Introduction
Bladder cancer (BCa) is one of the most common
cancers in Western countries and the first leading cause of death
among urinary malignancies (1). The
incidence and mortality rates of BCa vary substantially across
countries, in part due to different risk factors. In 2007, a
comprehensive review by the World Cancer Research Fund and the
American Institute for Cancer Research concluded that established
risk factors for BCa include tobacco consumption, Schistosoma
haematobium infection, and both occupational and environmental
exposure to carcinogens such as aromatic amines and polycyclic
aromatic hydrocarbons and arsenic in drinking water (2).
The role of nutrition as a protective factor in the
development of BCa remains unclear but many data indicate that a
regular consumption of fruit and vegetables appears to be linked to
a lower incidence of urothelial neoplasia (3). A recent study in a multiethnic cohort
showed that the intake of vegetables and some related
micronutrients such as vitamins A, C, E and carotenoids was
inversely associated with BCa risk only in women (4). Ros et al showed that a higher
plasma carotenoid concentration was associated with a lower
incidence of BCa, suggesting that specific compounds in fruit and
vegetables may exert protective effects on BCa risk (5). Moreover, data of the European
Prospective Investigation into Cancer and Nutrition (EPIC) study,
found an inverse association between the dietary intake of
flavanols and lignans and the risk of BCa (6).
Epidemiological evidence and many case-control
studies strongly support the hypothesis that adherence to the
Mediterranean diet reduces cancer risk and in particular olive oil
consumption is inversely related to cancer prevalence (7,8). Olive
oil is the main dietary fat of the Mediterranean area and its
health-promoting properties are well assessed by numerous studies
(9–12). Historically, the beneficial effects
of olive oil intake have been attributed to the high concentration
of monounsaturated fatty acids (MUFAs) such as oleic acid that
represents the main component. However, other oils rich in MUFA,
derived from the seeds of soybean or rapeseed, do not exert the
same health benefits as extra-virgin olive oil (EVOO). In the last
few years, attention has been focused on the minor phenolic
fraction mainly constituted of a complex mixture comprising at
least 36 distinct compounds (10).
The most represented phenolic molecules in EVOO are secoiridoids,
such as oleuropein and ligstroside, and phenolic alcohols, such as
hydroxytyrosol (HTy) and tyrosol (TY), accounting for ~90% of total
phenols. The remaining 10% of the mixture is mainly constituted by
flavonoids and lignans. Polyphenols have well-known antioxidant,
anti-inflammatory, cardioprotective, anti-atherogenic,
antithrombotic, neuroprotective and anticancer activities (13–15).
Recent findings suggest that in low amounts, polyphenols may exert
pharmacological activity within cells. In particular, polyphenols
have the potential to modulate intracellular signaling cascades, to
affect gene expression, to interact with mitochondria and to induce
antioxidant enzymes as well as to inhibit the expression of enzymes
involved in the generation of free radicals (16). By affecting such pathways they have
the ability to control cell survival, death and differentiation,
and to exhibit marked anti-inflammatory activity via modulation of
the expression of pro-inflammatory genes mainly acting through
nuclear factor-κB and mitogen-activated protein kinase signaling
(17,18). Owing to all of these properties,
polyphenols exert anticancer effects through the modulation of
genes and molecular signaling pathways associated with cell
survival, cell cycle progression, cell growth arrest and apoptosis,
as demonstrated in several tumor cell lines (19).
In a previous study, we demonstrated that very low
doses of EVOO phenols inhibited the invasive ability of a BCa cell
line by modulating the expression of MMP2 (20). The aim of the present study was to
investigate the antiproliferative activity of extra-virgin olive
oil extract (EVOOE) on BCa, with the attempt to clarify the
biological mechanisms that trigger cell death. Moreover, we also
evaluated the ability of low doses of EVOOE to modulate the in
vitro activity of paclitaxel or mitomycin C, two antineoplastic
drugs used in the management of different types of cancer.
Materials and methods
Materials
Acetonitrile (CH3CN), n-hexane,
dimethylsulphoxide (DMSO), acetic acid, methanol,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
paclitaxel, mitomycin C and propidium iodide (PI) were purchased
from Sigma-Aldrich (St. Louis, MO, USA). T24 and 5637 BCa cell
lines were purchased from CLS Cell Lines Service GmbH (Eppelheim,
Germany). All media and sera for cell culture were obtained from
Invitrogen (Carlsbad, CA, USA) and were endotoxin-free.
2′,7′-Dichlorodihydrofluorescein diacetate (H2DCFDA) was
from Molecular Probes, Inc. (Eugene, OR, USA). For the
immunofluorescence experiment, anti-α-tubulin antibody,
TRITC-labeled phalloidin and anti-mouse FITC-conjugated secondary
antibody were purchased from Sigma-Aldrich (cat. nos. t5168, p1951
and f0257, respectively). Protease inhibitor cocktail was purchased
from Sigma-Aldrich (cat. no. 11873580001) while Bradford reagent,
Any kD™ polyacrylamide gel, anti-mouse and anti-rabbit secondary
peroxidase-conjugated antibodies were obtained from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA) (cat. nos. 1706515 and
1706516, respectively). Primary antibodies for PARP-1 and GAPDH
were obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA) (cat. nos. 9542 and 2118, respectively), caspase-3 was from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) (cat. no.
sc-7148), and caspase-9 was from Sigma-Aldrich (cat. no.
c7729).
Extraction of the olive oil phenolic
fraction
EVOOE was obtained as previously described (21) with minor modifications, from three
different lots of an endemic monocultivar olive oil (Olea
europaea L. var. Itrana) within 1 month after production. Fifty
milliliters of each oil sample was extracted with 150 ml of
CH3CN/H2O (70:30 v/v). A defatting with
n-hexane was performed to completely remove the lipid
fraction. Aliquots of the raw hydrophilic extract were dried by
SpeedVac and stored at −80°C until use. The extract was dissolved
in DMSO at a stock concentration of 10 mg/ml immediately before
performing the experiments.
HPLC analysis of the phenolic
fraction
The HPLC analysis of the extract was carried out
with a Waters apparatus, equipped with a 600 F pump and pump
controller, a Rheodyne injection valve with a 50-µl loop, a
Symmetry300 column (C18 reversed-phase column, 4.6×250 mm, 5-µm
particle size, thermostated at 25°C, with a 3.9×20 mm guard column
of the same material matrix), and a Waters 2996 Photodiode Array
Detector. The elution was performed at a flow rate of 1 ml/min,
with solvent A being 2% acetic acid and solvent B being methanol.
The gradient consisted of an initial 5% B for 5 min, to 50% B in 35
min, 100% B in 10 min, 100% B for 10 min. Samples were filtered
through a 0.45-µm cellulose syringe filter before injection.
Elution was monitored at 280 nm and peak identification was
obtained by comparing retention times and spectral characteristics
with those of authentic standards.
Cell cultures
T24 and 5637 cells (human urinary bladder carcinoma)
were cultured in DMEM without phenol red supplemented with 10% FBS,
2 mM glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin.
Cell proliferation assays
Cell viability was assessed using the dye MTT. The
assay is based on the ability of living cells to convert MTT into
an insoluble purple-colored formazan, whose amount is proportional
to the number of living cells. Cells seeded in 96-well plates at a
density of 5,000 cells/well were exposed to different
concentrations of EVOOE, paclitaxel or mitomycin C. After 24 h, the
cells were treated with 20 µl of a 5 mg/ml solution of MTT in PBS
and incubated at 37°C for 4 h. After discarding the medium, the
formazan was extracted with DMSO, and absorbance was read at 570 nm
with a reference at 690 nm with a Tecan Sunrise™ microplate
reader.
Colony-forming ability
The cells were plated at a density of 20
cells/cm2 in tissue culture Petri dishes and exposed to
various concentrations of EVOOE. Cells were grown for 14 days with
a medium renewal every 2 days. After 2 weeks the cells were fixed
with ethanol, and stained with Giemsa stain to detect the
colonies.
Cell cycle analysis
Flow cytometric analysis of the cells was performed
using an Accuri C6 flow cytometer (BD Biosciences, Oxford, UK). To
analyze cell cycle distribution, the cells were initially treated
with various concentrations of EVOOE for 24 h, and were then
collected by trypsinization, fixed in 75% absolute ethanol, washed
in PBS, and resuspended in 1 ml of PBS containing 0.5 mg/ml of
RNase A and 0.01 mg/ml of PI in the dark for 30 min at room
temperature. The percentage of cells in the sub-G1, G0/G1, S, and
G2/M phases of the cell cycle was analyzed using the ModFit LT 3.0
software (Verity Software House, Inc., Topsham, ME, USA).
Immunofluorescence staining of fibrous
actin (F-actin) and α-tubulin
Cells were plated at a density of 2×104
cells/cm2 on a glass coverslip. After 24 h, the media
were removed and the cells were treated with media containing
different doses of EVOOE for 4 h. After the incubation, the media
were discarded and the glass coverslips were fixed for 20 min at
−20°C in methanol. After fixation, the cells were rinsed three
times with cold PBS and then permeabilized in PBS containing 0.5%
(v/v) Triton X-100 for 5 min, blocked in bovine serum albumin (BSA)
5% (w/v) for 1 h and then incubated overnight at 4°C with a human
monoclonal anti-α-tubulin antibody produced in mouse diluted at
1:1,000 in PBS containing 0.2% (w/v) Triton X-100. After
incubation, the coverslips were rinsed three times in PBS and
incubated for 45 min with a solution of TRITC-labeled phalloidin
which specifically binds to F-actin and anti-mouse FITC-conjugated
antibody. The cell nuclei were counterstained with DAPI and the
specimens were mounted on glass slides. The images were captured
with a Nikon ViCo.2 video confocal microscope (Nikon, Inc., Garden
City, NY, USA) at a magnification of ×600.
Measurement of intracellular ROS
generation
Intracellular ROS levels were evaluated by treating
cells with 2 µM H2DCFDA for 1 h at 37°C in the presence
of different doses of EVOOE. H2DCFDA, the acetylated
form of 2′,7′-dichlorofluorescein (DCF), is not fluorescent until
intracellular deacetylation and oxidation by peroxides (22). Fluorescent intensities were
quantified by flow cytometry.
Western blot analysis
T24 and 5637 cells were seeded in 10-cm Petri plates
at a concentration of 1×106 cells/plate. After 24 h, the
cells were treated with different concentrations of EVOOE for an
additional 24 h. The cells were scraped in culture medium, washed
twice with PBS, and finally lysed into 200 µl RIPA buffer [50 mM
Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% Na-deoxycholate, and 0.1%
sodium dodecyl sulfate (SDS)], containing a protease inhibitor
cocktail. After 20 min on ice, the lysates were centrifuged at
20,000 × g for 10 min. Protein concentration was determined with
the Bradford reagent. The lysates (20 µg of the total protein) were
resolved on Any kD™ polyacrylamide gel and transferred onto PVDF
membranes. Membranes were blocked for 1 h with 5% BSA, dissolved in
Tris-buffered saline (TBS) solution (10 mM Tris-HCl, pH 7.5, 150 mM
NaCl). Membranes were then successively incubated overnight at 4°C
with primary human antibodies in TBST (TBS with 0.1% Tween-20) with
5% BSA at the following dilution: PARP-1 1:1,000, caspase-9,
caspase-3 1:500 and GAPDH 1:4,000. After washing in TBST solution,
the secondary antibody [anti-rabbit or anti-mouse IgG horseradish
peroxidase (HRP)-conjugated antibody], diluted at 1:10,000 in
TBST/5% BSA, was added for 50 min at room temperature. Signals were
visualized by ECL reagent according to the manufacturer's
instructions.
Statistics
Each assay was replicated at least four times, and
statistical significance was determined using GraphPad Prism 4
statistical software package (GraphPad Software, Inc., San Diego,
CA, USA). Data are expressed as means ± SD. Comparison of the
groups was carried out by one-way ANOVA followed by Bonferroni's
post hoc test. Statistical significance was defined as
P<0.05.
Results
EVOOE chromatographic analysis
EVOOE was analyzed by HPLC-DAD to identify the main
phenolic compounds present in the extract. Analyses revealed a
chromatographic profile strictly similar to the one previously
obtained for the same olive oil extraction (20) which was characterized by a large
amount of secoiridoids such as the dialdehydic form of elenolic
acid linked to TY (p-HPEA-EDA; oleocanthal) and the
dialdehydic form of elenolic acid linked to HTy (3,4-DHPEA-EDA;
oleuropein aglycon, dialdehydic form), and of lignans such as
pinoresinol, whereas the simple phenolic fraction was scarcely
represented, with very low amounts of TY, HTy, vanillic acid,
vanillin, p-coumaric and ferulic acids. The low content of
simple phenols such as TY and HTy indicates that the olive oil
samples were obtained by correct production procedures giving rise
to a high quality EVOO (20).
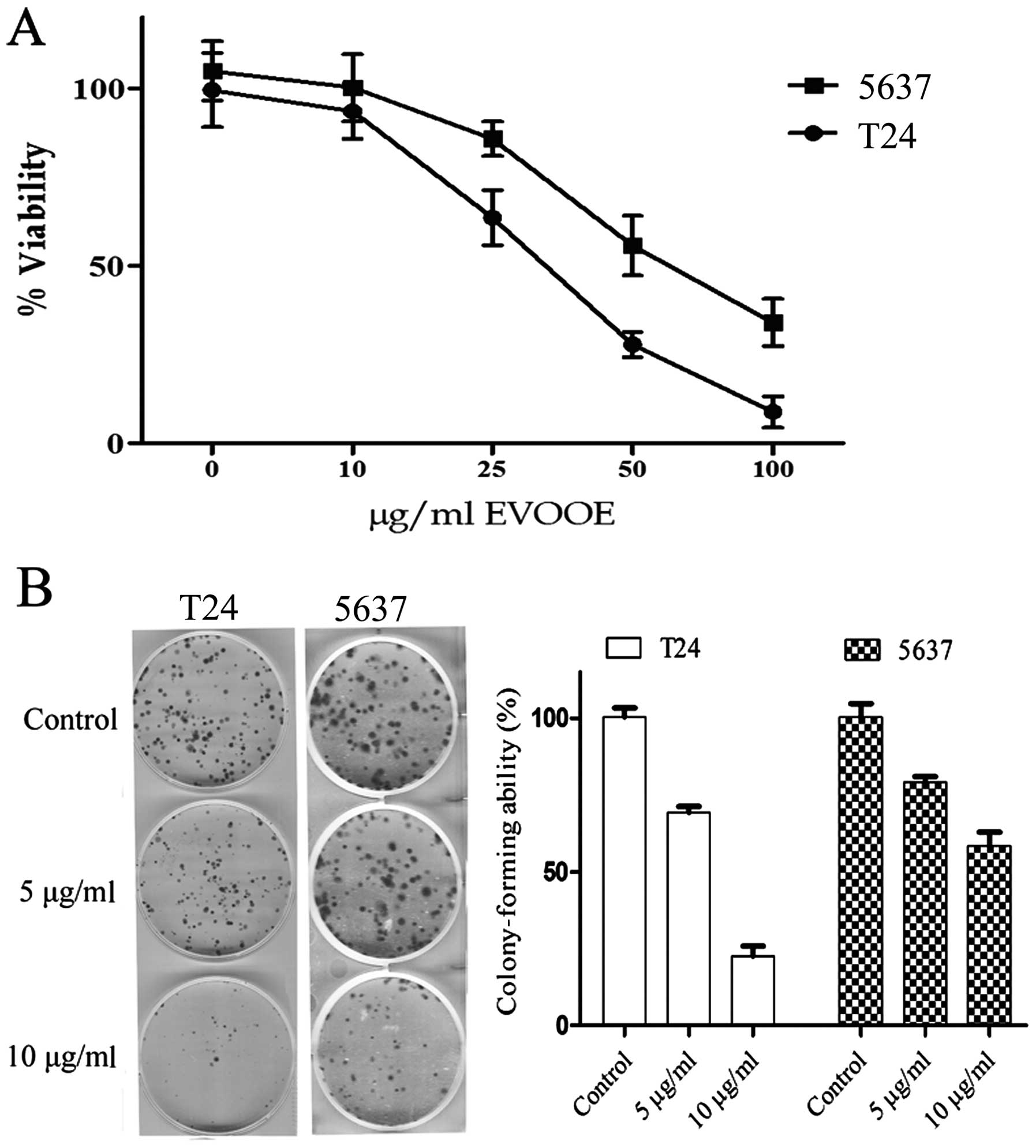
EVOOE inhibits cell growth and
clonogenic survival
The cytotoxic effect of EVOOE on BCa cells was
evaluated by MTT assay after 24 h of exposure to the phenolic
extract. As shown in Fig. 1A, EVOOE
treatment decreased T24 cell viability in a dose-dependent manner
with growth inhibition ranging from 40 to ~90%, at 25 and 100
µg/ml, respectively and an IC50 of 32±3 µg/ml. The
viability of 5637 cells was also inhibited by the treatment in a
dose-dependent manner with a higher IC50 of 55±7 µg/ml.
Proliferation of neither of the two cell lines was affected at
doses <10 µg/ml up to 72 h (data not shown).
We also examined the effect of EVOOE at subtoxic
doses on the clonogenic survival of T24 and 5637 cells. This assay
determines the ability of a cell to proliferate indefinitely,
retaining its reproductive ability to form a large colony or a
clone. Importantly, both cell lines showed a significant decrease
(P<0.001) in the ability to form colonies starting at a dose of
5 µg/ml with a marked inhibition of clonogenic activity at 10 µg/ml
(Fig. 1B).
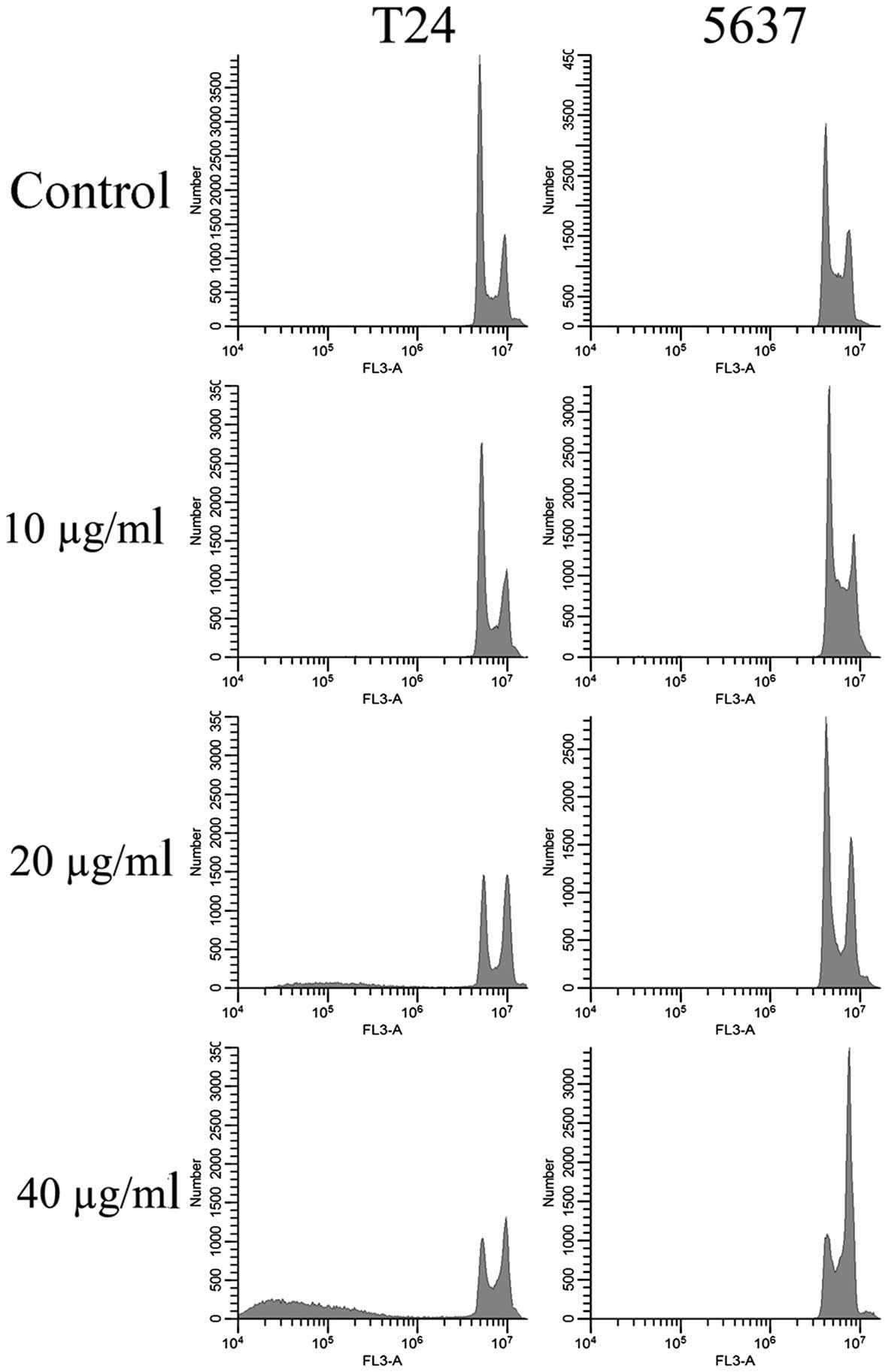
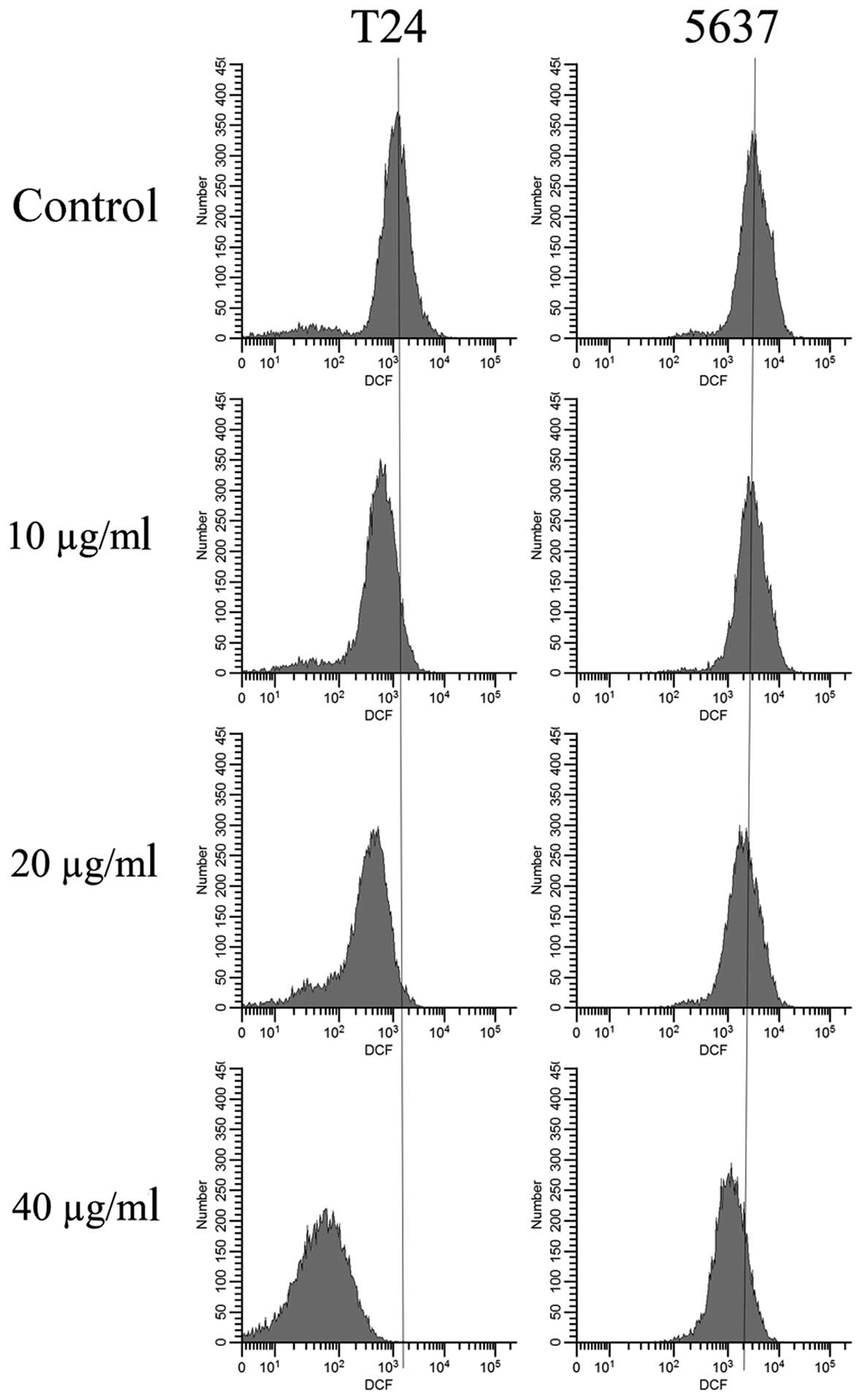
EVOOE blocks the cell cycle
progression at G2/M stage
To better understand the mechanism underlying the
cell growth impairment by EVOOE, cell cycle progression was
investigated. T24 and 5637 cells were treated with increasing doses
of EVOOE, and cell cycle distribution analysis was then performed
after 24 h of exposure. As shown in Table I, the G2/M population of the
EVOOE-treated T24 cells increased from 11.1% of the control to ~27%
at EVOOE doses of 20 and 40 µg/ml. The treatment also induced a
marked decrease in the G0/G1 population in a dose-dependent manner
with a subsequent increase in sub-G1 particles (Fig. 2). This result revealed that EVOOE
treatment caused marked accumulation of the G2/M population in the
T24 cells that led to an accumulation of the sub-G1 fraction
probably indicating induction of apoptosis. The cell cycle analysis
of 5637 cells after EVOOE treatment revealed a similar behavior to
that observed for the T24 cells, but with a consistently larger
increase in the G2/M population at 40 µg/ml (28% of T24 vs. 44% of
5637 cells). Notably, no increase in the sub-G1 fraction was
observed for this cell line.
 | Table I.Percentage of cells in each phase of
the cell cycle after 24 h of treatment with various doses of
EVOOE.a |
Table I.
Percentage of cells in each phase of
the cell cycle after 24 h of treatment with various doses of
EVOOE.a
|
| T24 cells | 5637 cells |
|---|
|
|
|
|
|---|
| EVOOE(µg/ml) | 0 | 10 | 20 | 40 | 0 | 10 | 20 | 40 |
|---|
| Sub-G1 | 6±2 | 8±0.5 | 18±0.3b | 28±3b | 3±0.4 | 2±0.5 | 4±1 | 5±1 |
| G1 | 72±4 | 70±6 | 41±4b | 30±2b | 60±5 | 64±6 | 59±5 | 34±2b |
| S | 12±3 | 10±3 | 16±4 | 14±1 | 24±1 | 22±3 | 16±0.7 | 17±3 |
| G2/M | 10±1 | 12±4 | 25±2b | 28±4b | 13±2 | 12±0.7 | 21±0.8b | 44±3b |
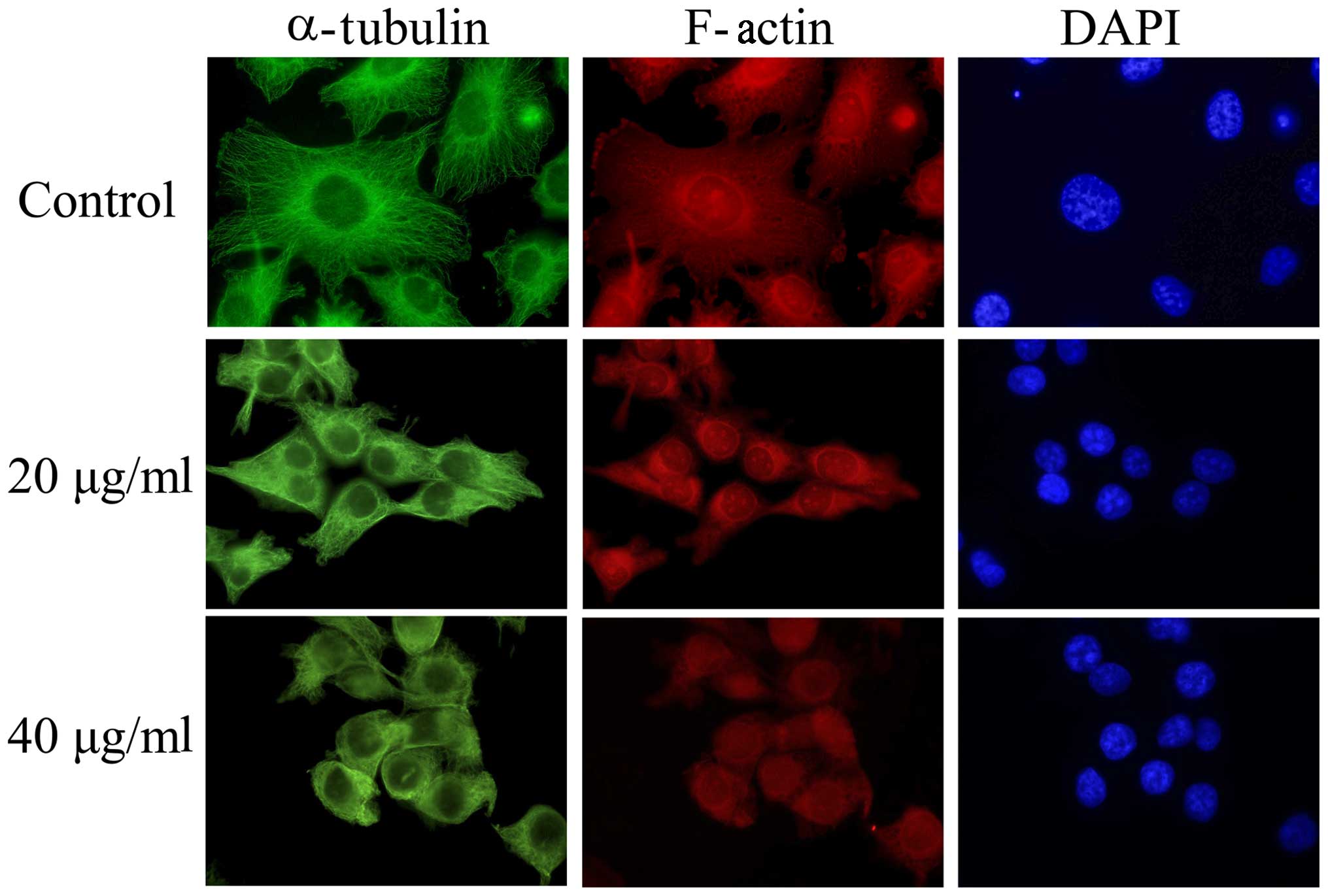
Short-time exposure to EVOOE alters
cell morphology
Interestingly, a consistent change in cell
morphology was noticeable in all the experiments performed. In
order to explain this phenomenon, we tested the hypothesis that
EVOOE could interfere with cytoskeleton remodeling and the
subsequent mitotic process. After treatment with 20 and 40 µg/ml of
EVOOE for 4 h, the cells acquired a rounded morphology. In order to
verify the alteration of the cytoskeleton, we performed
immunofluorescence experiments using phalloidin (that bind to
F-actin) and an antibody against α-tubulin. As clearly visible from
Fig. 3, the treatment induced a
change in T24 cell morphology with a marked variation of
cytoskeleton network arrangement and a perinuclear accumulation of
α-tubulin.
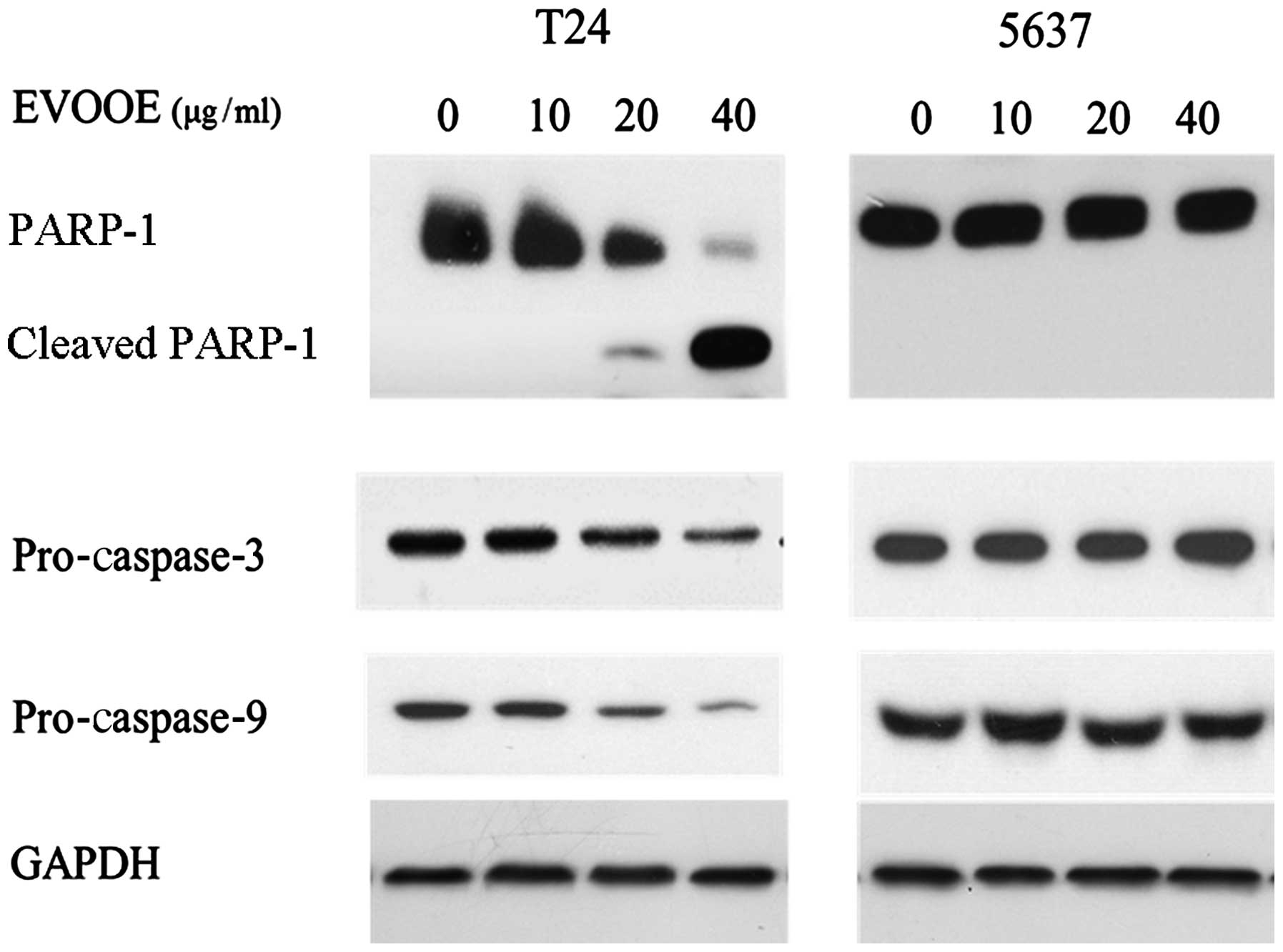
EVOOE induces apoptosis only in T24
cells
Despite the cell cycle blockade in the G2/M phase
that occurred in both cell lines, cell cycle analysis revealed an
accumulation of particles (sub-G1) with a small content of DNA only
in T24 cells (Fig. 2), indicating
that only in this cell line did the onset of apoptotic processes
occur. In order to confirm this finding, we performed western blot
analysis of PARP-1 and pro-caspase-3 and −9. Proteolytic cleavage
of specific proteins such as PARP-1 has been shown to occur in
cells exposed to a number of apoptotic stimuli (23–25).
EVOOE was found to induce apoptotic cell death only in T24 cells in
a dose-dependent manner. Indeed, western blot analysis (Fig. 4) of the cleavage of PARP-1 showed a
decrease in the full-size MW 116,000 fragment and an increase in
the cleaved form within 24 h after the T24 cell treatment in a
dose-dependent manner (Fig. 4).
Western blot analysis of caspase-3 and −9 also showed a marked
decrease in their protein levels at higher doses of the polyphenol
extract. Conversely to what was observed for T24 cells, the
treatment of 5637 cells did not induce apoptosis, as is clearly
visible from Fig. 4 that shows the
lack of any PARP-1 cleavage.
EVOOE treatment decreases
intracellular ROS production
The antioxidant effect of EVOOE was analyzed by
determining intracellular ROS levels using cytofluorimetric
analysis. After 1 h of treatment with different concentrations of
EVOOE, the basal ROS production decreased in a dose-dependent
manner as clearly evidenced by the leftward shift of fluorescence
that became greater at increasing doses of the polyphenol extract
(Fig. 5). The data analysis
revealed a decrease in the mean T24-cell fluorescence ranging from
12% at 10 µg/ml to 64% at 40 µg/ml. The same behavior was observed
for the 5637 cells with a lesser antioxidant activity that became
evident only at a dose of 40 µg/ml with a decrease in the mean cell
fluorescence of 35%.
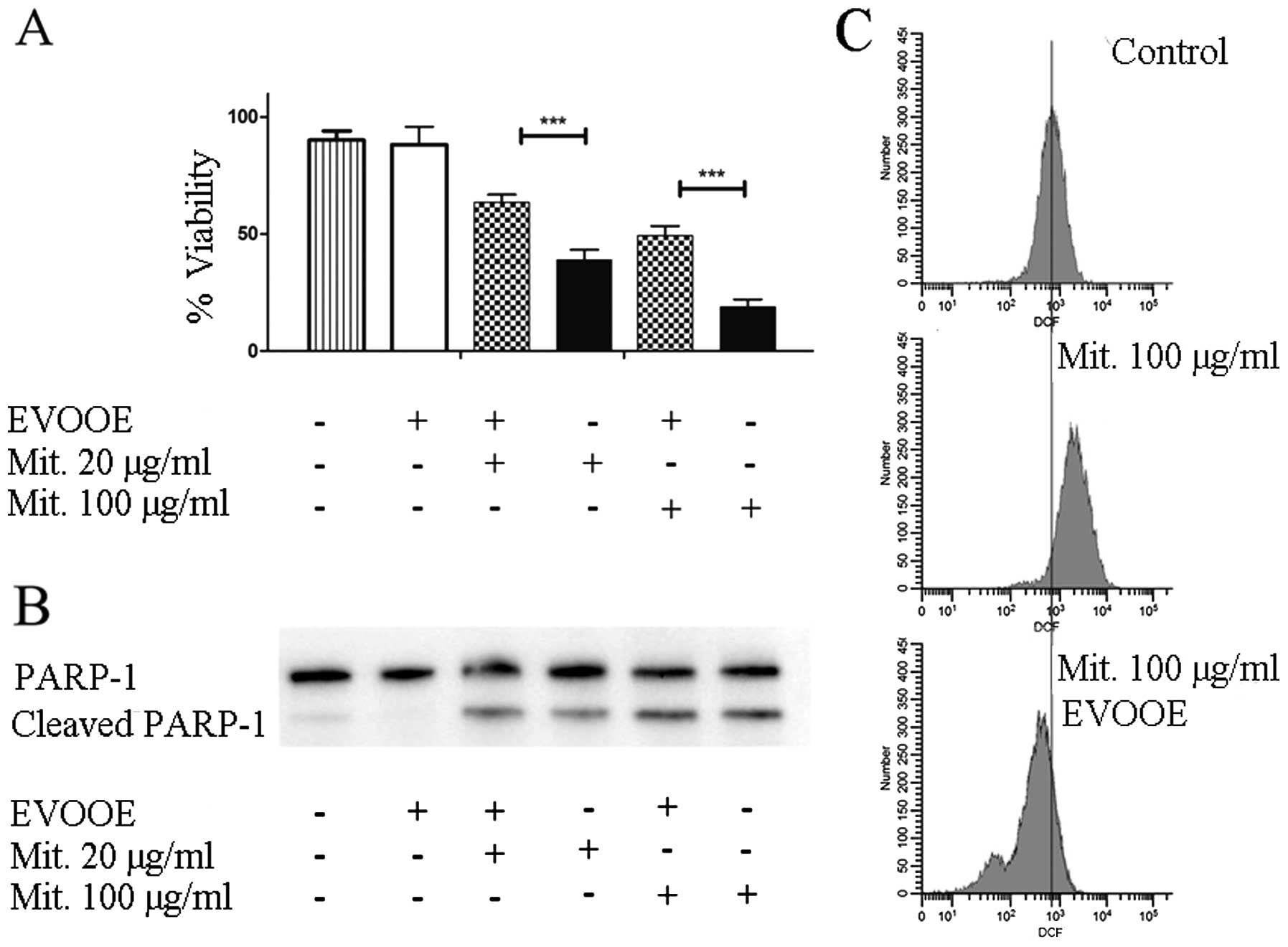
EVOOE negatively affects mitomycin
cytotoxicity
Mitomycin C is a methylazirinopyrroloindoledione
antineoplastic antibiotic isolated from the bacterium
Streptomyces caespitosus and other Streptomyces
bacterial species. Mitomycin C generates oxygen radicals, alkylates
DNA, and produces interstrand DNA cross-links, thereby inhibiting
DNA synthesis. This drug is often used in the chemotherapy of BCa
(26).
To evaluate the effect of the simultaneous exposure
to the drug and EVOOE on cell viability, T24 cells were treated for
24 h with different doses of mitomycin in the presence or absence
of the phenolic extract. In all the experiments, EVOOE was used at
a dose of 10 µg/ml which was found to be non-toxic.
The data shown in Fig.
6A demonstrated that, at each concentration tested, the
simultaneous treatment of EVOOE and mitomycin reduced the
chemotherapeutic cytotoxicity. At a drug concentration of 100 µg/ml
the cell viability increased from ~20% when exposed to mitomycin
alone to 50% with the co-treatment, and similar behavior was
observed at all of the concentrations tested. The data showed that
EVOOE reduced the mitomycin antiproliferative ability without
affecting PARP-1 cleavage (Fig.
6B). To clarify the possible involvement of ROS inhibition in
the decrease of the drug cytotoxicity we performed DCF assay and we
found that mitomycin at the dose of 100 µg/ml strongly increased
intracellular ROS production in T24 cells (Fig. 6C) whereas the co-treatment with
EVOOE strongly reduced the ROS production with a considerable
leftward shift of fluorescence.
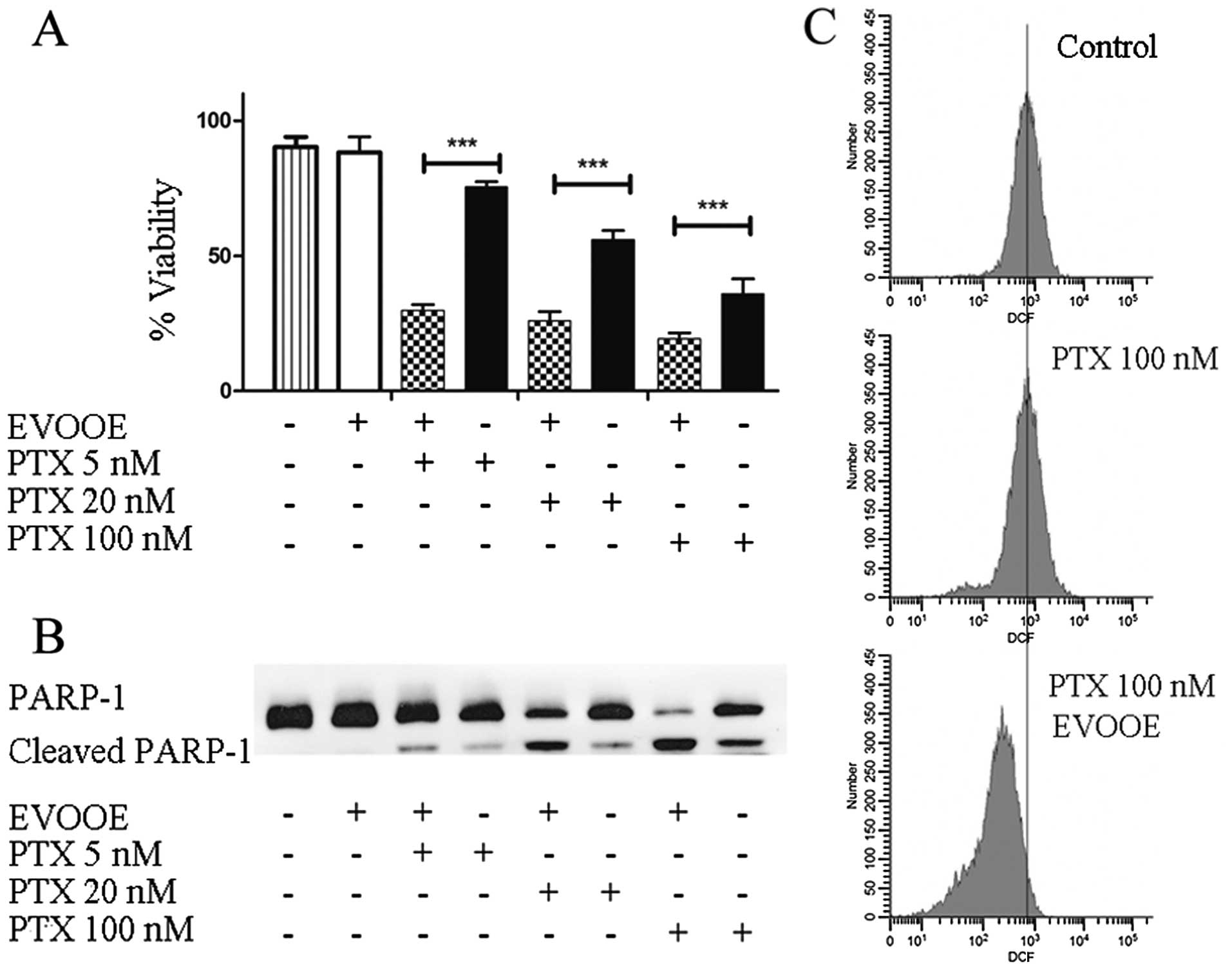
EVOOE positively affects paclitaxel
cytotoxicity
Paclitaxel is a mitotic inhibitor isolated from the
bark of the Pacific yew tree, Taxus brevifolia and is named
Taxol. This antineoplastic agent is indicated for the treatment of
advanced carcinoma of the ovary, and other types of cancer
including BCa (27). To perform our
experiments, we treated T24 cells for 24 h with three different
concentrations of paclitaxel alone or in association with 10 µg/ml
of EVOOE. The obtained data are shown in Fig. 7A. Importantly, at each tested
concentration, the addition of EVOOE strongly increased drug
cytotoxicity. When we treated the cells with 5 nM paclitaxel, the
cell viability decreased from 75 to ~25% in the presence of the
EVOOE, and a similar response was observed at 20 and 100 nM.
Notably, data analysis showed that exposure to 100 nM paclitaxel
exerted the same cytotoxicity as exposure to 5 nM of the same drug
when used in association with EVOOE. In order to evaluate the
mechanism underlying the increased cell death, we performed western
blot analyses to assess PARP-1 cleavage. As is clearly evident from
Fig. 7B, the simultaneous treatment
of paclitaxel and EVOOE strongly increased the protein cleavage at
each tested concentration. Moreover the data from the DCF assay
showed that paclitaxel alone did not induce oxidative stress,
whereas EVOOE co-treatment reduced basal ROS production (Fig. 7C).
Discussion
The relevance of polyphenols in nutrition and their
possible role as new drug candidates is well documented by the vast
body of literature in recent years. Many studies have evaluated the
effects of these molecules, either as single purified compounds or
as crude extracts, on various pathophysiological processes, in
particular as neuroprotective, cardioprotective, anti-inflammatory
and anticancer agents.
The antioxidant activity of polyphenols was
considered as the main mechanism of action of these compounds as
anticancer agents, given the evidence that oxidative stress acts as
a focal process in cancer development and progression (28). However, oxidative stress is only one
of the many aspects of cancer pathophysiology which is a complex
phenomenon and implies an alteration of many different signaling
pathways not strictly related to intracellular redox
equilibrium.
In recent years our group has focused on the effect
of olive oil polyphenolic extracts on various cancer cell cultures,
in particular on BCa cells. It is well known that after oral
ingestion, polyphenols are excreted in urine and may reach
concentrations high enough to exert a biological effect (29,30).
This phenomenon may explain the reduced incidence of BCa evidenced
by epidemiological studies (3,6).
Our previous study demonstrated that very low doses
of total polyphenol extract from olive oil strongly affect bladder
cell motility through a modulation of metalloprotease activity
(20). In the present study, we
evaluated whether the antiproliferative effect of EVOOE can
modulate the toxicity of chemotherapeutic drugs commonly used in
cancer treatment.
First, we evaluated the cell toxicity exerted by
EVOOE on two different BCa cell lines, T24 and 5637, which are
characterized by two different grades and are widely used as in
vitro models (31–33) as they cover the more frequent
subtypes of BCa. 5637 cells well represent the E2F3/RB1 pathway due
to amplification of 6p22.3, concomitant with loss of one copy of
RB1 and mutation of the other copy. The T24 cell line belongs to
the alternative pathway of FGFR3/CCND1 by mutated HRAS and
over-represented CCND1 (34).
Viability and clonogenic data indicated that T24 cells were more
prone to the toxic effect of EVOOE compared to 5367 cells, and
these data were further supported by cell cycle analyses which
highlighted that both cell lines were arrested in the G2/M phase
but with a different mechanism which resulted in apoptotic death
only for T24 cells, with a consistent increase in the sub-G1 peak
that was not evidenced in 5637 cells.
The data obtained from cell cycle analysis showed a
similar mechanism of action of EVOOE, in fact both of the cell
lines tested were subjected to growth arrest prior to mitosis.
While performing viability experiments, we observed a considerable
change in cell morphology, in both T24 and 5637 cells assuming a
rounded shape even after a very short treatment. We hence performed
immunofluorescence staining of the cytoskeletal proteins F-actin
and α-tubulin which are involved in the modulation of cell shape
and mitosis. These experiments confirmed that after treatment with
EVOOE there was a marked rearrangement of the cytoskeletal
proteins, with a perinuclear accumulation of the actin filament.
These data indicate that the arrest in the G2/M phase could be
explained by the hampering of normal dynamics of the cytoskeleton
and of the mitotic fuse. This evidence is in line with the findings
of Hamdi and Castellon (35) who
demonstrated that oleuropein treatment disrupted the organization
of actin filaments thus altering the shape of cancer cells and
their ability to proliferate. These data are also in line with the
findings of Monti et al (36), who evidenced an interaction between
oleocanthal and tau (τ) protein, a protein involved in the
stabilization of microtubules, thereby modulating the plasticity of
the cytoskeleton.
Apart from the arrest at the G2/M phase, the two
cell lines show a different behavior regarding the accumulation of
sub-G1 cell fragments, which are indicative of the onset of
apoptotic processes. In order to evaluate the possible activation
of apoptotic cell death, we investigated the cleavage of PARP-1 and
the protein level of pro-caspase-3 and −9 following EVOOE
treatment. Data confirmed the activation of apoptosis only in the
T24 cells and only at high EVOOE doses, confirming the
cytofluorimetric evidence. This different behavior of the two cell
lines in response to EVOOE treatment, can be explained by the
different mutational status in various key genes involved in cell
proliferation regulation (34). The
induction of programmed cell death only in T24 can also explain the
different susceptibility to the cytotoxic action of EVOOE, as
demonstrated by the difference in the decrease in viability
observed in our experiments (Fig.
1).
A wide body of literature indicates that apoptosis
may be responsible for polyphenol-mediated cell death. This effect
has been attributed to ROS production induced by polyphenols in
certain experimental conditions (37,38).
To clarify whether EVOOE may induce oxidative stress thus leading
to apoptosis, we evaluated intracellular ROS production via a
cytofluorimetric assay. Our data indicated that EVOOE was able to
decrease the basal level of oxidative stress in the T24 cells and
to a lower extent also in the 5637 cells. Hence, in our model the
induction of apoptosis was not due to ROS production but had to be
attributed to other molecular mechanisms such as the inhibition of
pro-survival cell signaling pathways (37). In the last part of this study we
evaluated the ability of low concentrations of EVOOE to improve the
activity of different chemotherapeutics used in common clinical
practice. It is of great interest to underline that current therapy
in the management of BCa consists of bladder instillation of
alchilant agents (39) that on the
other hand exert unfavorable effects (40). In the present study we used an
approach based on the comparison of viability since one of the two
treatments given in co-association was always non-toxic (EVOOE up
to 10 µg/ml). As expected, we obtained different results based on
the drug tested. Our data demonstrated that simultaneous treatment
with mitomycin and EVOOE markedly decreased the cytotoxicity of the
chemotherapeutic. These results are surprising only in part because
it is well known that mitomycin exerts its toxic effect through the
induction of oxidative stress and the antioxidant activity of the
phenolic molecules may dampen its efficacy.
Conversely to what was observed for mitomycin, the
addition of EVOOE to the paclitaxel treatment led to a strong
increase in the drug toxicity with a marked induction of apoptotic
cell death. These data are of great interest since the dose of
EVOOE used for this experiment was non-toxic per se to the
cells themselves, and this indicates a synergic effect in
combination with paclitaxel. It can be hypothesized that the
cytotoxicity enhancement of paclitaxel could be attributed to the
alteration in gene expression occurring at low doses of phenolic
extract, as observed in our previous study (20), that chemosensitize the cells by
altering pathways involved in cell growth and proliferation such as
platelet-activating factor (PAF) receptor signaling or PI3K
signaling (37,41,42).
There are controversial data concerning the role of
antioxidant supplements during chemotherapy (43–45),
however it is generally recommended to avoid the use of antioxidant
food supplements and herbal products. Based on our experimental
results, the polyphenolic extracts could be developed as potential
adjuvants in combination with certain chemotherapeutic drugs which
do not exert cytotoxicity through the induction of oxidative
stress.
Collectively, our findings suggest that the
potential adjuvant properties of EVOO phenols are related to the
class of drugs used according to previously published data that
point out how the adjunct of high doses of an antioxidant to
chemotherapy does not always improve the pharmacological treatment
(46). It is important to highlight
that even if the mechanism of action of many chemotherapeutics and
also radiotherapy is based in part on the production of free
radicals (44,45) there are also cytostatic drugs that
do not exert their effects through oxidative stress mechanisms such
as paclitaxel, vinca alkaloids, anthracycline and many others that
can be improved by the use of antioxidant-rich supplements
(43).
In conclusion, our data demonstrated that the
combination of EVOOE and paclitaxel exhibited a markedly higher
antiproliferative activity in vitro compared with each of
these alone. To the best of our knowledge this is the first report
that demonstrates how EVOO phenols markedly improve the activity of
paclitaxel, and this evidence may pave the way for the study of new
therapeutic strategies which exploit the synergy between drugs and
nutraceuticals.
Acknowledgements
This study was supported by L.I.L.T. ‘Lega Italiana
per la Lotta contro i Tumori’ section of Latina and ‘Fondazione
Terzo Pilastro - Italia e Mediterraneo’. The authors gratefully
acknowledge ‘CAPOL association’ for providing the EVOO samples.
Glossary
Abbreviations
Abbreviations:
|
BCa
|
bladder cancer
|
|
EVOOE
|
extra-virgin olive oil extract
|
|
MUFA
|
monounsaturated fatty acids
|
|
EPIC
|
European Prospective Investigation
into Cancer and Nutrition
|
|
HTy
|
hydroxytyrosol
|
|
TY
|
tyrosol
|
|
DCF
|
2′,7′-dichlorofluorescein
|
|
TBS
|
Tris-buffered saline
|
|
BSA
|
bovine serum albumin
|
References
|
1
|
Goodison S, Rosser CJ and Urquidi V:
Bladder cancer detection and monitoring: Assessment of urine- and
blood-based marker tests. Mol Diagn Ther. 17:71–84. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burger M, Catto JWF, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S, et al: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brinkman MT, Buntinx F, Kellen E, Van
Dongen MC, Dagnelie PC, Muls E and Zeegers MP: Consumption of
animal products, olive oil and dietary fat and results from the
Belgian case-control study on bladder cancer risk. Eur J Cancer.
47:436–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park SY, Ollberding NJ, Woolcott CG,
Wilkens LR, Henderson BE and Kolonel LN: Fruit and vegetable
intakes are associated with lower risk of bladder cancer among
women in the Multiethnic Cohort Study. J Nutr. 143:1283–1292. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ros MM, Bueno-de-Mesquita HB, Kampman E,
Aben KK, Büchner FL, Jansen EH, van Gils CH, Egevad L, Overvad K,
Tjønneland A, et al: Plasma carotenoids and vitamin C
concentrations and risk of urothelial cell carcinoma in the
European Prospective Investigation into Cancer and Nutrition. Am J
Clin Nutr. 96:902–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zamora-Ros R, Sacerdote C, Ricceri F,
Weiderpass E, Roswall N, Buckland G, St-Jules DE, Overvad K, Kyrø
C, Fagherazzi G, et al: Flavonoid and lignan intake in relation to
bladder cancer risk in the European Prospective Investigation into
Cancer and Nutrition (EPIC) study. Br J Cancer. 111:1870–1880.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Psaltopoulou T, Kosti RI, Haidopoulos D,
Dimopoulos M and Panagiotakos DB: Olive oil intake is inversely
related to cancer prevalence: A systematic review and a
meta-analysis of 13,800 patients and 23,340 controls in 19
observational studies. Lipids Health Dis. 10:1272011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giacosa A, Barale R, Bavaresco L, Gatenby
P, Gerbi V, Janssens J, Johnston B, Kas K, La Vecchia C, Mainguet
P, et al: Cancer prevention in Europe: The Mediterranean diet as a
protective choice. Eur J Cancer Prev. 22:90–95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bulotta S, Celano M, Lepore SM, Montalcini
T, Pujia A and Russo D: Beneficial effects of the olive oil
phenolic components oleuropein and hydroxytyrosol: Focus on
protection against cardiovascular and metabolic diseases. J Transl
Med. 12:2192014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cárdeno A, Sánchez-Hidalgo M and
Alarcón-de-la-Lastra C: An up-date of olive oil phenols in
inflammation and cancer: Molecular mechanisms and clinical
implications. Curr Med Chem. 20:4758–4776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nomikos T, Fragopoulou E and Antonopoulou
S: Food ingredients and lipid mediators. Curr Nutr Food Sci.
3:255–276. 2007. View Article : Google Scholar
|
|
12
|
Nasopoulou C, Gogaki V, Stamatakis G,
Papaharisis L, Demopoulos CA and Zabetakis I: Evaluation of the in
vitro anti-atherogenic properties of lipid fractions of olive
pomace, olive pomace enriched fish feed and gilthead sea bream
(Sparus aurata) fed with olive pomace enriched fish feed. Mar
Drugs. 11:3676–3688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Masci A, Coccia A, Lendaro E, Mosca L,
Paolicelli P and Cesa S: Evaluation of different extraction methods
from pomegranate whole fruit or peels and the antioxidant and
antiproliferative activity of the polyphenolic fraction. Food Chem.
202:59–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hernáez Á, Remaley AT, Farràs M,
Fernández-Castillejo S, Subirana I, Schröder H, Fernández-Mampel M,
Muñoz-Aguayo D, Sampson M, Solà R, et al: Olive oil polyphenols
decrease LDL concentrations and LDL atherogenicity in men in a
randomized controlled trial. J Nutr. 145:1692–1697. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martín-Peláez S, Covas MI, Fitó M, Kušar A
and Pravst I: Health effects of olive oil polyphenols: Recent
advances and possibilities for the use of health claims. Mol Nutr
Food Res. 57:760–771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jayasena T, Poljak A, Smythe G, Braidy N,
Münch G and Sachdev P: The role of polyphenols in the modulation of
sirtuins and other pathways involved in Alzheimer's disease. Ageing
Res Rev. 12:867–883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vauzour D, Rodriguez-Mateos A, Corona G,
Oruna-Concha MJ and Spencer JPE: Polyphenols and human health:
Prevention of disease and mechanisms of action. Nutrients.
2:1106–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao B, Ma Y, Xu Z, Wang J, Wang F, Wang
D, Pan S, Wu Y, Pan H, Xu D, et al: Hydroxytyrosol, a natural
molecule from olive oil, suppresses the growth of human
hepatocellular carcinoma cells via inactivating AKT and nuclear
factor-kappa B pathways. Cancer Lett. 347:79–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Casaburi I, Puoci F, Chimento A, Sirianni
R, Ruggiero C, Avena P and Pezzi V: Potential of olive oil phenols
as chemopreventive and therapeutic agents against cancer: A review
of in vitro studies. Mol Nutr Food Res. 57:71–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coccia A, Bastianelli D, Mosca L,
Monticolo R, Panuccio I, Carbone A, Calogero A and Lendaro E: Extra
virgin olive oil phenols suppress migration and invasion of T24
human bladder cancer cells through modulation of matrix
metalloproteinase-2. Nutr Cancer. 66:946–954. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Impellizzeri J and Lin J: A simple
high-performance liquid chromatography method for the determination
of throat-burning oleocanthal with probated antiinflammatory
activity in extra virgin olive oils. J Agric Food Chem.
54:3204–3208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bulotta S, Corradino R, Celano M,
D'Agostino M, Maiuolo J, Oliverio M, Procopio A, Iannone M,
Rotiroti D and Russo D: Antiproliferative and antioxidant effects
on breast cancer cells of oleuropein and its semisynthetic
peracetylated derivatives. Food Chem. 127:1609–1614. 2011.
View Article : Google Scholar
|
|
23
|
Chen H, Landen CN, Li Y, Alvarez RD and
Tollefsbol TO: Epigallocatechin gallate and sulforaphane
combination treatment induce apoptosis in paclitaxel-resistant
ovarian cancer cells through hTERT and Bcl-2 down-regulation. Exp
Cell Res. 319:697–706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang K, Zheng XY, Qin J, Wang YB, Bai Y,
Mao QQ, Wan Q, Wu ZM and Xie LP: Up-regulation of p21WAF1/Cip1 by
saRNA induces G1-phase arrest and apoptosis in T24 human bladder
cancer cells. Cancer Lett. 265:206–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan SSF, Chang HL, Chen HW, Kuo FC, Liaw
CC, Su JH and Wu YC: Selective cytotoxicity of squamocin on T24
bladder cancer cells at the S-phase via a Bax-, Bad-, and
caspase-3-related pathways. Life Sci. 78:869–874. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nargund VH, Tanabalan CK and Kabir MN:
Management of non-muscle-invasive (superficial) bladder cancer.
Semin Oncol. 39:559–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brighenti M, Passalacqua R, Arnaudi R,
Potenzoni M, Donini M, Liguigli W, Poli R, Lazzarelli S, Panni S
and Curti A: High rate of complete remission (CR) using two
sequential, dose-dense regimens of cisplatin, gemcitabine, and
paclitaxel (CGP) followed by HD-MVAC in patients with metastatic
bladder cancer (mBC). Eur J Cancer. 47:S5162011. View Article : Google Scholar
|
|
28
|
Mileo AM and Miccadei S: Polyphenols as
modulator of oxidative stress in cancer disease: New therapeutic
strategies. Oxid Med Cell Longev. 2016:64756242016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vissers MN, Zock PL, Roodenburg AJC,
Leenen R and Katan MB: Olive oil phenols are absorbed in humans. J
Nutr. 132:409–417. 2002.PubMed/NCBI
|
|
30
|
Miró-Casas E, Covas MI, Fitó M,
Farré-Albadalejo M, Marrugat J and de la Torre R: Tyrosol and
hydroxytyrosol are absorbed from moderate and sustained doses of
virgin olive oil in humans. Eur J Clin Nutr. 57:186–190. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou XU, Qi L, Tong S, Cui YU, Chen J,
Huang T, Chen Z and Zu XB: miR-128 downregulation promotes growth
and metastasis of bladder cancer cells and involves VEGF-C
upregulation. Oncol Lett. 10:3183–3190. 2015.PubMed/NCBI
|
|
32
|
Liao YX, Zeng JM, Zhou JJ, Yang GH, Ding K
and Zhang XJ: Silencing of RTKN2 by siRNA suppresses proliferation,
and induces G1 arrest and apoptosis in human bladder cancer cells.
Mol Med Rep. 13:4872–4878. 2016.PubMed/NCBI
|
|
33
|
Pacini L, De Falco E, Di Bari M, Coccia A,
Siciliano C, Ponti D, Pastore AL, Petrozza V, Carbone A, Tata AM,
et al: M2 muscarinic receptors inhibit cell proliferation and
migration in urothelial bladder cancer cells. Cancer Biol Ther.
15:1489–1498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pinto-Leite R, Carreira I, Melo J,
Ferreira SI, Ribeiro I, Ferreira J, Filipe M, Bernardo C,
Arantes-Rodrigues R, Oliveira P, et al: Genomic characterization of
three urinary bladder cancer cell lines: Understanding genomic
types of urinary bladder cancer. Tumour Biol. 35:4599–4617. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hamdi HK and Castellon R: Oleuropein, a
non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton
disruptor. Biochem Biophys Res Commun. 334:769–778. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Monti MC, Margarucci L, Tosco A, Riccio R
and Casapullo A: New insights on the interaction mechanism between
tau protein and oleocanthal, an extra-virgin olive-oil bioactive
component. Food Funct. 2:423–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan CM, Chai EQ, Cai HY, Miao GY and Ma W:
Oleuropein induces apoptosis via activation of caspases and
suppression of phosphatidylinositol 3-kinase/protein kinase B
pathway in HepG2 human hepatoma cell line. Mol Med Rep.
11:4617–4624. 2015.PubMed/NCBI
|
|
38
|
Sun L, Luo C and Liu J: Hydroxytyrosol
induces apoptosis in human colon cancer cells through ROS
generation. Food Funct. 5:1909–1914. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Raghavan D, Burgess E, Gaston KE, Haake MR
and Riggs SB: Neoadjuvant and adjuvant chemotherapy approaches for
invasive bladder cancer. Semin Oncol. 39:588–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Branchereau J, Luyckx F, Hitier M, Karam
G, Bouchot O and Rigaud J: Bladder necrosis after an immediate
post-operative mitomycin C instillation. Prog Urol. 21:151–153.
2011.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Onuchic AC, Machado CM, Saito RF, Rios FJ,
Jancar S and Chammas R: Expression of PAFR as part of a prosurvival
response to chemotherapy: A novel target for combination therapy in
melanoma. Mediators Inflamm. 2012:1754082012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Melnikova V and Bar-Eli M: Inflammation
and melanoma growth and metastasis: The role of platelet-activating
factor (PAF) and its receptor. Cancer Metastasis Rev. 26:359–371.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nechuta S, Lu W, Chen Z, Zheng Y, Gu K,
Cai H, Zheng W and Shu XO: Vitamin supplement use during breast
cancer treatment and survival: A prospective cohort study. Cancer
Epidemiol Biomarkers Prev. 20:262–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moss RW: Do antioxidants interfere with
radiation therapy for cancer? Integr Cancer Ther. 6:281–292. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
D'Andrea GM: Use of antioxidants during
chemotherapy and radiotherapy should be avoided. CA Cancer J Clin.
55:319–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gröber U: Antioxidants and other
micronutrients in complementary oncology. Breast Care (Basel).
4:13–20. 2009. View Article : Google Scholar : PubMed/NCBI
|