Introduction
Non-small cell lung cancer (NSCLC) is the leading
cause of cancer-related deaths worldwide. The treatment of advanced
NSCLC may be changing since the emergence of targeted therapy, but
the cisplatin-based doublet remains the foundation of treatment for
the majority of patients with advanced NSCLC (1). The resistance to chemotherapy has been
a major factor affecting the therapeutic efficacy in the treatment
of lung cancer. To overcome the resistance, second line or
combination chemotherapy regimens have been used, but the overall
survival benefits of various chemotherapies in NSCLC are not yet
satisfactory (2).
Transcriptional co-activator with PDZ binding motif
(TAZ), also known as WW domain-containing transcription
regulator-1, is a key transducer of the Hippo pathway. This pathway
is an essential regulator of organ size, stem cell maintenance, and
tumorigenesis. TAZ acts mainly through the TEAD family of
transcription factors to stimulate expression of genes that promote
cell proliferation and control organ size. TAZ has also been
suggested to be involved in other biological processes, including
mesenchymal stem cell differentiation (3), self-renewal of human embryonic stem
cells (4) and mechanotransduction
(5). In addition, TAZ may crosstalk
with different signaling pathways in different cell types (6).
Growing evidence has revealed that TAZ is an
oncogene in lung cancer, important for lung tumorigenesis and
metastasis (7). Clinical lung
cancer patient studies also reported that TAZ is overexpressed in
over 60% of NSCLC and its expression is significantly associated
with adenocarcinoma, poor differentiation, metastasis and poor
prognosis and survival (8). Recent
studies have consistently suggested that upregulation of TAZ and/or
its paralog YAP confers resistance against a diverse range of
cytotoxic agents such as anti-tubulin, antimetabolite and
DNA-damaging agents (9). TAZ
overexpression in breast cancer cells has been shown to induce
resistance to taxol and doxorubicin, and upregulation of its target
genes CYR61 and CTGF appears to contribute to resistance
development (10). YAP has been
reported to promote resistance to cisplatin in oral squamous cell
carcinoma (11), urothelial cell
carcinoma (12) and ovarian cancer
(13). In the present study, we
provided the first evidence that TAZ is also involved in cisplatin
sensitivity in lung adenocarcinoma.
Materials and methods
Reagents and antibodies
Cisplatin (APP Pharmaceuticals LLC, Schaumburg, IL,
USA) stock solution (3.3 mmol/l) prepared in sterile water was
provided by the outpatient pharmacy, The First Affiliated Hospital
of Nanjing Medical University (Jiangsu, China). TAZ, AKT, p-AKT
(Ser473/Thr308) and S6K antibodies were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The phospho-p70 S6
kinase (Thr389/412) antibody was purchased from Affinity
Biologicals (Ancaster, Canada). The GAPDH antibody was obtained
from Santa Cruz Biotechnology, Inc. (Santa Cruz, Califirnia,
USA).
Cell culture
Human bronchial epithelial cell line 16HBE and lung
adenocarcinoma cell lines A549 and H460 were purchased from the
Cell Resource Center (Shanghai Institutes for Biological Sciences,
Shanghai, China). The cisplatin-resistant A549 (A549/DDP) and H460
(H460/DDP) cell lines were kindly provided by Professor Zhou at the
Shanghai Pulmonary Hospital. All cell lines were maintained in
RPMI-1640 medium containing 10% fetal bovine serum (FBS) (both from
Life Technologies, Inc., Gaithersburg, MD, USA), 100 U/ml
penicillin, 100 U/ml streptomycin, 2 mM glutamine in a humidified
atmosphere with 5% CO2 at 37°C. To maintain drug
resistance, A549/DDP and H460/DDP cells were grown in RPMI-1640
medium containing 2 mg/ml DDP, and then in DDP-free RPMI-1640
medium two days before experiments.
Reverse transcription PCR
(RT-PCR)
Total RNA was extracted from cells with TRIzol
reagent (Invitrogen, San Diego, CA, USA) according to the
manufacturer's instructions and subjected to cDNA synthesis and
PCR. The primers used were as follows: The specific primers were as
follows: TAZ forward, 5′-AGTACCCTGAGCCAGCAGAA-3′ and reverse,
5′-GATTCTCTGAAGCCGCAGTT-3′; GAPDH forward,
5′-GGAGCCAAAAGGGTCATCAT-3′ and reverse,
5′-GTGATGGCATGGACTGTGGT-3′.
Western blot analysis
Cells were lysed in RIPA buffer (50 mmol/l Tris-HCl
buffer, pH 7.4, 150 mmol/l NaCl, 1% Triton X-100, 1% sodium
deoxycholate and 0.1% SDS) supplemented with 1X Halt protease
inhibitor cocktail and 1X Halt phosphatase inhibitor cocktail
(Pierce Biotechnology, Inc., Rockford, IL, USA). The proteins were
separated and western blot analysis was carried out as previously
described (14). The target protein
was visualized by chemiluminescence (Denville Scientific, Inc.,
Metuchen, NJ, USA).
Immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded tumor tissues were
obtained from the The First Affiliated Hospital of Nanjing Medical
University. IHC was performed using the protocol as previously
described (14), using the TAZ
antibody (Cell Signaling Technology, Inc., Danvers, MA, USA).
Immunostaining was classified on the basis of the intensity of
staining (no staining, 0; weak, 1; moderate, 2; and strong, 3
points). A final IHC score was obtained by multiplying the
intensity score and extent (%) of stained cells. The Institutional
Review Board (IRB) approved the present study.
Cell Counting Kit-8 assay
Cell Counting Kit-8 (CCK-8) assay (Beyotime
Institute of Biotechnology, Beijing, China) was used to detect cell
proliferation. According to the manufacturer's instructions, the
absorbance was measured at a wavelength of 450 nm using Bio-Tek
microplate reader ELx800 (Bio-Tek, Winooski, VT, USA) every 24 h
after transfection. The half maximal inhibitory concentration
(IC50) of lung adenocarcinoma cells were also determined
using the CCK-8 assay.
BrdUrd-labeling
Cells were labeled with 10 mmol/l bromodeoxyuridine
(BrdUrd; Sigma-Aldrich, St. Louis, MO, USA) in growth medium for 12
h at 37°C. BrdUrd-labeled DNA was detected with mouse monoclonal
anti-BrdUrd (Ruibo Biotech, Guangzhou, China) according to the
manufacturer's protocol. Cells were examined under Olympus IX-71
(Olympus, Tokyo, Japan) inverted microscope and photographed.
Flow cytometry
Cells were harvested and gently disaggregated to a
single cell suspension. Staining was made according to the
manufacturer's protocol. The rate of apoptosis induced by
anticancer regimens was analyzed by flow cytometry using an Annexin
V-FITC/PI kit (BD Biosciences, San Diego, CA, USA) following the
manufacturer's instructions.
Plasmid and transfection
The target sequences of shTAZ1 and shTAZ2 were:
GCGATGAATCAGCCTCTGAAT and AGGTACTTCCTCAATCACA. Cells reaching 80%
confluence were transfected with pGPU6-shTAZ in the presence of
Lipofectamine 2000 (Invitrogen). The plasmid of pEX2-TAZ and
negative control (pEX2), AKT-siRNA and siRNA negative control
(siNC) were purchased from GenePharma (Shanghai, China). The
sequence of siAKT and siNC were: 5′-GAACAAUCCGAUUCACGUATT-3′ and
5′-UUCUCCGAACGUGUCACGUTT-3′, respectively.
Statistical analysis
The data are expressed as the mean ± SD; Student's
t-tests and Chi-square tests were used to determine the
significance of differences in multiple comparisons. All data were
analyzed with SPSS statistical software (version 13.0; SPSS, Inc.,
Chicago, IL, USA) and a P-value of <0.05 was considered
statistically significant.
Results
High expression of TAZ in lung
adenocarcinoma
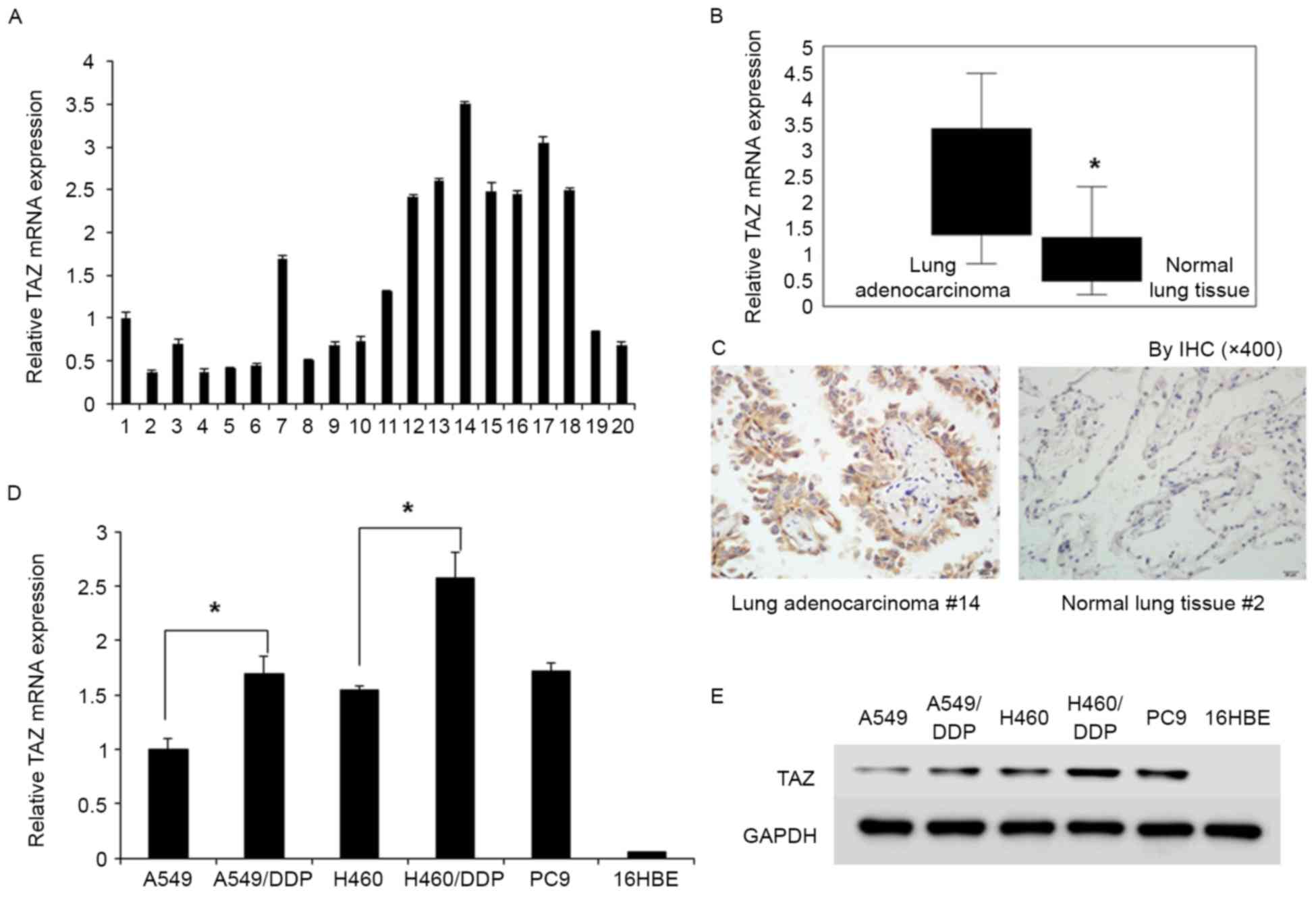
We investigated TAZ expression in 10 cases of lung
adenocarcinoma tissue and 10 cases of normal lung tissue by qRT-PCR
(Fig. 1A) and IHC. TAZ was
expressed at levels 3.15 times higher in lung adenocarcinoma than
those in normal lung tissue (Fig.
1B). TAZ-positive expression in tumor cells was observed in 8
out of 10 lung adenocarcinoma samples. Both cytoplasmic and nuclear
TAZ was present in lung adenocarcinoma tissue. TAZ overexpression
was not detected in non-neoplastic bronchial or alveolar epithelial
cells (Fig. 1C).
We examined the TAZ expression in lung cell lines by
qRT-PCR (Fig. 1D) and western
blotting (Fig. 1E). Specifically,
no TAZ expression was observed in non-tumorigenic human bronchial
epithelial cell line (16HBE), whereas overexpression of TAZ was
observed in tumorigenic lung adenocarcinoma cell lines (A549,
A549/DDP, A549/TR, H460 and PC9). The relative mRNA level of TAZ in
A549/DDP and H460/DDP cells was ~1.7 and 2.58 times higher than
that in A549 and H460 cells, respectively (Fig. 1D). Western blot assay revealed the
same trend in the protein expression of TAZ in cell lines.
TAZ knockdown regulates the
proliferation, apoptosis and cisplatin sensitivity in
cisplatin-resistant cells
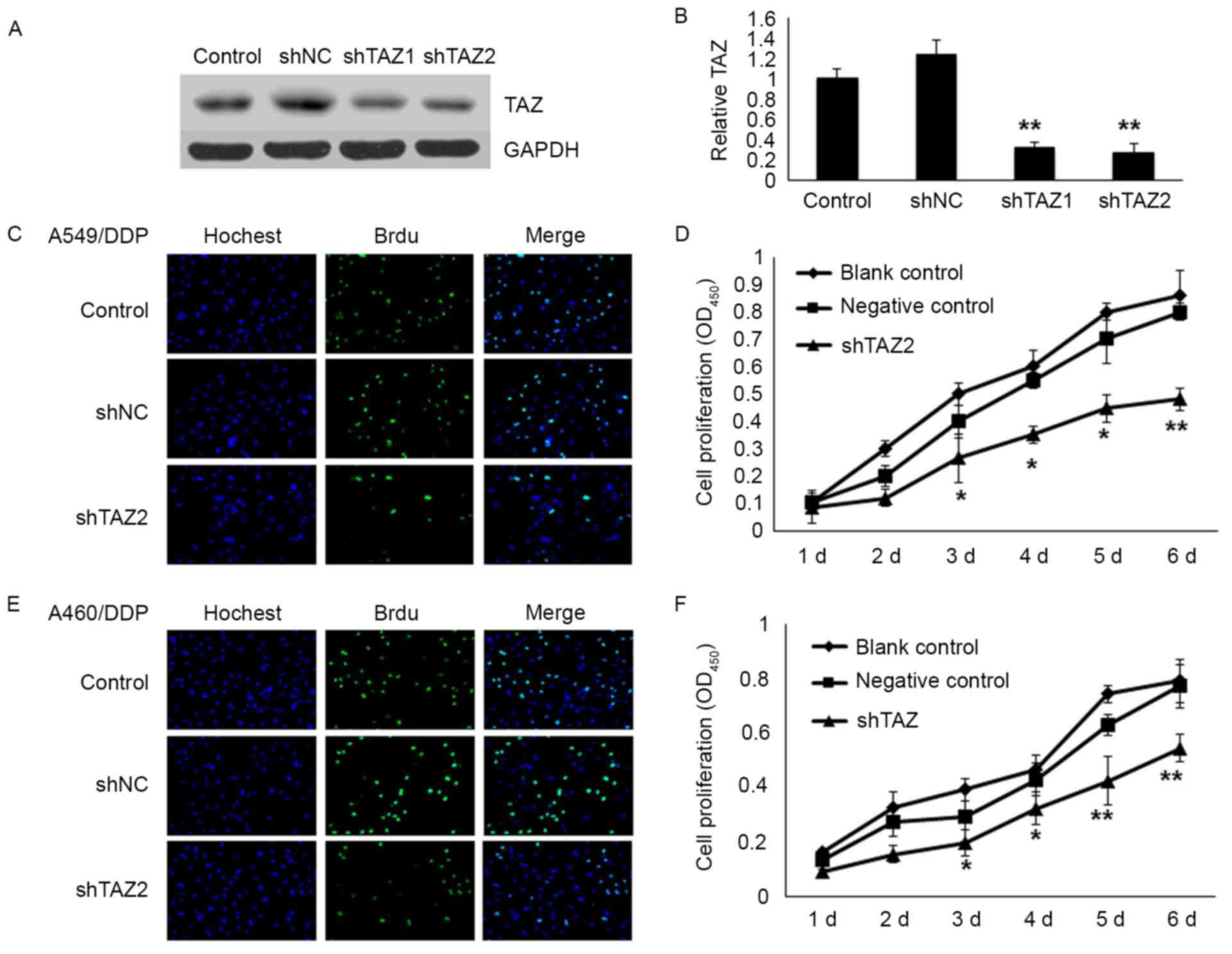
To further investigate the role of TAZ in the
resistance of the cells to chemotherapy, TAZ knockdown was
performed in A549/DDP and H460/DDP cells with specific shRNAs
(Fig. 2A and B). The results
demonstrated that the shTAZ2 exhibited the best interference
efficiency. In the present study, shTAZ2 was employed in some of
the following experiments. To elucidate the effect of TAZ on cell
proliferation, shTAZ2 was transfected into A549/DDP and H460/DDP
cells. After 48 h of transfection, the rate of cell proliferation
and growth was significantly decreased in both A549/DDP and
H460/DDP cells assessed by BrdU incorporation and CCK-8 assay. The
mean percentage of positive proliferative cells of the shTAZ2 group
decreased by 40% (P<0.05) in the A549/DDP cells and ~50% in the
H460/DDP cells. The result of the CCK-8 assay revealed that the
number of cells decreased by 40.0% in the A549/DDP cells (Fig. 2C and D) and 29.8% in the H460/DDP
cells (Fig. 2E and F) after
transfection on the 6th day.
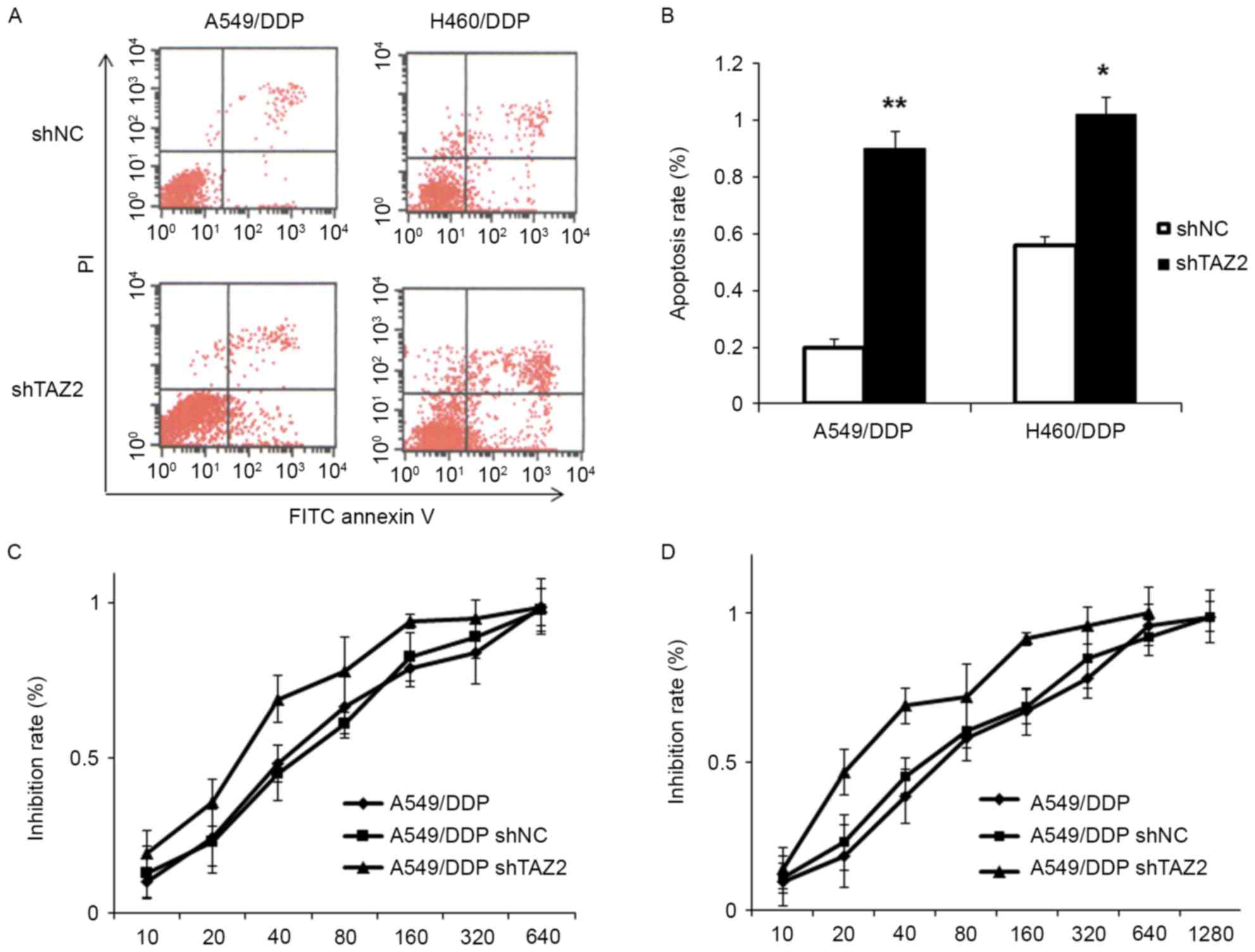
We subsequently explored the effect of TAZ knockdown
on cell apoptosis using by Annexin V-FITC/PI double staining. As
shown in Fig. 3A and B, the
apoptosis rate of the shTAZ2 group increased by 70% (P<0.05) in
the A549/DDP cells and 46% (P<0.05) in the H460/DDP cells,
indicating that TAZ knockdown induces apoptosis in
cisplatin-resistant lung adenocarcinoma cells.
Next, in order to assess the effect of TAZ knockdown
on the sensitivity of the resistant cells to cisplatin a CCK-8
assay was employed to examine the IC50 value of A549/DDP
and H460/DDP cells (Fig. 3C and D).
The results revealed that the IC50 value of the
shRNA-transfected cells had a pronounced decrease in the
IC50 value as compared to the negative control group.
The mean IC50 value of the A549/DDP cells transfected
shTAZ2 was 27.83±4.23 µM vs. 48.92±6.62 µM in the negative control
group. In the H460/DDP cells, the mean IC50 value for
the shTAZ2-transfection group was 30.53±5.76 µM vs. the negative
control group which was 58.62±7.54 µM.
TAZ regulates the AKT/mTOR signaling
pathway in lung adenocarcinoma cells
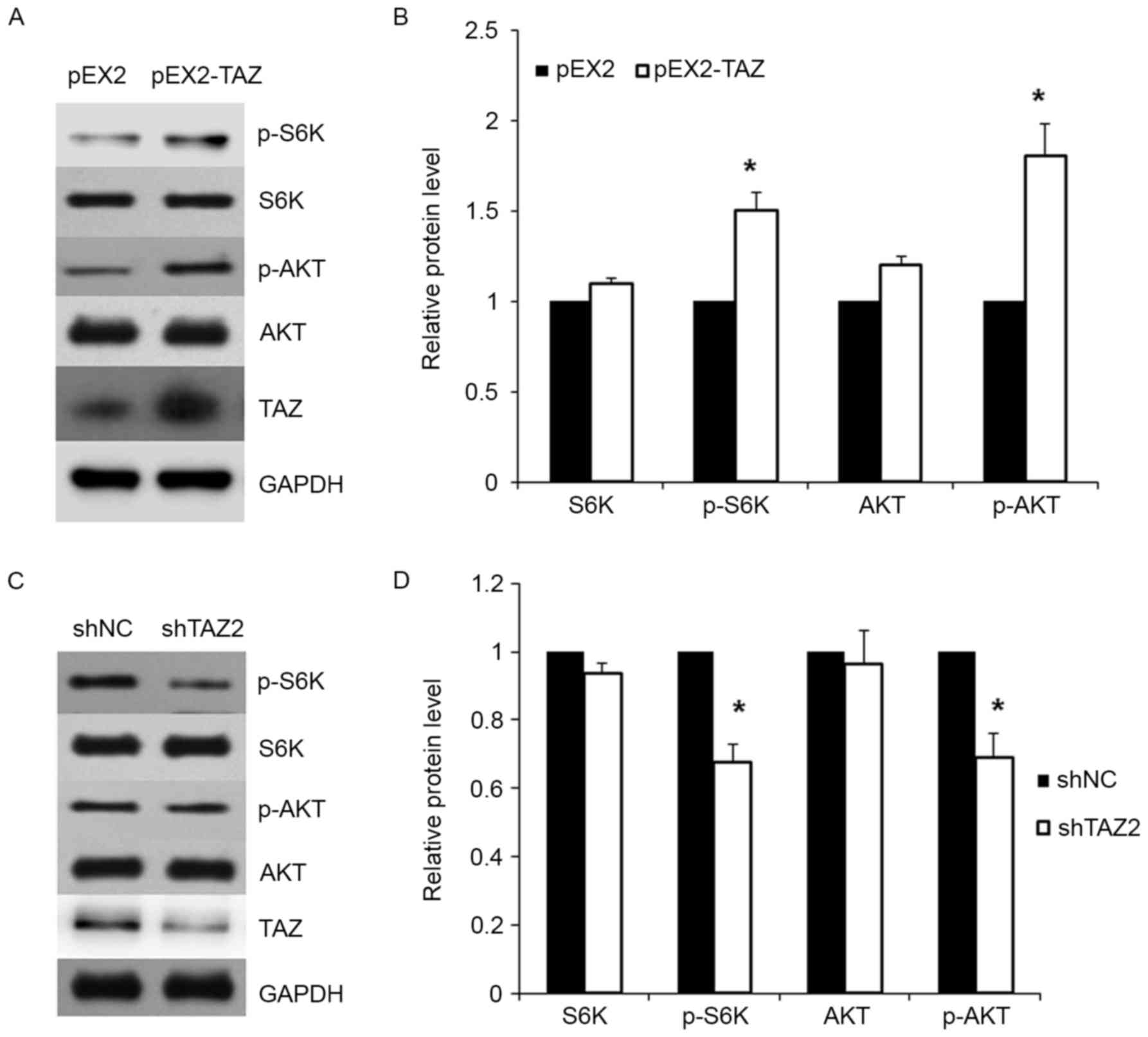
Previous studies demonstrated that TAZ affected cell
chemosensitivity by phosphorylation of AKT. To detect whether TAZ
affects lung adenocarcinoma cell sensitivity to cisplatin by
regulating the AKT/mTOR signaling pathway, we firstly overexpressed
TAZ in cancer cells. As shown in Fig.
4A, the protein expression of TAZ in A549 cell lines were
markedly increased after transfection. Moreover, the expression of
total AKT, phosphorylated AKT (p-AKT), total S6K and phosphorylated
S6K (p-S6K) were examined in transfected cells. Cells with TAZ
overexpression exhibited increased expression of p-AKT and p-S6K
when compared with the control group (Fig. 4A and B).
Furthermore, the expression of AKT, p-AKT, S6K and
p-S6K were examined in TAZ shRNA-transfected cells. Western blot
assay revealed that TAZ knockdown led to the significant decrease
in the expression of p-AKT and p-S6K (Fig. 4C and D).
TAZ affects the sensitivity of
cisplatin via AKT/mTOR signaling
We used AKT-specific siRNA (siAKT) to downregulate
the expression of AKT and to evaluate whether the effects of TAZ on
lung adenocarcinoma cells depended on the AKT/mTOR signaling
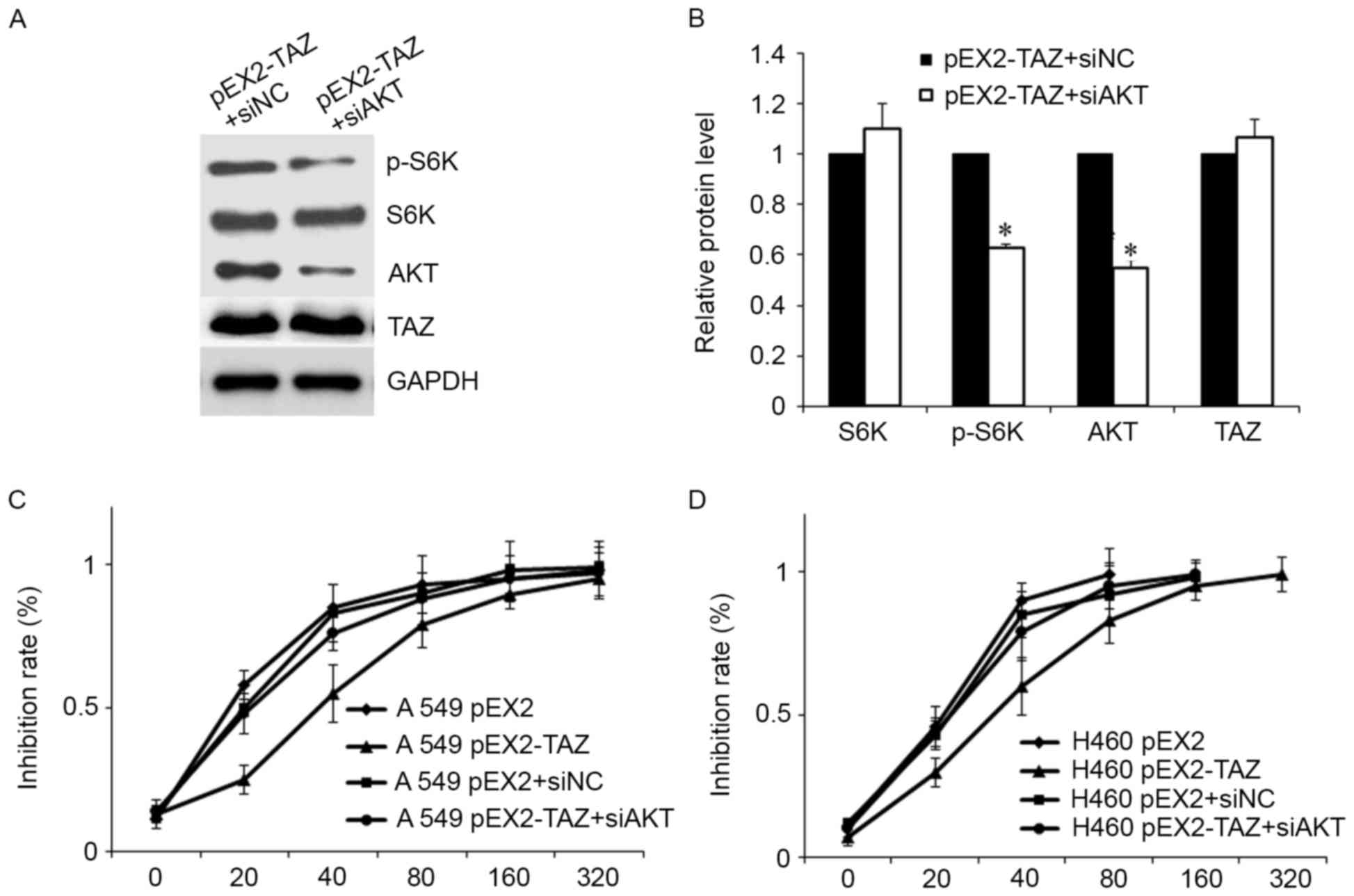
pathway. As is shown in Fig. 5A,
the level of p-S6K was markedly decreased after siAKT transfection,
in line with the previous experiments. Meanwhile, the increased
level of p-S6K-induced by TAZ overexpression was partially
abolished by AKT downregulation (Fig.
5A and B).
Furthermore, AKT downregulation restored the
sensitivity to cisplatin both in A549 and H460 cells (Fig. 5B and C). The increased
IC50 value induced by TAZ overexpression was eliminated
by siAKT.
Discussion
Although platinum-based chemotherapy alone or in
combination with other drugs has been used as a first-line therapy
in patients with advanced NSCLC, resistance to cisplatin, a
platinum compound, unfortunately, remains a major clinical problem.
The mechanisms of cisplatin resistance are not fully understood,
but they are believed to be multifactorial in nature (15). They include insufficient DNA
binding, increased detoxification and DNA repair, dysregulated
expression of transporters, and altered expression and activation
of genes involved in cell death pathways, such as p53, Bcl-2 and
AKT/mTOR. Among these pathways, the activation of the AKT/mTOR
pathway plays an important role in cisplatin resistance (16).
TAZ has emerged as a key player in organ growth and
tumorigenesis (17). It is tightly
regulated in the hippo pathway in a dependent and independent
manner in response to a wide range of extracellular and intrinsic
signals, including cell density, cell polarity, F-actin-related
mechanical stress, ligands of G protein-coupled receptors (GPCRs),
cellular energy status, hypoxia and osmotic stress (18). TAZ has been implicated to interact
with multiple transcription factors including RUNX2, MyoD, PPAR,
TTF1, PAX3, PAX8, Smads and TEADs (19).
Recent studies have consistently suggested that
TAZ/YAP upregulation confers resistance against a diverse range of
cytotoxic agents (9). The increased
levels of TAZ were found in human breast cancer cells which were
responsible for their resistance to Taxol though the
TAZ-TEAD-Cyr61/CTGF signaling pathway (10). Gene-expression profile studies
comparing metastagenic and non-metastagenic cells identified TAZ as
a central mediator of patient-derived breast cancer stem cell lines
metastatic ability involved in their chemoresistance and
tumorigenic potential (20).
Cisplatin-resistant oral squamous cancer cell lines exhibited
decreased phospho-YAP and increased nuclear YAP (11). Furthermore, YAP overexpression
protected while YAP knockdown sensitized urothelial carcinoma cells
to chemotherapy and radiation effects via increased accumulation of
DNA damage and apoptosis. Pharmacological YAP inhibition with
verteporfin inhibited tumor cell proliferation and restored
sensitivity to cisplatin (12).
Moreover, YAP signaling may regulate cisplatin resistance in
ovarian cancer cells by increasing cellular autophagic flux
(13).
We previously revealed that TAZ is upregulated in
lung adenocarcinoma cells with the EGFR T790M mutation, and TAZ
depletion inhibits tumorigenicity, epithelial mesenchymal
transition, migration, invasion of gefitinib-resistant NSCLC cells
and sensitizes their response to gefitinib (14). In the present study, we found that
TAZ was highly expressed in lung adenocarcinoma tissues and cell
lines, particularly in cisplatin-resistant cells. Knockdown of TAZ
confers decreased proliferation, increased apoptosis and cisplatin
sensitivity in cisplatin-resistant cells. We also elucidated the
possible signaling transduction mechanism of TAZ on the cisplatin
sensitivity in human lung adenocarcinoma and affirmed that TAZ
knockdown decreased the AKT/mTOR pathway expression. Overexpression
of TAZ increased p-AKT and p-S6K, which was inhibited by siAKT.
Furthermore, we found the that inhibition of the AKT/mTOR pathway
rescued the cells from chemoresistance caused by TAZ
overexpression. These results demonstrated that chemosensitivity
regulation of TAZ was, at least in part, AKT/mTOR signaling
pathway-dependent.
In summary, in the present study our data indicated
that inhibition of the transcription co-activator TAZ was linked to
cisplatin sensitivity in lung adenocarcinoma for the first time,
which was partly dependent on the AKT/mTOR signaling pathway. Thus,
TAZ may be a potent therapeutic target for NSCLC in combination
with conventional chemotherapy.
Acknowledgements
The present study was funded by Jiangsu Provincial
Key Discipline of Medicine (ZDXKA2016003), the Priority Academic
Program Development of Jiangsu Higher Education Institutions
(Jiangsu, China), and was also supported by grants from the
International Science and Technology Cooperation Program of China
(no. 2014DFA31940), the National Natural Science Foundation of
China (Beijing, China; nos. 81302014, 81572259 and 81402489), the
Six Talent Peaks Project (Jiangsu, China; no. 2015-WSN-038) and the
333 High-level Personnel Training Project of Jiangsu Province.
Glossary
Abbreviations
Abbreviations:
|
S6K
|
ribosomal protein S6 kinase
|
|
EGFR
|
epidermal growth factor receptor
|
|
CYR61
|
cysteine rich angiogenic inducer
61
|
|
CTGF
|
connective tissue growth factor
|
|
RUNX2
|
runt-related transcription factor
2
|
|
MyoD
|
class I myosin
|
|
PPAR
|
peroxisome proliferator-activated
receptor
|
|
TTF1
|
transcription termination factor 1
|
|
PAX3
|
paired box 3
|
References
|
1
|
Fennell DA, Summers Y, Cadranel J, Benepal
T, Christoph DC, Lal R, Das M, Maxwell F, Visseren-Grul C and Ferry
D: Cisplatin in the modern era: The backbone of first-line
chemotherapy for non-small cell lung cancer. Cancer Treat Rev.
44:42–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi Y and Sun Y: Medical management of
lung cancer: Experience in China. Thorac Cancer. 6:10–16. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hong JH, Hwang ES, McManus MT, Amsterdam
A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp
PA, et al: TAZ, a transcriptional modulator of mesenchymal stem
cell differentiation. Science. 309:1074–1078. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao B, Tumaneng K and Guan KL: The Hippo
pathway in organ size control, tissue regeneration and stem cell
self-renewal. Nat Cell Biol. 13:877–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dupont S, Morsut L, Aragona M, Enzo E,
Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M,
Bicciato S, et al: Role of YAP/TAZ in mechanotransduction. Nature.
474:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang K, Qi HX, Hu ZM, Chang YN, Shi ZM,
Han XH, Han YW, Zhang RX, Zhang Z, Chen T, et al: YAP and TAZ take
center stage in cancer. Biochemistry. 54:6555–6566. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Z, Hao Y, Liu N, Raptis L, Tsao MS
and Yang X: TAZ is a novel oncogene in non-small cell lung cancer.
Oncogene. 30:2181–2186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie M, Zhang L, He CS, Hou JH, Lin SX, Hu
ZH, Xu F and Zhao HY: Prognostic significance of TAZ expression in
resected non-small cell lung cancer. J Thorac Oncol. 7:799–807.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim MH and Kim J: Role of YAP/TAZ
transcriptional regulators in resistance to anti-cancer therapies.
Cell Mol Life Sci. 74:1457–1474. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai D, Ho KC, Hao Y and Yang X: Taxol
resistance in breast cancer cells is mediated by the hippo pathway
component TAZ and its downstream transcriptional targets
Cyr61 and CTGF. Cancer Res. 71:2728–2738. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshikawa K, Noguchi K, Nakano Y, Yamamura
M, Takaoka K, Hashimoto-Tamaoki T and Kishimoto H: The Hippo
pathway transcriptional co-activator, YAP, confers resistance to
cisplatin in human oral squamous cell carcinoma. Int J Oncol.
46:2364–2370. 2015.PubMed/NCBI
|
|
12
|
Ciamporcero E, Shen H, Ramakrishnan S, Ku
S Yu, Chintala S, Shen L, Adelaiye R, Miles KM, Ullio C, Pizzimenti
S, et al: YAP activation protects urothelial cell carcinoma from
treatment-induced DNA damage. Oncogene. 35:1541–1553. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao L, Shi XY, Zhang Y, Zhu Y, Zhu L,
Tian W, Zhu BK and Wei ZL: YAP induces cisplatin resistance through
activation of autophagy in human ovarian carcinoma cells. Onco
Targets Ther. 9:1105–1114. 2016.PubMed/NCBI
|
|
14
|
Xu W, Wei Y, Wu S, Wang Y, Wang Z, Sun Y,
Cheng SY and Wu J: Up-regulation of the Hippo pathway effector TAZ
renders lung adenocarcinoma cells harboring EGFR-T790M mutation
resistant to gefitinib. Cell Biosci. 5:72015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amable L: Cisplatin resistance and
opportunities for precision medicine. Pharmacol Res. 106:27–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu JL, Wang ZW, Hu LM, Yin ZQ, Huang MD,
Hu ZB, Shen HB and Shu YQ: Genetic variants in the
PI3K/PTEN/AKT/mTOR pathway predict platinum-based chemotherapy
response of advanced non-small cell lung cancers in a Chinese
population. Asian Pac J Cancer Prev. 13:2157–2162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Noguchi S, Saito A, Horie M, Mikami Y,
Suzuki HI, Morishita Y, Ohshima M, Abiko Y, Mattsson JS, König H,
et al: An integrative analysis of the tumorigenic role of TAZ in
human non-small cell lung cancer. Clin Cancer Res. 20:4660–4672.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moroishi T, Hansen CG and Guan KL: The
emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 15:73–79.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo L and Teng L: YAP/TAZ for cancer
therapy: Opportunities and challenges (Review). Int J Oncol.
46:1444–1452. 2015.PubMed/NCBI
|
|
20
|
Bartucci M, Dattilo R, Moriconi C,
Pagliuca A, Mottolese M, Federici G, Benedetto AD, Todaro M, Stassi
G, Sperati F, et al: TAZ is required for metastatic activity and
chemoresistance of breast cancer stem cells. Oncogene. 34:681–690.
2015. View Article : Google Scholar : PubMed/NCBI
|