Introduction
Heme oxygenase-1 (HO-1) is an inducible enzyme that
degrades heme to carbon monoxide, ferrous ions and biliverdin
(1). Accumulating evidence supports
that HO-1 could modulate the tumor growth and metastasis through
its regulation of apoptosis, angiogenesis and inflammatory
(2,3). Moreover, compared with the surrounding
healthy tissues, HO-1 expression is increased in various tumors,
such as glioblastoma, melanoma (4)
and hepatocellular carcinoma (5),
and decreased in non-small cell lung carcinoma (6). Previous study also confirmed that HO-1
inhibited the migratory ability of hepatocellular carcinoma (HCC)
cells (3,7). However, recent studies demonstrated
that HO-1 plays a contradictory role in several types of
malignancies including breast, lung and prostate cancer (8–10). The
molecular mechanism of HO-1 in HCC still require elucidating.
MicroRNAs (miRNAs), a class of small non-coding
RNAs, is one of the most abundant classes of gene regulatory
molecules in eukaryotic cells (11). miRNAs participate in various
biological processes of tumor progression including proliferation,
migration and angiogenesis by causing translational repression or
degradation of the mRNA. It is not surprising that HMOX1
expression can be regulated by miRNAs. It has been reported that
HO-1 protein level could be downregulated by miR-122 or miR-217/377
(12–14). Furthermore, Kozakowska et al
(15) revealed that both specific
miRNA expressions and global miRNA biogenesis could be also
regulated by HO-1. These studies implied that HO-1 plays a
complicated role by modulating miRNA. Moreover, the regulatory role
of HO-1 on miRNA is tissue-specific. The relation between HO-1 and
miRNAs remains unknown in HCC.
In the present study, our results showed that
overexpressing HO-1 could reduce the expression of both miR-30d and
miR-107. Furthermore, HO-1 could repress the proliferation and
migration of HCC both in vivo and in vivo which
depends on reducing the expression of miR-30d and miR-107. Iron,
one of HO-1 enzymatic products, plays an important role in
suppressing the expression of miR-30d and miR-107. During the
regulation of proliferation and migration, Akt and ERK pathways may
be involved in the function of HO-1/miR-30d/miR-107 in HCC.
Materials and methods
Cell culture, treatment and drug
preparation
The human hepatocellular cells HepG2 were maintained
in Dulbecco's modified Eagle's medium that was supplemented with
10% fetal bovine serum (FBS), 100 U/ml penicillin G and 100
µg/ml streptomycin at 37°C in a humidified incubator
containing 5% CO2. Cobalt protoporphyrin IX (CoPPIX),
tin protoporphyrin IX (SnPPIX), bilirubin and
tricarbonyldichlororuth enium(II) dimer (CORM-2) (Sigma, Shanghai,
China) stock solutions were prepared by dissolving in dimethyl
sulfoxide at a concentration of 20 µg/l of stock solution.
Ferricitrate and deferoxamine were respectively dissolved in
deionized water at concentration of 20 mM. iCORM is an inactive
form of CORM-2.
Cell transfection
HepG2 cells were seeded at a density of
2×105 cells in a 6-well plate and grown to 60–70%
confluency in growth media. Cells were transfected with pcDNA3.1
(+) containing human wild-type HO-1 (HepG2/HO-1) and empty vector
(HepG2/Mock) using Lipofectamine 2000 transfection reagent
(Invitrogen, Carlsbad, CA, USA). The stable cell lines were
selected with 500 µg/ml G418 (Sigma) and screened for HO-1
protein expression. For gene silencing, the pLL3.7,
pLL3.7-HO-1shRNA (4 µg), that were kindly provided by
Professor Hong Zhou (Academy of Military Medical Sciences, Beijing,
China), were used. miR-30d and miR-107 mimics (and their Nc mimics)
(100 pmol) (GenePharma, Shanghai, China) were transiently
transfected into HepG2/HO-1 or HepG2/Mock cells using Lipofectamine
2000 transfection reagent. After 24 h, the transfected cells were
used for further experiments. H1B and Mock were previously
described (3).
Cell viability assay
Cell viability was determined by
3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyl-tetrazolium bromide (MTT)
assay as previously described (3).
Cell migration assay
To detect the ability of cells to migrate in
vivo, we used the Transwell chamber assay. Briefly, HepG2 cells
(5×104) were placed in the upper compartment of a
24-well Transwell unit with 8 µm polycarbonate nucleopore
filters (Corning Costar, Cambridge, MA, USA). Medium containing 10%
FBS was added to the lower compartment. The cells were then
incubated for 24 h in a humidified atmosphere of 5% CO2
at 37°C. The cells were then fixed and counted as previously
described (3).
Colony formation assay
HepG2/HO-1 and HepG2/Mock cells, and HepG2 cells
that were transfected with miR-30d, miR-107 and NC mimics were
seeded in 3.5-cm dishes (1,000 cells/dish) and cultured for 2 weeks
to allow for colony formation. The colonies were fixed in methanol,
stained with 0.1% crystal violet and counted.
Real-time quantitative polymerase chain
reaction (qRT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen) according to the manufacturer's protocol, and the
concentration of total RNA was measured with a NanoDrop 2000c. RNA
(1 µg) was converted to cDNA using miR-30d- and
miR-107-specific stem-loop primer, and the cDNA and qRT-PCR with
miR-30d- and miR-107-specific primers was performed using a 7500
Real-Time PCR system (Applied Biosystems, Mannheim, Germany). For
relative quantification, the crossing point (Cp) value of miR-30d
or miR-107 was normalized to the Cp value of β-actin and U6 as a
control. miR-30d sense, 5′-CTTTCAGTCAGATGTTTGCTGC-3′ and antisense,
5′-ATTGCGTGTCGTGGAGTCG-3′; miR-107 sense,
5′-AGCAGCATTGTACAGGGCTATCA-3′ and antisense,
5′-ATTGCGTGTCGTGGAGTCG-3′; U6 sense,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and antisense,
5′-CGCTTCACGAATTTGCGTGTCAT-3′.
Western blot analysis
Whole cell and tissue extracts were prepared in cell
lysis buffer followed by immunoblotting with anti-HO-1 antibody
(1:4,000), β-actin (1:4,000), Akt (1:1,000), pAkt (1:1,000), ERK1/2
(1:1,000) and p-ERK1/2 (1:1,000) (Cell Signaling Technology) as
previously described (3).
In vivo tumor growth assays
BALB/c-nu nude mice (aged 4 weeks, male) were
purchased from the Shanghai Laboratory Animal Center (Shanghai,
China). The Institutional Animal Care and Use Committee of Harbin
Medical University approved all animal experiments. Mice housed
under identical conditions were allowed free access to a standard
diet and to tap water with a 12 h light: 12 h dark cycle.
Four-week-old male nude mice were anaesthetized by barbital sodium
at 70 mg/kg body weight and a laparotomy was performed. Ten mice
were randomly divided into two groups. These two groups of mice
were injected subcutaneously (s.c). with 1×106 stably
transfected HepG2/HO-1 and HepG2/Mock cells in the left flank.
After 19 days, the mice were sacrificed and photographed. Tumors
were harvested for paraffin embedding, sectioning and histological
examination after hematoxylin and eosin staining. Five animals were
included in each group. Data are shown for representative
experiments.
Statistical analysis
All data presented in the present study have been
repeated at least three times from three independent experiments
and are expressed as the mean ± standard error. Student's t-test
was performed to determine the significance of the respective group
for each experimental test condition. P<0.05, P <0.01 or
P<0.001 indicated a significant difference.
Results
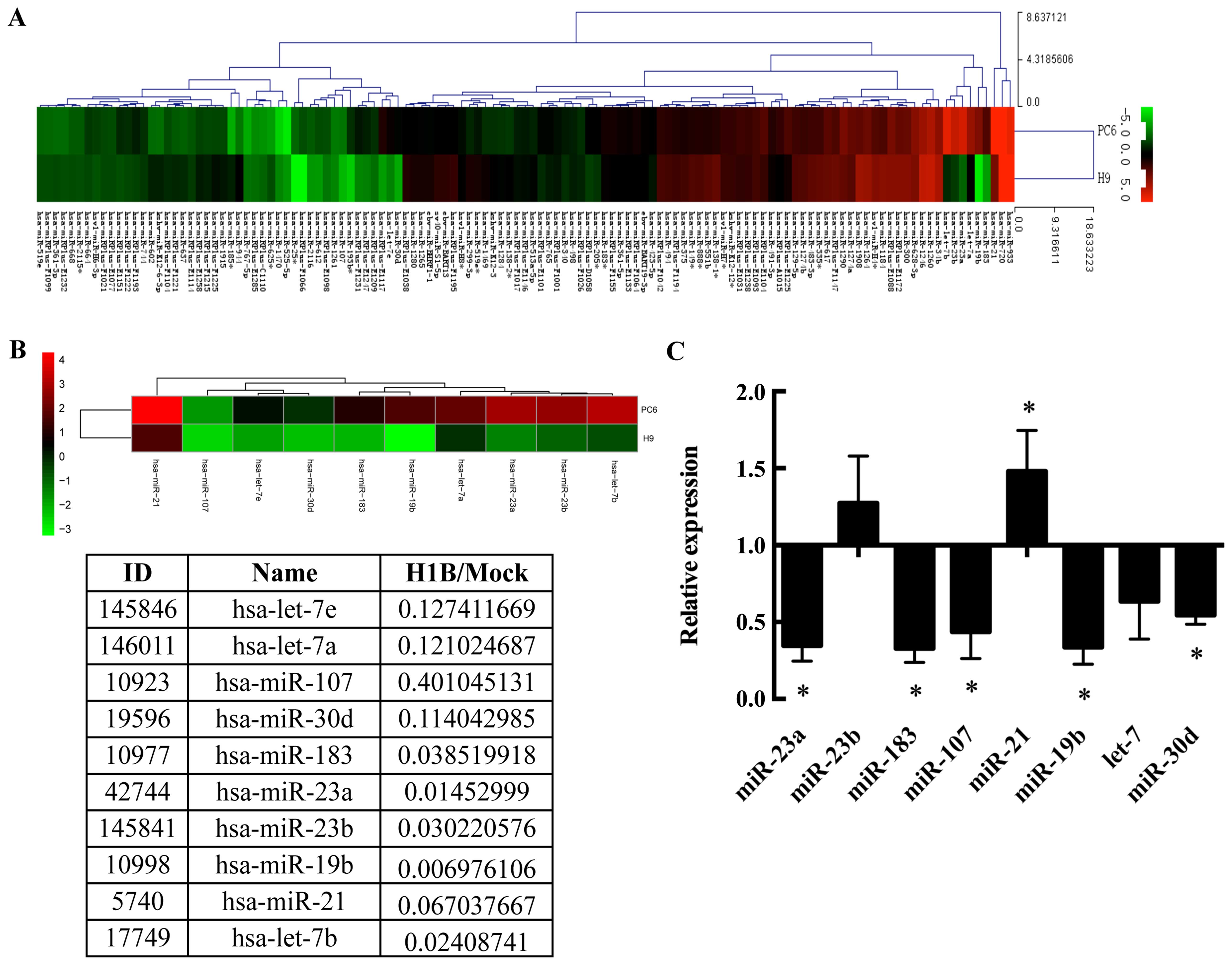
HO-1 modulates miRNA profile in HepG2
cells
In previous studies, we demonstrated that HO-1 could
inhibit the migratory ability of HepG2 cells (3). To further clarify the molecular
mechanism of HO-1 as a tumor regulator, microarray was used to
detect the difference of the miRNA profile between mouse HO-1
overexpressed HepG2 cells (H1B) and empty vector group (Mock). As
results show (Fig. 1A), there are
19 miRNAs upregulated and 23 downregulated at least two-fold by
HO-1 overexpression. Numerous downregulated miRNAs were also found
to relate to tumor progression such as hsa-let-7 and hsa-miR-19b
(Fig. 1A). Six miRNAs closely
related to tumor progression were chosen to verify their expression
by qPCR in H1B and Mock cells (Fig. 1B
and C).
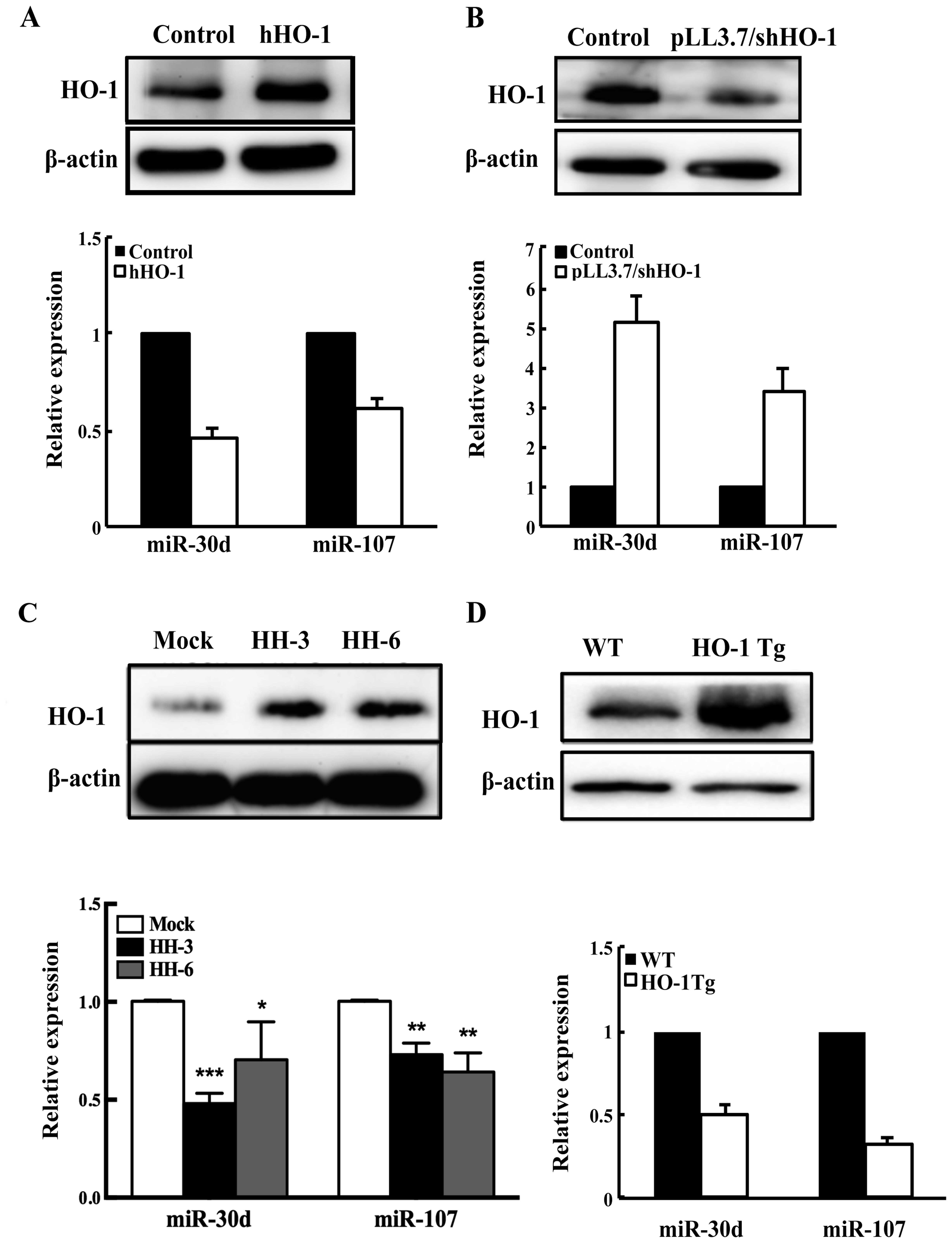
HO-1 reduces the expression of miR-30d
and miR-107 both in vitro and in vivo
In order to confirm the relation of HO-1 and miRNA
expression in human HCC, we altered the expression of HO-1 by
genetic manipulation in HepG2 cells and detected the expression of
HO-1 and above the six miRNA level. The results found that the
abundance of miR-30d and miR-107 were stably negatively correlated
with the expression of HO-1 (Fig. 2A
and B). To pinpoint critical relation between HO-1 and miRNAs,
we generated human HO-1 (hHO-1) stable overexpression HepG2 cell
lines (named HH-3 and HH-6). The expression of HO-1 in the
reconstituted HepG2 cell lines was verified by western blotting
(Fig. 2C). Next, we detected the
expression of HO-1 and miR-30d/miR-107 in HH-3 and HH-6. The
results show that the expression of miR-30d and miR-107 were stably
decreased (Fig. 2C). Furthermore,
we detected the expression of miR-30d/miR-107 in the livers of HO-1
Tg mice (Fig. 2D). All results
showed that HO-1 and miR-30d/miR-107 expressions were negatively
correlated.
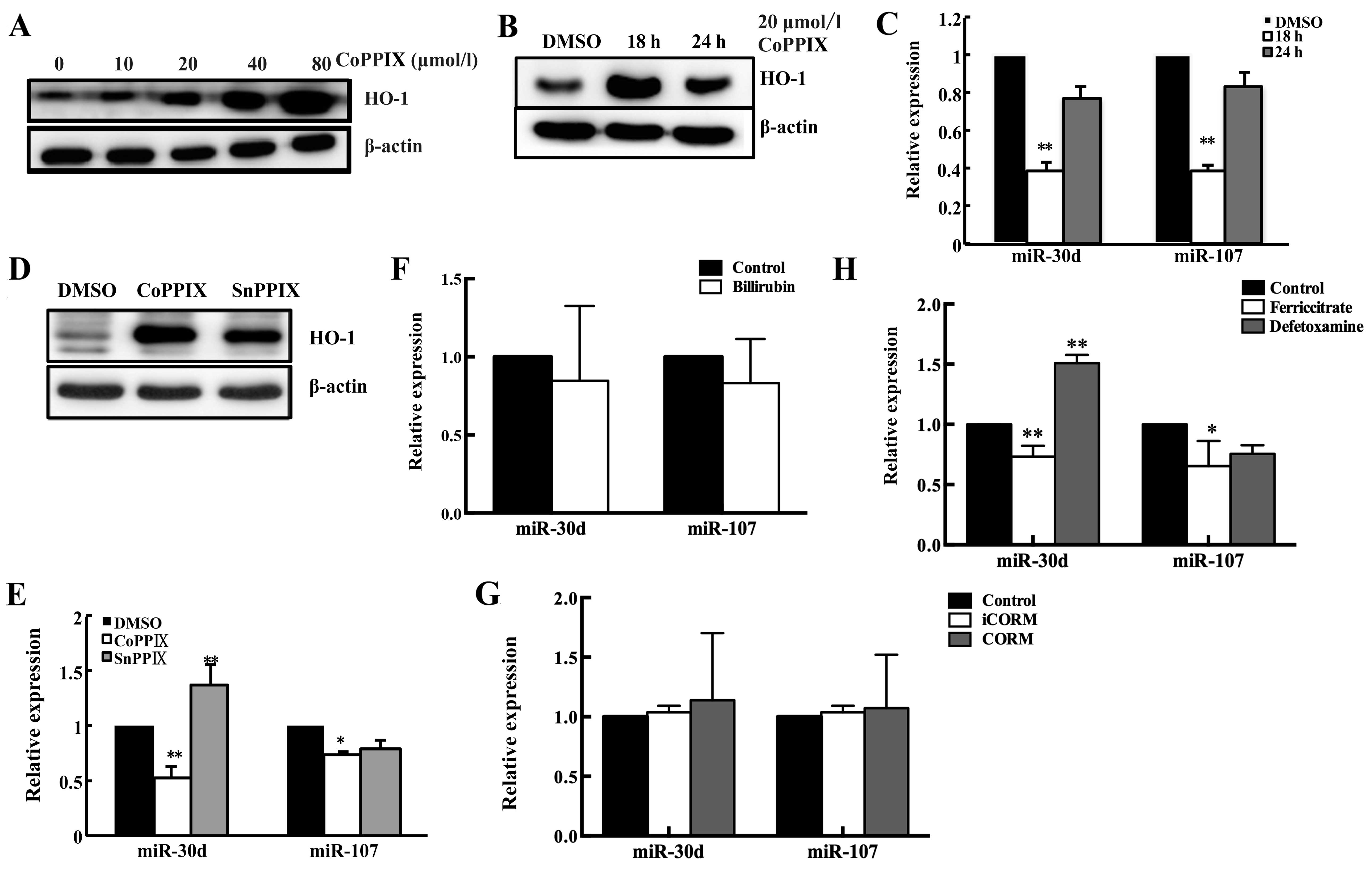
Moreover, the HO-1 and miR-30d/miR-107 expression
were also analyzed after treating cells by HO-1 inducer cobalt
protoporphyrin IX (CoPPIX) or HO-1 activity suppressor Sn
protoporphyrin IX (SnPP). First, we confirmed the induction of HO-1
by CoPPIX was both dose- and time-dependent (Fig. 3A). Notably, we found the regulatory
role of HO-1 in miR-30d/miR-107 expression was also time-dependent
(Fig. 3B and C). Moreover, the
variation of miR-30d/miR-107 after SnPPIX exposure was detected.
The results showed SnPPIX treatment restored the miR-30d/miR-107
expression (Fig. 3D and E).
Considering SnPPIX blocks the enzymatic activity of HO-1 without
influencing its expression, this suppressive effect of HO-1 on
miR-30d/miR-107 may be due to HO-1 activity.
HO-1 overexpression reduces the
expression of miR-30d and miR-107 via its metabolite iron
Although some researchers have found out new
functions of HO-1, we could not neglect that the main role of HO-1
is still as an enzyme functioning through its active products. Our
results also implied that HO-1 may regulate the level of
miR-30d/miR-107 by its metabolites. To determine which product of
HO-1 activity could be responsible for miR-30d/miR-107
downregulation, we treated HepG2 cells with ferricitrate,
deferoxamine, CO-releasing molecule (CORM), inactive CORM (iCORM)
and bilirubin (Fig. 3F–H). It
turned out that the effect of HO-1 on miR-30d/miR-107 could be
mimicked by only one of its products - iron. Moreover, the opposite
results can be received by treating deferoxamine (Fig. 3H). These results demonstrated that
iron maybe an important mediator in the procedure of HO-1
regulating miR-30d/miR-107.
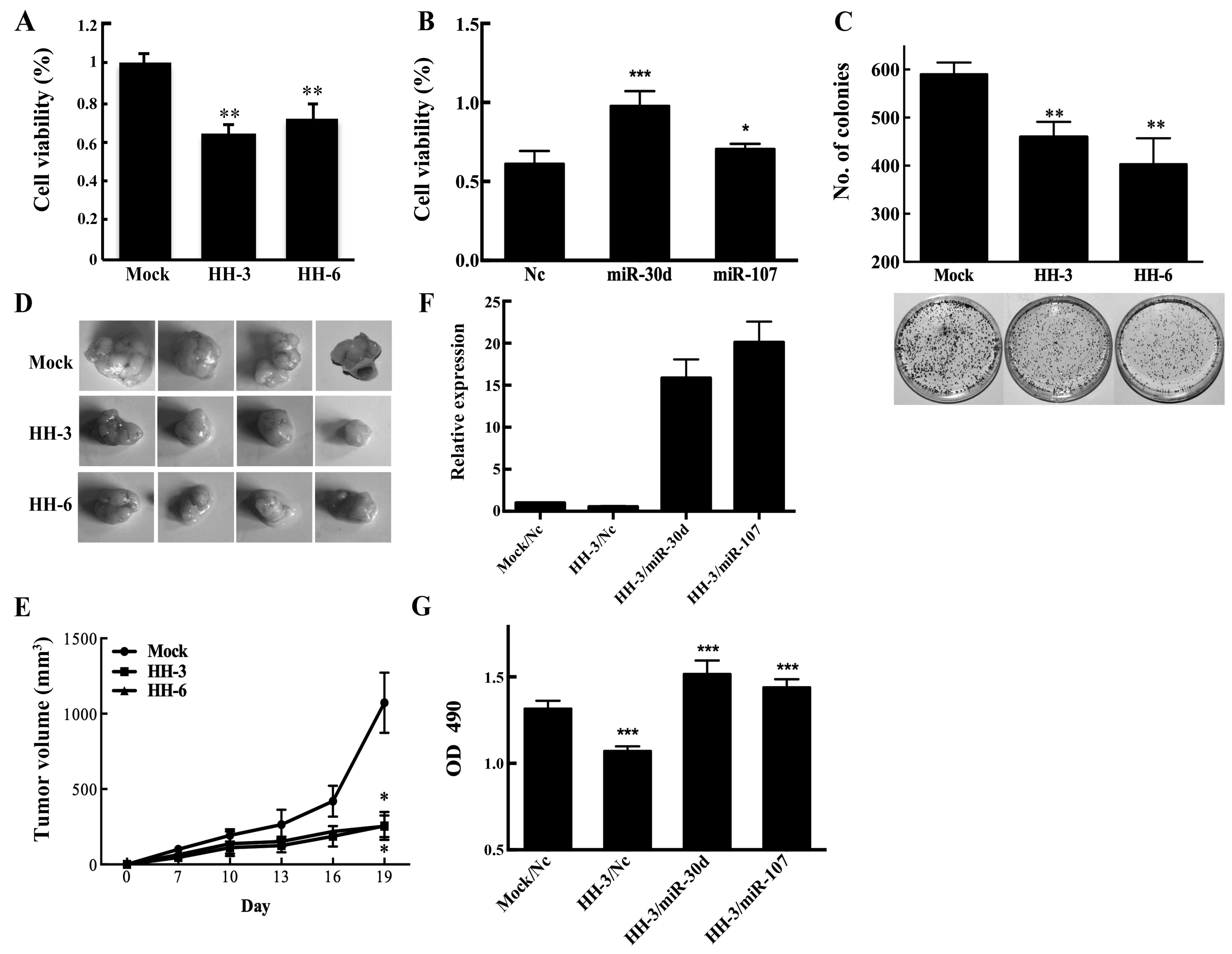
HO-1 inhibits the proliferation by
suppressing miR-30d and miR-107
To elucidate whether miR-30d/miR-107 is involved in
the function of HO-1 in HCC, MTT and colony formation assays were
performed different manipulations in HCC cells. MTT results showed
that the numbers of viable cells in HH-3 and HH6 were significantly
fewer than that of Mock cells (Fig.
4A). Opposite results were found through transient transfection
of HepG2 cells with miR-30d/miR-107 mimics (Fig. 4B). Colony formation assays also
showed that HO-1 overexpression could inhibit the proliferation of
HCC cells (Fig. 4C). In
vivo, we observed the growth rate of HepG2 cells subcutaneous
xenografts. Compared with the Mock, the tumor size in HH3 and HH6
group showed a marked reduction (Fig.
4D and E).
The results also showed that the beneficial effect
of HO-1 on HCC proliferation could be partially reversed by
increasing the expression of miR-30d and miR-107 (Fig. 4F and G). These data demonstrated
that HO-1 suppressed the proliferative ability of HepG2 cells via
inhibiting the expression of miR-30d/miR-107.
miR-30d/miR-107 is an important mediator
in antimetastasis function of HO-1
To investigate whether human HO-1 could inhibit
metastasis of HCC, similarly to mouse HO-1, we observed the
migratory ability of HH3 and HH6 by Transwell assays. The results
confirmed that hHO-1 could inhibit the migration of HepG2 cells.
HO-1 silencing enhanced the migration of HepG2 cells (Fig. 5A and B). As known, miR-30d is a
promoter of metastasis in Huh7 cells (16). Moreover, we demonstrated miR-107
could accelerate the migration of HCC cells (Fig. 5C and D) (3). Our further studies confirmed that
hHO-1 anti-migration effect could also be reversed by
overexpression of miR-30d and miR-107 (Fig. 5E and F). Therefore, we demonstrated
that HO-1 could suppress the migration of HCC by decreasing
miR-30d/miR-107 expression.
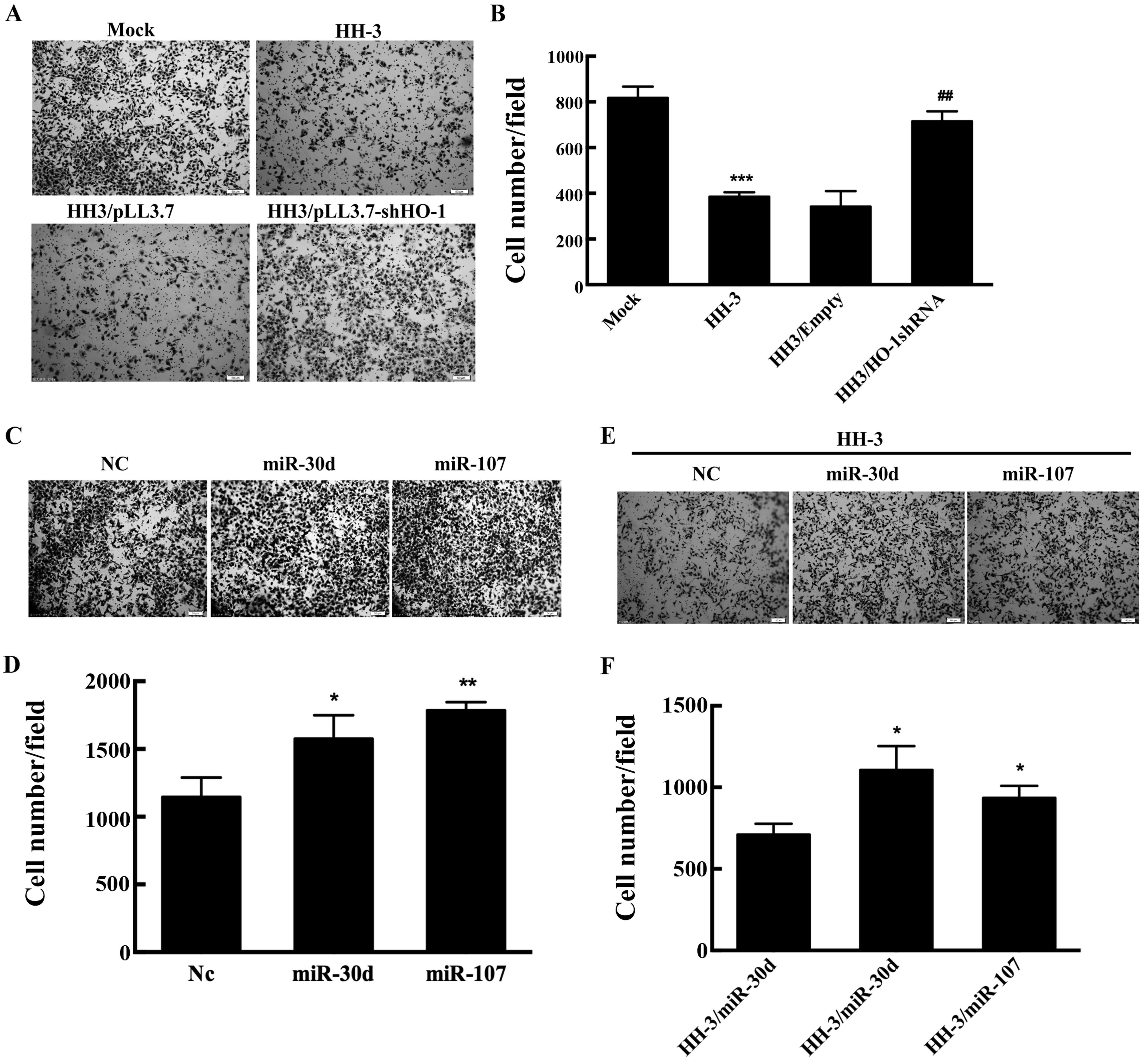 | Figure 5HO-1 and miR-30d/miR-107 regulate the
migration of HepG2 cells. (A-B) Mock and HH-3 cells were
transfected with the indicated plasmid for 24 h, and plated 50,000
cells/well on Transwell inserts and allowed to migrate for 24 h
toward media with 10% fetal bovine serum. (A) Representative images
showing the cell density on the filter, bottom (graph), and (B)
quantitative analysis for the cells migrating through the filter in
three independent experiments. (C and D) HepG2 cells were
transfected with the indicated oligonucleotides for 24 h, plated
50,000 cells/well were on Transwell inserts to perform the
Transwell assays. Top, (C) representative images showing the cell
density on the filter, and (D) quantitative analysis for the cells
migrating through the filter in three independent experiments. (E
and F) HH-3 cells were respectively transfected by the indicated
oligonucleotides for 24 h and following the migration assay. (E)
Top, representative images showing the cell density on the filter,
bottom (graph), and (F) quantitative analysis for the cells
migrating through the filter in three independent experiments;
*P<0.05, **P<0.01,
***P<0.001. |
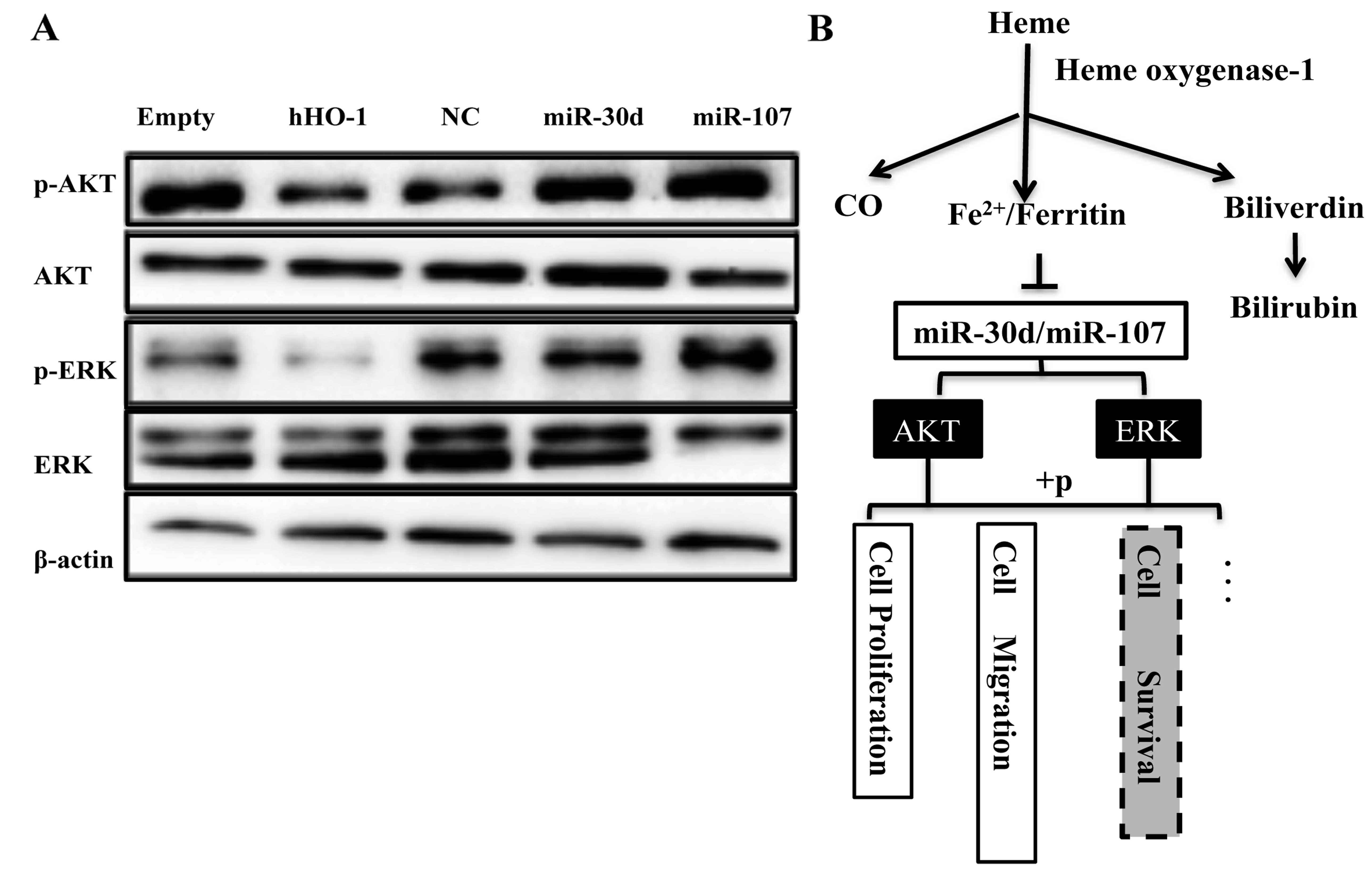
HO-1/miR-30d/miR-107 regulate the HCC
progression through PI3K/AKT and MAPK/ERK pathways
According to the above studies, we elucidated that
the proliferative and metastatic capability of HepG2 cells was
regulated by HO-1 and miR-30/miR-107 in backward directions. To
further investigate the specific factors involved in the
regulation, we transfected HepG2 cells with pcDNA3.1 (+)-hHO-1 or
the mimics of miR-30d/miR-107 to measure the activity of Akt and
ERK1/2 pathways. The results identified that HO-1 overexpression
could decrease the phosphorylation of AKT and ERK. miR-30d/miR-107
showed a crosscurrent in the regulation of the above factors
(Fig. 6A). These results suggested
that Akt and ERK pathways could be involved in the function of
HO-1/miR-30d/miR-107 axis (Fig.
6B).
Discussion
Heme oxygenase-1 (HO-1) is an inducible enzyme
catalyzing the first rate-limiting step in degradation of heme and
playing an important role in many pathophysiological processes.
HO-1 gene polymorphism leads to a correlation with cancer
susceptibility (17–19). Elevated HO-1 has been detected in
various tumors, including adenocarcinoma, glioblastoma, melanoma,
prostate and pancreatic cancer, thereby affecting tumor cell
apoptosis, proliferation, invasion and metastasis (2,3).
Notably, HO-1 has shown its influence on tumors is cell-type
specific and can be the opposite in different tissues (2). The mechanism of this complicated
character of HO-1 has not been clarified.
Recently, accumulated evidence demonstrates that
heme metabolism system is involved in microRNA biogenesis. Firstly,
Faller et al found that heme is involved in pri-miRNA processing
via promoting dimerization of DGCR8 which is essential for the
first step of miRNA processing (20). Subsequently, Li et al
verified that iron homeostasis regulates the activity of the
microRNA pathway through poly(C)-binding protein 2 (21). Several studies also observed
different interaction between HO-1 and miRNAs in different tissues.
For example, Skrzypek et al confirmed HO-1 could inhibit
growth, vascularization and distal metastasis through its
regulation on miR-378 in non-small cell lung carcinoma (22). Moreover, Kozakowska et al
attested that HO-1 inhibits myoblast differentiation by targeting
several myo-miRNAs (23). These
results showed that the regulation of HO-1 on miRNAs is
tissue-specific. In the present study, we investigated the
relationship between HO-1 and miR-30d/miR-107 and its mechanism in
hepatocellular carcinoma.
The mechanism of HO-1 regulating miRNAs is
disputable. In myoblasts, HO-1 regulates the miRNA biogenesis by
suppressing the DGCR8 level (23).
Overexpression of HO-1 in NCI-H292 lung cancer cells upregulates
DGCR8 and Drosha (22). DGCR8 and
Drosha are important microprocessors in regulating the homeostasis
of miRNAs pool. However, in the present study we found out that
HO-1 only impacted on several specific miRNA levels in HCC. The
disturbance of DGCR8 and Drosha expression or the fluctuation of
microRNA pool cannot explain these results. Considering the classic
role of HO-1, the catalytic activity, its catalysates of heme could
be the important candidates in the regulation between HO-1 and
miRNAs. In a previous study, CO has been demonstrated as an
important mediator in the interplay between HO-1 and miR-378 in
lung cancer (22). In the present
study, our results showed that another metabolite product, iron,
could mimic the inhibitory effect of HO-1 on miR-30d/miR-107.
Furthermore, this effect could be reversed by deferoxamine
treatment. Other products including CO and bilirubin did not mimic
the HO-1 effect on miR-30d/miR-107.
Furthermore, we also noted that miR-107 expression
was difficult to be upregulated significantly by SnPP or
deferoxamine, unlike miR-30d. Previous research reported that
miR-107 could directly target Dicer (24), which is a key enzyme of miRNAs
processing. There may be a feedback regulation between miR-107 and
Dicer, which could be the reason for the limited upregulation.
Growing number of studies show that the role of
miR-30d and miR-107 is cell or tissue-specific, which may be
dependent on cellular context or different downstream target genes
(16,25). A previous study, we firstly reported
that miR-107 played a pro-proliferation and pro-metastasis role in
HCC (26). In addition, consistent
with Yao et al (16), we
also confirmed that miR-30d promoted the progress of liver cancer.
In the present study, the results showed when overexpressing HO-1,
with transfected miR-30d/miR-107 mimic, the benefit of HO-1
overexpression on HCC inhibition may be counteracted. These data
confirmed that HO-1 restrains the HCC proliferation and migration
depending partly on downregulating miR-30d/miR-107. Moreover, the
specific pathway for the regulation needs to be clarified.
The phosphoinositide 3-kinase (PI3K)/Akt and
extracellular signal-regulated kinase (ERK) pathways are important
for many biological processes. Activation of PI3K/AKT and MAPK/ERK
pathways via phosphorylation of their variety substrates are widely
known to promote cell proliferation, survival, apoptosis and cell
migration (27,28). In the present study, we showed that
HO-1 could inhibit the phosphorylation of Akt and ERK, which may
lead to a suppression of the proliferation and migration of HCC.
Moreover, miR-30d and miR-107 could activate these signaling
pathways. These results together suggested that PI3K/Akt and
MAPK/ERK pathways maybe involved in the modulation of
HO-1/miR-30d/miR-107 in cancer progress.
In conclusion, the present study uncovers a new
mechanism of HO-1 function depending on the regulation of miRNAs in
HCC. HO-1 acts as a tumor-suppressor via downregulating the
expression of miR-30d/miR-107. The catalytic products of HO-1 play
important roles in this regulation. Moreover, PI3K/Akt and MAPK/ERK
signals may be the final effective pathway.
Acknowledgments
The present study was supported by the Natural
Science Foundation of China (81171997/81572347), the China
Postdoctoral Science Foundation (2015M581479), the Heilongjiang
Postdoctoral Science Foundation (LBH-Z15125), and the Natural
Science Foundation of Heilongjiang Province for youth
(QC2011C016).
References
|
1
|
Maines MD, Trakshel GM and Kutty RK:
Characterization of two constitutive forms of rat liver microsomal
heme oxygenase. Only one molecular species of the enzyme is
inducible. J Biol Chem. 261:411–419. 1986.PubMed/NCBI
|
|
2
|
Jozkowicz A, Was H and Dulak J: Heme
oxygenase-1 in tumors: Is it a false friend? Antioxid Redox Signal.
9:2099–2117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zou C, Zhang H, Li Q, Xiao H, Yu L, Ke S,
Zhou L, Liu W, Wang W, Huang H, et al: Heme oxygenase-1: A
molecular brake on hepatocellular carcinoma cell migration.
Carcinogenesis. 32:1840–1848. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deininger MH, Meyermann R, Trautmann K,
Duffner F, Grote EH, Wickboldt J and Schluesener HJ: Heme oxygenase
(HO)-1 expressing macrophages/microglial cells accumulate during
oligodendroglioma progression. Brain Res. 882:1–8. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doi K, Akaike T, Fujii S, Tanaka S, Ikebe
N, Beppu T, Shibahara S, Ogawa M and Maeda H: Induction of haem
oxygenase-1 nitric oxide and ischaemia in experimental solid
tumours and implications for tumour growth. Br J Cancer.
80:1945–1954. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Palma G, Mozzoni P, Acampa O,
Internullo E, Carbognani P, Rusca M, Goldoni M, Corradi M, Tiseo M,
Apostoli P, et al: Expression levels of some antioxidant and
epidermal growth factor receptor genes in patients with early-stage
non-small cell lung cancer. J Nucleic Acids. 2010:pii: 147528.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee SE, Yang H, Jeong SI, Jin YH, Park CS
and Park YS: Induction of heme oxygenase-1 inhibits cell death in
crotonaldehyde-stimulated HepG2 cells via the PKC-δ-p38-Nrf2
pathway. PLoS One. 7:e416762012. View Article : Google Scholar
|
|
8
|
Kim DH, Kim JH, Kim EH, Na HK, Cha YN,
Chung JH and Surh YJ: 15-Deoxy-Delta12,14-prostaglandin J2
upregulates the expression of heme oxygenase-1 and subsequently
matrix metalloproteinase-1 in human breast cancer cells: Possible
roles of iron and ROS. Carcinogenesis. 30:645–654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin CW, Shen SC, Hou WC, Yang LY and Chen
YC: Heme oxygenase-1 inhibits breast cancer invasion via
suppressing the expression of matrix metalloproteinase-9. Mol
Cancer Ther. 7:1195–1206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu PL, Tsai JR, Charles AL, Hwang JJ,
Chou SH, Ping YH, Lin FY, Chen YL, Hung CY, Chen WC, et al:
Resveratrol inhibits human lung adenocarcinoma cell metastasis by
suppressing heme oxygenase 1-mediated nuclear factor-kappaB pathway
and subsequently downregulating expression of matrix
metalloproteinases. Mol Nutr Food Res. 54(Suppl 2): S196–S204.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heo I and Kim VN: Regulating the
regulators: Posttranslational modifications of RNA silencing
factors. Cell. 139:28–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beckman JD, Chen C, Nguyen J, Thayanithy
V, Subramanian S, Steer CJ and Vercellotti GM: Regulation of heme
oxygenase-1 protein expression by miR-377 in combination with
miR-217. J Biol Chem. 286:3194–3202. 2011. View Article : Google Scholar :
|
|
13
|
Qiu L, Fan H, Jin W, Zhao B, Wang Y, Ju Y,
Chen L, Chen Y, Duan Z and Meng S: miR-122-induced down-regulation
of HO-1 negatively affects miR-122-mediated suppression of HBV.
Biochem Biophys Res Commun. 398:771–777. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shan Y, Zheng J, Lambrecht RW and
Bonkovsky HL: Reciprocal effects of micro-RNA-122 on expression of
heme oxygenase-1 and hepatitis C virus genes in human hepatocytes.
Gastroenterology. 133:1166–1174. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kozakowska M, Szade K, Dulak J and
Jozkowicz A: Role of heme oxygenase-1 in postnatal differentiation
of stem cells: A possible cross-talk with microRNAs. Antioxid Redox
Signal. 20:1827–1850. 2014. View Article : Google Scholar :
|
|
16
|
Yao J, Liang L, Huang S, Ding J, Tan N,
Zhao Y, Yan M, Ge C, Zhang Z, Chen T, et al: MicroRNA-30d promotes
tumor invasion and metastasis by targeting Galphai2 in
hepatocellular carcinoma. Hepatology. 51:846–856. 2010.PubMed/NCBI
|
|
17
|
Chin HJ, Cho HJ, Lee TW, Na KY, Yoon HJ,
Chae DW, Kim S, Jeon US, Do JY, Park JW, et al Progressive REnal
disease and Medical Informatics and gEnomics Research (PREMIER)
members: The heme oxygenase-1 genotype is a risk factor to renal
impairment of IgA nephropathy at diagnosis, which is a strong
predictor of mortality. J Korean Med Sci. 24(Suppl 1): S30–S37.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sunamura M, Duda DG, Ghattas MH, Lozonschi
L, Motoi F, Yamauchi J, Matsuno S, Shibahara S and Abraham NG: Heme
oxygenase-1 accelerates tumor angiogenesis of human pancreatic
cancer. Angiogenesis. 6:15–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vashist YK, Blessmann M, Trump F, Kalinin
V, Kutup A, Schneider C, Gawad K, Kaifi JT, Schmelzle R, Izbicki
JR, et al: Microsatellite GTn-repeat polymorphism in the promoter
of heme oxygenase-1 gene is an independent predictor of tumor
recurrence in male oral squamous cell carcinoma patients. J Oral
Pathol Med. 37:480–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Faller M, Matsunaga M, Yin S, Loo JA and
Guo F: Heme is involved in microRNA processing. Nat Struct Mol
Biol. 14:23–29. 2007. View
Article : Google Scholar
|
|
21
|
Li Y, Lin L, Li Z, Ye X, Xiong K, Aryal B,
Xu Z, Paroo Z, Liu Q, He C, et al: Iron homeostasis regulates the
activity of the microRNA pathway through poly(C)-binding protein 2.
Cell Metab. 15:895–904. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Skrzypek K, Tertil M, Golda S, Ciesla M,
Weglarczyk K, Collet G, Guichard A, Kozakowska M, Boczkowski J, Was
H, et al: Interplay between heme oxygenase-1 and miR-378 affects
non-small cell lung carcinoma growth, vascularization, and
metastasis. Antioxid Redox Signal. 19:644–660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kozakowska M, Ciesla M, Stefanska A,
Skrzypek K, Was H, Jazwa A, Grochot-Przeczek A, Kotlinowski J,
Szymula A, Bartelik A, et al: Heme oxygenase-1 inhibits myoblast
differentiation by targeting myomirs. Antioxid Redox Signal.
16:113–127. 2012. View Article : Google Scholar :
|
|
24
|
Martello G, Rosato A, Ferrari F, Manfrin
A, Cordenonsi M, Dupont S, Enzo E, Guzzardo V, Rondina M, Spruce T,
et al: A MicroRNA targeting dicer for metastasis control. Cell.
141:1195–1207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Finnerty JR, Wang WX, Hébert SS, Wilfred
BR, Mao G and Nelson PT: The miR-15/107 group of microRNA genes:
Evolutionary biology, cellular functions, and roles in human
diseases. J Mol Biol. 402:491–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou CD, Zhao WM, Wang XN, Li Q, Huang H,
Cheng WP, Jin JF, Zhang H, Wu MJ, Tai S, et al: MicroRNA-107: A
novel promoter of tumor progression that targets the CPEB3/EGFR
axis in human hepatocellular carcinoma. Oncotarget. 7:266–278.
2016.
|
|
27
|
Cheng P, Alberts I and Li X: The role of
ERK1/2 in the regulation of proliferation and differentiation of
astrocytes in developing brain. Int J Dev Neurosci. 31:783–789.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu J, Lv H, Guan H, Ma X, Ji M, He N, Shi
B and Hou P: Metallothionein 1G functions as a tumor suppressor in
thyroid cancer through modulating the PI3K/Akt signaling pathway.
BMC Cancer. 13:4622013. View Article : Google Scholar : PubMed/NCBI
|