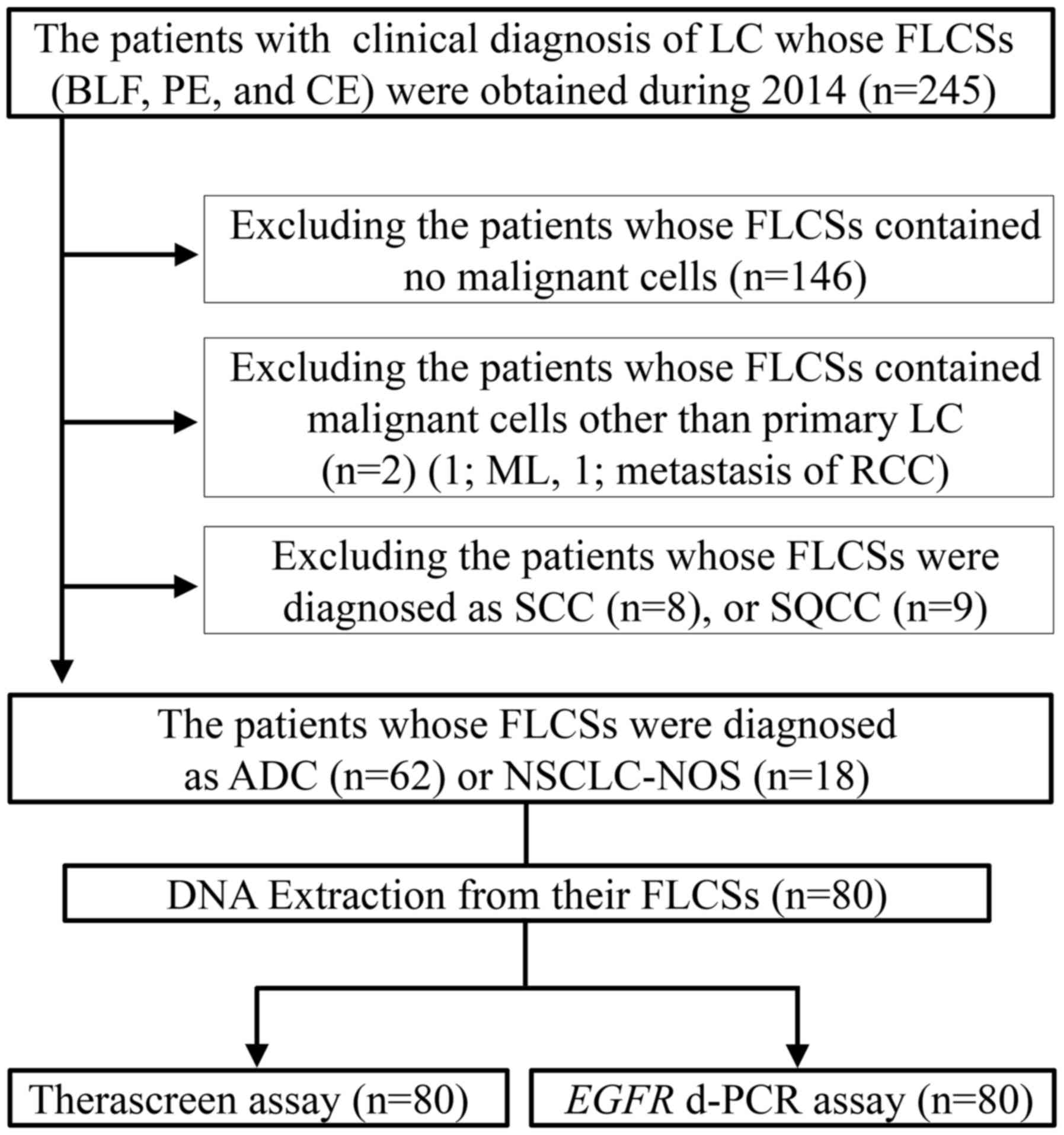
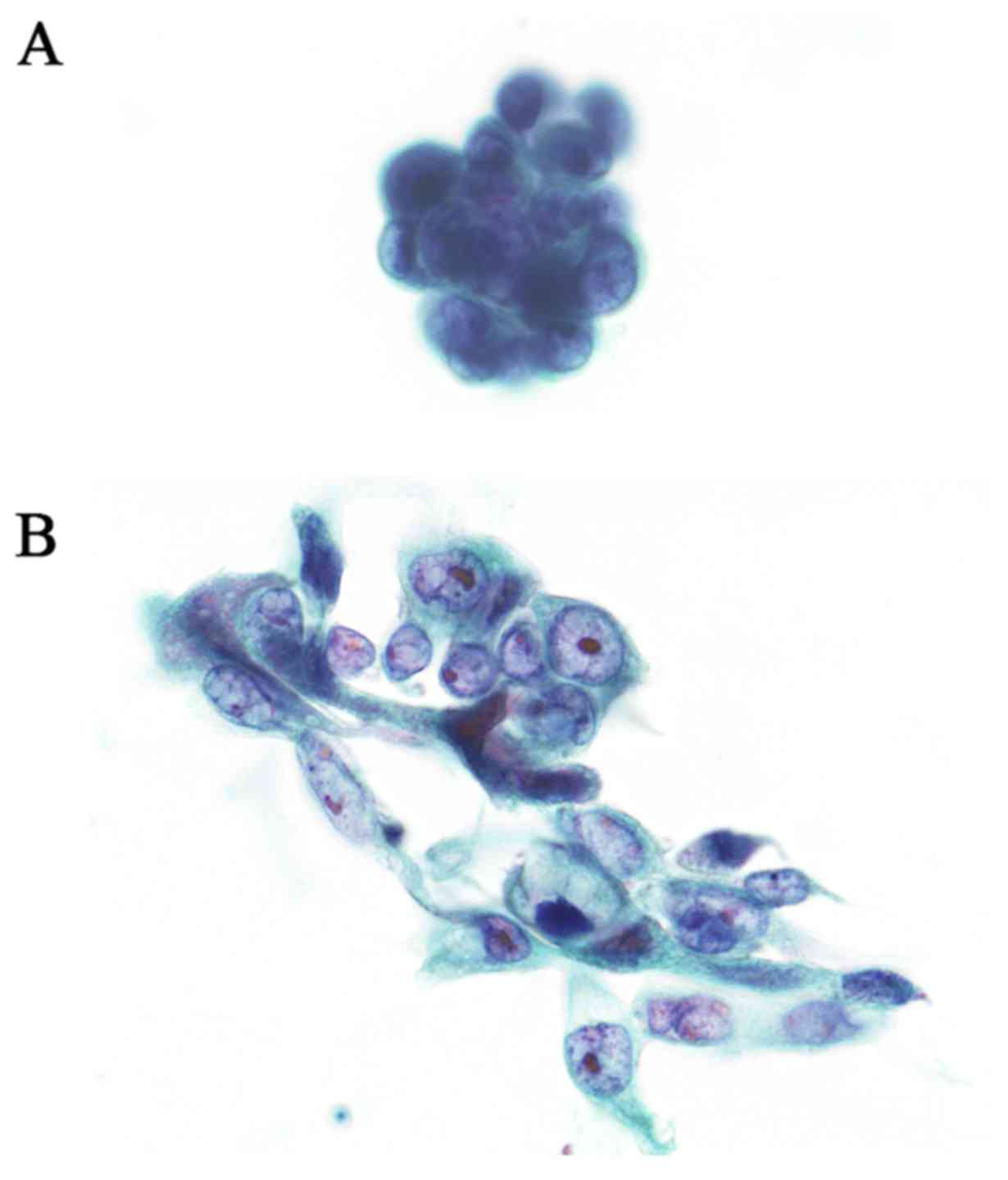
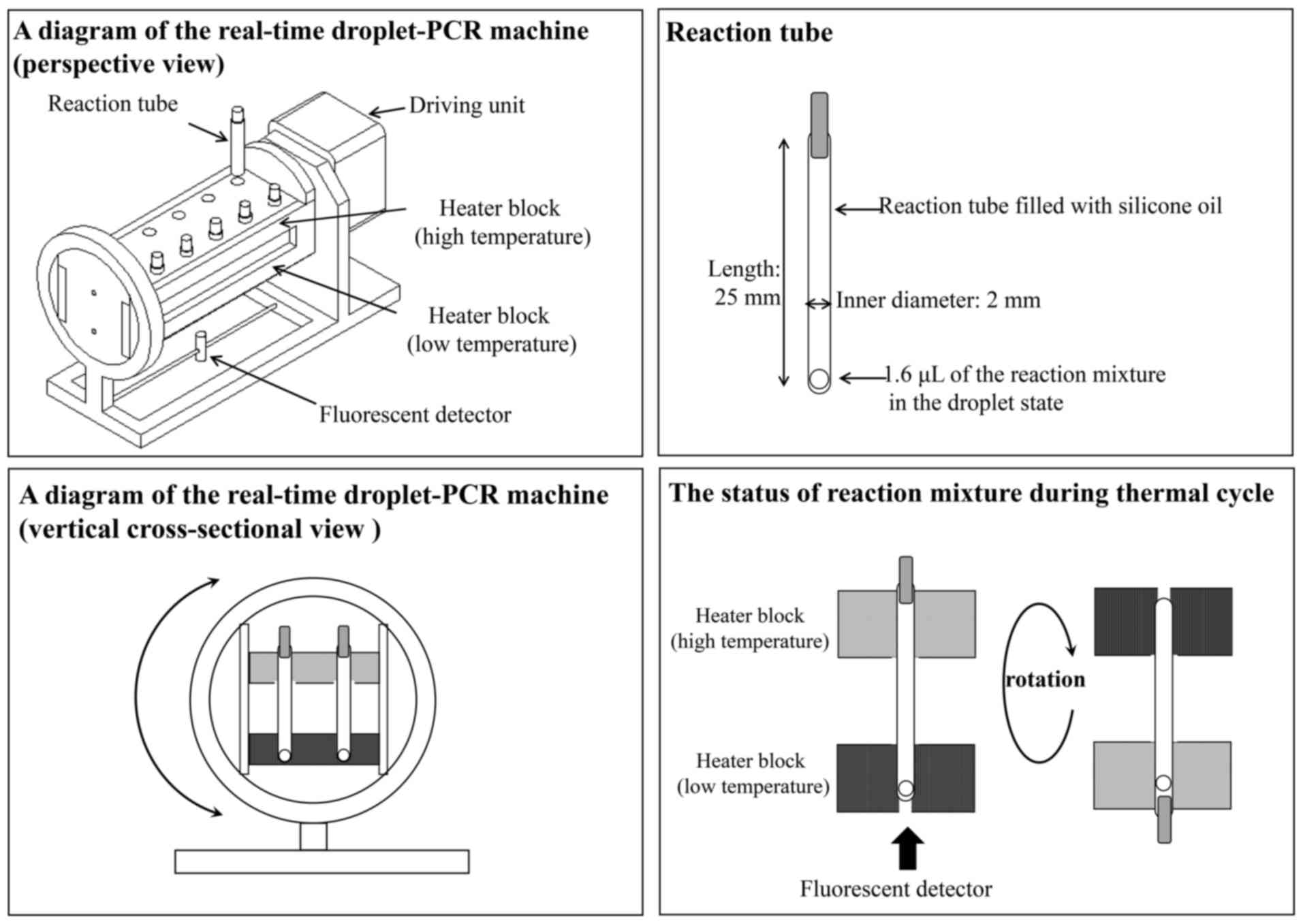
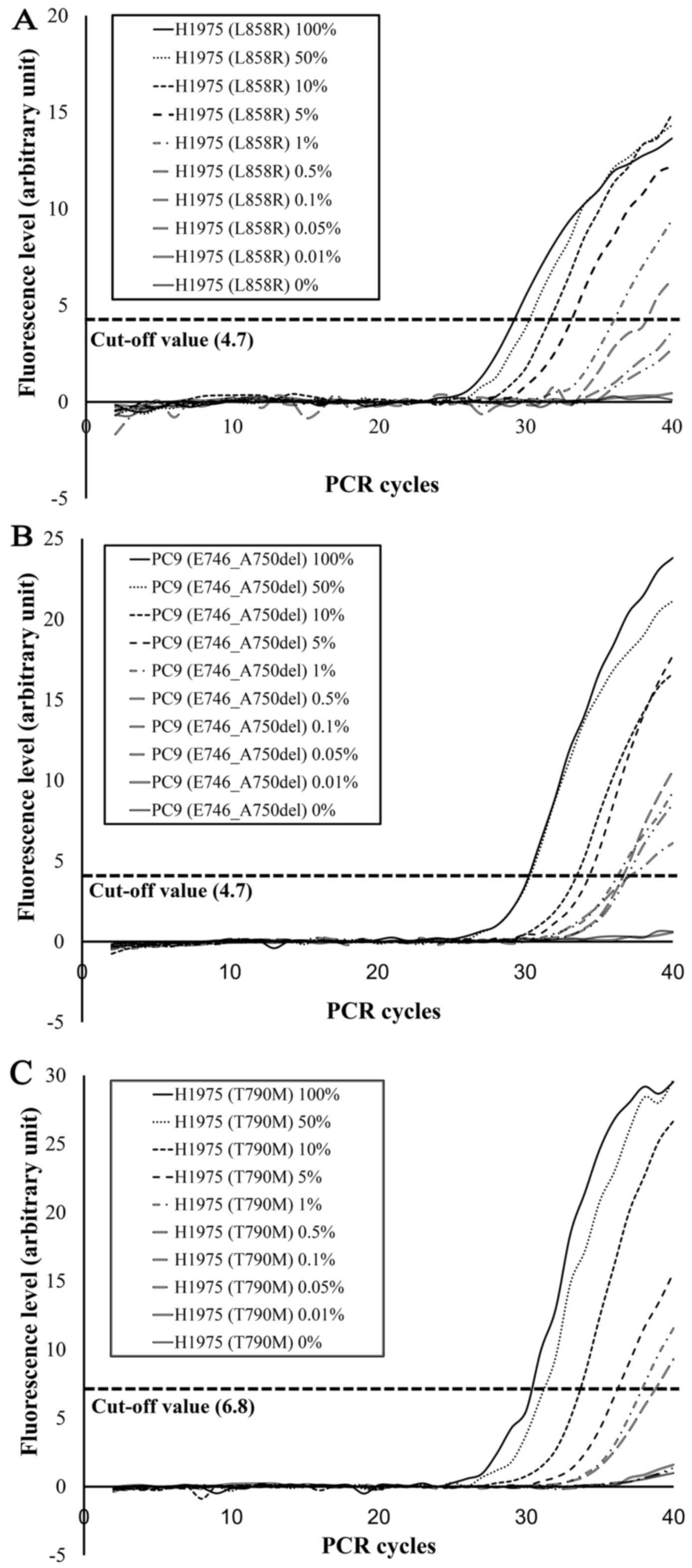
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mano H: Non-solid oncogenes in solid
tumors: EML4-ALK fusion genes in lung cancer. Cancer Sci.
99:2349–2355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravon P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: North-East Japan Study Group: Gefitinib or chemotherapy for
non-small-cell lung cancer with mutated EGFR. N Engl J Med.
362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Spanish Lung Cancer Group in
collaboration with Groupe Français de Pneumo-Cancérologie and
Associazione Italiana Oncologia Toracica: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pao W and Ladanyi M: Epidermal growth
factor receptor mutation testing in lung cancer: Searching for the
ideal method. Clin Cancer Res. 13:4954–4955. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuda K, Yamaguchi A, Taira C, Sueki A,
Koeda H, Takagi F, Sugano M and Honda T: A novel high-speed
droplet-polymerase chain reaction can detect human influenza virus
in less than 30 min. Clin Chim Acta. 413:1742–1745. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sueki A, Matsuda K, Yamaguchi A, Uehara M,
Sugano M, Uehara T and Honda T: Evaluation of saliva as diagnostic
materials for influenza virus infection by PCR-based assays. Clin
Chim Acta. 453:71–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sueki A, Matsuda K, Taira C, Yamaguchi A,
Koeda H, Takagi F, Kobayashi Y, Sugano M and Honda T: Rapid
detection of PML-RARA fusion gene by novel high-speed
droplet-reverse transcriptase-polymerase chain reaction:
Possibility for molecular diagnosis without lagging behind the
morphological analyses. Clin Chim Acta. 415:276–278. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shigeto S, Matsuda K, Yamaguchi A, Sueki
A, Uehara M, Sugano M, Uehara T and Honda T: Rapid diagnosis of
acute promyelocytic leukemia with the PML-RARA fusion gene using a
combination of droplet-reverse transcription-polymerase chain
reaction and instant-quality fluorescence in situ hybridization.
Clin Chim Acta. 453:38–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taira C, Matsuda K, Yamaguchi A, Sueki A,
Koeda H, Takagi F, Kobayashi Y, Sugano M and Honda T: Novel
high-speed droplet-allele specific-polymerase chain reaction:
Application in the rapid genotyping of single nucleotide
polymorphisms. Clin Chim Acta. 424:39–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taira C, Matsuda K, Yamaguchi A, Uehara M,
Sugano M, Okumura N and Honda T: Rapid single nucleotide
polymorphism based method for hematopoietic chimerism analysis and
monitoring using high-speed droplet allele-specific PCR and
allele-specific quantitative PCR. Clin Chim Acta. 445:101–106.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uehara M, Matsuda K, Sugano M and Honda T:
A new high-speed droplet-real-time polymerase chain reaction method
can detect bovine respiratory syncytial virus in less than 10 min.
J Vet Med Sci. 76:477–480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamaguchi A, Matsuda K, Sueki A, Taira C,
Uehara M, Saito Y and Honda T: Development of a rapid and sensitive
one-step reverse transcription-nested polymerase chain reaction in
a single tube using the droplet-polymerase chain reaction machine.
Clin Chim Acta. 448:150–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vallée A, Le Loupp AG and Denis MG:
Efficiency of the Therascreen® RGQ PCR kit for the
detection of EGFR mutations in non-small cell lung carcinomas. Clin
Chim Acta. 429:8–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mitsudomi T, Kosaka T and Yatabe Y:
Biological and clinical implications of EGFR mutations in lung
cancer. Int J Clin Oncol. 11:190–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumor specimens at the time of acquired resistance to
EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marchetti A, Martella C, Felicioni L,
Barassi F, Salvatore S, Chella A, Camplese PP, Iarussi T, Mucilli
F, Mezzetti A, et al: EGFR mutations in non-small-cell lung cancer:
Analysis of a large series of cases and development of a rapid and
sensitive method for diagnostic screening with potential
implications on pharmacologic treatment. J Clin Oncol. 23:857–865.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagai Y, Miyazawa H, Huqun Tanaka T,
Udagawa K, Kato M, Fukuyama S, Yokote A, Kobayashi K, Kanazawa M,
et al: Genetic heterogeneity of the epidermal growth factor
receptor in non-small cell lung cancer cell lines revealed by a
rapid and sensitive detection system, the peptide nucleic
acid-locked nucleic acid PCR clamp. Cancer Res. 65:7276–7282. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hall JG, Eis PS, Law SM, Reynaldo LP,
Prudent JR, Marshall DJ, Allawi HT, Mast AL, Dahlberg JE,
Kwiatkowski RW, et al: Sensitive detection of DNA polymorphisms by
the serial invasive signal amplification reaction. Proc Natl Acad
Sci USA. 97:8272–8277. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yatabe Y, Hida T, Horio Y, Kosaka T,
Takahashi T and Mitsudomi T: A rapid, sensitive assay to detect
EGFR mutation in small biopsy specimens from lung cancer. J Mol
Diagn. 8:335–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kimura H, Kasahara K, Kawaishi M, Kunitoh
H, Tamura T, Holloway B and Nishio K: Detection of epidermal growth
factor receptor mutations in serum as a predictor of the response
to gefitinib in patients with non-small-cell lung cancer. Clin
Cancer Res. 12:3915–3921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawahara A, Taira T, Azuma K, Tominaga M,
Hattori S, Kawahara M, Abe H, Yamaguchi T, Akiba J, Takamori S, et
al: A diagnostic algorithm using EGFR mutation-specific antibodies
for rapid response EGFR-TKI treatment in patients with non-small
cell lung cancer. Lung Cancer. 78:39–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lewandowska MA, Jóźwicki W, Jochymski C
and Kowalewski J: Application of PCR methods to evaluate EGFR, KRAS
and BRAF mutations in a small number of tumor cells in cytological
material from lung cancer patients. Oncol Rep. 30:1045–1052.
2013.PubMed/NCBI
|
|
28
|
Tuononen K, Mäki-Nevala S, Sarhadi VK,
Wirtanen A, Rönty M, Salmenkivi K, Andrews JM, Telaranta-Keerie AI,
Hannula S, Lagström S, et al: Comparison of targeted
next-generation sequencing (NGS) and real-time PCR in the detection
of EGFR, KRAS, and BRAF mutations on formalin-fixed,
paraffin-embedded tumor material of non-small cell lung carcinoma -
superiority of NGS. Genes Chromosomes Cancer. 52:503–511. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lindeman NI, Cagle PT, Beasley MB, Chitale
DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS,
Squire J, et al: College of American Pathologists International
Association for the Study of Lung Cancer and Association for
Molecular Pathology: Molecular testing guideline for selection of
lung cancer patients for EGFR and ALK tyrosine kinase inhibitors:
Guideline from the College of American Pathologists, International
Association for the Study of Lung Cancer, and Association for
Molecular Pathology. J Mol Diagn. 15:415–453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smouse JH, Cibas ES, Jänne PA, Joshi VA,
Zou KH and Lindeman NI: EGFR mutations are detected comparably in
cytologic and surgical pathology specimens of nonsmall cell lung
cancer. Cancer. 117:67–72. 2009.PubMed/NCBI
|
|
31
|
Reynolds JP, Tubbs RR, Minca EC, MacNamara
S, Almeida FA, Ma PC, Pennell NA and Cicenia JC: EGFR mutational
genotyping of liquid based cytology samples obtained via fine
needle aspiration (FNA) at endobronchial ultrasound of non-small
cell lung cancer (NSCLC). Lung Cancer. 86:158–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lozano MD, Labiano T, Echeveste J, Gurpide
A, Martín- Algarra S, Zhang G, Sharma A and Palma JF: Assessment of
EGFR and KRAS mutation status from FNAs and core-needle biopsies of
non-small cell lung cancer. Cancer Cytopathol. 123:230–236. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sakamoto T, Kodani M, Takata M, Chikumi H,
Nakamoto M, Nishii-Ito S, Ueda Y, Izumi H, Makino H, Touge H, et
al: A novel point-of-care system for high-speed real-time
polymerase chain reaction testing for epidermal growth factor
receptor mutations in bronchial lavage fluids after transbronchial
biopsy in patients with non-small cell lung cancer. Int J Oncol.
46:1473–1480. 2015.PubMed/NCBI
|
|
34
|
Takata M, Chikumi H, Matsunami K, Kodani
M, Sakamoto T, Hashimoto K, Nakamoto M, Okada K, Kitaura T,
Matsumoto S, et al: A new rapid method for detecting epidermal
growth factor receptor mutations in non-small cell lung cancer.
Oncol Rep. 33:1040–1048. 2015.PubMed/NCBI
|
|
35
|
Angulo B, Conde E, Suárez-Gauthier A,
Plaza C, Martínez R, Redondo P, Izquierdo E, Rubio-Viqueira B,
Paz-Ares L, Hidalgo M, et al: A comparison of EGFR mutation testing
methods in lung carcinoma: Direct sequencing, real-time PCR and
immunohistochemistry. PLoS One. 7:e438422012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Naidoo J, Sima CS, Rodriguez K, Busby N,
Nafa K, Ladanyi M, Riely GJ, Kris MG, Arcila ME and Yu HA:
Epidermal growth factor receptor exon 20 insertions in advanced
lung adenocarcinomas: Clinical outcomes and response to erlotinib.
Cancer. 121:3212–3220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee VHF, Tin VPC, Choy TS, Lam KO, Choi
CW, Chung LP, Tsang JW, Ho PP, Leung DK, Ma ES, et al: Association
of exon 19 and 21 EGFR mutation patterns with treatment outcome
after first-line tyrosine kinase inhibitor in metastatic
non-small-cell lung cancer. J Thorac Oncol. 8:1148–1155. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY
and Yang PC: Effectiveness of tyrosine kinase inhibitors on
‘uncommon’ epidermal growth factor receptor mutations of unknown
clinical significance in non-small cell lung cancer. Clin Cancer
Res. 17:3812–3821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Otsuka T, Mori M, Yano Y, Uchida J,
Nishino K, Kaji R, Hata A, Hattori Y, Urata Y, Kaneda T, et al:
Effectiveness of tyrosine kinase inhibitors in Japanese patients
with non-small cell lung cancer harboring minor epidermal growth
factor receptor mutations: Results from a multicenter retrospective
study (HANSHIN Oncology Group 0212). Anticancer Res. 35:3885–3891.
2015.PubMed/NCBI
|