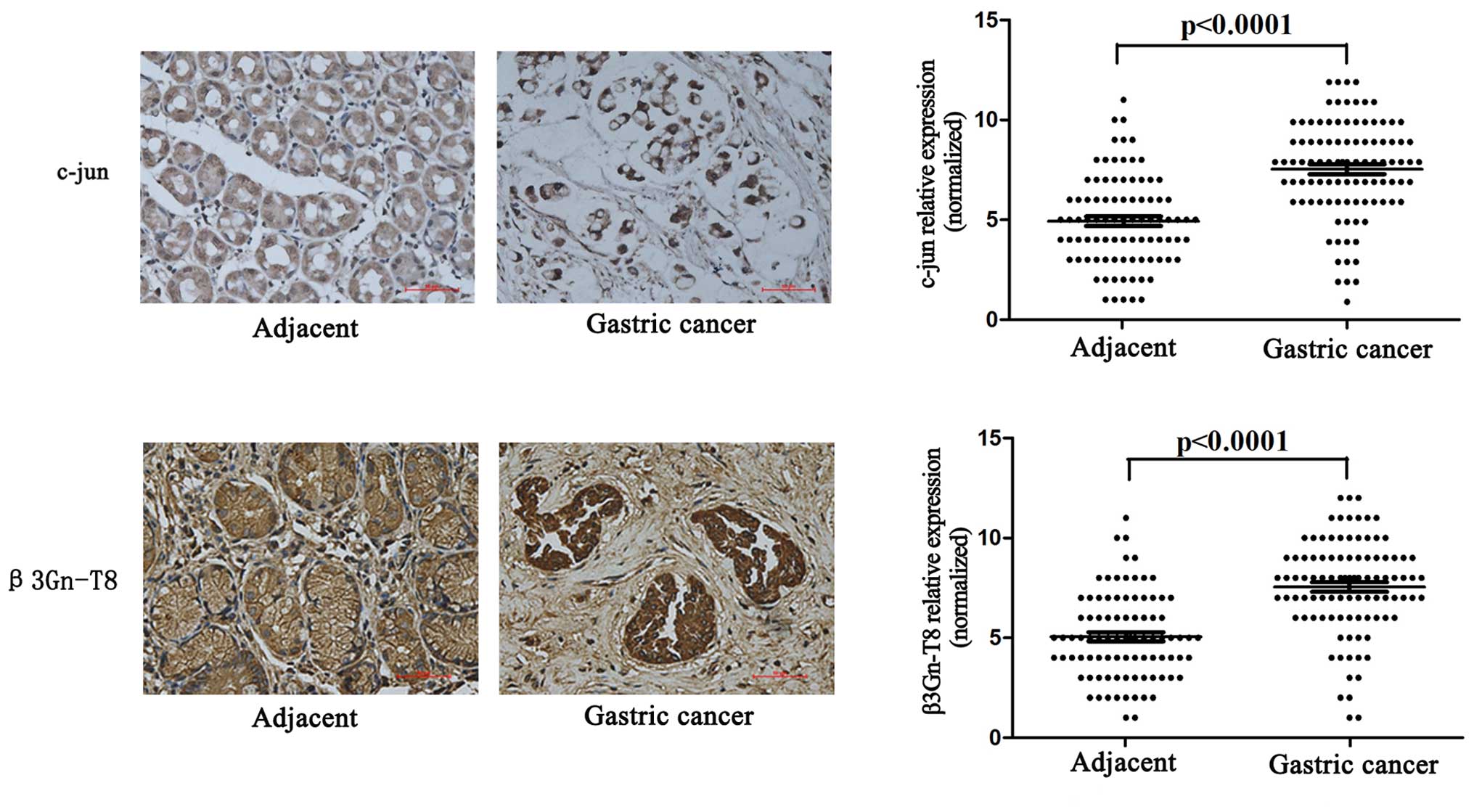
tissues (eight adjacent tissues were lost). As shown in Fig. 5, the expression of c-jun and β3Gn-T8
in gastric cancer tissues was significantly higher than that noted
in the adjacent tissues (p<0.001). Furthermore, c-jun and
β3Gn-T8 expression was related to clinicopathological features. The
characteristics of the 97 patients included in this study are
described in Table II. c-jun and
β3Gn-T8 expression were positively correlated with TNM stage
(AJCC), depth of invasion and lymph node metastasis (p<0.05).
There was no significant association between c-jun and β3Gn-T8
expression when comparing age, gender, histological grade and
Lauren type (p>0.05).
 | Table IIRelationship between expression of
β3Gn-T8 and c-jun and clinicopathological parameters of the gastric
cancer cases. |
Table II
Relationship between expression of
β3Gn-T8 and c-jun and clinicopathological parameters of the gastric
cancer cases.
| Clinicopathological
parameters | No. | High expression of
c-jun n (%) | P-value | High expression of
β3Gn-T8 (%) n (%) | P-value |
|---|
| Age (years) | | | 0.662 | | 0.879 |
| ≥60 | 63 | 54 (86) | | 53 (84) | |
| <60 | 34 | 28 (82) | | 29 (85) | |
| Gender | | | 0.516 | | 0.752 |
| Female | 29 | 25 (86) | | 24 (82) | |
| Male | 68 | 57 (84) | | 58 (85) | |
| TNM stage
(AJCC) | | | 0.000b | | 0.001b |
| I–II | 33 | 21 (64) | | 22 (66) | |
| III–IV | 64 | 61 (95) | | 60 (93) | |
| Depth of
invasion | | | 0.001a | | 0.011a |
| T1 to T2 | 21 | 13 (62) | | 14 (66) | |
| T3 to T4 | 76 | 69 (91) | | 68 (89) | |
| Lymph node
metastasis | | | 0.017b | | 0.003b |
| Yes | 70 | 63 (90) | | 64 (91) | |
| No | 27 | 19 (70) | | 18 (66) | |
| Histological
grade | | | 0.107 | | 0.305 |
| High or
moderate | 34 | 26 (76) | | 27 (79) | |
| Low | 63 | 56 (89) | | 55 (87) | |
| Lauren type | | | 0.280 | | 0.633 |
| Intestinal | 66 | 54 (82) | | 55 (83) | |
| Diffuse | 31 | 28 (90) | | 27 (87) | |
We further investigated whether the expression of
β3Gn-T8 was correlated with that of c-jun and invasion-related
proteins MMP2 and TIMP2 in the gastric cancer tissue samples. As
shown in Table III, the β3Gn-T8
protein expression level was significantly correlated with those of
c-jun (r=0.842; P=0.01), MMP2 (r=0.703; P=0.000), and TIMP2
(r=−0.298; P=0.021).
 | Table IIICorrelation between β3Gn-T8 and
c-jun, MMP2 and TIMP2 expression levels in gastric cancer. |
Table III
Correlation between β3Gn-T8 and
c-jun, MMP2 and TIMP2 expression levels in gastric cancer.
| | c-jun | MMP2 | TIMP2 |
|---|
| β3Gn-T8 | r | 0.842 | 0.703 | −0.298 |
| P-value | 0.011 | 0.000 | 0.021 |
Discussion
Nearly all proteins that are expressed on the plasma
membrane or secreted carry glycans that are involved in cell
adhesion, recognition, molecular trafficking, clearance, and
signaling (27). Aberrant
glycosylation occurs in essentially all types of human cancer and
appears to be an early event as well as a key factor in the
induction of invasion and metastasis (1–5,28).
Changes in glycosylation that occur in cancer can also alter
molecular interactions with the immune system (29) and receptor signaling. Thus,
increased expression of β3Gn-T8, which catalyzes the formation of
poly-LacNAc glycans, may play an important role in the promotion
and progression of cancer. β3Gn-T8 was found to be expressed in
various human tissues. Notably, Ishida et al (21) reported that expression of β3Gn-T8 is
quite low in normal colon tissues, but increases markedly in colon
cancer tissues. Our results indicated that the enzyme was expressed
significantly higher in some tumor tissues than in normal tissues
(30). Knockdown of β3Gn-T8
expression by RNAi reduced the tumorgenicity of gastric cancer
cells in nude mice (31). Moreover,
overexpression of β3Gn-T8 promoted cancer invasion and metastasis
ability in AGS gastric cancer (22), U251 glioma (23), LS-174T and LoVo colon cancer cells
(24).
To date, little is known concerning the regulation
of β3Gn-T8 expression in gastric cancer cells. Analysis of the
promoter region of β3Gn-T8 identified binding sites for the
ubiquitous transcription factor c-jun predicted by three
bioinformatics softwares, AliBaba 2.1, TESS and PATCH (data not
shown). AP-1 is a sequence-specific transcriptional factor composed
of Fos and Jun family members, which form homodimers or
heterodimers to recognize the AP-1 site or related sequence. As one
of the major subunits of the AP-1 complex, c-jun was reported to be
upregulated in various human cancers (32). Recent studies suggest that the AP-1
signaling pathway plays an important role in the regulation of cell
proliferation, apoptosis and malignant transformation, and is also
involved in tumor formation, invasion and metastasis (33–35).
In the present study, luciferase assay and ChIP analysis showed
that β3Gn-T8 promoter activity was regulated by c-jun in a
dose-dependent manner in the region of −561/+8 (Fig. 1). Furthermore, to investigate
whether c-jun actually regulates β3Gn-T8 transcription, c-jun
expression was upregulated or downregulated and the β3Gn-T8
expression was also increased or decreased accordingly (Fig. 2). In addition, this change in
expression also led to changes in the formation of poly-LacNAc
chains on glycoconjugates (Fig. 3).
All these results suggest that β3Gn-T8 expression may be regulated
by c-jun.
It has been reported that CD147 is a cell surface
trans-membrane glycoprotein carrying β1,6 branched poly-LacNAc
chains on its N-glycans (36) and may act as the substrate for
β3Gn-T8 in colon cancer cells (24). CD147 is highly expressed in various
human carcinoma tissues and cell lines, and is correlated with
tumor progression under experimental and clinical conditions
(37). It has been confirmed that
all CD147 glycosylation is N-linked. A high-glycosylated
form HG-CD147 (~40–60 kDa) contains complex-type carbohydrates,
while the low-glycosylated form LG-CD147 (~32 kDa) contains the
high-mannose form (36). It has
been reported that HG-CD147 plays an important role in the
induction of MMPs, thereby leading to extracellular matrix
degradation and increased tumor growth and metastasis (38). In addition, HG-CD147 was found to
contribute to lymphatic metastasis potential in mouse
hepatocarcinoma cells by altering the level of N-glycans
(39). Moreover, N-glycans
of HG-CD147 mainly carry β1,6-branched structures, which are formed
by GnT-V. The GnT-V product is the preferred substrate for
extension with poly-LacNAc chains (40). In the present study, the level of
glycosylation on HG-CD147 was greatly reduced with silencing of
β3Gn-T8 expression when compared to the wild-type and mock group in
the SGC-7901 cell line (p<0.05) (Fig. 4A), indicating that the
N-glycans of CD147 contain β1,6-branched poly-LacNAc
catalyzed by β3Gn-T8 in SGC-7901 cells. Notably, N-glycans
of HG-CD147 were decreased with silenced c-jun expression compared
with the control cells but increased in the c-jun-upregulated cells
(Fig. 4B). All of these results
suggest that c-jun affects N-glycans of HG-CD147 through the
regulation of β3Gn-T8 expression in the SGC-7901 cells.
In summary, the present study demonstrated that the
transcription factor c-jun could bind to the β3Gn-T8 promoter and
activate β3Gn-T8 expression, and further regulate the
N-glycans of HG-CD147 in the SGC-7901 cell line.
Furthermore, c-jun and β3Gn-T8 were both upregulated in the gastric
cancer tissues, and their expression also had a positive
correlation with each other. Therefore, it can be concluded that
the significance of c-jun in malignant potential such as tumor cell
invasion can be ascribed at least partially to the increased
expression of β3Gn-T8. Prevention of β3Gn-T8 as well as c-jun
activity would provide a novel strategy for gastric cancer
therapy.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (nos. 31170772 and 31400688) and Suzhou
Municipal Natural Science Foundation (SYS201208).
References
|
1
|
Hakomori S: Glycosylation defining cancer
malignancy: New wine in an old bottle. Proc Natl Acad Sci USA.
99:10231–10233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burchell JM, Mungul A and
Taylor-Papadimitriou J: O-linked glycosylation in the mammary
gland: Changes that occur during malignancy. J Mammary Gland Biol
Neoplasia. 6:355–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ono M and Hakomori S: Glycosylation
defining cancer cell motility and invasiveness. Glycoconj J.
20:71–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Picco G, Julien S, Brockhausen I, Beatson
R, Antonopoulos A, Haslam S, Mandel U, Dell A, Pinder S,
Taylor-Papadimitriou J, et al: Overexpression of ST3Gal-I promotes
mammary tumorigenesis. Glycobiology. 20:1241–1250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mungul A, Cooper L, Brockhausen I, Ryder
K, Mandel U, Clausen H, Rughetti A, Miles DW, Taylor-Papadimitriou
J and Burchell JM: Sialylated core 1 based O-linked glycans enhance
the growth rate of mammary carcinoma cells in MUC1 transgenic mice.
Int J Oncol. 25:937–943. 2004.PubMed/NCBI
|
|
6
|
Seko A and Yamashita K: Activation of
beta1,3-N-acetylglu-cosaminyltransferase-2 (beta3Gn-T2) by
beta3Gn-T8. Possible involvement of beta3Gn-T8 in increasing
poly-N-acetyllactosamine chains in differentiated HL-60 cells. J
Biol Chem. 283:33094–33100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dennis JW, Laferté S and Vanderelst I:
Asparagine-linked oligosaccharides in malignant tumour growth.
Biochem Soc Trans. 17:29–31. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dennis JW and Laferté S: Oncodevelopmental
expression of -GlcNAc beta 1-6Man alpha 1-6Man beta 1- branched
asparagine-linked oligosaccharides in murine tissues and human
breast carcinomas. Cancer Res. 49:945–950. 1989.PubMed/NCBI
|
|
9
|
Seberger PJ and Chaney WG: Control of
metastasis by Asn-linked, beta1-6 branched oligosaccharides in
mouse mammary cancer cells. Glycobiology. 9:235–241. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Granovsky M, Fata J, Pawling J, Muller WJ,
Khokha R and Dennis JW: Suppression of tumor growth and metastasis
in Mgat5-deficient mice. Nat Med. 6:306–312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zamze S, Harvey DJ, Chen YJ, Guile GR,
Dwek RA and Wing DR: Sialylated N-glycans in adult rat brain tissue
- a widespread distribution of disialylated antennae in complex and
hybrid structures. Eur J Biochem. 258:243–270. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niemelä R, Natunen J, Penttilä L, Salminen
H, Helin J, Maaheimo H, Costello CE and Renkonen O: Isolation and
characterization of linear polylactosamines containing one and two
site-specifically positioned Lewis x determinants: WGA agarose
chromatography in fractionation of mixtures generated by random,
partial enzymatic alpha3-fucosylation of pure polylactosamines.
Glycobiology. 9:517–526. 1999. View Article : Google Scholar
|
|
13
|
Nishihara S, Iwasaki H, Kaneko M, Tawada
A, Ito M and Narimatsu H: Alpha1,3-fucosyltransferase 9 (FUT9;
Fuc-TIX) preferentially fucosylates the distal GlcNAc residue of
polylactosamine chain while the other four alpha1,3FUT members
preferentially fucosylate the inner GlcNAc residue. FEBS Lett.
462:289–294. 1999. View Article : Google Scholar
|
|
14
|
Dennis JW, Granovsky M and Warren CE:
Glycoprotein glycosylation and cancer progression. Biochim Biophys
Acta. 1473:21–34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamoto S, Oka S, Inoue M, Shimuta M,
Manabe T, Takahashi H, Miyamoto M, Asano M, Sakagami J, Sudo K, et
al: Mice deficient in nervous system-specific carbohydrate epitope
HNK-1 exhibit impaired synaptic plasticity and spatial learning. J
Biol Chem. 277:27227–27231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Castronovo V, Luyten F, van den Brûle F
and Sobel ME: Identification of a 14-kDa laminin binding protein
(HLBP14) in human melanoma cells that is identical to the 14-kDa
galactoside binding lectin. Arch Biochem Biophys. 297:132–138.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dennis JW, Carver JP and Schachter H:
Asparagine-linked oligosaccharides in murine tumor cells:
Comparison of a WGA-resistant (WGAr) nonmetastatic mutant and a
related WGA-sensitive (WGAs) metastatic line. J Cell Biol.
99:1034–1044. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saitoh O, Wang WC, Lotan R and Fukuda M:
Differential glycosylation and cell surface expression of lysosomal
membrane glycoproteins in sublines of a human colon cancer
exhibiting distinct metastatic potentials. J Biol Chem.
267:5700–5711. 1992.PubMed/NCBI
|
|
19
|
Ujita M, McAuliffe J, Hindsgaul O, Sasaki
K, Fukuda MN and Fukuda M: Poly-N-acetyllactosamine synthesis in
branched N-glycans is controlled by complemental branch specificity
of I-extension enzyme and beta1,4-galactosyltransferase I. J Biol
Chem. 274:16717–16726. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang C, Zhou J, Wu S, Shan Y, Teng S and
Yu L: Cloning and tissue distribution of the human B3GALT7 gene, a
member of the beta1,3-glycosyltransferase family. Glycoconj J.
21:267–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishida H, Togayachi A, Sakai T, Iwai T,
Hiruma T, Sato T, Okubo R, Inaba N, Kudo T, Gotoh M, et al: A novel
beta1,3-N-acetylglucosaminyltransferase (beta3Gn-T8), which
synthesizes poly-N-acetyllactosamine, is dramatically upregulated
in colon cancer. FEBS Lett. 579:71–78. 2005. View Article : Google Scholar
|
|
22
|
Shen L, Liu Z, Tu Y, Xu L, Sun X and Wu S:
Regulation of MMP-2 expression and activity by
β-1,3-N-acetylglucosamin yltransferase-8 in AGS gastric cancer
cells. Mol Biol Rep. 38:1541–1550. 2011. View Article : Google Scholar
|
|
23
|
Liu J, Shen L, Yang L, Hu S, Xu L and Wu
S: High expression of β3GnT8 is associated with the metastatic
potential of human glioma. Int J Mol Med. 33:1459–1468.
2014.PubMed/NCBI
|
|
24
|
Ni J, Jiang Z, Shen L, Gao L, Yu M, Xu X,
Zou S, Hua D and Wu S: β3GnT8 regulates the metastatic potential of
colorectal carcinoma cells by altering the glycosylation of CD147.
Oncol Rep. 31:1795–1801. 2014.PubMed/NCBI
|
|
25
|
Mitsui Y, Yamada K, Hara S, Kinoshita M,
Hayakawa T and Kakehi K: Comparative studies on glycoproteins
expressing polylactosamine-type N-glycans in cancer cells. J Pharm
Biomed Anal. 70:718–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen GY, Osada H, Santamaria-Babi LF and
Kannagi R: Interaction of GATA-3/T-bet transcription factors
regulates expression of sialyl Lewis X homing receptors on Th1/Th2
lymphocytes. Proc Natl Acad Sci USA. 103:16894–16899. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohtsubo K and Marth JD: Glycosylation in
cellular mechanisms of health and disease. Cell. 126:855–867. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Julien S, Ivetic A, Grigoriadis A, QiZe D,
Burford B, Sproviero D, Picco G, Gillett C, Papp SL, Schaffer L, et
al: Selectin ligand sialyl-Lewis x antigen drives metastasis of
hormone-dependent breast cancers. Cancer Res. 71:7683–7693. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Napoletano C, Rughetti A, Agervig Tarp MP,
Coleman J, Bennett EP, Picco G, Sale P, Denda-Nagai K, Irimura T,
Mandel U, et al: Tumor-associated Tn-MUC1 glycoform is internalized
through the macrophage galactose-type C-type lectin and delivered
to the HLA class I and II compartments in dendritic cells. Cancer
Res. 67:8358–8367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang Z, Ge Y, Zhou J, Xu L and Wu SL:
Subcellular localization and tumor distribution of human
beta3-galactosyltransferase by beta3GalT7 antiserum. Hybridoma
(Larchmt). 29:141–146. 2010. View Article : Google Scholar
|
|
31
|
Liu Z, Shen L, Xu L, Sun X, Zhou J and Wu
S: Down-regulation of β-1,3-N-acetylglucosaminyltransferase-8 by
siRNA inhibits the growth of human gastric cancer. Mol Med Rep.
4:497–503. 2011.PubMed/NCBI
|
|
32
|
Szabo E, Riffe ME, Steinberg SM, Birrer MJ
and Linnoila RI: Altered cJUN expression: An early event in human
lung carcinogenesis. Cancer Res. 56:305–315. 1996.PubMed/NCBI
|
|
33
|
Hess J, Angel P and Schorpp-Kistner M:
AP-1 subunits: Quarrel and harmony among siblings. J Cell Sci.
117:5965–5973. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fujioka S, Niu J, Schmidt C, Sclabas GM,
Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL and Chiao PJ:
NF-kappaB and AP-1 connection: Mechanism of NF-kappaB-dependent
regulation of AP-1 activity. Mol Cell Biol. 24:7806–7819. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Das R, Mahabeleshwar GH and Kundu GC:
Osteopontin induces AP-1-mediated secretion of urokinase-type
plasminogen activator through c-Src-dependent epidermal growth
factor receptor transactivation in breast cancer cells. J Biol
Chem. 279:11051–11064. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang W, Chang SB and Hemler ME: Links
between CD147 function, glycosylation, and caveolin-1. Mol Biol
Cell. 15:4043–4050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Riethdorf S, Reimers N, Assmann V,
Kornfeld JW, Terracciano L, Sauter G and Pantel K: High incidence
of EMMPRIN expression in human tumors. Int J Cancer. 119:1800–1810.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun J and Hemler ME: Regulation of MMP-1
and MMP-2 production through CD147/extracellular matrix
metalloproteinase inducer interactions. Cancer Res. 61:2276–2281.
2001.PubMed/NCBI
|
|
39
|
Fan J, Wang S, Yu S, He J, Zheng W and
Zhang J: N-acetylglucosaminyltransferase IVa regulates metastatic
potential of mouse hepatocarcinoma cells through glycosylation of
CD147. Glycoconj J. 29:323–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Srinivasan N, Bane SM, Ahire SD, Ingle AD
and Kalraiya RD: Poly N-acetyllactosamine substitutions on N- and
not O-oligosaccharides or Thomsen-Friedenreich antigen facilitate
lung specific metastasis of melanoma cells via galectin-3.
Glycoconj J. 26:445–456. 2009. View Article : Google Scholar
|