Introduction
Glioblastoma multiforme (GBM) is the most frequently
encountered primary central nervous system neoplasm in adults, and
is associated with high morbidity and mortality. Despite multimodal
treatment options, including surgical resection, radiotherapy, and
chemotherapy, the median survival of patients with glioblastoma is
12–15 months and 2–5 years for patients with anaplastic gliomas
(1). GBM arises as a result of
various genetic alterations and the deregulation of growth-factor
signaling pathways, resulting in abnormal cellular proliferation,
malignant differentiation, and increased invasiveness of malignant
cells (2,3).
Human epidermal growth factor receptor 2 (HER2) is a
cell membrane receptor with tyrosine kinase activity. HER2 plays
critical roles in cell proliferation, differentiation, adhesion,
and motility (4). The gene
amplification and protein overexpression of HER2 in human tumors is
associated with a poor prognosis (5). According to previous studies, HER2 is
expressed by up to 80% of GBM cases (6,7). The
frequent overexpression of HER2 in GBMs (8–10) and
its rare expression in the adult central nervous system (11) make HER2 an ideal therapeutic target
in GBMs.
Apoptosis, a process in which cells actively
participate in their own death, is often disrupted in tumor cells
(12). Therefore, the introduction
of proapoptotic molecules may be an effective approach for the
treatment of tumors, including malignant gliomas. Caspases, a
family of cysteine proteases, play a vital role in transducing
apoptosis signals and executing apoptosis in mammalian cells
(13). Constitutively active
recombinant caspase-6 is capable of autocatalytic processing in
vitro and in vivo, and can induce apoptosis independent
of endogenous apoptosis signals (14). Therefore recombinant caspase-6 could
be used at very low concentrations to induce apoptosis in tumor
cells. It has been reported that tumor specific delivery of
recombinant caspase-6 to gliomas triggered apoptosis independent of
upstream apoptotic signaling (15).
In our previous studies, we generated a novel immuno-caspase-6
(immuno-casp6) containing a HER2-specific single-chain antibody
(e23sFv), a furin cleavage sequence from diphtheria toxin (Fdt),
and a constitutively active caspase-6 molecule (casp6) (16). Following receptor-mediated
endocytosis and furin-mediated cleavage in the endosome, the
e23sFv-Fdt-casp6 proteins engaged in direct translocation of the
released C-terminal fragment (active caspase-6) and induced
apoptosis in HER2-overexpressing gastric tumor cells. The purpose
of the present study is to investigate the proapoptotic effects of
the novel immuno-casp6 (e23sFv-Fdt-casp6) and its therapeautic
efficacy in HER2-overexpressing malignant gliomas.
Materials and methods
Cell culture
The human GBM cell lines A172, U251MG, and U87MG
[American Type Culture Collection (ATCC), Manassas, VA, USA], and a
Chinese hamster ovary (CHO) cell line (Chinese Academy of Sciences
Cell Bank, Shanghai, China) were cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS) (both from Gibco, Grand Island, NY, USA) at 37°C in 5%
CO2.
PCR amplification
Genomic DNA was extracted from CHO cells that were
stably transfected with an empty pCMV vector or
pCMV-e23sFv-Fdt-casp6, after G418 selection, by using 5′-TTT GCG
GCC GCG AAA GCC GGC AAT AGA GTG AGG AGA TCT GTG GGC GCA GCC TCC GTT
TAC-3′ as the upstream primer and 5′-TTT TCT AGA TTA ATC TAC TAC
ATC CAA AGG AAT-3′ as the downstream primer. A fusion gene that
encompassed the sequence encoding Fdt and active caspase-6 was
amplified from the genomic templates.
Western blot analysis
CHO culture supernatants were concentrated by
centrifugation for 30 min at 4°C and 3,000 × g in Amicon Ultra
concentrators (30,000 MWCO; Millipore Corp., Billerica, MA, USA).
Phosphate-buffered saline (PBS) was added to the device, and
centrifugation was performed again as above to concentrate the
supernatant and change the buffers (17). The proteins from the whole-cell
lysate and concentrated supernatants were blotted onto PVDF
membranes. The membranes were then incubated overnight at 4°C with
primary antibodies against active caspase-6 (1:500; Abcam,
Cambridge, UK), HER2 (1:500; NeoMarkers, Fremont, CA, USA), and
then incubated with horseradish peroxidase-conjugated secondary
antibody (1:2,000; Zhongshan Golden Bridge Biotechnology Co.,
Beijing, China) for 2 h at room temperature. The blots were
visualized using an enhanced chemiluminescence kit (Pierce
Biotechnology, Inc., Rockford, IL, USA).
Expression of HER2 examined by flow
cytometry
Cells from the human GBM cell lines A172, U251MG,
and U87MG were washed with PBS, blocked with rabbit serum, and then
incubated with mouse anti-HER2 antibody (1:100; NeoMarkers) for 30
min. The appropriate isotype antibody was used as a negative
control. Cells were extensively washed with PBS and incubated with
PE-conjugated rabbit anti-mouse IgG (1:200; BD Biosciences, San
Diego, CA, USA) for 30 min. Finally, samples were analyzed via flow
cytometry (Becton-Dickinson, San Diego, CA, USA).
Immunofluorescent staining
Stably transfected CHO cells were cultured on cover
slips in DMEM containing 10% FBS and then fixed in a freshly
prepared 4% paraformaldehyde solution for 10 min at room
temperature. Then, they were permeabilized with 0.1% Triton X-100
for 10 min, and incubated overnight at 4°C with rabbit anti-active
caspase-6 (1:200; Abcam). Cells were washed extensively with PBS
and incubated with Cy3-labelled goat anti-rabbit secondary
antibodies (1:100; Boster Inc., Wuhan, China) for 30 min at room
temperature followed by further rinsing. DAPI (Invitrogen,
Carslbad, CA, USA) was used for nuclear staining. Finally, cells
were washed with PBS, mounted on slides, and observed under a
fluorescence microscope (Olympus, Tokyo, Japan).
A172, U251MG, and U87MG cells were cultured on cover
slips. The supernatant was aspirated and replaced with freshly
collected medium from stably transfected CHO cell cultures every 24
h. Immunofluorescent staining was carried out 2 days later, as
described above. DAPI was used for nuclear staining. The cells were
observed under a fluorescence microscope (Olympus).
Analysis of apoptosis by flow
cytometry
A172, U251MG, and U87MG cells were cultured for 2
days with freshly collected culture supernatant from stably
transfected CHO cells. The medium was aspirated and replaced every
24 h. After washing twice with ice-cold PBS, the cells were stained
with FITC-labeled Annexin V staining solution and propidium iodide
(PI) (Roche, Basel, Switzerland) according to the manufacturer's
instructions and analyzed using a flow cytometer.
Cell proliferation assay
GBM cells A172, U251MG, and U87MG were cultured in
96-well plates at a density of 5×103 cells/well with
freshly collected culture supernatant from stably transfected CHO
cells. The medium was aspirated and replaced every 24 h. The cells
were then incubated with 20 μl of 1.5 mg/ml MTT per well for
4 h, followed by the addition of 150 ml DMSO at each indicated
time-point. A490 nm values were determined using a Sunrise
microplate reader (Tecan Austria GmbH, Salzburg, Austria). Each
assay was performed in triplicate on at least 3 independent
occasions.
In vivo antitumor activity of the
e23sFv-Fdt-casp6 protein
Severe combined immunodeficient (SCID) mice aged 6–7
weeks were purchased from the Vital River Laboratory Animal
Technology Co. (Beijing, China) and were cared for and used in
compliance with the guidelines of Animal Center of the Fourth
Military Medical University. The mice were inoculated
subcutaneously with 5×106 U251MG cells in the right
posterior limb. When tumors reached a diameter of 5–7 mm (day 0),
mice were randomly assigned to 2 groups, 15 mice/group. The mice in
each group were further randomly classified into 2 subgroups, in
which 7 mice were used for immunohistochemical staining and the
TUNEL assay, and 8 mice for survival analysis. The treatment group
received 6 doses of 10 μg Lipofectamine-encapsulated
pCMV-e23sFv-Fdt-casp6 every 3 days by intramuscular injection into
the right posterior limb. Control mice were injected with
Lipofectamine mixed with the empty pCMV vector. Tumor growth was
monitored using a caliper to measure 2 perpendicular tumor
diameters every 4 days. The volume of the tumor was then calculated
using the formula: tumor volume = (width) 2 × (length/2). On day 28
mice were sacrificed by cervical dislocation, and dissected to
collect organ tissues. Tissues were embedded in paraffin for
further analysis. For the survival analysis, the mice received the
same treatment as before and their survival time was recorded.
Distribution of e23sFv-Fdt-casp6 via
immunohistochemical analysis
Tissues were fixed in 10% formalin and embedded in
paraffin, sectioned at 4 μm thickness, then dewaxed and
blocked with 0.3% H2O2. The sections were
incubated with trypsin and normal goat serum, followed by rabbit
anti-active caspase-6 (1:50; Abcam). The appropriate isotype
antibody was used as a negative control. Secondary biotinylated
goat anti-rabbit IgG antibody (Dako, Glostrup, Denmark) was added,
followed by streptavidin-horseradish peroxidase (Sigma-Aldrich, St.
Louis, MO, USA). The slides were stained with diaminobenzidine
(Sigma-Aldrich) and counterstained with hematoxylin.
TUNEL assay
DNA fragmentation was detected using the TUNEL
assay. The TUNEL assay was performed according to the
manufacturer's instructions (Roche). DAPI was used for nuclei
staining. The cells were observed under a fluorescent microscope
(Olympus).
Statistical analysis
All data were analyzed using SPSS v19 and are
presented as means ± SD of at least 3 independent experiments.
Student's t-test was used to analyze the difference between groups.
For the in vivo experiments, the survival times were
analyzed using the Kaplan-Meier method and the log-rank test was
used to assess the difference between groups. A value of P<0.05
was considered statistically significant.
Results
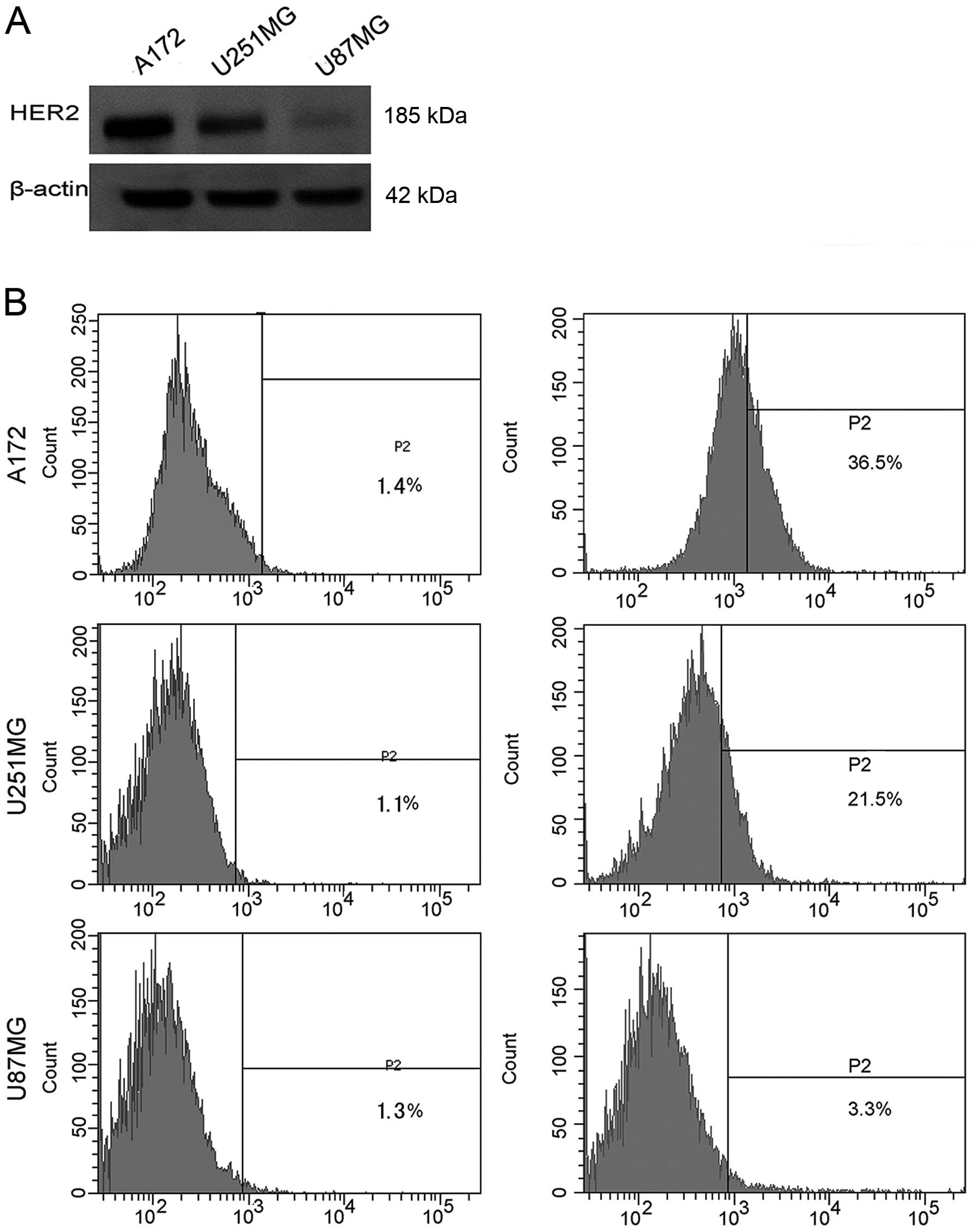
Expression of HER2 in GBM cell lines
HER2 is expressed by up to 80% of GBMs (6,7). To
gain further information on the expression status of HER2 in GBM
cell lines, we used western blot analysis and flow cytometry
analyses to determine the protein expression of HER2 in A172,
U251MG, and U87MG. High levels of HER2 protein were detected in the
A172 and U251MG cells, and low expression was observed in U87MG
cells (Fig. 1A). HER2 expression on
the cell surface of A172, U251MG, and U87MG cells was detected via
flow cytometry, and percent of cells expressing HER2 was 36.5, 21.5
and 3.3%, respectively (Fig. 1B).
Based on these results, in subsequent experiments A172 and U251MG
cell lines were chosen for in vitro and in vivo
studies, whereas U87MG was employed as a negative control.
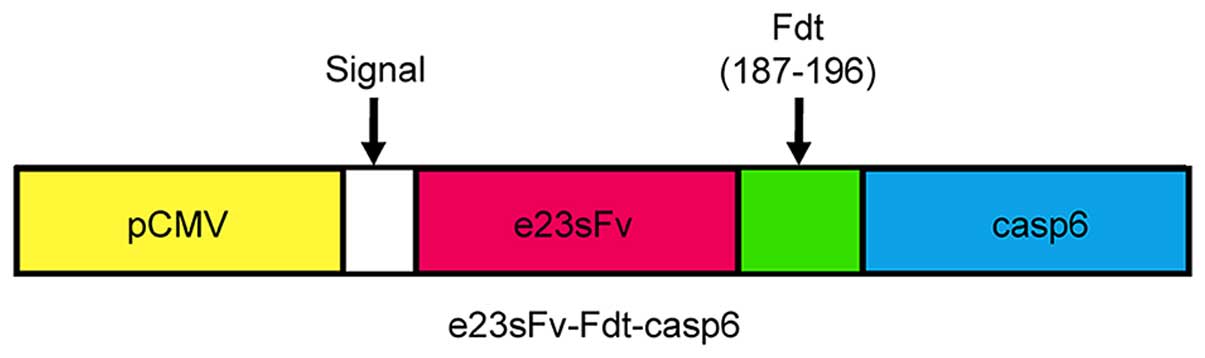
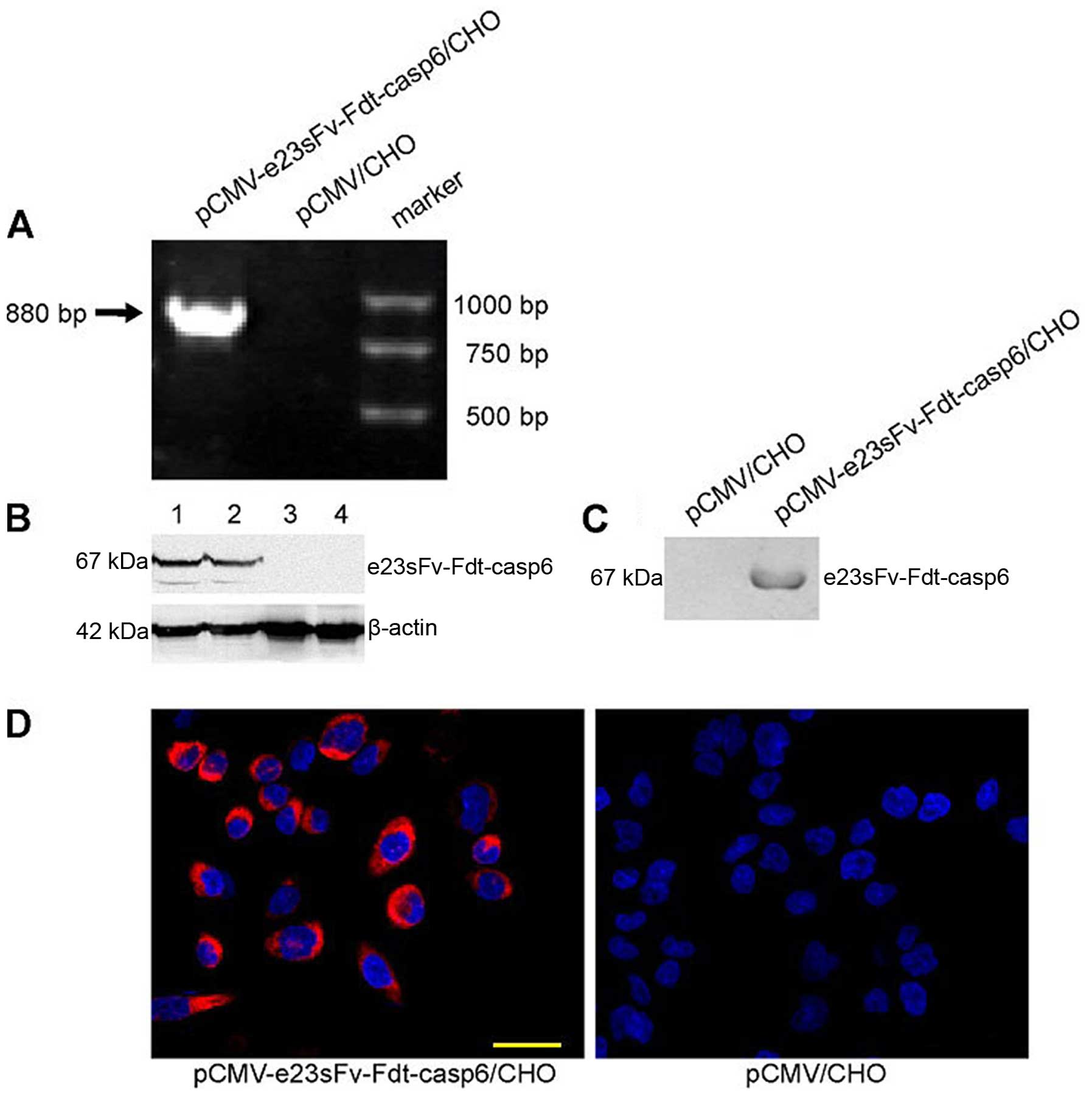
Expression and secretion of the
recombinant protein in CHO cells
The immuno-casp6 gene was cloned downstream and in
frame with the DNA encoding a signal sequence in the expression
vector pCMV (Fig. 2). CHO cells are
important host cells for the industrial production of
pharmaceutical proteins owing to their capacity for correct
folding, assembly, and post-translational modification of
recombinant proteins (18). To
obtain continuous and high yields of the recombinant proteins, CHO
cells were stably transfected with the empty pCMV or the
pCMV-e23sFv-Fdt-casp6 vector and selected for with G418. The
chimeric gene was specifically amplified from the transduced cells
for genomic PCR analysis (Fig. 3A),
indicating that the e23sFv-Fdt-casp6 gene was integrated into the
host CHO cell's genome. The expression of the e23sFv-Fdt-casp6
protein was confirmed via western blot analysis both in the
transfected CHO cells and in the cell culture supernatant (Fig. 3B and C). Furthermore, the
recombinant proteins were stained strongly in the cytoplasm of the
stably transfected CHO cells (Fig.
3D), indicating a high expression efficiency of the recombinant
protein in the modified CHO cells. The CHO cells producing the
e23sFv-Fdt-casp6 protein showed a normal morphology and
proliferation relative to the parental CHO cells (Fig. 3D), suggesting that they did not
experience toxicity from the fusion proteins.
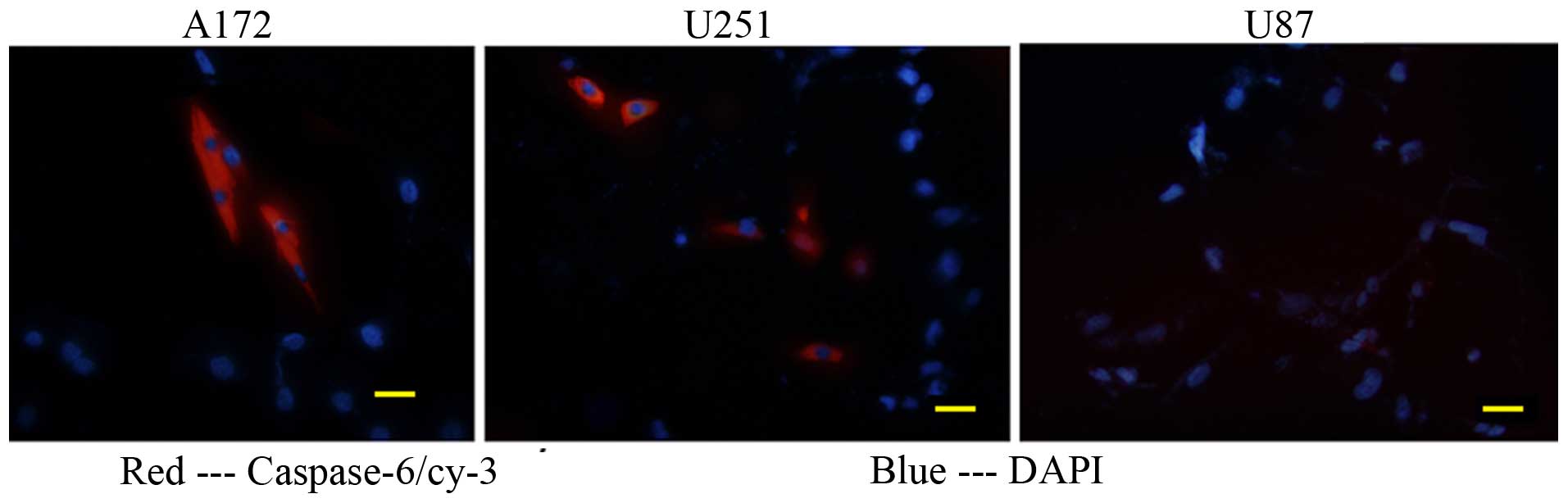
Binding and internalization of
e23sFv-Fdt-casp6 in HER2-overexpressing GBM cells
To confirm whether the secreted e23sFv-Fdt-casp6
recombinant protein can specifically bind to and be subsequently
internalized by HER2-overexpressing GBM cells, we cultured A172,
U251MG, and U87MG cells with freshly collected supernatants from
the modified CHO cells. After 2 days of incubation,
e23sFv-Fdt-casp6 recombinant proteins produced by the modified CHO
cells were concentrated in many of the A172 and U251MG cells, but
not in the HER2-negative U87MG cells, indicating the specific
binding and internalization of the recombinant protein by the
HER2-overexpressing GBM cells (Fig.
4).
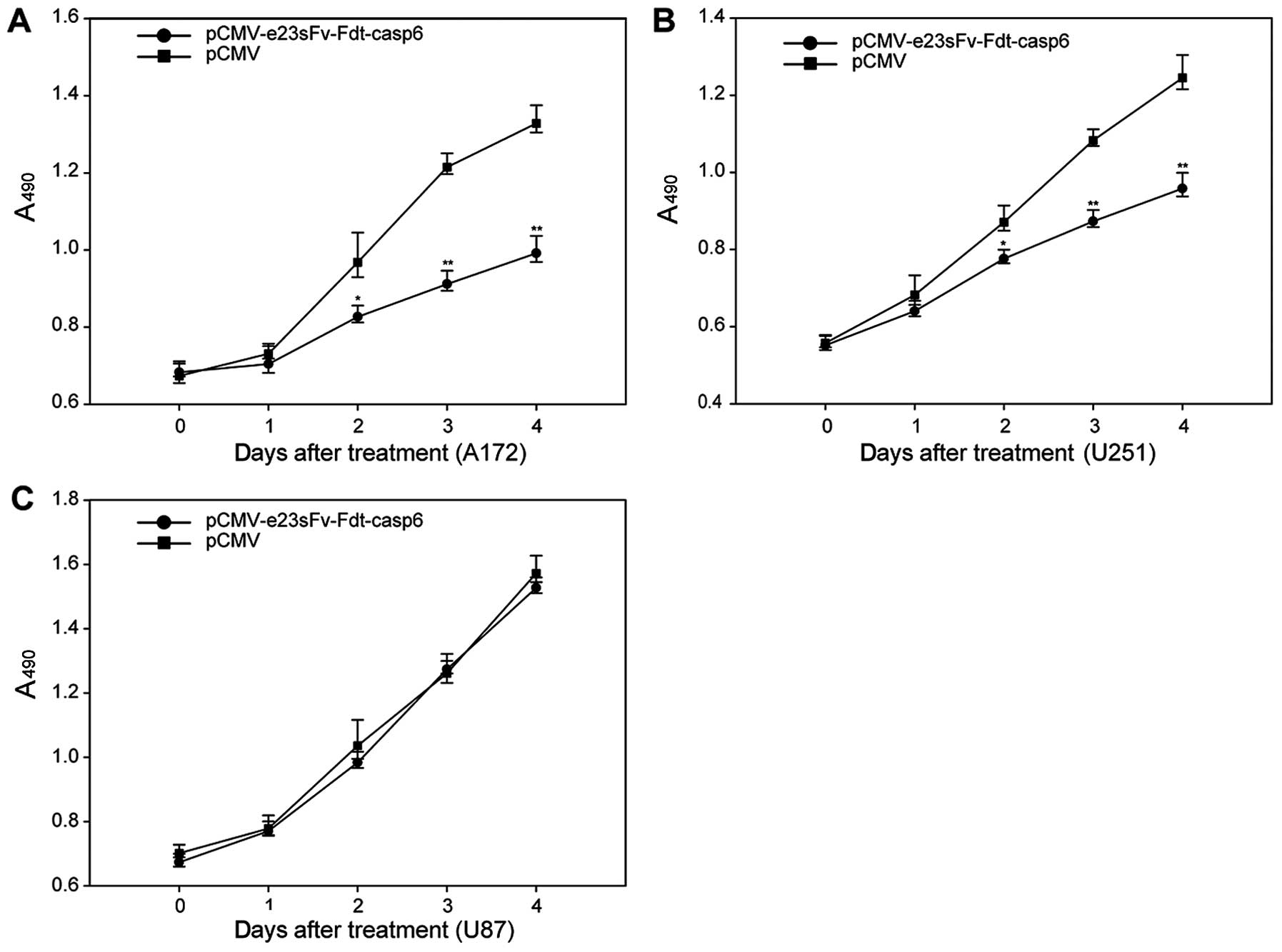
Proliferation inhibition, and apoptosis
induction in HER2-overexpressing GBM cells by e23sFv-Fdt-casp6
A172, U251MG, and U87MG cells were cultured with
supernatants from the modified CHO cells. Cells were cultured with
supernatants from the normal CHO cells as the negative control.
After 48 h of culture, abundant cell death was observed in the A172
and U251MG cells. The MTT assay showed inhibition of proliferation
in the A172 and U251MG cells. By contrast, no difference in
proliferation was observed in the U87MG cells, suggesting that the
inhibition of proliferation depends on the overexpression of HER2
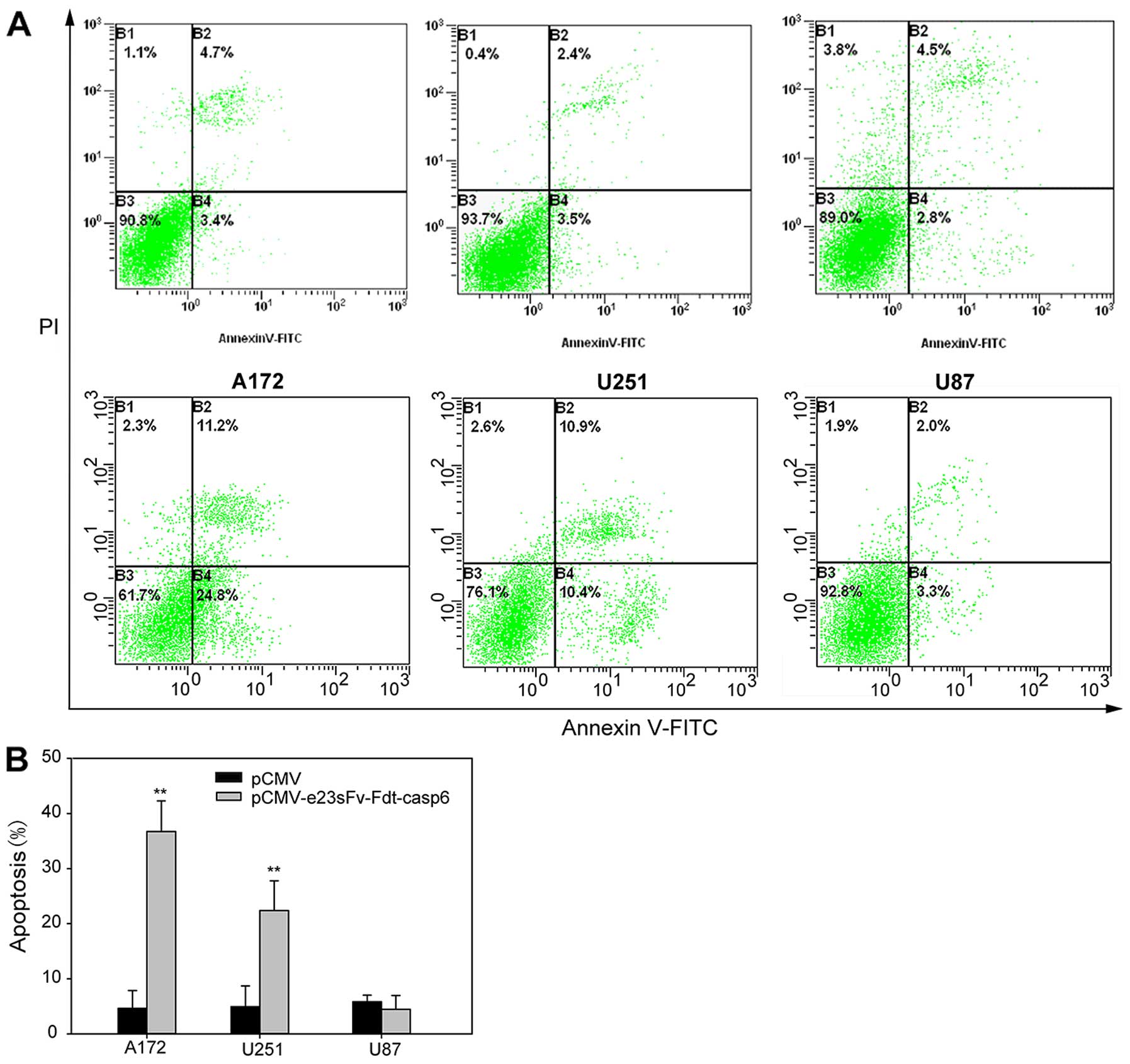
on cell surfaces (Fig. 5). To
investigate whether the inhibition of proliferation was induced by
cell apoptosis, flow cytometry was performed in A172, U251MG, and
U87MG cells cultured with the supernatants of stably transfected
CHO cells. After 48 h, Annexin V-FITC staining revealed that 36%
and 21.3% of A172 and U251MG cells respectively were apoptotic,
higher than that in the control cells. No obvious difference was
observed in the U87MG cells (Fig.
6).
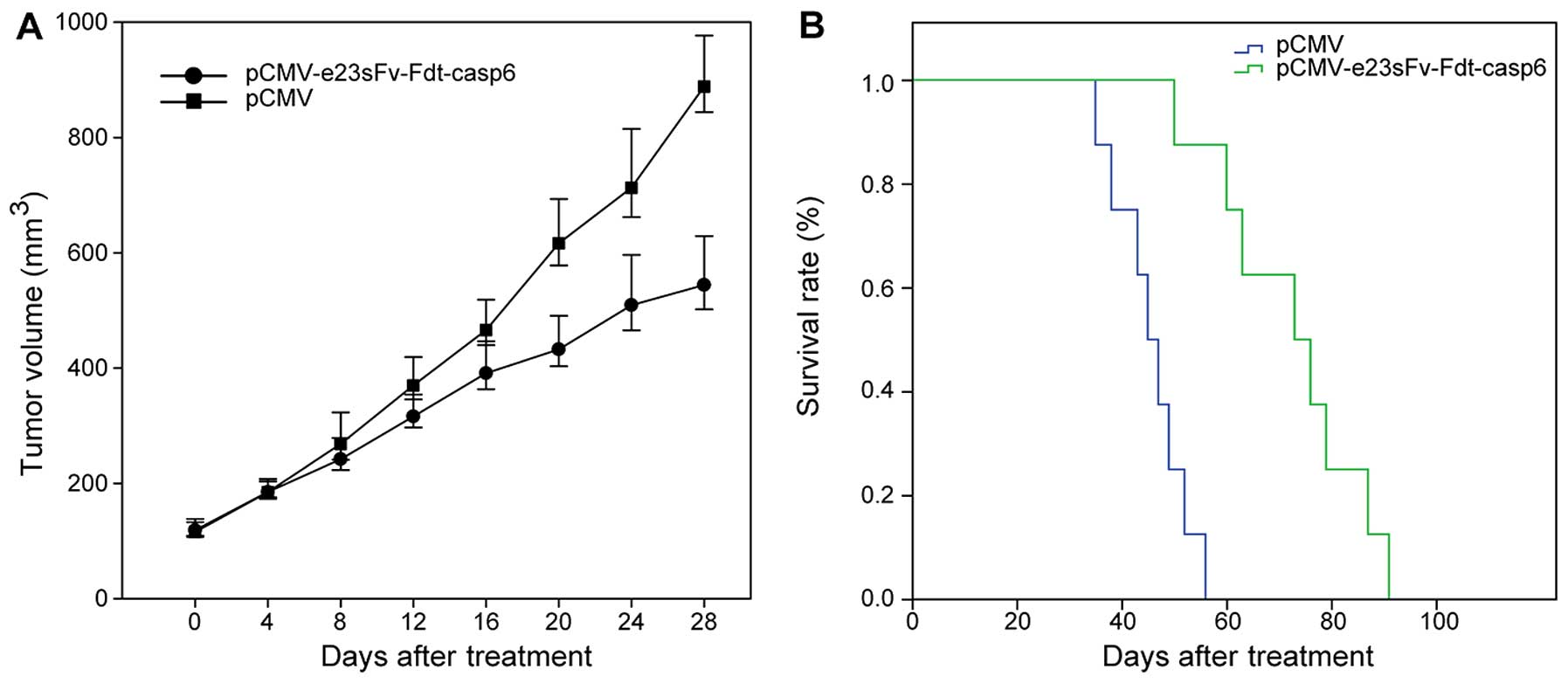
Tumor growth suppression in SCID mice and
distribution of e23sFv-Fdt-casp6 in xenograft tissues
The in vivo therapeutic effect of the fusion
protein e23sFv-Fdt-casp6 was evaluated in SCID mice bearing human
glioblastoma xenografts. After the establishment of glioblastoma
xenografts, the mice were randomly assigned to treatment and
control treatment groups, with each mouse receiving 6 doses of 10
μg of Lipofectamine-encapsulated pCMV-e23sFv-Fdt-casp6 or
pCMV plasmid, respectively. Plasmids were injected intramuscularly
in the right posterior limb every 3 days. The tumor growth and
survival rates were monitored and analyzed. The tumors in mice
receiving the Lipofectamine-pCMV-e23sFv-Fdt-casp6 complex grew more
slowly than those in the control mice, suggesting that the
e23sFv-Fdt-casp6 protein secreted by the genetically modified
muscle cells suppressed the growth of HER2-positive GBM cells. The
average tumor size in mice receiving the
Lipofectamine-pCMV-e23sFv-Fdt-casp6 complex at the time they were
sacrificed was approximately two third the size of the tumors in
the control mice (544.3±42.5 vs. 888.7±44.2 mm3,
P<0.05) (Fig. 7A). The mice
treated with the pCMV-e23sFv-Fdt-casp6 construct exhibited a
prolonged mean survival time compared with those treated with the
control vector (72.4±4.9 vs. 47.0±3.1 days, P<0.01) (Fig. 7B).
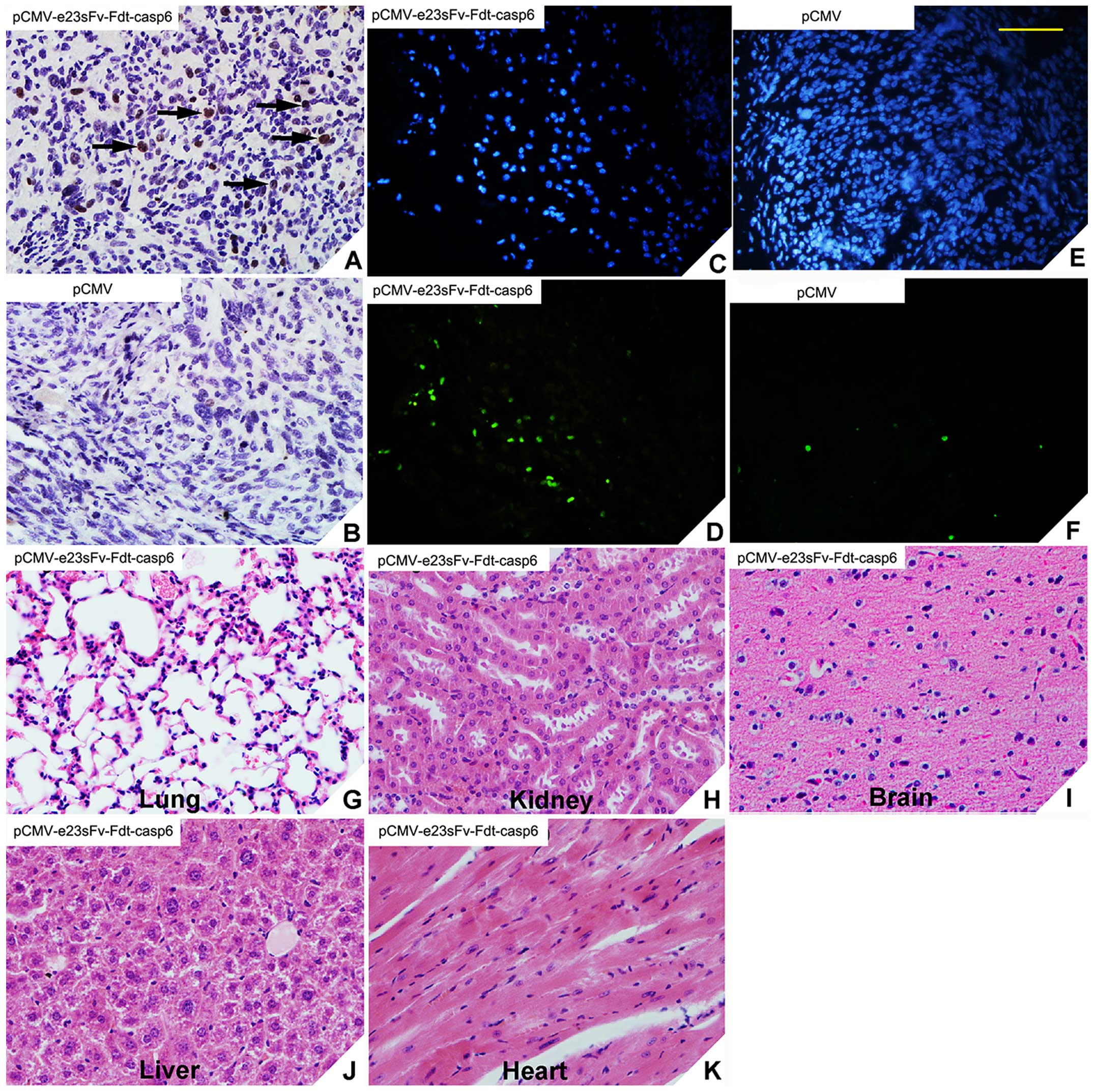
The distribution of the e23sFv-Fdt-casp6 recombinant
proteins was determined in tumor tissues and normal tissues via
immunohistochemistry. Positive staining for caspase-6 was observed
in the tumor tissues from the treatment mice but not in those from
control (Fig. 8A and B). No
positive staining was observed in the lung, kidney, brain, liver,
or heart tissues of mice treated with the
Lipofectamine-pCMV-e23sFv-Fdt-casp6 complex (data not shown).
Therefore, e23sFv-Fdt-casp6 protein produced and secreted by the
modified muscle cells specifically localizes to HER2-positive tumor
cells. Similarly, in the TUNEL assay, an abundance of apoptotic
cells were detected in tumors from mice treated with the
Lipofectamine -pCMV-e23sFv-Fdt-casp6 complex, but not in the tumors
from the control mice treated with the Lipofectamine-pCMV complex
(Fig. 8C–F). Hematoxylin and eosin
staining was performed to evaluate the toxicity of the recombinant
protein on other important organs, such as lung, kidney, brain,
liver, and heart. We found no signs of massive cell injury
(Fig. 8G–K), which suggested the
recombinant protein e23sFv-Fdt-casp6 can effectively target and
induce apoptosis in HER2-overexpressing tumor cells with no obvious
side-effects to normal tissues.
Discussion
Malignant gliomas, such as GBM, are the most common
and lethal intracranial tumors. The standard therapy for newly
diagnosed malignant gliomas involves surgical resection (when
feasible), radiotherapy, and chemotherapy. Malignant gliomas cannot
be eliminated completely because of their infiltrative nature, and
their resistance to conventional therapies (1,2).
Therefore novel therapeutic strategies are necessary. The past two
decades have witnessed striking advances in GBM immunotherapy. HER2
is not expressed in the adult central nervous system, however, its
expression increases with the degree of astrocytoma anaplasia. It
is estimated that HER2 is expressed by up to 80% of GBM cases
(6,7), and therefore is ideal target for
therapeutic approaches. Herceptin was reported to induce cellular
apoptosis in GBMs overexpressing HER2 in vitro (19). In our study, the novel
e23sFv-Fdt-casp6 specifically targeted HER2-overexpressing GBM
cells and exhibited its killing activity in vitro and in
vivo.
To investigate the specific killing activities of
e23sFv-Fdt-casp6 protein, we preformed subcutaneous implantation to
establish animal model in SCID mice, and the e23sFv-Fdt-casp6 gene
was introduced intramuscularly to the xenograft mouse models by
liposome encapsulation. Cells were transfected with the
immuno-casp6 plasmid and were expected to exert their
apoptosis-inducing effects in a paracrine manner. To our knowledge,
one of the differences between the subcutaneous and intracranial
implantations is the blood-brain barrier (BBB) that is usually very
efficient in blocking most of the molecules outside the central
nervous system (19). Proteins (150
kDa), such as IgG, are thought to cross the abnormal GBM BBB, and
an intravital fluorescence microscopical approach demonstrated that
tumoral microvascularization produces an abnormal BBB (20). The molecular weight of recombinant
e23sFv-Fdt-casp6 protein (67 kDa) is smaller than that of
Herceptin, and is therefore likely to cross the BBB. We are
conducting further experiments to validate this. In addition,
subcutaneous tumors can be repetitively measured through the skin.
Although intracranial implantations are more clinically relevant,
the tumor sizes can be measured only when the animals are
sacrificed, making it difficult to evaluate the anti-tumor activity
of e23sFv-Fdt-casp6.
There are already many strategies of using HER2 as
the therapy target for treating GBM, including adoptive cell
therapies with chimeric antigen receptor (CAR) expressing T cells
(21), activated T cells ATC armed
with bispecific antibodies, and activated dendritic cell based
immunotherapy (22,23), which are already into the phase I
trial. In the present study, we used a DNA based method to
introduce the functional therapeutic elements into the tumor cell
lines and also the animal tumor model, both were effective on
successful treatment of GBM. Nucleic acid vaccines are an
alternative to attenuated bacterial antigens or protein or peptide
vaccines. The advantage of DNA therapeutic vaccine include:
inducing humoral and cellular immune response at the same time,
various promoters, enhancers, and other elements could be chosen to
control the expression of the encoded protein, easy production,
safer, more stable, and inexpensive (24–26).
However, the major problem is that DNA vaccines may have a
relatively poor immunogenicity (27). In our results, the immunogenicity is
proven enough to eliminate the tumor, both in vitro to
induce apoptosis, and in vivo to eliminate the tumor size in
the SCID mouse model. Thus, our approach has potential to further
develop to clinical use.
In conclusion, our evidence of e23sFv-Fdt-casp6's
ability to induce apoptosis or cytotoxicity in HER2-overexpressing
GBM cells makes it a promising therapeutic alternative for GBM
treatment.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 30872978 and no.
81301702). We thank the research staff who supported this study,
including Jin-Xiang Cheng and Xiao-Liang Yang.
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Furnari FB, Fenton T, Bachoo RM, Mukasa A,
Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al:
Malignant astrocytic glioma: Genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohgaki H and Kleihues P: Genetic pathways
to primary and secondary glioblastoma. Am J Pathol. 170:1445–1453.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lupu R, Colomer R, Kannan B and Lippman
ME: Characterization of a growth factor that binds exclusively to
the erbB-2 receptor and induces cellular responses. Proc Natl Acad
Sci USA. 89:2287–2291. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koka V, Potti A, Forseen SE, Pervez H,
Fraiman GN, Koch M and Levitt R: Role of Her-2/neu overexpression
and clinical determinants of early mortality in glioblastoma
multiforme. Am J Clin Oncol. 26:332–335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahmed N, Salsman VS, Kew Y, Shaffer D,
Powell S, Zhang YJ, Grossman RG, Heslop HE and Gottschalk S:
HER2-specific T cells target primary glioblastoma stem cells and
induce regression of autologous experimental tumors. Clin Cancer
Res. 16:474–485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu G, Ying H, Zeng G, Wheeler CJ, Black
KL and Yu JS: HER-2, gp100, and MAGE-1 are expressed in human
glioblastoma and recognized by cytotoxic T cells. Cancer Res.
64:4980–4986. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bian XW, Shi JQ and Liu FX: Pathologic
significance of proliferative activity and oncoprotein expression
in astrocytic tumors. Anal Quant Cytol Histol. 22:429–437.
2000.
|
|
9
|
Forseen SE, Potti A, Koka V, Koch M,
Fraiman G and Levitt R: Identification and relationship of
HER-2/neu overexpression to short-term mortality in primary
malignant brain tumors. Anticancer Res. 22:1599–1602.
2002.PubMed/NCBI
|
|
10
|
Mineo JF, Bordron A, Quintin-Roué I,
Loisel S, Ster KL, Buhé V, Lagarde N and Berthou C: Recombinant
humanised anti-HER2/neu antibody (Herceptin) induces cellular death
of glioblastomas. Br J Cancer. 91:1195–1199. 2004.PubMed/NCBI
|
|
11
|
Press MF, Cordon-Cardo C and Slamon DJ:
Expression of the HER-2/neu proto-oncogene in normal human adult
and fetal tissues. Oncogene. 5:953–962. 1990.PubMed/NCBI
|
|
12
|
Bold RJ, Termuhlen PM and McConkey DJ:
Apoptosis, cancer and cancer therapy. Surg Oncol. 6:133–142. 1997.
View Article : Google Scholar
|
|
13
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srinivasula SM, Ahmad M, MacFarlane M, Luo
Z, Huang Z, Fernandes-Alnemri T and Alnemri ES: Generation of
constitutively active recombinant caspases-3 and -6 by
rearrangement of their subunits. J Biol Chem. 273:10107–10111.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Komata T, Kondo Y, Kanzawa T, Hirohata S,
Koga S, Sumiyoshi H, Srinivasula SM, Barna BP, Germano IM, Takakura
M, et al: Treatment of malignant glioma cells with the transfer of
constitutively active caspase-6 using the human telomerase
catalytic subunit (human telomerase reverse transcriptase) gene
promoter. Cancer Res. 61:5796–5802. 2001.PubMed/NCBI
|
|
16
|
Ren JL, Meng YL, Hu B, Jia LT, Zhang R, Xu
YM, Xie QS, Zhang YQ, Jin BQ, Chen SY, et al: The effect of direct
translocation across endosomes on the cytotoxicity of the
recombinant protein e23sFv-Fdt-casp6 to HER2 positive gastric
cancer cells. Biomaterials. 32:7641–7650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang T, Zhao J, Ren JL, Zhang L, Wen WH,
Zhang R, Qin WW, Jia LT, Yao LB, Zhang YQ, et al: Recombinant
immunoproapoptotic proteins with furin site can translocate and
kill HER2-positive cancer cells. Cancer Res. 67:11830–11839. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Omasa T, Onitsuka M and Kim WD: Cell
engineering and cultivation of chinese hamster ovary (CHO) cells.
Curr Pharm Biotechnol. 11:233–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mineo JF, Bordron A, Quintin-Roué I,
Maurage CA, Buhé V, Loisel S, Dubois F, Blond S and Berthou C:
Increasing of HER2 membranar density in human glioblastoma U251MG
cell line established in a new nude mice model. J Neurooncol.
76:249–255. 2006. View Article : Google Scholar
|
|
20
|
Vajkoczy P, Schilling L, Ullrich A,
Schmiedek P and Menger MD: Characterization of angiogenesis and
microcirculation of high-grade glioma: An intravital
multifluorescence microscopic approach in the athymic nude mouse. J
Cereb Blood Flow Metab. 18:510–520. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hegde M, Corder A, Chow KK, Mukherjee M,
Ashoori A, Kew Y, Zhang YJ, Baskin DS, Merchant FA, Brawley VS, et
al: Combinational targeting offsets antigen escape and enhances
effector functions of adoptively transferred T cells in
glioblastoma. Mol Ther. 21:2087–2101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akiyama Y, Oshita C, Kume A, Iizuka A,
Miyata H, Komiyama M, Ashizawa T, Yagoto M, Abe Y, Mitsuya K, et
al: α-type-1 polarized dendritic cell-based vaccination in
recurrent high-grade glioma: A phase I clinical trial. BMC Cancer.
12:6232012. View Article : Google Scholar
|
|
23
|
Phuphanich S, Wheeler CJ, Rudnick JD,
Mazer M, Wang H, Nuño MA, Richardson JE, Fan X, Ji J, Chu RM, et
al: Phase I trial of a multi-epitope-pulsed dendritic cell vaccine
for patients with newly diagnosed glioblastoma. Cancer Immunol
Immunother. 62:125–135. 2013. View Article : Google Scholar :
|
|
24
|
Sasaki S, Takeshita F, Xin KQ, Ishii N and
Okuda K: Adjuvant formulations and delivery systems for DNA
vaccines. Methods. 31:243–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robinson HL and Pertmer TM: DNA vaccines
for viral infections: Basic studies and applications. Adv Virus
Res. 55:1–74. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun Y, Hu YH, Liu CS and Sun L:
Construction and analysis of an experimental Streptococcus iniae
DNA vaccine. Vaccine. 28:3905–3912. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim D, Hung CF, Wu TC and Park YM: DNA
vaccine with α-galactosylceramide at prime phase enhances
anti-tumor immunity after boosting with antigen-expressing
dendritic cells. Vaccine. 28:7297–7305. 2010. View Article : Google Scholar : PubMed/NCBI
|