Introduction
It is estimated that approximately 277,000 cases of
pancreatic cancer are diagnosed worldwide every year, accounting
for 2.2% of all cancers (1).
Pancreatic cancer is a multifactorial disease and the seventh
leading cause of cancer-related death in China (2). Moreover, pancreatic cancer has lower
rates of diagnosis and the 5-year survival rate is <5% (3). Pancreatic cancer has limited treatment
options. At present, surgery is considered as the only effective
therapeutic measure. However, only 20% of the patients are suited
for this approach (4). Therefore,
more effective therapies are needed.
Melatonin is a pineal gland hormone and adjusts
sleep and circadian rhythm (5).
Melatonin also plays a part in biological processes including
immunomodulation, antioxidative stress and anti-inflammation
(6–9). Researchers have revealed that
melatonin is an anticancer hormone (10). The anticancer mechanisms of
melatonin involve activation of antioxidation stress, inhibition of
migration and induction of cell apoptosis (11–13).
Researchers showed that melatonin had an apoptotic effect on the
hepatocarcinoma HepG2 cell line (14). Research has found that using
melatonin has no significant side-effects (15). In conclusion, we postulated that
melatonin may exert a protective effect against cancer.
The mitogen-activated protein kinase (MAPK) pathway
plays an important role in cell survival and proliferation
(16). The MAPK family has been
categorized into three groups: c-jun N-terminal kinase (JNK),
extracellular-regulated kinase 1/2 (ERK1/2) and p38MAPK. Some
studies have suggested that the JNK substrate is involved in cell
growth and apoptosis (17), which
take part in tumor progression and are affected by melatonin
(18).
Nuclear factor-κB (NF-κB) of the Rel family includes
p50, p52, p65 (RelA), c-Rel and Rel B. NF-κB, as a transcription
factor, stimulates the expression level of many genes closely
related to oxidative stress and anti-apoptosis (19). Tamura et al found that
melatonin suppressed the NF-κB pathway in rat endothelial cells
(20). Gilad et al also
revealed that melatonin suppressed the NF-κB pathway in murine
macrophages (21).
At present, it is unclear whether melatonin has an
effect on the apoptosis of the human pancreatic carcinoma cell line
MIA PaCa-2 through the JNK and ERK pathways. Therefore, we examined
the function of melatonin on the viability and apoptosis of MIA
PaCa-2 cells via the MAPK signaling pathways and we investigated
whether melatonin induces cell apoptosis through a decrease in
NF-κB.
Materials and methods
Cell culture and reagents
Human pancreatic carcinoma cell line MIA PaCa-2 was
purchased from the Cell Bank of the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) (Sigma, St.
Louis, MO, USA) supplemented with 1% penicillin/streptomycin and
10% heat-inactivated fetal bovine serum (FBS; Sigma) in 5%
CO2 in a humidified incubator. Cells were seeded at a
density of 5×105 cells/100-mm dish. Melatonin (Sigma)
was dissolved in 0.2% dimethyl sulphoxide (DMSO) and cells were
treated with different doses of melatonin (0–4 mM) from 0 to 72
h.
Colony-forming assays
MIA PaCa-2 cells were seeded into 6-well plates
(5×105 cells/well) at 37°C with 5% CO2 in a
humidified environment. On the second day, the cells were treated
with 1 and 2 mM melatonin for 8 days. Then each well was washed
twice with PBS and stained with crystal violet. During the
incubation period of 8 days, the culture medium was replaced every
3 days in all wells.
Cell viability assay
The Cell Count Kit-8 (CCK-8; Dojindo, Japan) was
used to evaluate the effects of melatonin on cell viability. For
the CCK-8 assays, MIA PaCa-2 cells were seeded into a 96-well
culture plate (3×103 cells/well) in 200 μl of
complete medium. The plating medium was replaced with new culture
medium after 24 h. Then melatonin was dissolved in the new medium
at different doses (0.25–4 mM). Vehicle control cells were cultured
in DMEM with 0.2% DMSO. Each group included three parallel wells.
After exposurure for 12, 24, 48 and 72 h, CCK-8 was added to the
culture media. The supernatant of each well was measured at a
450-nm wavelength using a plate reader (Infinite® 200
PRO NanoQuant; Tecan Austria GmbH, Austria).
Wound-healing assay
MIA PaCa-2 cells were seeded into 12-well plates in
complete medium at a density of 3×104 cells/well, at
37°C with 5% CO2. When the cells formed a monolayer, a
scratch was created in the middle of the well with a 100-μl
pipette tip. Subsequently, the debris was washed away and the wells
were cultured with fresh media and 1% FBS with different
concentrations of melatonin (0–2 mM) and 0.2% DMSO. After
incubation at 0, 24 and 48 h, images of the cells were captured
(phase-contrast microscope). Each experiment was performed in
triplicate. The initial migration of the scratch in the field of
view was determined by the area divided by the length devoid of
cells using Image-Pro Plus software (Media Cybernetics, Inc.,
Bethesda, MD, USA). Results are expressed as the difference in the
migration distance between 0 and 48 h of treatment.
Apoptosis assay using Annexin V
FITC/PI
To observe early apoptosis and necrosis, the cells
were stained with FITC-conjugated Annexin V and propidium iodide
(PI) (MultiSciences Biotech Co., Ltd., Hangzhou, China). The cells
(4×105) were plated in 6-well plates and treated with 0
and 2 mM melatonin for 0 to 72 h. Cells were harvested by
trypsinization, cleared with PBS, centrifuged at 1,000 rpm for 5
min and the supernatant was discarded. The pellet was resuspended
in 1X binding buffer. A total of 500 μl of the sample
solution was added to 5 μl of FITC-conjugated Annexin V and
10 μl of PI and the solution was incubated for 5 min in the
dark at room temperature. The cells were visualized and sorted
using FACS (Becton Dickinson, San Jose, CA, USA) and quantified
using FlowJo software. Positioning of quadrants on the Annexin V
FITC/PI dot plots was used to distinguish living cells (Annexin V
FITC−/PI−), early apoptotic cells (Annexin V
FITC+/PI−) and late apoptotic/secondary
necrotic cells (Annexin V FITC+/PI+)
(18).
Western blot analysis
After 2 mM melatonin treatment, the cells were
washed three times with cold PBS and lysed by adding ice-cold RIPA
buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 1%
sodium deoxycholate, 0.1% SDS, sodium orthovanadate, sodium
fluoride, EDTA, leupeptin and PMSF and PhosSTOP (Roche, Basel,
Switzerland) for 15 min on the table concentrator minus four
degrees. Then cells were scraped off the plate. The extracts were
transferred to a microfuge tube and centrifuged at 12,000 × g for
15 min. The protein concentration was determined using the BCA
assay (Beyotime). Equal amounts of total protein (40 μg)
were separately subjected to 10% SDS-PAGE and transferred to a PVDF
membrane (Bio-Rad, Hercules, CA, USA). The membranes were blocked
at room temperature for 1 h in blocking buffer and the proteins
were incubated with primary antibodies targeted against:
phospho-ERK (1:1,000), ERK (1:1,000), phospho-JNK (1:1,000), JNK
(1:1,000), phospho-p65 (1:1,000), p65 (1:1,000) (Cell Signaling
Technology, Beverly, MA, USA) and GAPDH (1:1,000) (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 12–16 h. After
washing with TBST three times, the membranes were incubated for 1 h
at room temperature with the secondary HRP-conjugated antibody
(1:5,000; Bioworld Technology, Inc., USA) and visualized using
WesternBright ECL detection kit (Advansta, Menlo Park, CA, USA).
The density of the specific bands was quantified using Image Lab
software with an imaging densitometer (Bio-Rad ChemiDoc MP) (both
from Bio-Rad).
Statistical analysis
The results were analyzed using SPSS software
(version 13) (SPSS, Inc., Chicago, IL, USA) and are presented as
the mean values ± SEM. Data comparisons were performed using
analysis of variance (ANOVA). When the analysis suggested the
presence of a significant difference, the means were compared with
the Newman-Keuls test. Statistical significance was accepted at
P<0.05.
Results
Melatonin affects MIA PaCa-2 cell
viability, colony formation, invasion and apoptosis
We used the human pancreatic carcinoma cell line MIA
PaCa-2 to evaluate the antitumour effects of melatonin on
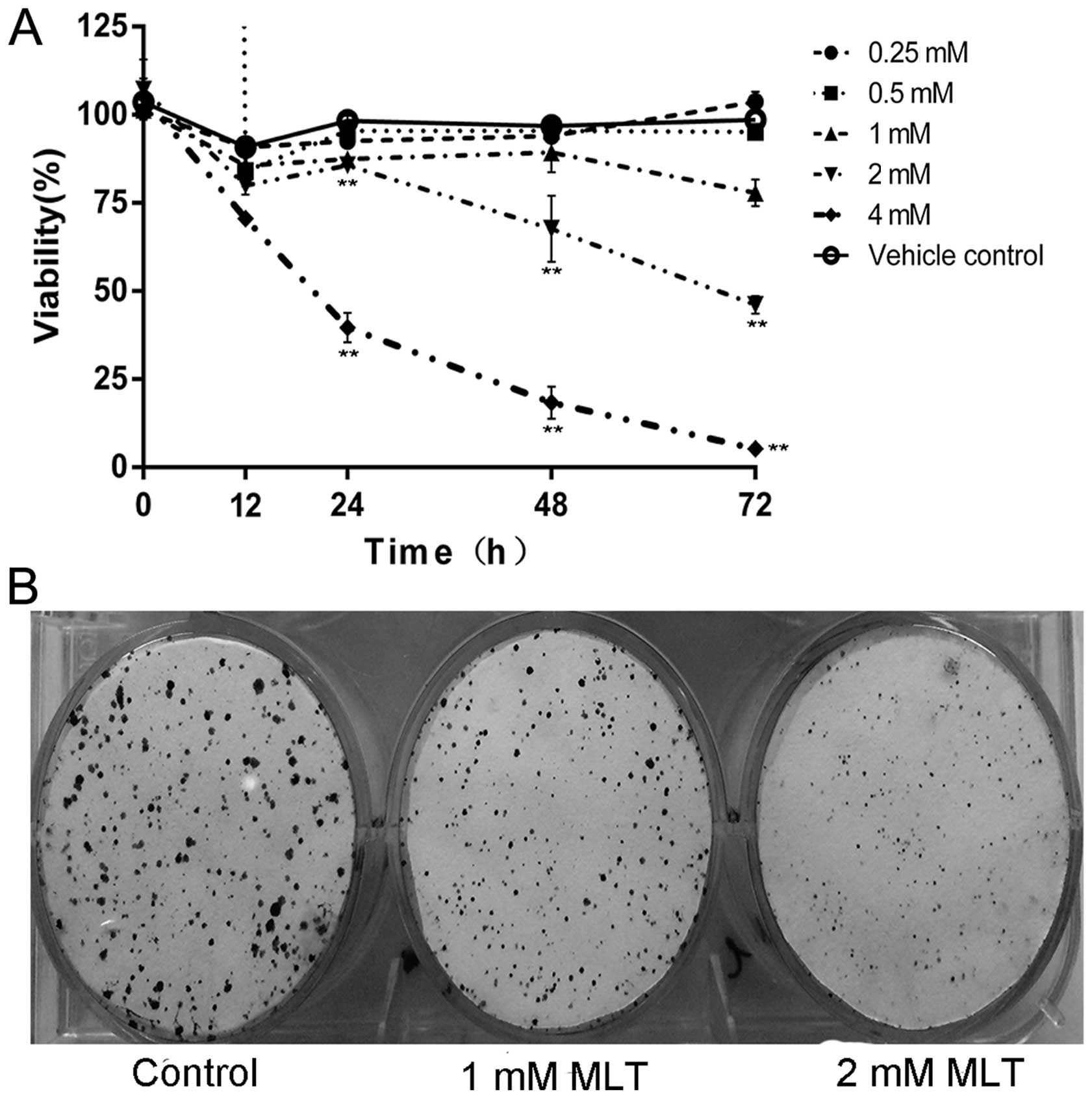
pancreatic cancer. The effect of melatonin on MIA PaCa-2 cell
viability was observed by the CCK-8 assay and the results showed
that cell viability was significantly reduced by 2 and 4 mM
melatonin after 12–72 h of treatment (Fig. 1A). When analysing the number of
colonies formed, melatonin treatment (1 and 2 mM) caused a
significant decline in colony formation (Fig. 1B).
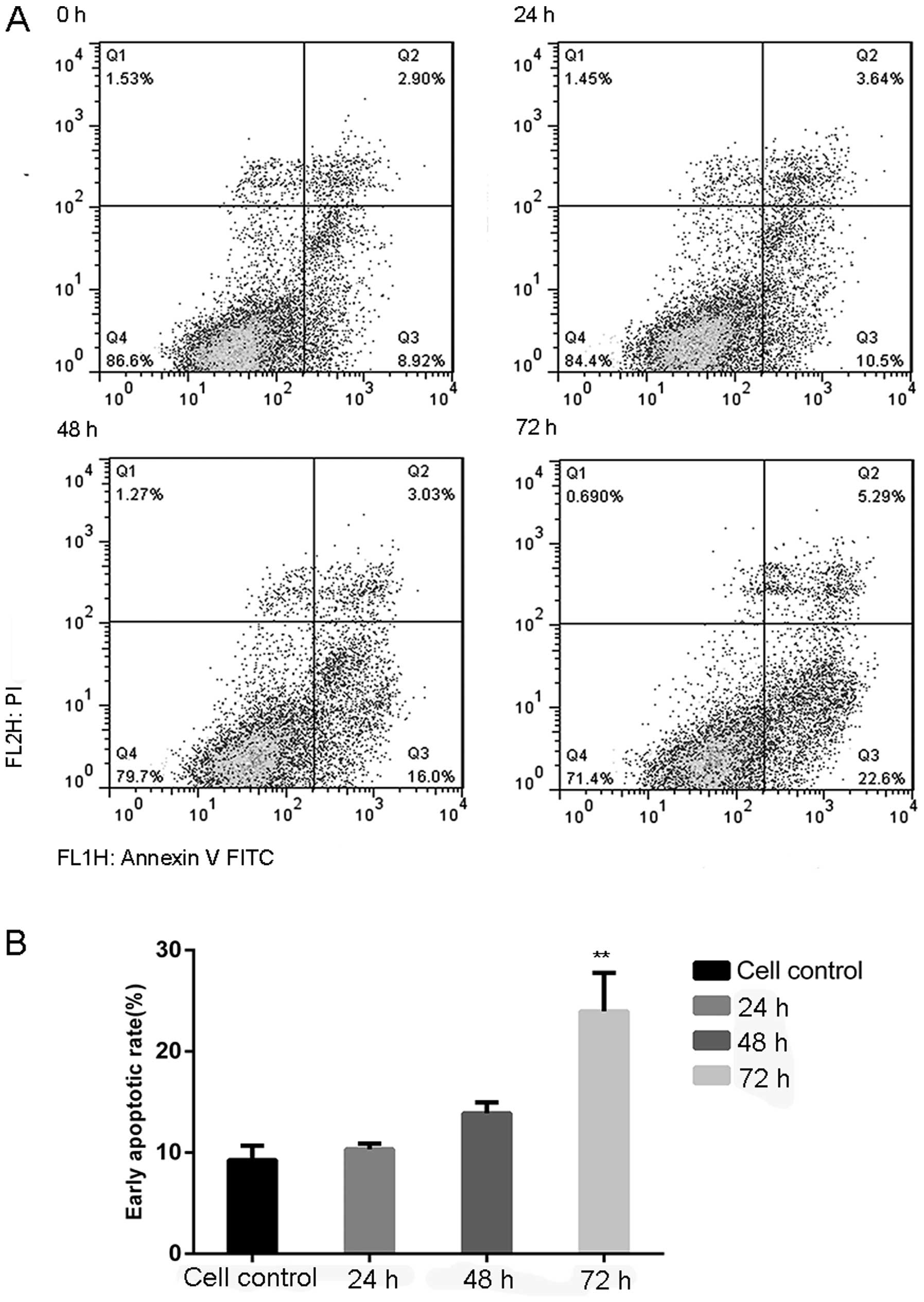
Flow cytometry and Annexin V FITC/PI staining, which
can identify early apoptotic cells, were used to assess apoptosis
in the human pancreatic carcinoma cell line MIA PaCa-2 after
exposure to 2 mM melatonin for 0, 24, 48 and 72 h. As shown in
Fig. 2A, after treatment for 48 h
the percentage of early apoptotic cells was 1.79-fold of that noted
in the control cells. The early apoptotic rate (Annexin V FITC/PI
staining) was significantly increased after 72 h (23.9%, Fig. 2B).
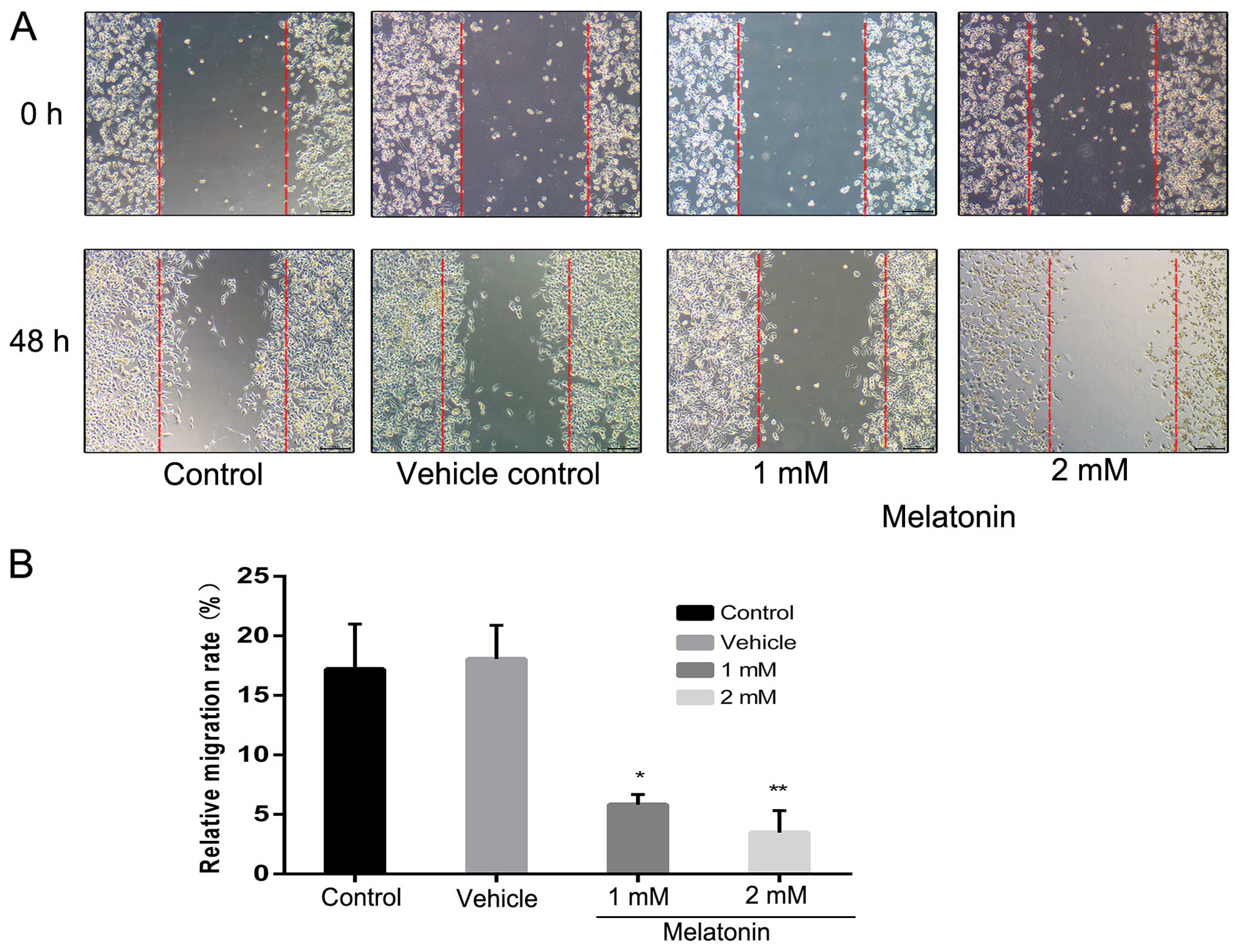
MIA PaCa-2 cells were treated with various
concentrations of melatonin at various times to assess the effects
of melatonin on cell migration. As shown in Fig. 3, 2
mM of melatonin markedly reduced MIA PaCa-2 cell migration (20.1%
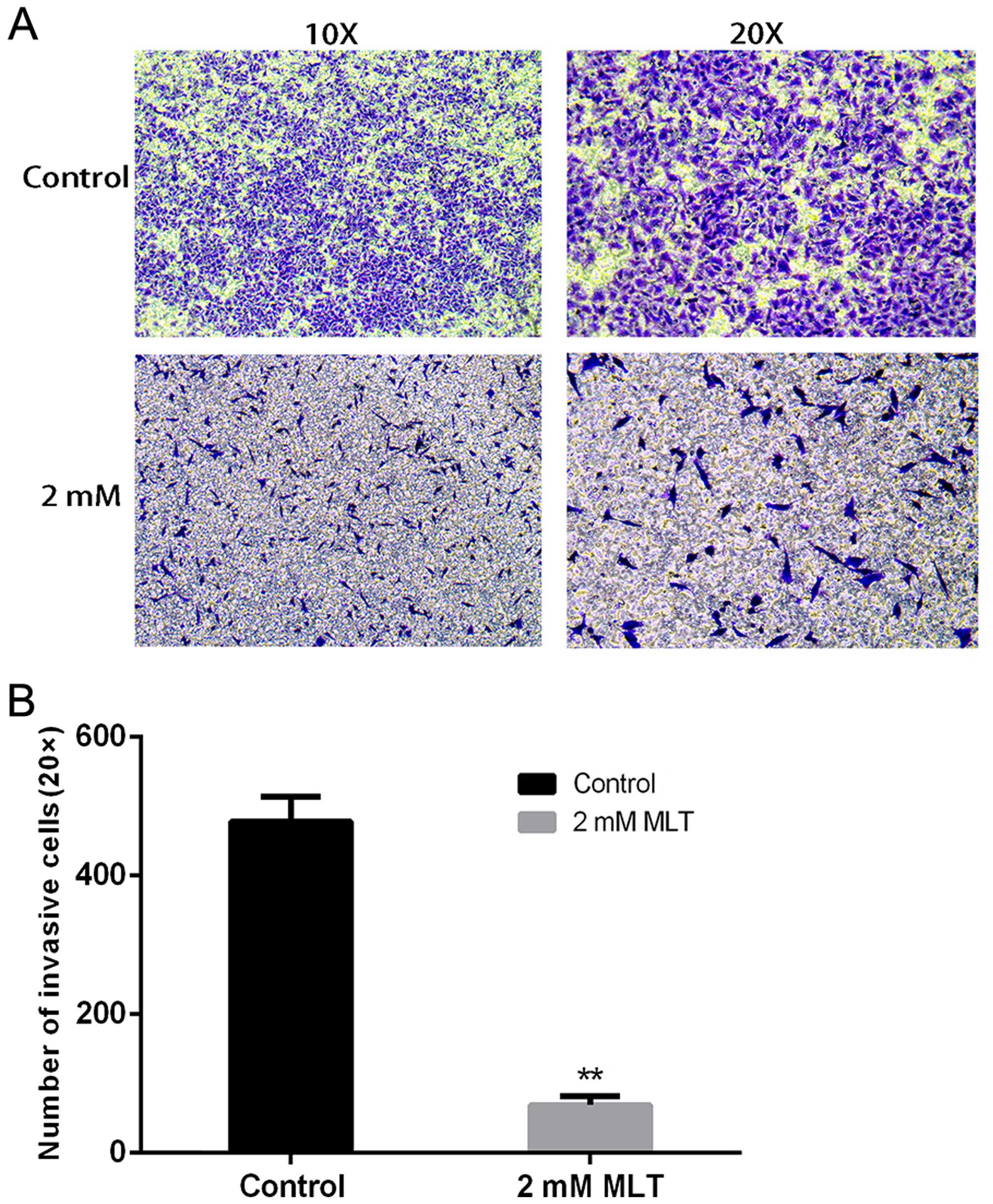
of the control at 48 h). To examine the inhibition of cell motility
by melatonin by invasion assay, we found that melatonin also
suppressed cell invasion compared with the control group (Fig. 4).
Effects of melatonin on the
phosphorylation of MAPK pathway components
Melatonin was found to inhibit cell viability and
migration and induce cell apoptosis in the MIA PaCa-2 cells.
Considering the possible mechanisms, we evaluated the function on
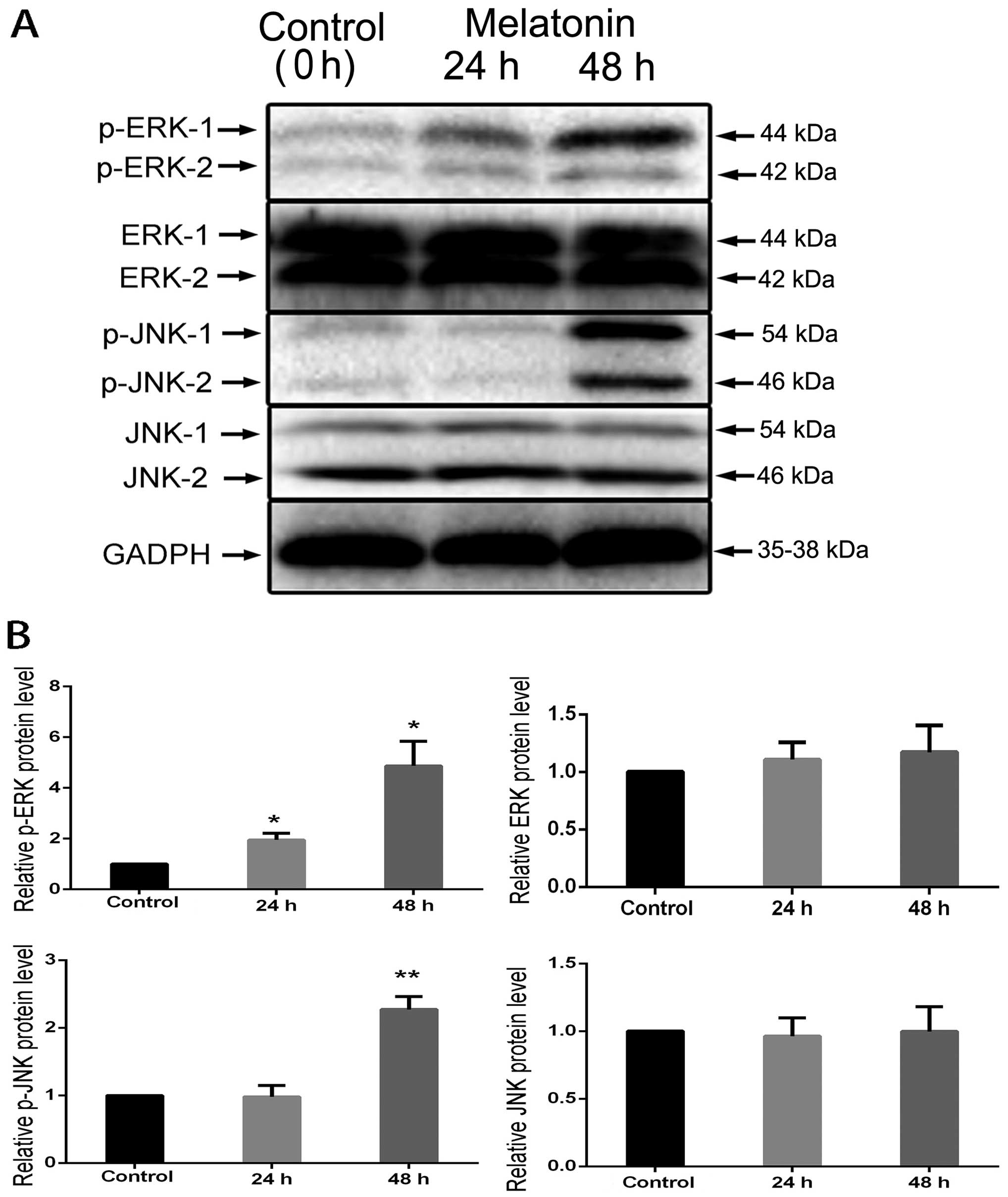
the elementary activation status of MAPKs. JNK and ERK
phosphorylation was significantly induced at 24 and 48 h (Fig. 5A); the levels of JNK and ERK were
used as internal controls and the phosphorylated proteins were
quantified using the control. The results revealed that the levels
of p-JNK and p-ERK were increased at 24 and 48 h (Fig. 5B).
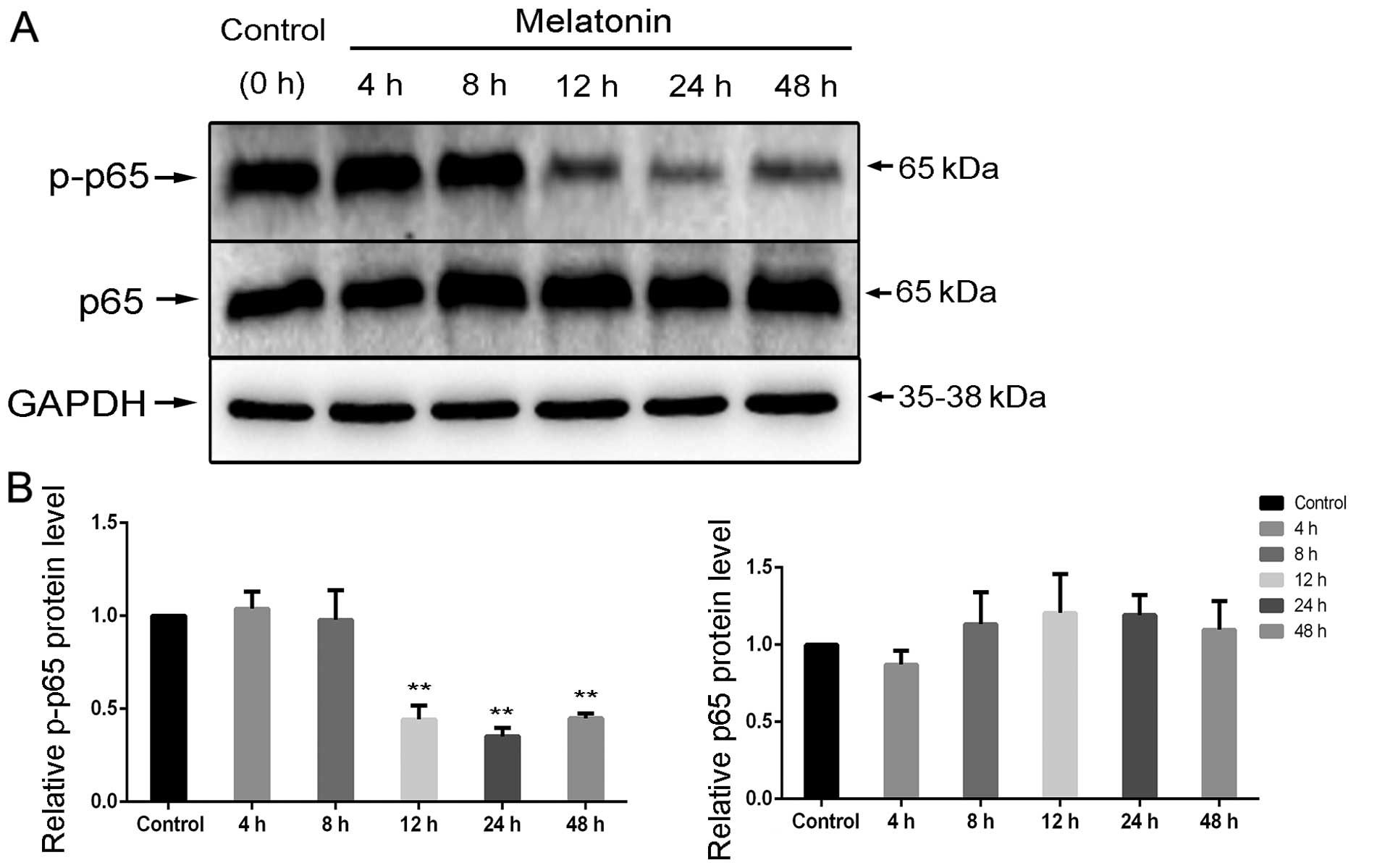
Melatonin inhibits NF-κB activation
Following treatment with 2 mM melatonin, p65
phosphorylation was significantly induced in a time-dependent
manner (Fig. 6A); the levels of p65
were used as internal controls and the phosphorylated proteins were
quantified using the control. The results presented a clear
decrease in p-p65 activity at 12, 24 and 48 h (Fig. 6B).
Discussion
Pancreatic cancer originates from pancreas tissue
and has a high rate of metastasis (22). Pancreatic cancer is not only
difficult to diagnose at an early stage, but also lacks effective
therapeutic strategies (23).
Recently, studies have revealed that melatonin has anticancer
properties. However, the mechanisms of melatonin in regards to its
antitumour properties are poorly understood. Induction of apoptosis
is a potential antitumour mechanism and it is a basic step in the
regulation of different cell types. Therefore, we studied melatonin
which induced cell apoptosis in the human pancreatic carcinoma cell
line MIA PaCa-2.
We found that melatonin inhibited MIA PaCa-2 cell
viability, migration and invasion (Figs. 1, 3
and 4). Moreover, we revealed that
melatonin induced cell apoptosis at various concentrations. Joo
et al demonstrated that apoptosis activates-caspases which
are related to cell viability (18). Pro-apoptosis is viewed as the most
suitable strategy for treating cancer. Some reports suggest that
ERK pathway activation influences a survival signal that weakens
pro-apoptotic effects via activating JNK (24,25).
Cisplatin through ERK pathway activation, slows down cell growth
and causes apoptotic cell death (26).
In summary, we considered whether melatonin through
MAPK (JNK, ERK) pathways induces apoptosis in MIA PaCa-2 cells. We
hypothesized that melatonin acts as a potential apoptotic inducer.
Our western blot analysis of MAPK pathway components showed that
phosphorylation of JNK and ERK was enhanced at 24 h and
significantly increased at 48 h by melatonin (Fig. 5). Recent research suggests an
important role for JNK and ERK in pathways related to apoptosis and
growth-inhibitory pathways (27,28).
In addition, JNK induces caspase-3 activation and JNK is necessary
for the phosphorylation of proteins related to apoptosis in cancer
cells, including Bcl-2 and Bax (29). In conclusion, we consider that
melatonin caused MIA PaCa-2 cell apoptosis by activating JNK and
ERK which promote caspase-3 overexpression and caspase-3-induced
cell apoptosis.
The transcription factor NF-κB family is comprised
of related protein dimers (30).
Upon activation, NF-κB p-65 is liberated from the IκB compound and
NF-κB p-65 translocates to the nucleus, where it causes the
expression of a series of genes encoding different proteins which
take part in suppressing apoptosis and causing inflammation,
cellular invasion and proliferation (31). These target genes play key roles in
malignant development and include apoptosis-suppressor proteins,
such as Bcl-2 and Bcl-XL, and cell-cycle regulatory proteins, such
as cyclin D1 (32). In malignant
cells, stimulation of cell proliferation and protection against
apoptosis are connected with abnormal NF-κB activation (33). Curcumin (34) and flavopiridol (35) have been suggested to inhibit NF-κB.
In this study, we found that melatonin may suppress the
phosphorylation of NF-κB p-65 (Fig.
6).
In summary, melatonin may possess anticancer effects
in several types of cancer, including gastric cancer. Research
suggests that melatonin exerts an antitumour effect (36). The potential to target mechanisms
that inhibit NF-κB p65 and promote ERK and JNK may offer effective
strategies for chemotherapy. By analyzing the present and previous
data, we established that melatonin induces apoptosis and
suppresses migration and invasion via modulation of signaling
mediated by the JNK and ERK MAPKs and NF-κB p65 pathways. The
present study suggests the requirement for additional or adjunct
therapies in combination with melatonin treatment to fully inhibit
cancer progression. Our results suggest that melatonin may act as a
potential anticancer agent against human pancreatic cancer.
Abbreviations:
|
MLT
|
melatonin
|
|
NF-κB
|
nuclear factor-κB
|
|
JNK
|
c-jun N-terminal kinase
|
|
MAPK
|
mitogen-activated protein kinases
|
|
ERK1/2
|
extracellular-regulated kinase 1/2
|
Acknowledgments
This study was supported by the Science and
Technology Bureau of Wenzhou, Zhejiang Province, China (no.
2014S0192).
References
|
1
|
Raimondi S, Maisonneuve P and Lowenfels
AB: Epidemiology of pancreatic cancer: An overview. Nat Rev
Gastroenterol Hepatol. 6:699–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo L, Fan L, Pang Z, Ren J, Ren Y, Li J,
Chen J, Wen Z and Jiang X: TRAIL and doxorubicin combination
enhances anti-glioblastoma effect based on passive tumor targeting
of liposomes. J Control Release. 154:93–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peixoto RD, Ho M, Renouf DJ, Lim HJ, Gill
S, Ruan JY and Cheung WY: Eligibility of metastatic pancreatic
cancer patients for first-line palliative intent nab-paclitaxel
plus gemcitabine versus FOLFIRINOX. Am J Clin Oncol. 12015.
View Article : Google Scholar
|
|
4
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: Groupe Tumeurs Digestives of Unicancer;
PRODIGE Intergroup: FOLFIRINOX versus gemcitabine for metastatic
pancreatic cancer. N Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lerner AB, Case JD, Takahashi Y, Lee TH
and Mori W: Isolation of melatonin, the pineal gland factor that
lightens melanocytes. J Am Chem Soc. 80:2587. 1958. View Article : Google Scholar
|
|
6
|
Berra B and Rizzo AM: Melatonin: Circadian
rhythm regulator, chronobiotic, antioxidant and beyond. Clin
Dermatol. 27:202–209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cardinali DP, Esquifino AI, Srinivasan V
and Pandi- Perumal SR: Melatonin and the immune system in aging.
Neuroimmunomodulation. 15:272–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ambriz-Tututi M, Rocha-González HI, Cruz
SL and Granados-Soto V: Melatonin: A hormone that modulates pain.
Life Sci. 84:489–498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fischer TW, Kleszczyński K, Hardkop LH,
Kruse N and Zillikens D: Melatonin enhances antioxidative enzyme
gene expression (CAT, GPx, SOD), prevents their UVR-induced
depletion, and protects against the formation of DNA damage
(8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J Pineal Res.
54:303–312. 2013. View Article : Google Scholar
|
|
10
|
Shiu SY, Li L, Xu JN, Pang CS, Wong JT and
Pang SF: Melatonin-induced inhibition of proliferation and G1/S
cell cycle transition delay of human choriocarcinoma JAr cells:
Possible involvement of MT2 (MEL1B) receptor. J Pineal Res.
27:183–192. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu L, Liu H, Zhang H, Wang RX, Song J and
Zhou RX: Growth-inhibitory activity of melatonin on murine
foregastric carcinoma cells in vitro and the underlying molecular
mechanism. Anat Rec (Hoboken). 296:914–920. 2013. View Article : Google Scholar
|
|
12
|
Xu L, Jin QD, Gong X, Liu H and Zhou RX:
Anti-gastric cancer effect of melatonin and Bcl-2, Bax, p21 and p53
expression changes. Sheng Li Xue Bao. 66:723–729. 2014.In Chinese.
PubMed/NCBI
|
|
13
|
Ordoñez R, Carbajo-Pescador S,
Prieto-Dominguez N, García-Palomo A, González-Gallego J and Mauriz
JL: Inhibition of matrix metalloproteinase-9 and nuclear factor
kappa B contribute to melatonin prevention of motility and
invasiveness in HepG2 liver cancer cells. J Pineal Res. 56:20–30.
2014. View Article : Google Scholar
|
|
14
|
Martín-Renedo J, Mauriz JL, Jorquera F,
Ruiz-Andrés O, González P and González-Gallego J: Melatonin induces
cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line.
J Pineal Res. 45:532–540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vijayalaxmi, Reiter RJ, Tan DX, Herman TS
and Thomas CR Jr: Melatonin as a radioprotective agent: A review.
Int J Radiat Oncol Biol Phys. 59:639–653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsieh MH and Nguyen HT: Molecular
mechanism of apoptosis induced by mechanical forces. Int Rev Cytol.
245:45–90. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Joo SS and Yoo YM: Melatonin induces
apoptotic death in LNCaP cells via p38 and JNK pathways:
Therapeutic implications for prostate cancer. J Pineal Res.
47:8–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crawford LJ, Walker B and Irvine AE:
Proteasome inhibitors in cancer therapy. J Cell Commun Signal.
5:101–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tamura EK, Cecon E, Monteiro AWA, Silva
CLM and Markus RP: Melatonin inhibits LPS-induced NO production in
rat endothelial cells. J Pineal Res. 46:268–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gilad E, Wong HR, Zingarelli B, Virág L,
O'Connor M, Salzman AL and Szabó C: Melatonin inhibits expression
of the inducible isoform of nitric oxide synthase in murine
macrophages: Role of inhibition of NFkappaB activation. FASEB J.
12:685–693. 1998.PubMed/NCBI
|
|
22
|
Hidalgo M, Cascinu S, Kleeff J, Labianca
R, Löhr JM, Neoptolemos J, Real FX, Van Laethem JL and Heinemann V:
Addressing the challenges of pancreatic cancer: Future directions
for improving outcomes. Pancreatology. 15:8–18. 2015. View Article : Google Scholar
|
|
23
|
Xu B, Zhang K and Huang Y: Lin28 modulates
cell growth and associates with a subset of cell cycle regulator
mRNAs in mouse embryonic stem cells. RNA. 15:357–361. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsiang C-H, Tunoda T, Whang YE, Tyson DR
and Ornstein DK: The impact of altered annexin I protein levels on
apoptosis and signal transduction pathways in prostate cancer
cells. Prostate. 66:1413–1424. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mukherjee JJ, Gupta SK, Sikka H and Kumar
S: Inhibition of benzopyrene-diol-epoxide (BPDE)-induced bax and
caspase-9 by cadmium: Role of mitogen activated protein kinase.
Mutat Res. 661:41–46. 2009. View Article : Google Scholar
|
|
26
|
Sainz RM, Reiter RJ, Tan DX, Roldan F,
Natarajan M, Quiros I, Hevia D, Rodriguez C and Mayo JC: Critical
role of glutathione in melatonin enhancement of tumor necrosis
factor and ionizing radiation-induced apoptosis in prostate cancer
cells in vitro. J Pineal Res. 45:258–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang HL, Hsieh MJ, Chien MH, Chen HY,
Yang SF and Hsiao PC: Glabridin mediate caspases activation and
induces apoptosis through JNK1/2 and p38 MAPK pathway in human
promyelocytic leukemia cells. PLoS One. 9:e989432014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou Y, Zhao W, Xie G, Huang M, Hu M,
Jiang X, Zeng D, Liu J, Zhou H, Chen H, et al: Induction of
Nur77-dependent apoptotic pathway by a coumarin derivative through
activation of JNK and p38 MAPK. Carcinogenesis. 35:2660–2669. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu J, Wu N, Ma L-N, Zhong JT, Liu G,
Zheng LH and Lin XK: p38 MAPK signaling mediates mitochondrial
apoptosis in cancer cells induced by oleanolic acid. Asian Pac J
Cancer Prev. 15:4519–4525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deorukhkar A, Krishnan S, Sethi G and
Aggarwal BB: Back to basics: How natural products can provide the
basis for new therapeutics. Expert Opin Investig Drugs.
16:1753–1773. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahn KS and Aggarwal BB: Transcription
factor NF-kappaB: A sensor for smoke and stress signals. Ann NY
Acad Sci. 1056:218–233. 2005. View Article : Google Scholar
|
|
33
|
Yuan L, Wei S, Wang J and Liu X:
Isoorientin induces apoptosis and autophagy simultaneously by
reactive oxygen species (ROS)-related p53, PI3K/Akt, JNK, and p38
signaling pathways in HepG2 cancer cells. J Agric Food Chem.
62:5390–5400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bharti AC, Donato N, Singh S and Aggarwal
BB: Curcumin (diferuloylmethane) down-regulates the constitutive
activation of nuclear factor-κB and IkappaBalpha kinase in human
multiple myeloma cells, leading to suppression of proliferation and
induction of apoptosis. Blood. 101:1053–1062. 2003. View Article : Google Scholar
|
|
35
|
Takada Y and Aggarwal BB: Flavopiridol
inhibits NF-kappaB activation induced by various carcinogens and
inflammatory agents through inhibition of IkappaBalpha kinase and
p65 phosphorylation: Abrogation of cyclin D1, cyclooxygenase-2, and
matrix metalloprotease-9. J Biol Chem. 279:4750–4759. 2004.
View Article : Google Scholar
|
|
36
|
Rondanelli M, Faliva MA, Perna S and
Antoniello N: Update on the role of melatonin in the prevention of
cancer tumorigenesis and in the management of cancer correlates,
such as sleep-wake and mood disturbances: Review and remarks. Aging
Clin Exp Res. 25:499–510. 2013. View Article : Google Scholar : PubMed/NCBI
|