Introduction
Fibroblast growth factors (FGFs) are a large family
of growth factors that signal through FGF receptors (FGFRs) to
regulate fundamental developmental pathways, controlling events
such as cell proliferation, differentiation, cell survival,
angiogenesis, and wound healing (1–3).
Aberrant FGF signaling can promote tumor development by increasing
tumor cell proliferation, invasion, survival and angiogenesis
(3). Fibroblast growth factor 8
(FGF8) was first identified in the Shionogi mouse mammary carcinoma
cell line SC-3 and its expression was induced by androgen (4,5). FGF8
is rarely detected in normal adult tissues, but upregulated in
several human cancers, including HCC (HCC) (6). It was reported that autocrine
overexpression of FGF8 in phosphatase and tensin homolog (PTEN)
deficiency of prostate epithelium could lead to prostate
adenocarcinoma (7). Overexpression
of FGF8 in prostate cancer is associated with decreased patient
survival and persists in androgen-independent diseases (8).
The Hippo signaling pathway was initially defined as
a regulator of proliferation, organ size, and cell differentiation
(9,10). Yes-associated protein 1 (YAP1) is a
transcriptional coactivator in the Hippo signaling pathway
(11,12). Increased YAP1 expression or activity
can promote the growth of tumor cells (13). It was reported that β-catenin-driven
cancers require a YAP1 transcriptional complex for survival and
tumorigenesis (14). Enforced YAP1
expression can enable tumor maintenance upon KrasG12D
extinction in a human pancreatic ductal adenocarcinoma model
(15). Moreover, YAP1 amplification
has been described as an essential oncogene in various types of
human cancers, including human HCC (16).
Epidermal growth factor receptor (EGFR) signaling
plays an important role in various cancers, including HCC (17). Overexpression of EGFR was also found
in HCC, and hence EGFR represents a valuable therapeutic target for
the treatment of HCC (18,19). EGFR antagonists were effective in
human HCC cells (18). Gefitinib
and erlotinib, two clinically approved EGFR chemical inhibitors,
are used for the treatment of advanced HCC (20–25).
However, some studies have demonstrated the resistance to EGFR
inhibitors in human HCC cells (26–29).
The aim of the present study was to determine the
effect of FGF8 in human HCC cells. Our data revealed that FGF8
promoted cell growth and the resistance to EGFR inhibition via
upregulation of EGFR in human HCC cells.
Materials and methods
Cell lines and culture
HepG2 and HCC-LM3 cell lines were purchased from the
American Type Culture Collection (ATCC; Dallas, TX, USA). These
cells were maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), penicillin (100
U/ml) and streptomycin (100 µg/ml) in a humidified atmosphere of 5%
CO2 at 37°C.
Reagents
5-Fluorouracil (5-FU), doxorubicin, paclitaxel,
oxaliplatin, erlotinib, gefitinib and RAD001 (Everolimus) were
purchased from Selleck Chemicals LLC (Houston, TX, USA). Human
recombinant FGF8 was purchased from PeproTech (Rocky Hill, NJ,
USA).
Cell viability assay
Cells were seeded into white walled clear-bottom
96-well plates and grown for 24 h before treatment with various
concentrations of drugs. Cell viability was assessed after 24-h
treatment by measuring the cellular ATP levels using Luminescent
CellTiter-Glo assay (Promega, Madison, WI, USA). All drug
treatments were performed at least in triplicate and the data was
normalized to the vehicle-treated control and presented as the mean
± SEM.
Lentivirus transduction
The lentivirus of the YAP1 shRNAs (PLKO.1 vector,
target sequences: sh1-CAGGTGATACTATC AACCAAA;
sh2-CAGGTGATACTATCAACCAAA) and FGF8 overexpressing (pCDH vector) or
their vector control lentivirus were obtained from Genesent
(Shanghai, China). Viral infection was performed in 6-well plates
using 2×106 HepG2 or HCC-LM3 cells in a total volume of
2 ml of lentiviral supernatant containing 8 µg/ml of Polybrene
(Sigma-Aldrich, St. Louis, MO, USA). Two days after infection, 1
µg/ml of puromycin (Sigma-Aldrich) was added to the media and
selected for one week. The expression levels of the target genes
were determined by western blot analysis.
Real-time PCR
Gene expression levels were determined with
real-time RT-PCR. Total RNA was isolated using TRIzol (Invitrogen,
Carlsbad, CA, USA) and procedures were carried out as recommended
by the manufacturer. Then, extracted RNA was subjected to reverse
transcription using the PrimeScript II First Strand cDNA Synthesis
kit (Takara, Dalian, China) according to the manufacturer's
instructions. The primers for human VEGFR were: forward,
5′-AGGCACGAGTAACAAGCTCAC-3′ and reverse,
5′-ATGAGGACATAACCAGCCACC-3′. Real-time PCR was performed with a
7500 Fast Real-Time PCR system (Applied Biosystems, Foster City,
CA, USA) using SYBR-Green Gene expression analysis (Tiangen,
Shanghai, China). EGFR expression was normalized to GAPDH. Ct
values of the target genes were subtracted by that of the internal
control GAPDH. The relative expression levels of EGFR were assessed
by the 2−ΔΔCt method.
Colony formation assays
HepG2 or HCC-LM3 human HCC cell lines were seeded
into 6-well plates with 500 cells/well in triplicate and cultured
for 24 h. Then the cells were treated with exogenous recombinant
FGF8 or erlotinib and cultured for 14 days. The colonies were fixed
with 10% formaldehyde, stained with crystal violet (0.4 g/l; Sigma,
St. Louis, MO, USA). The number of colonies was counted using grid.
Three independent experiments were performed and the data are
presented as the mean ± SD.
Tumor sphere formation assay
Single shNC or sh1-YAP1 of HepG2 cells were plated
at a density of 1,000 cells/ml onto a 6-well ultralow attachment
plate (500 cells/well; Corning, Lowell, MA, USA). Cells were grown
in serum-free DMEM/F12 medium supplemented with 4 µg/ml of insulin
(Sigma-Aldrich), B27, 20 ng/ml of EGF, and 20 ng/ml of basic FGF
(Invitrogen, Carlsbad, CA, USA). The cells were then incubated at
37°C in a humidified atmosphere containing 5% CO2 for 14
days.
Western blot analysis
Cells were trypsinized and washed 3 times with
phosphate-buffered saline (PBS) before being lysed on ice for 30
min with RIPA lysis buffer containing and complete
protease/phosphatase inhibitor cocktail (Roche Life Science,
Mannheim, Germany). The lysate was centrifuged at 13,000 rpm for 20
min at 4°C and the supernatant was collected. Protein concentration
was determined by Bradford protein assay. The proteins were
separated by SDS-PAGE gel electrophoresis and then transferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore Inc.,
Billerica, MA, USA). The membranes were blocked with 5% skimmed
milk in TBST for 1 h and then were incubated with primary
antibodies overnight at 4°C. Primary antibodies: rabbit polyclonal
to FGF8 (1:1,000, ab81384), rabbit monoclonal (EP38Y) to EGFR
(1:1,000, ab52894), rabbit monoclonal (EP1674Y) to YAP1 (1:1,000,
ab52771), mouse monoclonal to β-actin (1:3,000, ab8226) were all
obtained from Abcam (Cambridge, MA, USA). After being washed with
TBST 3 times, the membranes were probed with HRP-conjugated
secondary antibodies in TBST for 1 h at room temperature, and then
washed with PBST three times. The results were analyzed using a
chemiluminescence method (ECL; Millipore Inc.).
Statistical analysis
Data are presented as the means ± SD and analyzed
using Student's t-test. P-values of <0.05 were considered to
indicate a statistically significant result.
Results
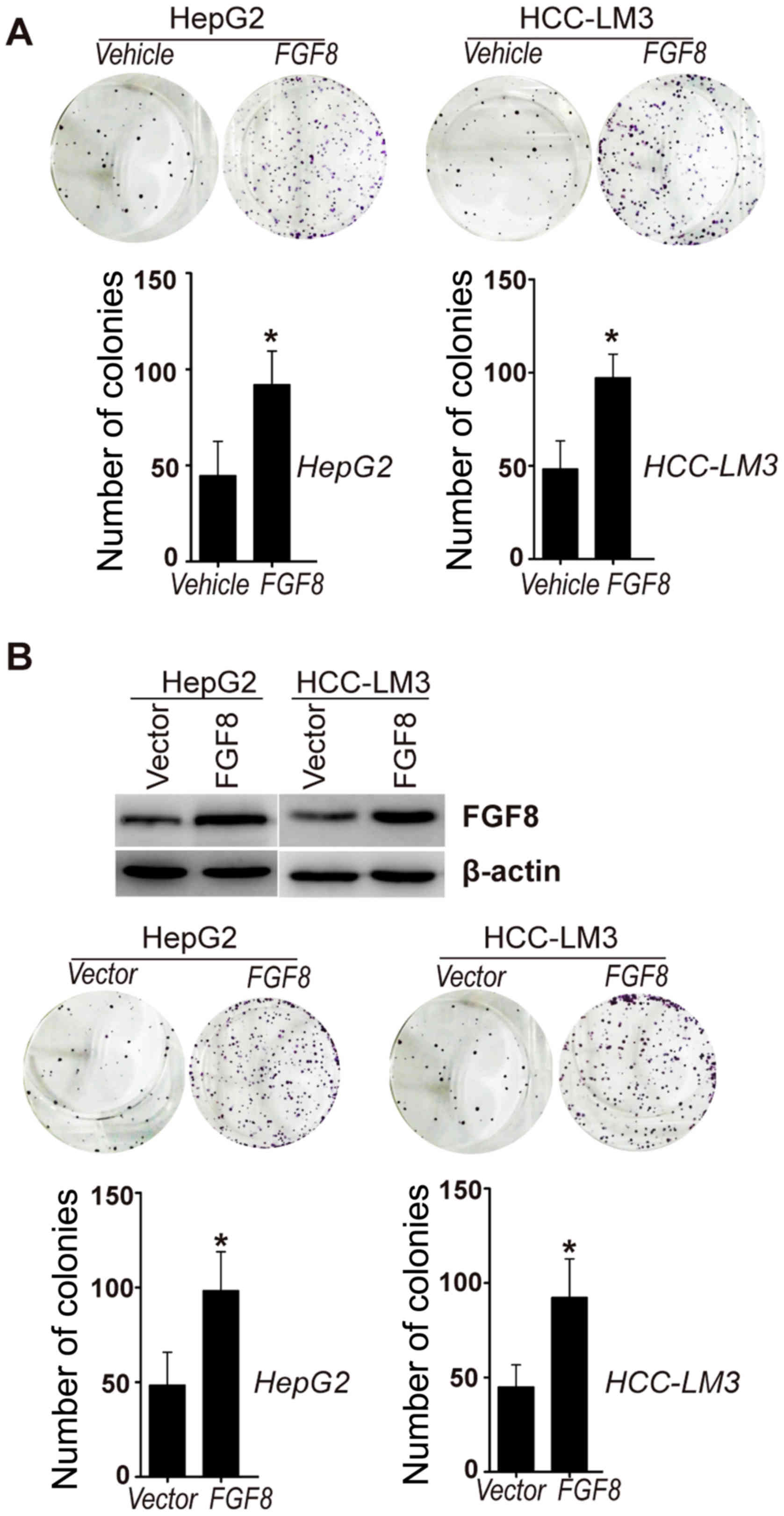
FGF8 enhances the proliferation of
human HCC cells
To determine the potential significance of FGF8 in
the proliferation of human HCC cells, exogenous recombinant human
FGF8 was added to HepG2 and HCC-LM3 human HCC cell lines. Colony
formation assays were used to examine the proliferation of human
HCC cells affected by exogenous human recombinant FGF8. As shown in
Fig. 1A, colony formation assays
revealed that exogenous recombinant FGF8 increased the
proliferation of HepG2 and HCC-LM3 cells in vitro. To extend
this analysis, we also generated stable HepG2 or HCC-LM3 cell lines
that stably expressed human FGF8 (Fig.
1B). We found that stable overexpression of FGF8 also increased
the proliferation of HepG2 or HCC-LM3 cells in vitro
(Fig. 1B).
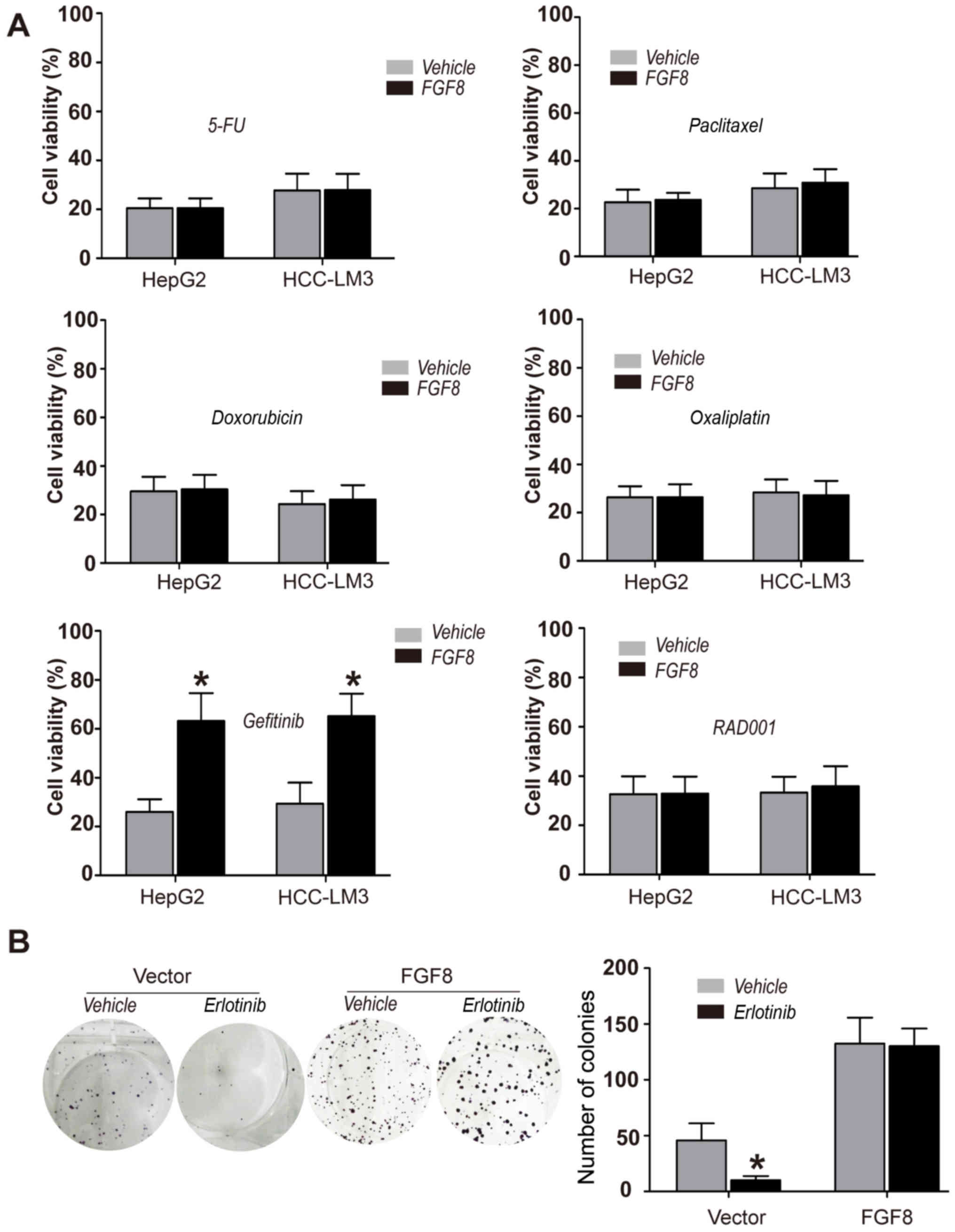
FGF8 promotes the resistance to EGFR
inhibitors of human HCC cells
To further determine the function of FGF8 in human
HCC cells, HepG2 and HCC-LM3 HCC cells were treated with various
anticancer chemotherapeutic drugs, such as 5-FU, doxorubicin,
paclitaxel, oxaliplatin, gefitinib (EGFR inhibitor) and RAD001
(Everolimus; mTOR inhibitor). CellTiter-Glo assays were used for
the determination of cell viability. We found that exogenous
recombinant FGF8 promoted the resistance to EGFR inhibitor
gefitinib in HepG2 or HCC-LM3 HCC cells, but not to several other
drugs (Fig. 2A). To confirm the
effect of FGF8 on the resistance to EGFR inhibitors in human HCC
cells, we performed colony formation assays to determine the effect
of FGF8 in HepG2 or HCC-LM3 cells with another EGFR inhibitor
erlotinib. As shown in Fig. 2B,
erlotinib could not inhibit the proliferation of HepG2 cells with
the treatment of exogenous recombinant FGF8.
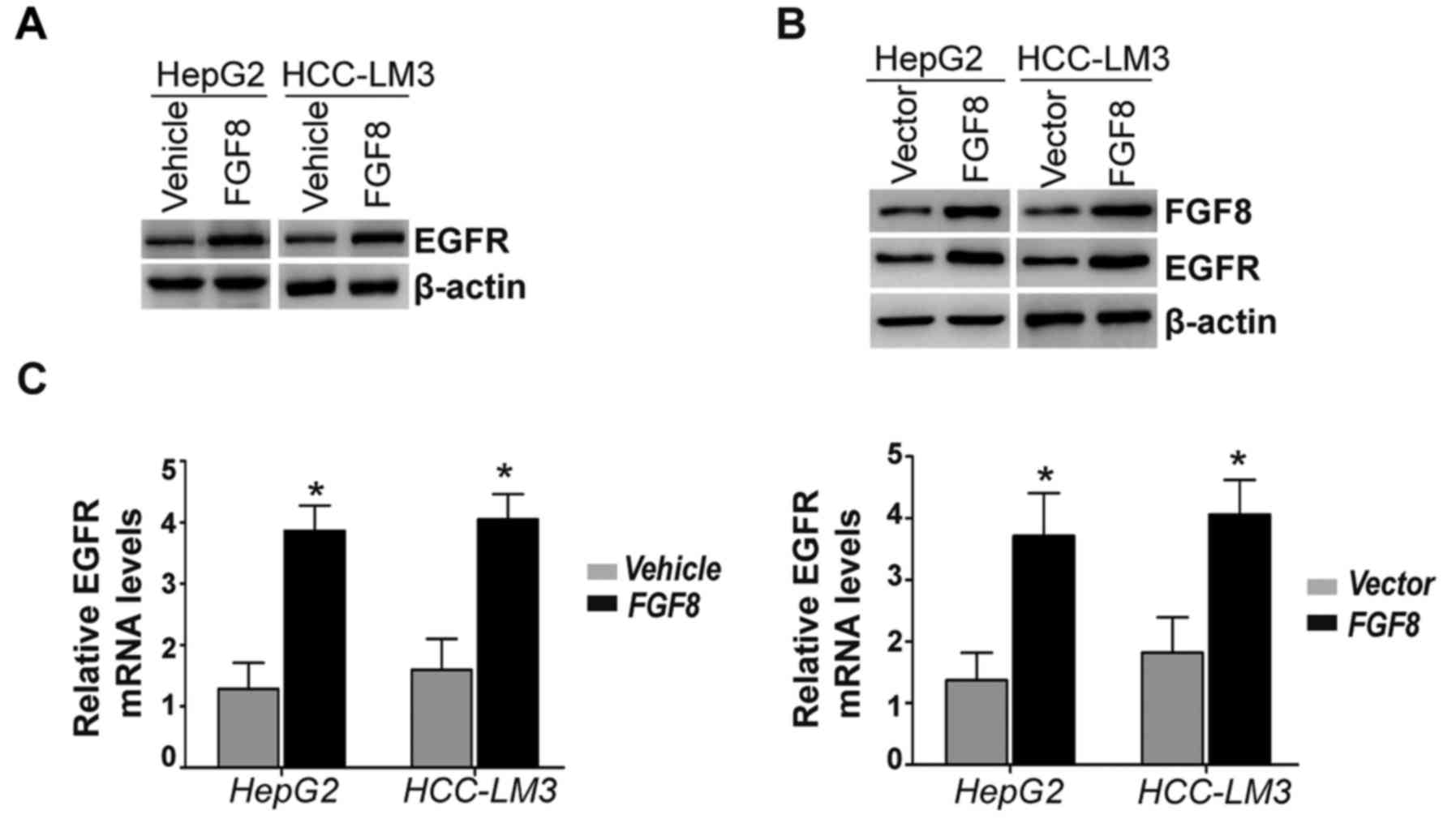
FGF8 increases the expression of EGFR
in human HCC cells
Gefitinib is a selective EGFR inhibitor and EGFR is
found to be overexpressed in human HCC cells. Thus, we detected the
expression levels of the EGFR protein with the treatment of
exogenous recombinant FGF8 in HepG2 and HCC-LM3 HCC cells. The
results revealed that the expression levels of the EGFR protein
were increased by the stimulation of exogenous recombinant FGF8 in
HepG2 and HCC-LM3 cells (Fig. 3A).
In addition, HepG2 and HCC-LM3 cells stably overexpressing FGF8
also exhibited significantly increased expression levels of the
EGFR protein compared with the vector control (Fig. 3B). Moreover, we determined the
expression levels of EGFR mRNA by real-time PCR. Real-time PCR
revealed significantly increased expression levels of EGFR mRNA in
exogenous recombinant FGF8-treated HepG2 or HCC-LM3 cells (Fig. 3C; left panel). Consistently, the
effect of stable overexpression of FGF8 was similar to the effect
of exogenous recombinant FGF8 in HepG2 and HCC-LM3 cells (Fig. 3C; right panel).
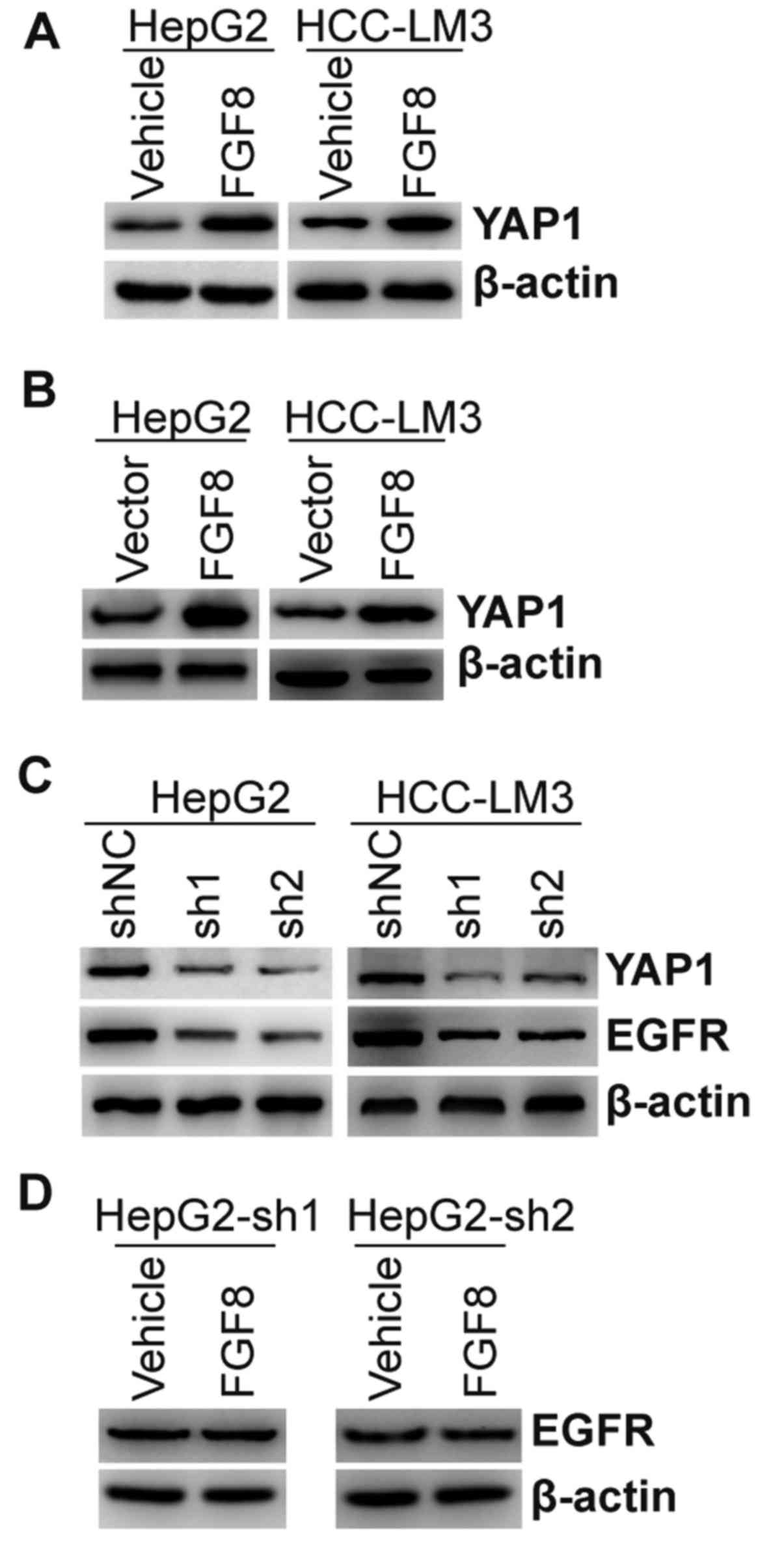
FGF8 enhances the expression of EGFR
via upregulation of YAP1
YAP1 is a transcriptional coactivator in the Hippo
signaling pathway. Increased YAP1 expression can promote the growth
of tumor cells. Moreover, YAP1 was demonstrated to mediate EGFR
overexpression and confer chemoresistance in esophageal cancer
cells (30). Thus, we detected the
expression of YAP1 in HepG2 and HCC-LM3 cells with the treatment of
exogenous recombinant FGF8. As shown in Fig. 4A, exogenous recombinant FGF8
upregulated the expression of the YAP1 protein. HepG2 and HCC-LM3
cells stably overexpressing FGF8 also exhibited upregulated
expression of the YAP1 protein compared with the vector control
(Fig. 4B). To further confirm the
relationship between YAP1 and EGFR, endogenous expression of YAP1
was stably knocked down by lentivirus shRNAs in HepG2 or HCC-LM3
cells (Fig. 4C). We found that the
expression of EGFR was inhibited in YAP1-knockdown cells (Fig. 4C). To determine whether FGF8-induced
upregulation of EGFR was dependent upon its regulation to YAP1,
HepG2 cells with stable knockdown of YAP1 were treated with
exogenous recombinant FGF8. As shown in Fig. 4D, FGF8 could not regulate the
expression of EGFR in HepG2 cells with stable knockdown of
YAP1.
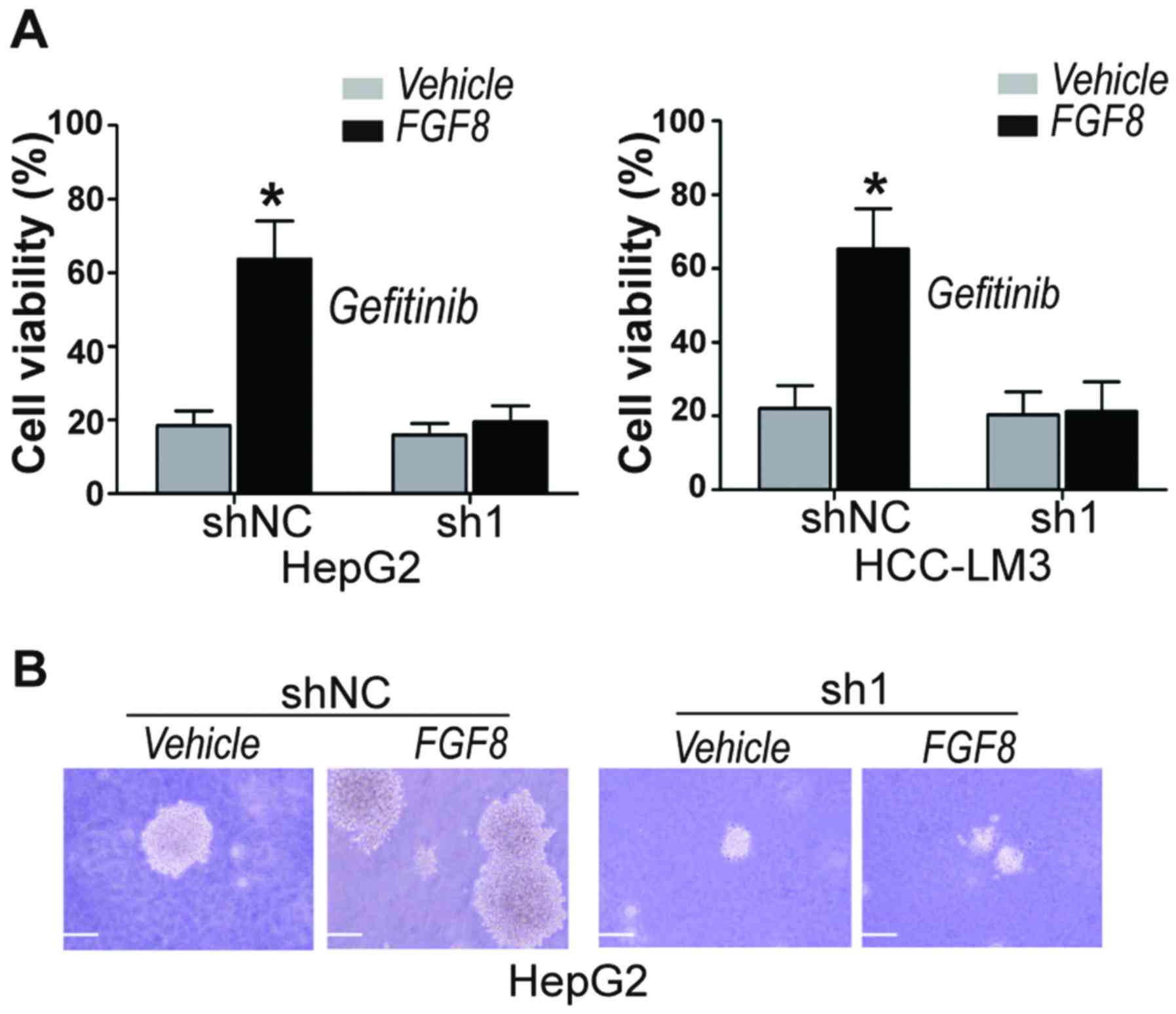
YAP1 is essential for FGF8 induced
gefitinib resistance and CSC properties
Upon determination that FGF8-induced upregulation of
EGFR was dependent upon its regulation to YAP1, we then determined
whether FGF8-induced gefitinib resistance in human HCC cells was
dependent upon YAP1. CellTiter-Glo assays were used for the
determination of cell viability after treatment with gefitinib. We
found that exogenous recombinant FGF8 significantly increased the
cell viability of gefitinib-treated vector control HepG2 and
HCC-LM3 cells, but not YAP1-knockdown cells (Fig. 5A). Cancer stem cells (CSCs) are
considered as the origin of cancer cells and one of the causes of
multidrug resistance (31–34). The effects of EGFR on the
maintenance of CSC properties has been demonstrated by other
studies (35). Here, we
investigated the effect of FGF8 on the CSC properties of HCC cells
by tumor sphere formation assay. As shown in Fig. 5B, exogenous recombinant FGF8
significantly increased the tumor sphere formation of vector
control HepG2 cells, but not YAP1-knockdown cells.
Discussion
HCC is one of the most commonly observed
malignancies in the world, especially in developing countries
(36,37). Systemic treatments with chemotherapy
(single agents or combination) have been used to treat advanced
HCC, but these show no clear impact on overall survival (38,39).
Notably, HCCs are recognized as inherently chemotherapy-resistant
(40). Additionally, CSCs are
considered as the origin of cancer cells and one of the causes of
multidrug resistance (39). Thus,
some factors that determine CSC properties may play important roles
in drug resistance to cancer chemotherapy.
FGF8 is a member of a large family of growth factors
that signal through FGF tyrosine kinase receptors to regulate
embryonic development, cell proliferation, cell differentiation and
cell migration (5,6,8). FGF8
is rarely detected in normal adult tissues, but is found
overexpressed in several human tumors, including HCC. In the
present study we first determined whether FGF8 could affect the
proliferation of human HCC cells. We found that stably
overexpressing or exogenous recombinant FGF8 increased the
proliferation of human HCC cells. 5-FU, doxorubicin, paclitaxel,
oxaliplatin, gefitinib and RAD001 have been used in the treatment
of cancer as anticancer chemotherapeutic drugs. We determined that
exogenous recombinant FGF8 promoted the resistance to EGFR
inhibitor gefitinib in HCC cells, but not to several other drugs.
Additionally, the expression of EGFR was also found increased by
exogenous recombinant or stably overexpressing FGF8.
YAP1 is a transcriptional coactivator in the Hippo
signaling pathway (15). Increased
YAP1 expression or activity can promote the growth of tumor cells,
including human HCC (14,16). In a previous study, FGF8 was found
to promote colorectal cancer growth and metastasis by activating
YAP1 signaling by increasing the transcription of YAP1 (6). Accordingly, expression of YAP1 was
found to be increased in HCC cells by the stable overexpression or
the exogenous addition of recombinant FGF8.
These findings imply that FGF8 enhances the
resistance to EGFR inhibitors and the expression of EGFR via
upregulation of YAP1. In the present study, stable knockdown of
YAP1 by lentivirus shRNA inhibited FGF8-induced upregulation of
EGFR and increased resistance to EGFR inhibitor gefinitib in HCC
cells. In addition, we also found that FGF8 promoted the tumor
sphere formation of HepG2 cells. Conversely, FGF8 could not
increase the tumor sphere formation in YAP1-knockdown cells.
In conclusion, we demonstrated that FGF8 promoted
the proliferation of human HCC cells via upregulation of the
YAP1/EGFR axis, thereby contributing to the resistance to EGFR
inhibitors and CSC properties. Our results suggest an important
role of FGF8 in the resistance to EGFR inhibition in human HCC
cells.
Acknowledgements
This study was funded by the Shanxi Health
Department Research Project 201201003, and the Cooperation Project
of Shandong University-Susceptible Gene of Breast Cancer of
Chinese.
References
|
1
|
Sandhu DS, Baichoo E and Roberts LR:
Fibroblast growth factor signaling in liver carcinogenesis.
Hepatology. 59:1166–1173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yun YR, Won JE, Jeon E, Lee S, Kang W, Jo
H, Jang JH, Shin US and Kim HW: Fibroblast growth factors: Biology,
function, and application for tissue regeneration. J Tissue Eng.
2010:2181422010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Turner N and Grose R: Fibroblast growth
factor signalling: From development to cancer. Nat Rev Cancer.
10:116–129. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Linscott ML and Chung WC: Fibroblast
growth factor 8 expression in GT1-7 GnRH-secreting neurons is
androgen-independent, but can be upregulated by the inhibition of
DNA methyltransferases. Front Cell Dev Biol. 4:342016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen N, Ma J, Zhao Y, Wu M, Yang H, Gong
W, Chao J and Li X: Expression of functional recombinant human
fibroblast growth factor 8b and its protective effects on
MPP+-lesioned PC12 cells. Appl Microbiol Biotechnol.
100:625–635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu R, Huang S, Lei Y, Zhang T, Wang K,
Liu B, Nice EC, Xiang R, Xie K, Li J, et al: FGF8 promotes
colorectal cancer growth and metastasis by activating YAP1.
Oncotarget. 6:935–952. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong C, Saribekyan G, Liao CP, Cohen MB
and Roy-Burman P: Cooperation between FGF8b overexpression and PTEN
deficiency in prostate tumorigenesis. Cancer Res. 66:2188–2194.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dorkin TJ, Robinson MC, Marsh C, Bjartell
A, Neal DE and Leung HY: FGF8 over-expression in prostate cancer is
associated with decreased patient survival and persists in androgen
independent disease. Oncogene. 18:2755–2761. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mo JS, Park HW and Guan KL: The Hippo
signaling pathway in stem cell biology and cancer. EMBO Rep.
15:642–656. 2014.PubMed/NCBI
|
|
10
|
Zhao B, Li L, Lei Q and Guan KL: The
Hippo-YAP pathway in organ size control and tumorigenesis: An
updated version. Genes Dev. 24:862–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Pasolli HA and Fuchs E:
Yes-associated protein (YAP) transcriptional coactivator functions
in balancing growth and differentiation in skin. Proc Natl Acad Sci
USA. 108:2270–2275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nguyen Q, Anders RA, Alpini G and Bai H:
Yes-associated protein in the liver: Regulation of hepatic
development, repair, cell fate determination and tumorigenesis. Dig
Liver Dis. 47:826–835. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kowalik MA, Saliba C, Pibiri M, Perra A,
Ledda-Columbano GM, Sarotto I, Ghiso E, Giordano S and Columbano A:
Yes-associated protein regulation of adaptive liver enlargement and
HCC development in mice. Hepatology. 53:2086–2096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosenbluh J, Nijhawan D, Cox AG, Li X,
Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, et
al: β-catenin-driven cancers require a YAP1 transcriptional complex
for survival and tumorigenesis. Cell. 151:1457–1473. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kapoor A, Yao W, Ying H, Hua S, Liewen A,
Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, et al: Yap1 activation
enables bypass of oncogenic Kras addiction in pancreatic cancer.
Cell. 158:185–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lorenzetto E, Brenca M, Boeri M, Verri C,
Piccinin E, Gasparini P, Facchinetti F, Rossi S, Salvatore G,
Massimino M, et al: YAP1 acts as oncogenic target of 11q22
amplification in multiple cancer subtypes. Oncotarget. 5:2608–2621.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Panvichian R, Tantiwetrueangdet A,
Sornmayura P and Leelaudomlipi S: Missense mutations in exons 18–24
of EGFR in HCC tissues. Biomed Res Int. 2015:1718452015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lanaya H, Natarajan A, Komposch K, Li L,
Amberg N, Chen L, Wculek SK, Hammer M, Zenz R, Peck-Radosavljevic
M, et al: EGFR has a tumour-promoting role in liver macrophages
during HCC formation. Nat Cell Biol. 16:972–981. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Badawy AA, El-Hindawi A, Hammam O, Moussa
M, Gabal S and Said N: Impact of epidermal growth factor receptor
and transforming growth factor-α on hepatitis C virus-induced
hepatocarcinogenesis. APMIS. 123:823–831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thomas MB, Morris JS, Chadha R, Iwasaki M,
Kaur H, Lin E, Kaseb A, Glover K, Davila M and Abbruzzese J: Phase
II trial of the combination of bevacizumab and erlotinib in
patients who have advanced HCC. J Clin Oncol. 27:843–850. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yau T, Wong H, Chan P, Yao TJ, Pang R,
Cheung TT, Fan ST and Poon RT: Phase II study of bevacizumab and
erlotinib in the treatment of advanced HCC patients with
sorafenib-refractory disease. Invest New Drugs. 30:2384–2390. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Zong Y, Xu GZ and Xing K:
Erlotinib for advanced HCC. A systematic review of phase II/III
clinical trials. Saudi Med J. 37:1184–1190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Llovet JM and Bruix J: Testing molecular
therapies in HCC: The need for randomized phase II trials. J Clin
Oncol. 27:833–835. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gu HR, Park SC, Choi SJ, Lee JC, Kim YC,
Han CJ, Kim J, Yang KY, Kim YJ, Noh GY, et al: Combined treatment
with silibinin and either sorafenib or gefitinib enhances their
growth-inhibiting effects in HCC cells. Clin Mol Hepatol. 21:49–59.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei Z, Doria C and Liu Y: Targeted
therapies in the treatment of advanced HCC. Clin Med Insights
Oncol. 7:87–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ezzoukhry Z, Louandre C, Trécherel E,
Godin C, Chauffert B, Dupont S, Diouf M, Barbare JC, Mazière JC and
Galmiche A: EGFR activation is a potential determinant of primary
resistance of HCC cells to sorafenib. Int J Cancer. 131:2961–2969.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu HC, Chen HJ, Chang YL, Liu CY, Shiau
CW, Cheng AL and Chen KF: Inhibition of CIP2A determines
erlotinib-induced apoptosis in HCC. Biochem Pharmacol. 85:356–366.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huether A, Höpfner M, Sutter AP, Schuppan
D and Scherübl H: Erlotinib induces cell cycle arrest and apoptosis
in hepatocellular cancer cells and enhances chemosensitivity
towards cytostatics. J Hepatol. 43:661–669. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao J, Kelnar K and Bader AG: In-depth
analysis shows synergy between erlotinib and miR-34a. PLoS One.
9:e891052014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song S, Honjo S, Jin J, Chang SS, Scott
AW, Chen Q, Kalhor N, Correa AM, Hofstetter WL, Albarracin CT, et
al: The Hippo coactivator YAP1 mediates EGFR overexpression and
confers chemoresistance in esophageal cancer. Clin Cancer Res.
21:2580–2590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Feng D, Liu D, Wang S, Yu X, Dai E,
Wang J, Wang L and Jiang W: Dissecting the origin of breast cancer
subtype stem cell and the potential mechanism of malignant
transformation. PLoS One. 11:e01650012016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song K, Wu J and Jiang C: Dysregulation of
signaling pathways and putative biomarkers in liver cancer stem
cells (Review). Oncol Rep. 29:3–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Di C and Zhao Y: Multiple drug resistance
due to resistance to stem cells and stem cell treatment progress in
cancer (Review). Exp Ther Med. 9:289–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma L, Zhang G, Miao XB, Deng XB, Wu Y, Liu
Y, Jin ZR, Li XQ, Liu QZ, Sun DX, et al: Cancer stem-like cell
properties are regulated by EGFR/AKT/β-catenin signaling and
preferentially inhibited by gefitinib in nasopharyngeal carcinoma.
FEBS J. 280:2027–2041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu RX, Seto WK, Lai CL and Yuen MF:
Epidemiology of HCC in the Asia-Pacific Region. Gut Liver.
10:332–339. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gong XL and Qin SK: Progress in systemic
therapy of advanced HCC. World J Gastroenterol. 22:6582–6594. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Deng GL, Zeng S and Shen H: Chemotherapy
and target therapy for HCC: New advances and challenges. World J
Hepatol. 7:787–798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brown KS: Chemotherapy and other systemic
therapies for HCC and liver metastases. Semin Intervent Radiol.
23:99–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pardee AD and Butterfield LH:
Immunotherapy of HCC: Unique challenges and clinical opportunities.
Oncoimmunology. 1:48–55. 2012. View Article : Google Scholar : PubMed/NCBI
|