Introduction
Laryngeal squamous cell carcinoma (LSCC) is the
second most common malignant cancer type of the head and neck
(1,2). Approximately 650,000 cases are newly
diagnosed and more than 350,000 deaths are attributed to this
disease every year in the US (1,3). Due
to high exposure to tobacco and alcohol risk factors, middle-aged
and elderly men often suffer from this disease, and its incidence
has been rising in recent years (4). Therapeutic measures for LSCC include
surgery, chemotherapy or radiotherapy and comprehensive surgery,
radiotherapy and chemotherapy (5–8). The
clinical prognosis of this disease, however, is poor, and LSCC
continues to be one of the major causes of cancer-related deaths
(9). An epidemiological survey
(2) indicated that the clinical
outcome of LSCC has not obviously improved over the past 20 years,
despite considerable improvements in technologies related to LSCC
detection and diagnosis. Therefore, it is necessary to develop
valuable biomarkers to identify patients with a poor prognosis and
the risk factors for recurrence, which can serve as a guide for
determining the surgical approach and the combination treatment
scheme.
Heat shock protein 47 (HSP47), also known as
colligin-2, is a type of endoplasmic reticulum resident collagen
protein and specific molecular chaperone, which is essential for
the synthesis and secretion of procollagens (10). HSP47 is a product of the CBP2
gene, located at chromosome 11q13.5 (11), which is a region frequently involved
in the biological behaviour of malignant tumours, including oral
tongue cancer, nasopharyngeal and esophageal squamous cell
carcinoma, lung diseases, pancreatic ductal adenocarcinoma,
cervical squamous cell and ulcerative colitis-associated carcinoma
(12–17). As a classical serpin, HSP47 has been
proven to exert inhibitory effects on tumour proliferation,
invasion and motility (18,19). However, the profile and role of
HSP47 expression in LSCC patients and the potential mechanisms
underlying the generation, development and metastasis of tumours
have not been fully explored.
In the present study, we examined the HSP47
expression and its prognostic significance in LSCC patients.
Moreover, the effects of HSP47 on cell viability, invasion,
chemotherapeutic sensitivity and apoptosis were evaluated in
vitro. We further explored the potential apoptotic mechanisms
of HSP47, which may be related to the regulation of PARP and
cleaved caspase-7/-8/-9. We conclude that HSP47 may act as an
important prognostic biomarker and an attractive therapeutic target
in LSCC.
Materials and methods
LSCC tissue collection
A total of 62 LSCC patients who received no
radiotherapy or chemotherapy and who underwent surgery between 2011
and 2015 at the First Affiliated Hospital of Wenzhou Medical
University were examined in this retrospective research. All
samples were from 61 males and 1 female with ages ranging from 43
to 79 years (mean, 64.5 years). The present study was approved by
the Clinical Medicine Ethics Committee of The First Affiliated
Hospital of Wenzhou Medical University, and consent for enrollment
in the study was provided by the patients. The study protocol was
carried out according to the principles of the Helsinki
Declaration. In total, 50 formalin-fixed, paraffin-embedded tumour
sections were obtained from the pathology department for
immunohistochemical analysis, and 7 samples of histologically
normal laryngeal tissues surrounding the tumours were treated as
control tissues. Another 12 pairs of fresh-frozen LSCC tissues
which were used for western blot analysis, including cancerous and
matched adjacent non-cancerous tissues from the same patients, were
maintained at −80°C. Patient follow-up was performed via telephone
calls until October 2016. The outcome event was defined according
to the date of death.
Cell culture and reagents
The human LSCC, Hep-2 cell line, was obtained from
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). In brief, cells were cultivated in a complete
medium containing RPMI-1640 medium, 10% fetal bovine serum (FBS)
(both from HyClone, Logan, UT, USA) and 1% antibiotics (100 U/ml
penicillin/streptomycin) in a humidified incubator at 37°C with 5%
CO2. Cisplatin was obtained from Qilu Pharmaceutical
Co., Ltd. (Shandong, China).
Western blot analysis
Briefly, total protein was extracted and the
concentration was measured with a bicinchoninic acid (BCA;
Beyotime, Jiangsu, China) protein assay kit. For each sample,
~30–50 µg of protein were run on a 10% polyacrylamide gel, and then
transferred to polyvinylidene fluoride (PVDF) membranes. The
membranes were immunoblotted with the primary antibodies, followed
by the secondary HRP-conjugated antibody. Immunoreactive proteins
were visualized using ECL western blot detection kit (Advansta,
Menlo Park, CA, USA) and quantified employing Image Lab software
(Bio-Rad, Hercules, CA, USA). Information concerning the antibodies
was as follows: rabbit anti-GAPDH polyclonal antibody (ab-p-r001;
1:1,000; Xianzhi, Zhejiang, China); rabbit anti-HSP47 monoclonal
antibody (ab109117; 1:3,000; Abcam, Cambridge, UK); rabbit
anti-Bcl-2 monoclonal antibody (#4223; 1:1,000); rabbit anti-Bax
monoclonal antibody (#5023; 1:1,000); rabbit anti-PARP monoclonal
antibody (#9532; 1:1,000); rabbit anti-cleaved caspase-7 monoclonal
antibody (#8438; 1:1,000); rabbit anti-cleaved caspase-8 monoclonal
antibody (#9496; 1:1,000); rabbit anti-cleaved caspase-9 monoclonal
antibody (#9505; 1:1,000) (all from Cell Signaling Technology,
Danvers, MA, USA).
Immunohistochemistry
Fifty formalin-fixed, paraffin-embedded tumour
sections and 7 control tissues were stained with HSP47-specific
antibody raised against the amino acid sequence of human HSP47
(ab109117; 1:300; Abcam). Briefly, the sections (5-µm) were
deparaffinized in dimethylbenzene, dehydrated in graded alcohols,
subjected to peroxidase blocking and then retrieved with citrate
buffer. The sections were immunoblotted with the anti-HSP47
antibody at a dilution of 1:300 overnight at 4°C, followed by
incubation with the secondary HRP-conjugated antibody. The sections
were then counterstained with haematoxylin and observed under a
biological imaging microscope (BX53; Olympus, Tokyo, Japan), which
also was used to obtain images. All of the immunohistochemically
stained sections were evaluated via a double-blind
semi-quantitative analysis to avoid artificial errors. Cytoplasmic
staining was regarded as positive. In total, 10 high-power fields
(magnification, ×200) within the stained tumour cytoplasm were
selected to analyse the expression of HSP47. In the present study,
to evaluate prognosis, we divided the extent of staining into 2
groups: a high-score group (staining extent ≥50%) and a low-score
group (staining extent <50%). These cut-off values have been
used in past studies (20,21).
Quantitative RT-PCR
According to the protocol of the manufacturer, total
RNA from the Hep-2 cells was extracted using Trizol (Invitrogen,
Carlsbad, CA, USA). The first-strand cDNAs were synthesized using a
reverse transcription kit (Takara, Dojindo, Japan). The synthesized
cDNA (2 µl) was amplified (final volume, 20 µl) using a SYBR-Green
PCR kit (Takara) and loaded on the ABI 7500 sequence detection
system (Applied Biosystems, Foster City, CA, USA). DNA
amplification conditions were as follows: 95°C for 30 sec and
subsequent 40 cycles, 95°C for 5 sec, 60°C for 34 sec. The primer
information was as follows: HSP47 (human forward)
5′-CACCGCCTTTGAGTTGGACAC-3′ and HSP47 (human reverse)
5′-GGCGCCCAATGAATAGCAG-3′; GAPDH (human forward)
5′-ATGAGCCCCAGCCTTCTCCAT-3′ and GAPDH (human reverse)
5′-GGTCGGAGTCAACGGATTTG-3′. The 2−ΔΔCt analysis was used
to calculate gene expression levels.
Gene overexpression and silencing
According to the reagent protocol, the cells were
transiently transfected with plasmid DNA or small interfering RNA
(siRNA) using Lipofectamine 3000 (Invitrogen). In regards to the
plasmid transfection, Lipofectamine 3000 was diluted in Opti-MEM
medium after the cells were seeded at 80–90% confluence. Meanwhile
plasmid DNA and P3000 reagent were diluted in Opti-MEM medium. Each
was incubated for 5 min, respectively, and then mixed together for
20 min. Finally, the DNA-lipid complex was added to the cells. Gene
transfection of siRNA was the same, except for the addition of
P3000 reagent when diluting the siRNA. Quantitative RT-PCR and
western blotting were used to compare transfection efficiency.
(pCDH)-HSP47, (pCDH)-control, siHSP47 and siControl were all
obtained from GenePharma (Shanghai, China). Information regarding
the siRNAs was as follows: siHSP47, 5′-GCAGCAAGCAGCACUACAATT-3′ and
siControl, 5′-TTCTCCGAACGTGTCACGTTT-3′.
Cell viability assay
Cell viability was assessed using Cell Counting
Kit-8 (CCK-8; Dojindo, Kumamoto, Japan). After transfection for 24
h, the cells were plated into 96-well plates at a density of
1×103 cells/well, and then subjected to cell
proliferation assay by CCK-8 at 24, 48, 72 and 96 h. For
chemosensitivity analysis, the cells were transfected with
(pCDH)-HSP47 or siHSP47 for 24 h, and then plated into 96-well
plates at a density of 4×103 cells/well. The cells were
subsequently treated with cisplatin (9 µM/l) for 24–96 h, and
subjected to cell proliferation assay by CCK-8 at 24, 48, 72 and 96
h. Each group contained 3 parallel wells. CCK-8 (10 µl) was added
to the cell culture medium (90 µl) according to the manufacturers
instructions. The cells were subsequently cultured for 2 h in an
incubator at 37°C and the absorbance was measured at 450 nm.
Transwell invasion assay
The cells on the lower surface of the membrane were
quantified to assess the invasive potential. As follows, Matrigel
matrix gel (BD Biosciences, San Jose, CA, USA) was diluted with
serum-free culture medium at 1:8, for coating of the 24-well
transwell chambers (Corning Costar, USA) which were placed in an
incubator at 37°C overnight. The upper chamber was filled with the
transfected cells (1×105 with 200 µl of serum-free
culture medium), and meanwhile the lower chamber was filled with
600 µl of complete medium that contained 10% FBS. Following 18 h of
cultivation, the cells in the upper chamber were wiped off with a
cotton swab gently, and the invading cells that adhered to the
lower membrane were fixed in paraformaldehyde for 15 min, followed
by crystal violet staining for 1 h.
Wound-healing assay
The relative distance of Hep-2 cells moving into a
wounded space was used to evaluate cell migration ability. Briefly,
cells (5×105) were plated into 6-well plates in
serum-free medium for 24 h. When achieving a monolayer, the
confluent monolayers were scratched using a 10 µl pipette tip and
the floating cells were removed by phosphate-buffered saline (PBS).
Finally, fresh culture medium was added to the wells. Phase
contrast microscopy was performed to measure the relative distance
of the wound.
Apoptosis assay and cell cycle
analysis
Cells (4×105) were seeded into 6-well
plates and transfected with the plasmid. After 48 h of culture, the
cells were trypsinized, washed twice with PBS, and then either
incubated with fluorescein isothiocyanate (FITC)-Annexin
V/propidium iodide (PI) or only stained with PI (both from
MultiSciences Biotech Co., Ltd., Zhejiang, China) in the dark
according to the reagent protocol. A flow cytometer (FCM;
Becton-Dickinson, San Jose, CA, USA) was used to calculated the
percentages of apoptotic and necrotic cells and the DNA
content.
Statistical analysis
All analyses were carried out using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). Results are presented as
mean values ± SD. The distinct expression of HSP47 protein between
tumour tissues and controls was examined using paired-sample
t-tests. The difference in overall survival (OS) between 2 groups
was detected by the long-rank test. The prognostic value of HSP47
protein expression in LSCC patients was analysed by Kaplan-Meier
survival curve. Tumour cell viability and invasion assays were
assessed using independent t-tests. P<0.05 was considered to
indicate a statistical significant result.
Results
Low expression of HSP47 protein is
associated with a poor LSCC patient prognosis
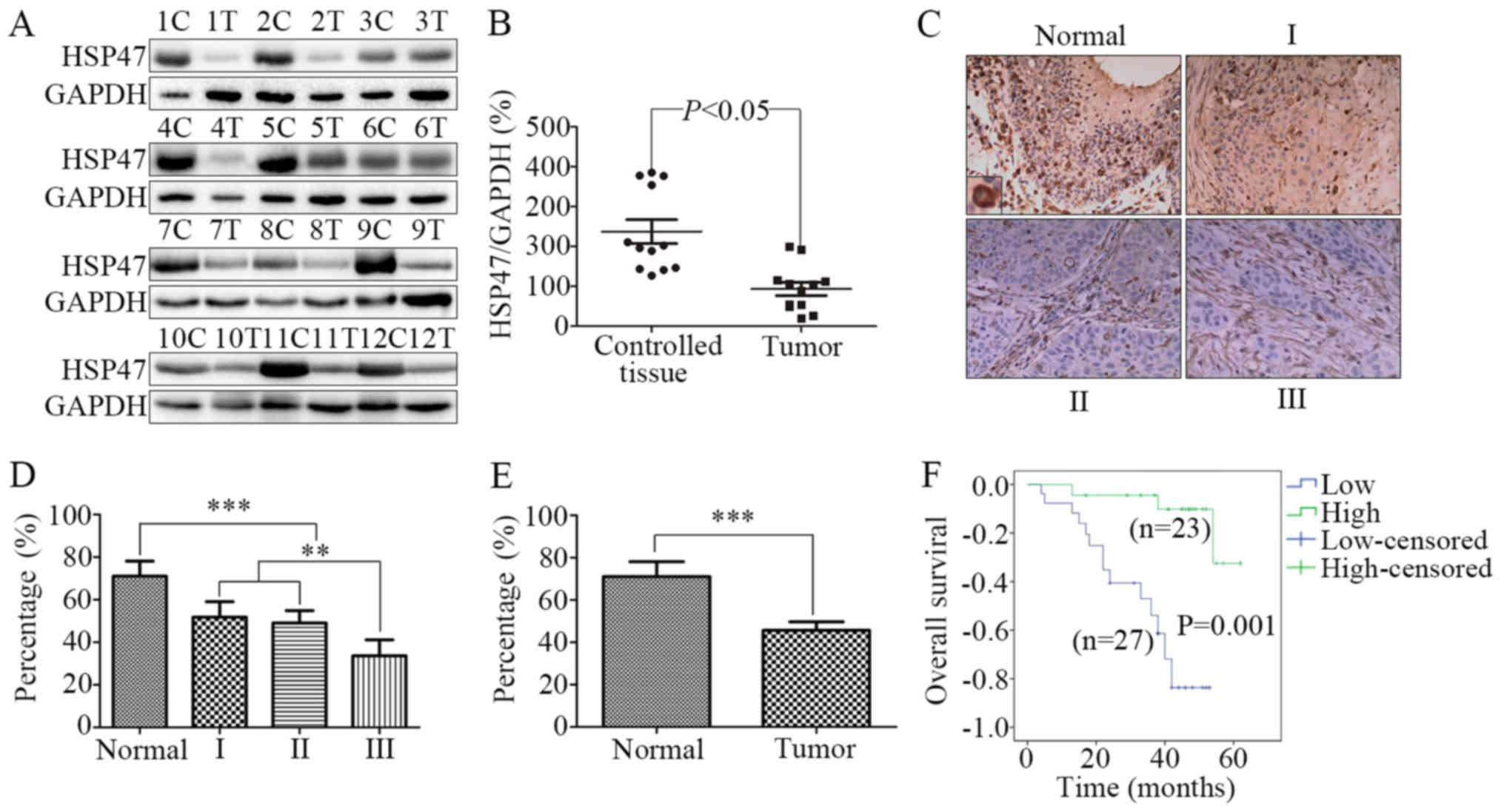
To explore the expression of HSP47 in LSCC, we
quantified the protein levels of HSP47 in matched cancerous and
adjacent non-cancerous tissues from 12 LSCC patients by immunoblot
analysis. As shown in Fig. 1A and
B, in contrast to the controls, the level of HSP47 was
significantly decreased in the tumour tissues (P<0.05).
Furthermore, immunohistochemistry was performed on 50
formalin-fixed, paraffin-embedded LSCC and 7 adjacent normal
laryngeal tissues. Based on the histopathologic grade, we
classified the samples into 4 groups: normal, high differentiation,
medium differentiation and low differentiation. As shown in
Fig. 1C, the expression of HSP47
protein in the normal control tissues was obviously higher than
that in the LSCC tissues with low to high differentiation, and the
HSP47 protein was mainly localized in the cytoplasm. Moreover, the
average immunohistochemical scores for HSP47 expression were
observed to decrease as the tumour pathological grade of LSCC
increased (Fig. 1D). Consistent
with the results of the western blotting, LSCC tissues showed lower
expression of HSP47 protein compared with that noted in the
adjacent normal laryngeal tissues (Fig.
1E; 71.0±7.1 vs. 45.7±4.0%, respectively; P<0.001).
We used a Kaplan-Meier survival curve to analyse the
prognostic value of HSP47 protein expression in LSCC patients. We
divided patients according to HSP47 protein expression levels into
a low-score group (32.8±2.4; 95% CI, 27.9–37.7) and a high-score
group (70.0±2.4; 95% CI, 65.0–85.0). As shown in Fig. 1F, the low-score group had a shorter
OS time compared with the high-score group. The median OS time in
the low-score group was 38±2.5 months, while in the high-score
group was 46±2.9 months (P=0.001).
Taken together, these results indicate that HSP47
may be an important prognostic factor for LSCC patients.
HSP47 overexpression inhibits the
growth of Hep-2 cells
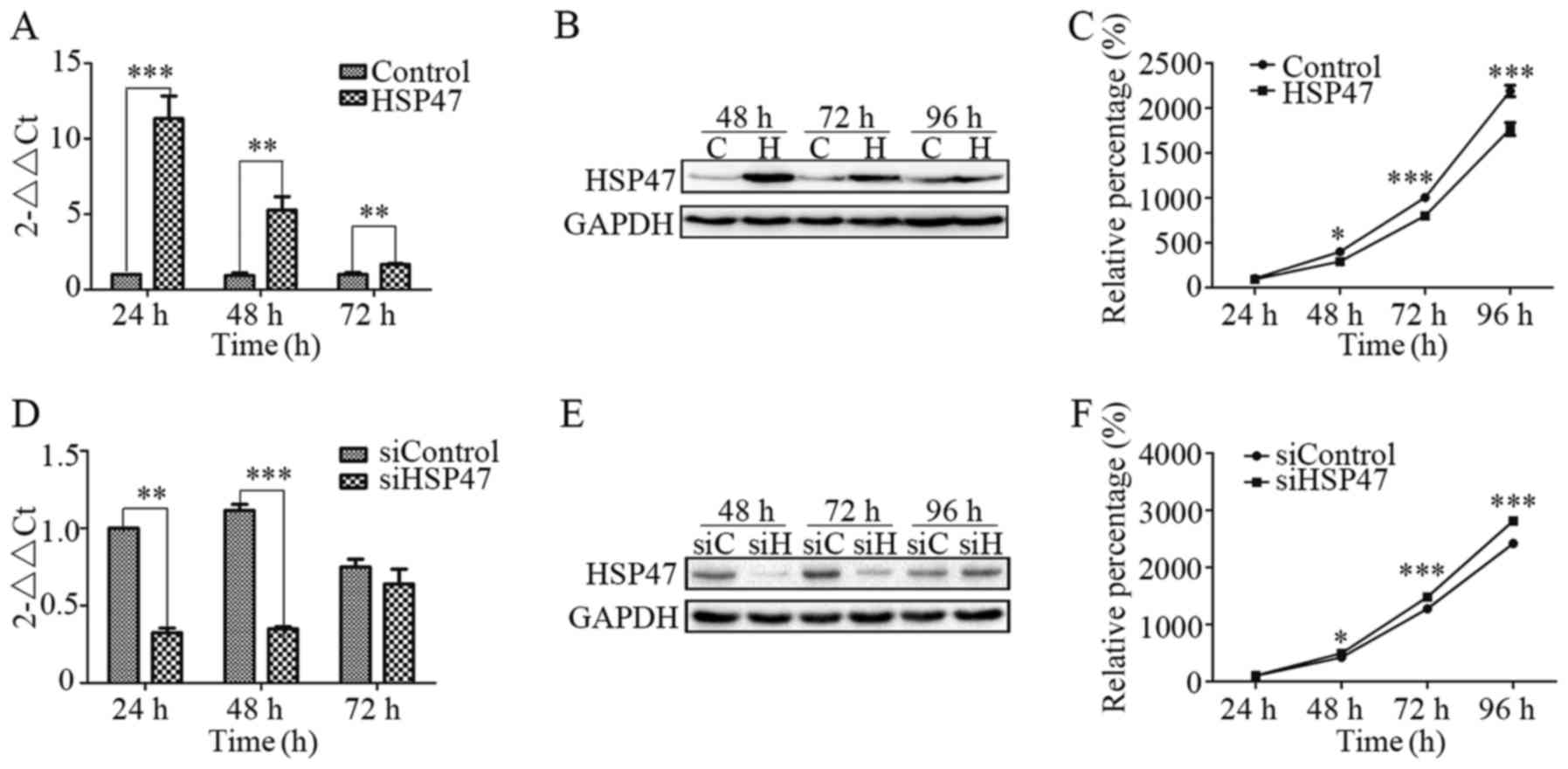
To analyze the effects of HSP47 on Hep-2 cells, cell
proliferation analysis was performed after transfection with either
a plasmid encoding HSP-47 [(pCDH)-HSP47)] or HSP47-specific siRNA
(siHSP47) to upregulate or knockdown HSP47 levels in Hep-2 cells.
As shown in Fig. 2A,
(pCDH)-HSP47-transfected cells showed a marked increase in HSP47
mRNA expression as confirmed by real-time RT-PCR, while
siHSP47-transfected cells exhibited a significant decrease
(Fig. 2D). After transfection for
24 or 48 h, HSP47 mRNA expression was significantly increased, by
10.3 and 4.6 times, respectively, in the (pCDH)-HSP47-transfected
cells compared with the (pCDH)-control cells. In contrast,
siHSP47-transfected cells respectively exhibited 68 and 69% lower
expression than that noted in the siControl cells. The level of
HSP47 protein expression was also detected by immunoblot analysis,
which revealed an increase in the Hep-2-HSP47 cells (Fig. 2B) and a decrease in the
Hep-2-siHSP47 cells (Fig. 2E).
Furthermore, we assessed the effect of HSP47
expression on cell viability using CCK-8 assay. Compared with the
control, Hep-2 cells exhibited a marked reduction (Fig. 2C) in cell viability when HSP47 was
upregulated, while there was a significant increase (Fig. 2F) when HSP47 was silenced. At 96 h
after transfection, the cells with upregulation of HSP47 were 19.3%
less viable when compared with the (pCDH)-control cells. Meanwhile,
HSP47-knockdown cells were 16.4% more viable than the siControl
cells. The present study indicated that upregulation of HSP47
inhibited the growth of Hep-2 cells, whereas HSP47 downregulation
stimulated proliferation.
HSP47 overexpression suppresses the
migration and invasion abilities of the Hep-2 cells
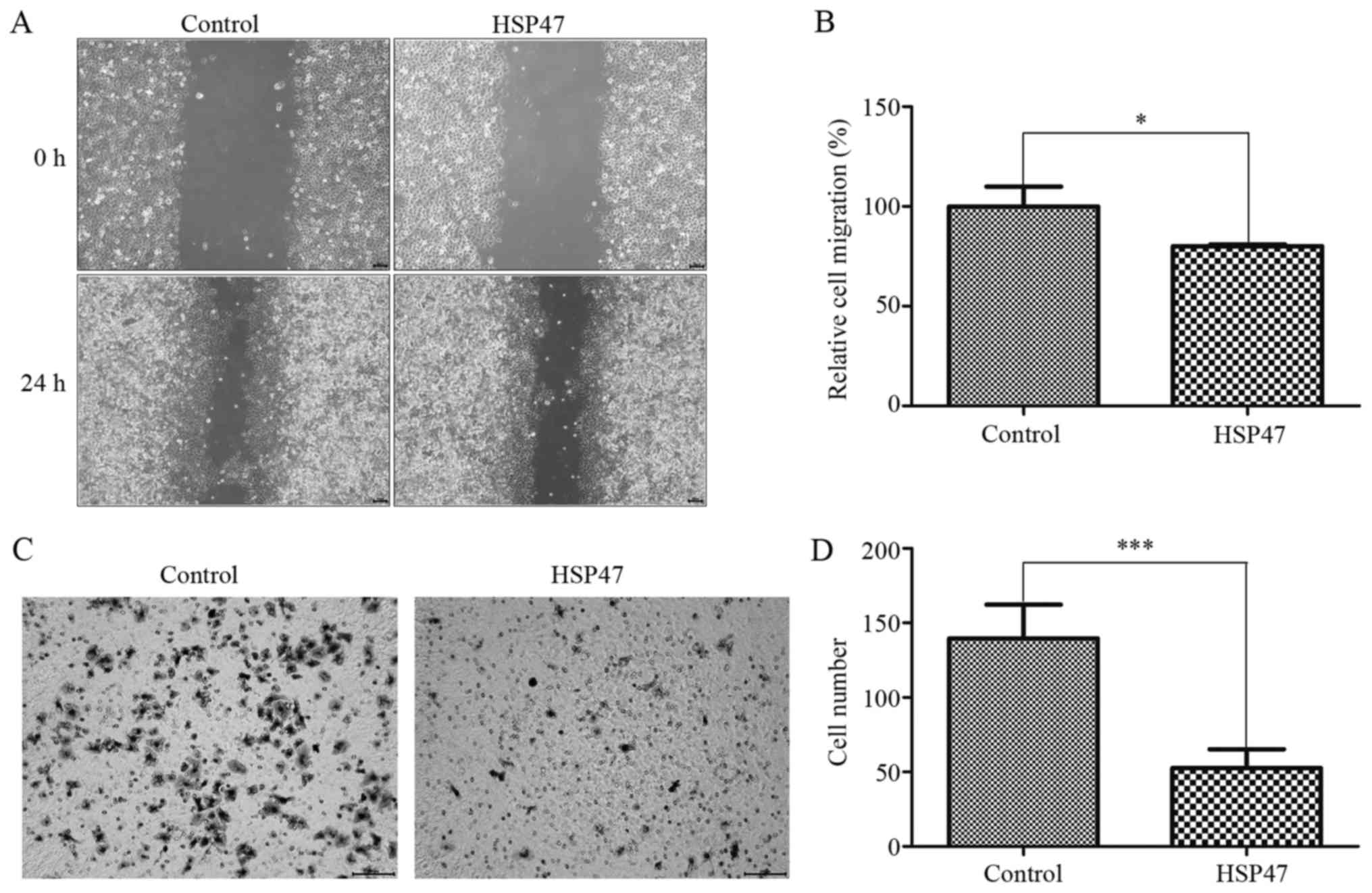
We also investigated whether HSP47 upregulation
inhibits cell migration and invasive abilities by wound-healing and
transwell invasion assays, respectively. HSP47 upregulation
markedly diminished wound-healing migration in the Hep-2 cells
(Fig. 3A), and the ability to
migrate was decreased by 20% (Fig.
3B; P<0.05). Moreover, upregulation of HSP47 resulted in
notably decreased migration (by 62.3%) into the lower chamber in
triplicate independent assays (Fig. 3C
and D; P<0.001). From these assays, we demonstrated that
HSP47 upregulation suppressed Hep-2 cell mobility.
HSP47 modulates chemosensitivity to
cisplatin in Hep-2 cells
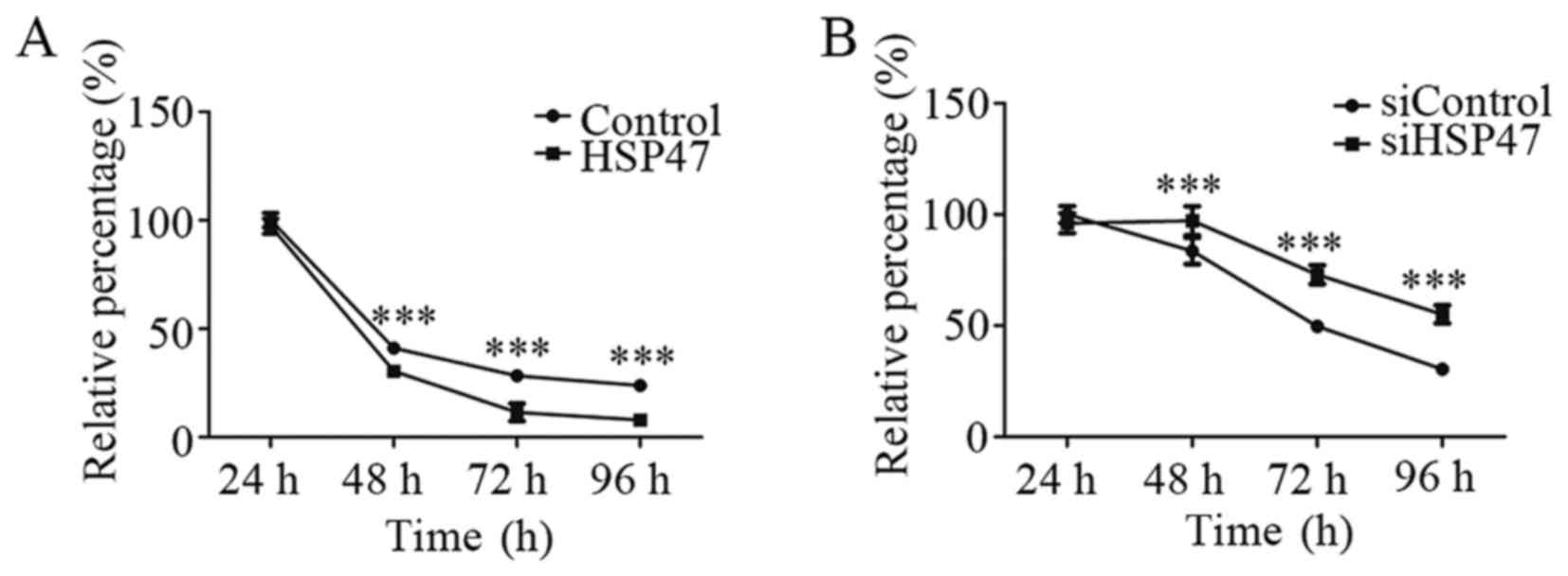
We next evaluated the potential effect on
sensitivity to chemotherapy by HSP47 in LSCC cells. As shown in
Fig. 4A, upregulation of HSP47
significantly increased the cell chemosensitivity to cisplatin, by
25.9%, at 48 h. In contrast, knockdown of HSP47 led to more
resistance to cisplatin chemotherapy, with a 16.2% increase
compared with the negative-control group at 48 h (Fig. 4B). These results revealed that HSP47
overexpression sensitized the Hep-2 cell line to cisplatin
chemotherapy, whereas HSP47 silencing protected cells against
cisplatin.
HSP47 upregulation enhances the
apoptosis of Hep-2 cells
To investigate the mechanisms underlying the growth
inhibition and chemosensitivity enhancement noted in the
HSP47-upregulated cells, we assessed cell cycle distribution and
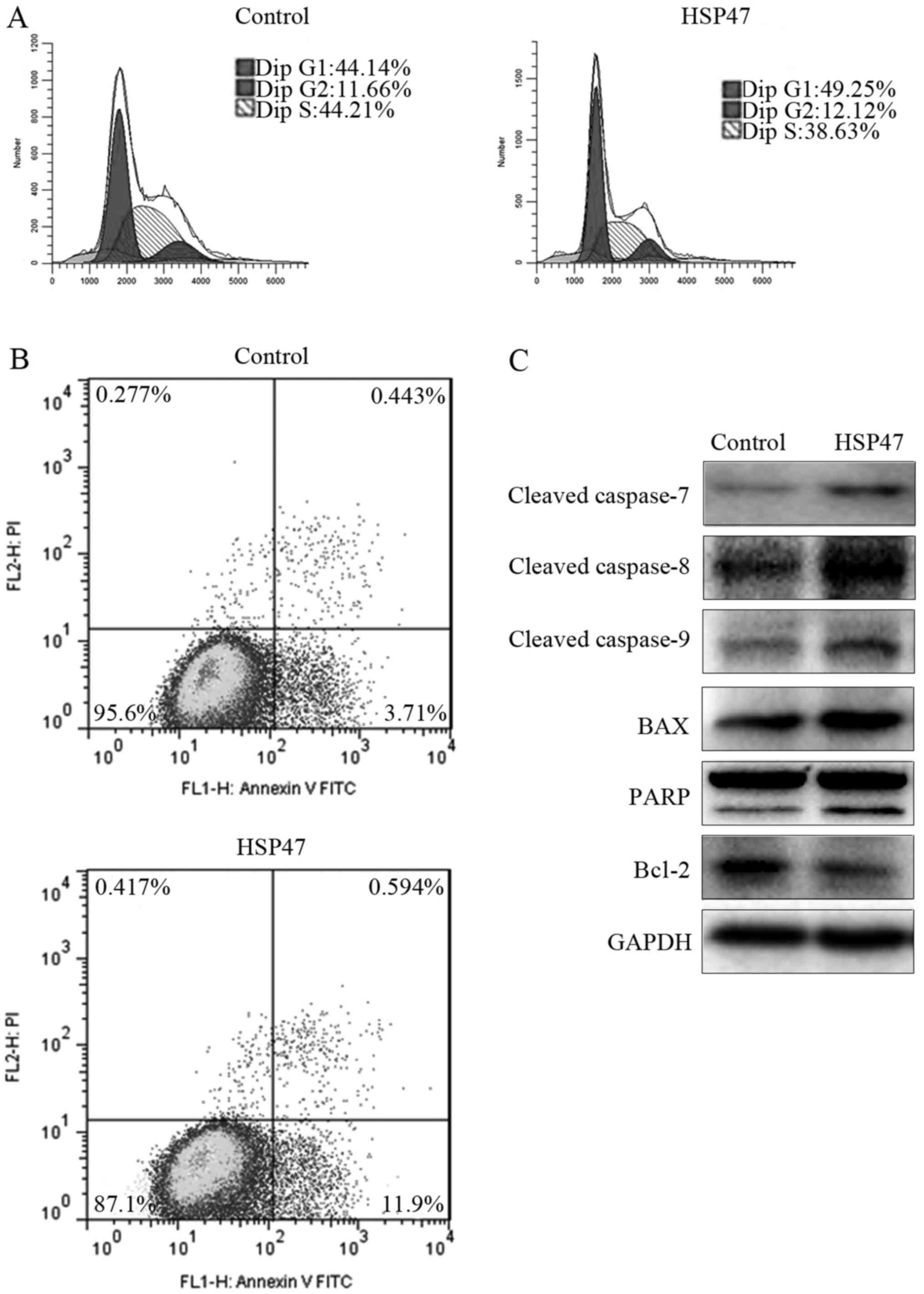
apoptosis by flow cytometric assay at 48 h after transfection. As
shown in Fig. 5A, upregulation of
HSP47 resulted in marked G1 phase arrest, with an increase of
11.6%, compared with the cell cycle distribution of the control
cells. This G1 phase arrest was accompanied by a 12.6% decrease in
the percentage of S phase cells. Meanwhile, the percentage of cells
in the lower right quadrant, which represent early apoptotic cells,
increased by 221% compared with the control (Fig. 5B). However, no obvious difference in
late apoptotic cells or necrotic cells was noted. These findings
indicated that overexpression of HSP47 mainly arrested Hep-2 cells
in the G1 phase and promoted early apoptosis.
To further elucidate the molecules downstream of
HSP47, the expression of apoptosis-related biomarkers was detected
via western blotting, including PARP, cleaved caspase-7/-8/-9,
Bcl-2 and Bax. As shown in Fig. 5C,
the above-mentioned biomarkers were markedly upregulated in the
(pCDH)-HSP47-transfected cells compared with these levels noted in
the (pCDH)-control cells, except for Bcl-2 protein, which was
notably downregulated.
Discussion
Overexpression of heat shock proteins (HSPs) has
previously been demonstrated to be associated with poor prognosis
in cancer patients, such as HSP70 overexpression in colorectal
cancer (22), HSP27 overexpression
in osteosarcoma (23) and breast
cancer (24), HSP72 overexpression
in renal cancer (25), and HSP90
overexpression in breast cancer (26). Moreover, malignant glioma patients
with low expression of HSP73 had significantly longer
progression-free survival than those with high HSP73 expression
(27). In contrast, HSP27
overexpression was associated with a more favourable prognosis in
malignant fibrous histiocytoma (28). These findings indicated that the
prognostic signature of HSPs may be dependent on the cancer type.
Our present study found that the low expression of HSP47 was
significantly correlated with poor prognosis in LSCC patients by
promoting Hep-2 cell proliferation and enhancing the resistance to
cisplatin chemotherapy.
Previous research found that HSP47 was overexpressed
in several human diseases and was highly related to tumourigenesis
and poor prognosis (15,29,30).
However, subsequent research from multiple groups found that the
expression of HSP47 is not always positively associated with
unfavourable prognosis and progressive stages of disease. In
particular, diabetic foot ulcer patients showed a significant
decrease in HSP47 expression compared with wound patients without
diabetes (31), and HSP47 inhibited
the proliferation and collagen synthesis of keloid fibroblast cells
(32). In the present study, we
discovered that HSP47 protein expression in LSCC was markedly lower
compared to that noted in the adjacent non-cancerous tissues by
both immunoblot analysis and immunohistochemical staining.
Moreover, an elevated tumour pathological grade of LSCC coupled
with decreased expression of HSP47 protein was observed, and lower
expression of HSP47 protein was associated with the poorer
prognosis of the LSCC patients, indicating that HSP47 may act as a
prognostic factor in LSCC. The cause of that outcome may be closely
related to tissue-specific expression. HSP27 has been reported to
be overexpressed and associated with poor prognosis in many human
cancers, including breast, rectal, gastric, lung, liver and
prostate cancers (33–36). Contrary to the findings in most
cancers, in human esophageal squamous cell carcinoma (HESCC), the
expression of HSP27 was low, and this low expression was correlated
with poor prognosis (37,38).
Furthermore, we assessed the biological significance
of HSP47 in LSCC. We found that higher expression of HSP47
consistently resulted in inhibition of Hep-2 cell proliferation and
promotion of sensitivity to cisplatin chemotherapy in vitro.
In addition, upregulation of HSP47 expression also reduced the
invasive ability, notably promoted the apoptosis of Hep-2 cells,
and blocked the cells in the G1 phase. These observations indicated
that HSP47 may be a potential therapeutic target in LSCC and
indirectly supported the concept that low HSP47 expression is
correlated with short survival and poor prognosis in LSCC. As shown
in previous studies, HSP47 plays an important regulatory role in
various types of tumours (39–41)
and has a close relationship with tumour apoptosis (40,42).
However, the mechanism of HSP47-mediated Hep-2 cell apoptosis is
still unknown. Previous research has indicated that apoptosis is
regulated by changing the expression of a series of
apoptosis-associated downstream genes, such as Bcl-2 family
(proapoptotic Bax and antiapoptotic Bcl-2) and caspase family
(caspase-7/-8/-9) proteins (43–45).
As the above proteins are involved in tumour apoptosis, we also
explored whether the levels of apoptosis-associated proteins were
changed when HSP47 was upregulated in Hep-2 cells to further
elucidate the molecules downstream of HSP47. The results showed
that Bax was markedly increased in the (pCDH)-HSP47-transfected
cells compared with that in the (pCDH)-control cells, whereas Bcl-2
was decreased, indicating that HSP47 overexpression promoted tumour
apoptosis. We speculate that upregulation of HSP47 may suppress the
expression of Bcl-2, which plays a key role in apoptotic signal
transduction through inhibition of intracellular signalling
proteins and the expression of Bax (46–48).
In accordance with the results for Bax/Bcl-2, HSP47 overexpression
also resulted in increased expression of cleaved caspase-7/-8/-9
and PARP. Caspase-8 and caspase-9 both play a role in the extrinsic
apoptotic pathway and the intrinsic mitochondrial apoptotic
pathway, respectively, given that both of them can activate
downstream caspase family proteins, such as caspase-7 (44,49,50).
Therefore, it appears that HSP47 induces apoptosis via both the
extrinsic and the intrinsic mitochondrial apoptosis pathways.
In conclusion, the present study demonstrated that
there is a significant positive correlation between HSP47 and
prognosis in LSCC patients, as the low expression of HSP47 was
significantly correlated with poor prognosis. Moreover, in
vitro, it was confirmed that HSP47 protein plays an important
role in the biological behaviour of Hep-2 cells by inhibiting cell
proliferation and invasion, enhancing sensitivity to cisplatin
chemotherapy and promoting apoptosis. In brief, the present study
revealed that HSP47 may be a potential prognostic biomarker and an
attractive therapeutic target in LSCC. Further studies regarding
the role of HSP47 expression in more LSCC patients may be
worthwhile and the precise mechanism and regulatory pathways remain
to be elucidated.
Acknowledgements
We thank the patients who participated in the
present study, and Dr Guorong Chen, Fang Wang and Yi Jin for
assistance in preparing the paraffin sections and
immunohistochemistry analysis. This work was supported by funds
from the National Natural Science Foundation of China (81201687),
the Zhejiang Provincial Natural Science Foundation of China
(LY14H130003, LY16H160051 and LY13H090009), and the Zhejiang
Province Medical and Health Science and Technology Plan Project
(2012RCA042).
Glossary
Abbreviations
Abbreviations:
|
LSCC
|
laryngeal squamous cell carcinoma
|
|
HSP47
|
heat shock protein 47
|
|
FBS
|
fetal bovine serum
|
|
BCA
|
bicinchoninic acid
|
|
PVDF
|
polyvinylidene fluoride
|
|
siRNA
|
small interfering RNA
|
|
CCK-8
|
Cell Counting Kit-8
|
|
PBS
|
phosphate-buffered saline
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
propidium iodide
|
|
FCM
|
flow cytometry
|
|
OS
|
overall survival
|
|
HSPs
|
heat shock proteins
|
|
HESCC
|
human esophageal squamous cell
carcinoma
|
References
|
1
|
Farhadieh RD, Rees CG, Yang JL, Salardini
A, Russell P and Smee R: Radiotherapy in larynx squamous cell
carcinoma is not associated with an increased diagnosis of second
primary tumours. Clin Oncol (R Coll Radiol). 21:315–319. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yilmaz M, Karatas OF, Yuceturk B, Dag H,
Yener M and Ozen M: Alpha-B-crystallin expression in human
laryngeal squamous cell carcinoma tissues. Head Neck. 37:1344–1348.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tai P, Yu E, Shiels R and Tonita J:
Long-term survival rates of laryngeal cancer patients treated by
radiation and surgery, radiation alone, and surgery alone: Studied
by lognormal and Kaplan-Meier survival methods. BMC Cancer.
5:132005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varghese BT, Sebastian P and Mathew A:
Treatment outcome in patients undergoing surgery for carcinoma
larynx and hypopharynx: A follow-up study. Acta Otolaryngol.
129:1480–1485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghaffar S, Akhtar S, Ikram M, Imam SZ and
Sepah YJ: Comparison of different treatment modalities in advanced
laryngeal hypopharyngeal squamous cell carcinoma. J Coll Physicians
Surg Pak. 20:171–174. 2010.PubMed/NCBI
|
|
8
|
Belcher R, Hayes K, Fedewa S and Chen AY:
Current treatment of head and neck squamous cell cancer. J Surg
Oncol. 110:551–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cooper JS, Zhang Q, Pajak TF, Forastiere
AA, Jacobs J, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, et
al: Long-term follow-up of the RTOG 9501/intergroup phase III
trial: Postoperative concurrent radiation therapy and chemotherapy
in high-risk squamous cell carcinoma of the head and neck. Int J
Radiat Oncol Biol Phys. 84:1198–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sauk JJ, Nikitakis N and Siavash H: Hsp47
a novel collagen binding serpin chaperone, autoantigen and
therapeutic target. Front Biosci. 10:107–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bekri S, Adélaïde J, Merscher S,
Grosgeorge J, Caroli-Bosc F, Perucca-Lostanlen D, Kelley PM,
Pébusque MJ, Theillet C, Birnbaum D, et al: Detailed map of a
region commonly amplified at 11q13->q14 in human breast
carcinoma. Cytogenet Cell Genet. 79:125–131. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li D, Guang W, Abuzeid WM, Roy S, Gao GP,
Sauk JJ and O'Malley BW Jr: Novel adenoviral gene delivery system
targeted against head and neck cancer. Laryngoscope. 118:650–658.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwon YJ, Lee SJ, Koh JS, Kim SH, Kim YJ
and Park JH: Expression patterns of aurora kinase B, heat shock
protein 47, and periostin in esophageal squamous cell carcinoma.
Oncol Res. 18:141–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reese C, Lee R, Bonner M, Perry B, Heywood
J, Silver RM, Tourkina E, Visconti RP and Hoffman S: Fibrocytes in
the fibrotic lung: Altered phenotype detected by flow cytometry.
Front Pharmacol. 5:1412014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao D, Jiang X, Yao C, Zhang L, Liu H,
Xia H and Wang Y: Heat shock protein 47 regulated by miR-29a to
enhance glioma tumor growth and invasion. J Neurooncol. 118:39–47.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamamoto N, Kinoshita T, Nohata N, Yoshino
H, Itesako T, Fujimura L, Mitsuhashi A, Usui H, Enokida H, Nakagawa
M, et al: Tumor-suppressive microRNA-29a inhibits cancer
cell migration and invasion via targeting HSP47 in cervical
squamous cell carcinoma. Int J Oncol. 43:1855–1863. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Araki K, Mikami T, Yoshida T, Kikuchi M,
Sato Y, Oh-ishi M, Kodera Y, Maeda T and Okayasu I: High expression
of HSP47 in ulcerative colitis-associated carcinomas: Proteomic
approach. Br J Cancer. 101:492–497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen HX and Cleck JN: Adverse effects of
anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol.
6:465–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tseng MY, Liu SY, Chen HR, Wu YJ, Chiu CC,
Chan PT, Chiang WF, Liu YC, Lu CY, Jou YS, et al: Serine protease
inhibitor (SERPIN) B1 promotes oral cancer cell motility and is
over-expressed in invasive oral squamous cell carcinoma. Oral
Oncol. 45:771–776. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Catzavelos C, Bhattacharya N, Ung YC,
Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I,
Kapusta L, et al: Decreased levels of the cell-cycle inhibitor
p27Kip1 protein: Prognostic implications in primary breast cancer.
Nat Med. 3:227–230. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chappuis PO, Donato E, Goffin JR, Wong N,
Bégin LR, Kapusta LR, Brunet JS, Porter P and Foulkes WD: Cyclin E
expression in breast cancer: Predicting germline BRCA1
mutations, prognosis and response to treatment. Ann Oncol.
16:735–742. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lazaris AC, Theodoropoulos GE, Davaris PS,
Panoussopoulos D, Nakopoulou L, Kittas C and Golematis BC: Heat
shock protein 70 and HLA-DR molecules tissue expression. Prognostic
implications in colorectal cancer. Dis Colon Rectum. 38:739–745.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uozaki H, Horiuchi H, Ishida T, Iijima T,
Imamura T and Machinami R: Overexpression of resistance-related
proteins (metallothioneins, glutathione-S-transferase pi, heat
shock protein 27, and lung resistance-related protein) in
osteosarcoma. Relationship with poor prognosis. Cancer.
79:2336–2344. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vargas-Roig LM, Gago FE, Tello O, Aznar JC
and Ciocca DR: Heat shock protein expression and drug resistance in
breast cancer patients treated with induction chemotherapy. Int J
Cancer. 79:468–475. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Santarosa M, Favaro D, Quaia M and
Galligioni E: Expression of heat shock protein 72 in renal cell
carcinoma: Possible role and prognostic implications in cancer
patients. Eur J Cancer. 33:873–877. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pick E, Kluger Y, Giltnane JM, Moeder C,
Camp RL, Rimm DL and Kluger HM: High HSP90 expression is associated
with decreased survival in breast cancer. Cancer Res. 67:2932–2937.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hermisson M, Strik H, Rieger J, Dichgans
J, Meyermann R and Weller M: Expression and functional activity of
heat shock proteins in human glioblastoma multiforme. Neurology.
54:1357–1365. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Têtu B, Lacasse B, Bouchard HL, Lagacé R,
Huot J and Landry J: Prognostic influence of HSP-27 expression in
malignant fibrous histiocytoma: A clinicopathological and
immunohistochemical study. Cancer Res. 52:2325–2328.
1992.PubMed/NCBI
|
|
29
|
Lee HW, Kwon J, Kang MC, Noh MK, Koh JS,
Kim JH and Park JH: Overexpression of HSP47 in esophageal squamous
cell carcinoma: Clinical implications and functional analysis. Dis
Esophagus. 29:848–855. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu J, Xiong G, Fu H, Evers BM, Zhou BP
and Xu R: Chaperone Hsp47 drives malignant growth and invasion by
modulating an ECM gene network. Cancer Res. 75:1580–1591. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh K, Agrawal NK, Gupta SK, Mohan G,
Chaturvedi S and Singh K: Decreased expression of heat shock
proteins may lead to compromised wound healing in type 2 diabetes
mellitus patients. J Diabetes Complications. 29:578–588. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen J, Zhao S, Liu Y, Cen Y and Nicolas
C: Effect of captopril on collagen metabolisms in keloid fibroblast
cells. ANZ J Surg. 86:1046–1051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang SH, Kang KW, Kim KH, Kwon B, Kim SK,
Lee HY, Kong SY, Lee ES, Jang SG and Yoo BC: Upregulated HSP27 in
human breast cancer cells reduces Herceptin susceptibility by
increasing Her2 protein stability. BMC Cancer. 8:2862008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tweedle EM, Khattak I, Ang CW, Nedjadi T,
Jenkins R, Park BK, Kalirai H, Dodson A, Azadeh B, Terlizzo M, et
al: Low molecular weight heat shock protein HSP27 is a prognostic
indicator in rectal cancer but not colon cancer. Gut. 59:1501–1510.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Voll EA, Ogden IM, Pavese JM, Huang X, Xu
L, Jovanovic BD and Bergan RC: Heat shock protein 27 regulates
human prostate cancer cell motility and metastatic progression.
Oncotarget. 5:2648–2663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ciocca DR and Calderwood SK: Heat shock
proteins in cancer: Diagnostic, prognostic, predictive, and
treatment implications. Cell Stress Chaperones. 10:86–103. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xue L, Yang L, Jin ZA, Gao F, Kang JQ, Xu
GH, Liu B, Li H, Wang XJ, Liu LJ, et al: Increased expression of
HSP27 inhibits invasion and metastasis in human esophageal squamous
cell carcinoma. Tumour Biol. 35:6999–7007. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Langer R, Ott K, Specht K, Becker K,
Lordick F, Burian M, Herrmann K, Schrattenholz A, Cahill MA,
Schwaiger M, et al: Protein expression profiling in esophageal
adenocarcinoma patients indicates association of heat-shock protein
27 expression and chemotherapy response. Clin Cancer Res.
14:8279–8287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yohannes E, Chang J, Tar MT, Davies KP and
Chance MR: Molecular targets for diabetes mellitus-associated
erectile dysfunction. Mol Cell Proteomics. 9:565–578. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kawasaki K, Ushioda R, Ito S, Ikeda K,
Masago Y and Nagata K: Deletion of the collagen-specific molecular
chaperone Hsp47 causes endoplasmic reticulum stress-mediated
apoptosis of hepatic stellate cells. J Biol Chem. 290:3639–3646.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Miyata S, Mizuno T, Koyama Y, Katayama T
and Tohyama M: The endoplasmic reticulum-resident chaperone heat
shock protein 47 protects the Golgi apparatus from the effects of
O-glycosylation inhibition. PLoS One. 8:e697322013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zeng YL, Zhang XJ, Shang J, Ding GQ and
Kang Y: Single-chain human anti-EGFR antibody/truncated protamine
fusion protein carrying Hsp47 siRNA can induce apoptosis of human
hepatic stellate cells. Zhonghua Gan Zang Bing Za Zhi. 22:843–848.
2014.(In Chinese). PubMed/NCBI
|
|
43
|
Pećina-Slaus N: Wnt signal transduction
pathway and apoptosis: A review. Cancer Cell Int. 10:222010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Faião-Flores F, Coelho PR, Toledo
Arruda-Neto JD, Maria-Engler SS, Tiago M, Capelozzi VL, Giorgi RR
and Maria DA: Apoptosis through Bcl-2/Bax and cleaved caspase
up-regulation in melanoma treated by boron neutron capture therapy.
PLoS One. 8:e596392013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kiefer MC, Brauer MJ, Powers VC, Wu JJ,
Umansky SR, Tomei LD and Barr PJ: Modulation of apoptosis by the
widely distributed Bcl-2 homologue Bak. Nature. 374:736–739. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chung TK, Cheung TH, Lo WK, Yim SF, Yu MY,
Krajewski S, Reed JC and Wong YF: Expression of apoptotic
regulators and their significance in cervical cancer. Cancer Lett.
180:63–68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharma H, Sen S, Mathur M, Bahadur S and
Singh N: Combined evaluation of expression of telomerase, survivin,
and anti-apoptotic Bcl-2 family members in relation to loss of
differentiation and apoptosis in human head and neck cancers. Head
Neck. 26:733–740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kleinberg L and Davidson B: Cell survival
and apoptosis-related molecules in cancer cells in effusions: A
comprehensive review. Diagn Cytopathol. 37:613–624. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Olsson M and Zhivotovsky B: Caspases and
cancer. Cell Death Differ. 18:1441–1449. 2011. View Article : Google Scholar : PubMed/NCBI
|