Introduction
Hepatocellular carcinoma (HCC) is the leading cause
of cancer-related deaths worldwide (1). Ultrasound, CT and MRI have been
recommended as effective examination methods for the diagnosis of
HCC, but the diagnostic efficacy of these traditional imaging
methods is decreased when the lesions are smaller than 1 cm
(2–4). Fluorine-18-labeled fluorodeoxyglucose
positron emission tomography computed tomography
(18F-FDG PET/CT) is an effective technique for early
detection and precise staging of a variety of malignant diseases,
based on the fact that most malignant tumors are glucose-avid
(5). However, 18F-FDG
PET/CT does not exhibit a good efficiency in detecting HCCs,
particularly when they are well-differentiated (6–7).
Clinical studies have revealed that the total sensitivity of
18F-FDG PET/CT in HCC is only 39–64%, and the average
false-negative rate is up to 40–50% (8–9).
Hence, the current guidelines do not recommend the use of
18F-FDG PET/CT imaging for the detection of HCC
(1). Novel methods, such as
18F-FDG dual phase delay imaging (10), and 18F-FDG +
11C-acetate double tracer imaging (11,12),
have been proposed as alternative methods to PET/CT for the
detection of HCC. However, 18F-FDG dual phase delay
imaging is complicated to operate and time-consuming; the
11C isotope has a short radioactive half-life and must
be produced with a cyclotron, which greatly limits the clinical
application of these methods (10,11).
Hence, the applicability and effectiveness of these approaches are
still controversial (13,14).
The uptake of various molecular imaging tracers by
the tumor is mainly determined by the differential expression of
the biomarkers (15). In
well-differentiated HCC, it is widely accepted that the low uptake
of 18F-FDG mainly results from the overexpression of
glucose-6-phosphatase (G6Pase), which leads to the release of
18F-FDG from the tumor cells by converting the
6-phosphoric acid-FDG back to the FDG prototype (12,16).
However, the low expression of glucose transporter 1 (GLUT1), which
serves as the primary transporter of FDG, also decreases the uptake
of 18F-FDG to some degree (17). Therefore, a new PET/CT tracer that
targets other molecular biomarkers rather than those related to
glucose metabolism should be developed to improve the clinical
applications of PET/CT for the detection of well-differentiated
HCC.
CD13 is a membrane-bound exopeptidase which has been
shown to be overexpressed in a variety of tumor cells and
neovascular endothelial cells (18–21).
Therefore, it is a potential target for cancer diagnosis and
therapy (22). The high expression
rate of CD13 in a variety of HCC cell lines and clinical samples
has already been confirmed (23–25).
The asparagine-glycine-arginine (NGR) motif has been identified as
an adequate ligand for CD13, displaying high affinity and
specificity (26). It has been well
established that, by labelling NGR peptide with 64Cu or
68Ga isotopes, the efficiency and specificity of NGR
probes for the PET/CT imaging of CD13-positive tumors can be
improved (27–30). Therefore, we questioned whether the
radio-labeled NGR probe could non-invasively image
well-differentiated HCC tumors. To address this issue, we performed
a direct quantitative comparative preclinical study between
radio-labeled NGR and FDG probes in well-differentiated HCC
tumors.
Herein, the uptakes of 68Ga-NGR and
18F-FDG by the malignancies were compared in mouse
xenograft models, bearing SMMC-7721-derived well-differentiated HCC
tumors (positive for both CD13 and G6Pase). The models bearing
HT-1080 tumors (CD13-positive and G6Pase-negative) and HT-29 tumors
(negative for both CD13 and G6Pase) were also analyzed for
comparison.
Materials and methods
Materials
The NGR peptide with the chelator
1,4,7,10-tetraazacyclododecan-N,N′,N″,N′″-tetraacetic acid
(DOTA-GGGCNGRC, Cys4 and Cys8 being
conjugated by a disulfide bridge) was synthesized by GL Biochem
Ltd. (Shanghai, China), with a purity >95%, as confirmed by
high-performance liquid chromatography (HPLC; Agilent Technologies,
Santa Clara, CA, USA). 68GaCl3 was obtained
from the 68Ge-68Ga radionuclide generator
(ITG Co., Munich, Germany). The cell lines SMMC-7721, HT-1080 and
HT-29 were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA) and cultured in RPMI or high glucose
Dulbecco's modified Eagle's medium (DMEM) culture supplemented with
10% fetal bovine serum (FBS) (all from HyClone, Logan, UT, USA) in
37°C humidified atmosphere containing 5% CO2. All other
reagents were commercially purchased and used without further
purification.
68Ga-NGR synthesis
68Ga-NGR was prepared using the following
protocol: 68GaCl3 (200 µl, 111–148 MBq) was
added into DOTA-NGR (2 µg), which was previously dissolved in
deionized water (2 µl). The pH value was adjusted to 4.0 by adding
sodium acetate (10 µl, 1.25 M). The mixture obtained was
subsequently incubated at 90–100°C for 10 min.
The radiochemical purity (RCP) was analyzed by the
mean of HPLC. The analytic HPLC experiment was performed using a RP
C18 column (Zorbax; 5 µm, 4.6×250 mm). The HPLC method employed is
described below: solvent A consisted of 0.05% trifluoroacetic acid
(TFA) in water, and solvent B consisted of 0.05% TFA in
acetonitrile. The flow rate was set to 1 ml/min; and the gradient
was 0–3 min, 5–5% solvent B; 3–20 min, 5–65% solvent B. All
experiments were repeated four times under the same conditions.
Western blot analysis
After the cultures (HT-1080, SMMC-7721 and HT-29)
were subjected to the lysis process, the protein samples were
quantified by bicinchoninic acid (BCA) assay and heated up at 95°C
for 10 min, followed by the loading buffer addition. The samples,
each containing 40 µg proteins, were loaded onto sodium dodecyl
sulfate-polyacrylamide gel in the electrophoresis gels and
transferred to polyvinylidene fluoride membrane filters (Life
Technologies, Foster City, CA, USA). The membranes were blocked for
2 h in Tris-buffered saline containing 0.01% Tween-20 (TBST) and 5%
bovine serum albumin. Afterwards, an overnight incubation process
at 4°C was accomplished, having the appropriate primary antibodies
in TBST with 2.5% bovine serum albumin as following: mouse
anti-CD13 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
rabbit anti-G6Pase (1:1,000), rabbit anti-GLUT1 (1:1,000) (both
from Abcam, Cambridge, MA, USA) and anti-β-actin antibodies
(loading control; Beijing Biosynthesis Biotechnology Co., Ltd.,
Beijing, China). After 1 h of incubation with the
peroxidase-conjugated secondary antibody (1:5,000; ZSGB-BIO,
Beijing, China), the membranes were washed with TBST. The blots
were developed with an enhanced chemiluminescence kit (Thermo
Scientific), and images were acquired using a ChemiDOC XRS+
instrument (Bio-Rad, Hercules, CA, USA).
The relative expression levels of detected proteins
were quantified in accordance with a previously reported method
(31). The statistics were acquired
based on four repeated experiments.
Cell uptake studies
For the cellular uptake studies, the incubation of
SMMC-7721, HT-1080 and HT-29 cells was carried out in plastic
tubes, with a volume of 450 µl containing 5×105 cells.
This was followed by the addition of 50 µl (37 kBq)
68Ga-NGR or 18F-FDG into each tube. The
incubation process was carried out at 37°C for 30, 60, 90 and 120
min. Afterwards, cold phosphate-buffered saline (PBS) was added to
the mixture and centrifuged to discard the supernatant. For the
cell blocking studies, SMMC-7721 cultures were incubated along with
68Ga-NGR (50 µl, 37 kBq) and unlabeled DOTA-NGR (2 µl,
10 µg/µl). The dosage of unlabeled DOTA-NGR was performed as
previously reported (32,33). The radioactivity of the precipitate
was counted using a γ-counter (with decay correction, the same
below). These experiments were also repeated four times under the
same conditions.
In vivo micro-PET/CT imaging
studies
All the animal studies were performed according to
the protocol approved by the Institutional Animal Care and Use
Committee of the Fourth Military Medical University. Fifty-four
male BALB/c nude mice (4–6 weeks old, with a body weight of 20–25
g) were used. The xenograft models were generated by administering
a subcutaneously injection containing 2.5×106 tumor
cells into the upper flanks of nude mice; the HT-1080 xenografts
were constructed on the left scapular region of nude mice, the
HT-29 and SMMC-7721 xenografts were both on the right side. The
number of each type of xenograft model was 18. The tumors were
allowed to grow for 2–3 weeks until they reached a volume of 0.5–1
cm3, as required for the micro-PET/CT imaging and
biodistribution experiments. The nude mice bearing SMMC-7721 tumors
were randomly divided into three groups, each group including six
mice, and marked as group 1, 2 and 3; the same treatments were
administered to HT-1080 and HT-29 xenografts. The mice in group 1
were injected with 7.4 MBq 18F-FDG by the tail vein, and
the images were eventually acquired at 30, 60 and 90 min
post-injection using a micro-PET/CT scanner (Mediso Medical Imaging
Systems, Budapest, Hungary). In contrast, the mice composing group
2 were injected with 7.4 MBq 68Ga-NGR; while the mice in
group 3 were administered 7.4 MBq 68Ga-NGR as well as an
excessive unlabeled amount of NGR (20 mg/kg). The scanning time
(30, 60 and 90 min) and blocking dosage were based on previously
reported procedures (27,32); the rest of the steps involved were
similar as for group 1. The regions of interest (ROIs) over tumors
on the coronal images were carefully delineated, and the average
signal levels in the ROIs were expressed as % ID/g (percentage of
injected dose per gram of tissue).
Immunofluorescence staining and
immunohistostaining studies
HT-1080, SMMC-7721 and respectively HT-29 cells were
planted in a 24-well plate at a density of 5×104
cells/well. After overnight incubation at 37°C in 5%
CO2, the cells were washed three times with cold PBS and
fixed with 4% paraformaldehyde for 15 min. The cells were then
blocked with 3% bovine serum albumin at room temperature for 1 h
and subsequently incubated with mouse anti-CD13 for 12–16 h at 4°C
(1:200; Abcam). The cultures were washed with PBS and further
incubated with a fluorescent secondary goat anti-mouse
FITC-antibody (1:200; ZSGB-BIO) for 2 h at ambient temperature.
This was followed by repeated washings (five times) and continued
by the addition of 4,6-diamidino-2-phenylindole (DAPI) to the cells
which were further incubated for 10 min to stain the nuclei. The
cells were analyzed using an Olympus IX71 fluorescence microscope
(Olympus, Tokyo, Japan).
The mice were sacrificed by cervical dislocation,
then HT-1080, SMMC-7721 and HT-29 cell-derived tumor tissues were
fixed with 4% of paraformaldehyde, embedded in paraffin and
sectioned at 0.3 µm. Then, the sections were blocked with 3% serum
for 30 min and underwent antigen retrieval by heat mediation in a
citrate buffer; this was followed by incubation with mouse
anti-CD13 antibody (1:100; Abcam) at 4°C for 16–18 h. The sections
were subsequently washed three times with PBS and incubated with
the peroxidase-conjugate secondary antibody (1:500; goat anti-mouse
IgG for mouse anti-CD13; ZSGB-BIO) at room temperature for 1 h,
washed again with PBS, stained using 0.025% 3,3′-diaminobenzidine
solution in PBS, and eventually counterstained with hematoxylin.
The sections were visualized by the aid of an Olympus BX51
microscope (Olympus).
Biodistribution studies
The xenograft models bearing SMMC-7721 tumors were
randomly divided into two groups (n=6), which were individually
injected with 7.4 MBq of either 68Ga-NGR or
18F-FDG via the tail vein. At 30 min post-injection, the
mice were sacrificed by cervical dislocation and their blood,
tumors and the major organs (heart, lung, liver, spleen, pancreas,
stomach, intestine, kidney, muscle, bone and brain) were harvested
and weighed. The radioactivity of each sample was measured by
γ-counter. The data collected are expressed as % ID/g.
Statistical analysis
Quantitative data are expressed as mean ± SD. Means
were compared using one-way ANOVA and Student's t-test. P values
<0.05 were considered to indicate a statistically significant
result.
Results
68Ga-NGR radiochemistry
properties and cell uptake studies
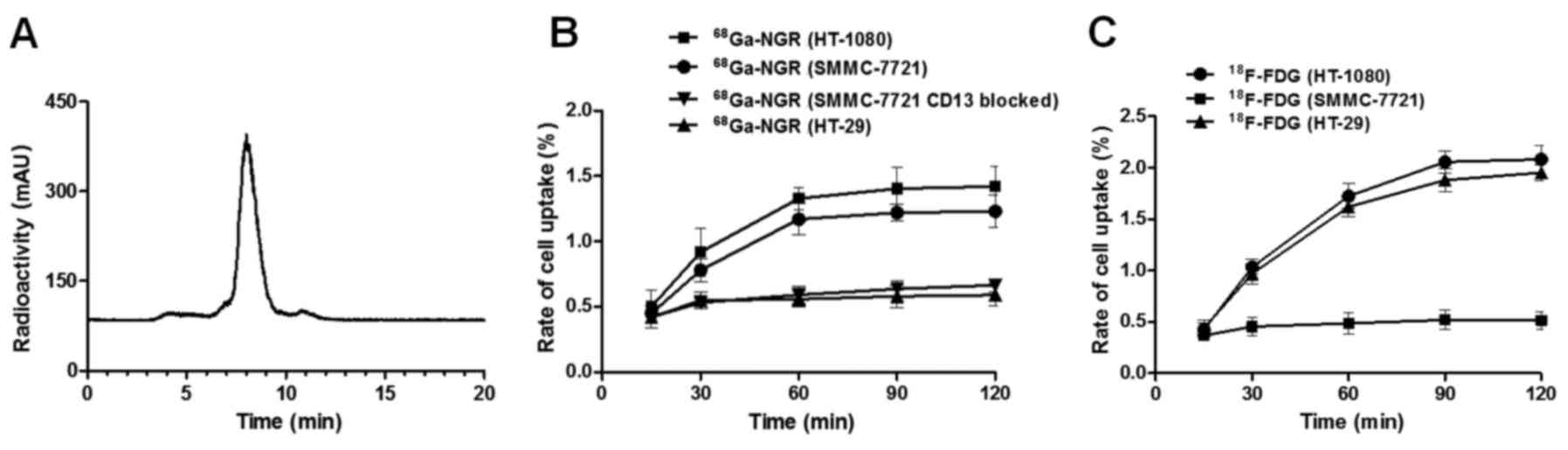
68Ga-NGR was successfully synthesized
with an RCP as high as 98.2±0.9% (Fig.
1A). The specific activity of the tracer was found to be
between 55–80 MBq/nmol.
The cellular uptake studies revealed that
68Ga-NGR could bind to both SMMC-7721 and HT-1080 cells,
but not to HT-29 cells and the CD13 blocked SMMC-7721 cells
(Fig. 1B). However,
18F-FDG could be absorbed by HT-1080 and HT-29 cells,
but not by SMMC-7721 cells (Fig.
1C). For SMMC-7721 cells, after 2 h of incubation, the uptake
of 68Ga-NGR was significantly higher than that of
18F-FDG (1.23±0.11 vs. 0.515±0.14%, P=0.0024 <0.01).
Furthermore, after being blocked by non-radioactive NGR (CD13
blocked), the uptake of 68Ga-NGR in SMMC-7721
(0.59±0.08%) cells was significantly lower than that in non-blocked
SMMC-7721 cells (1.23±0.11%, P=0.0013 <0.01), which demonstrated
that 68Ga-NGR was selectively targeting the CD13
receptor.
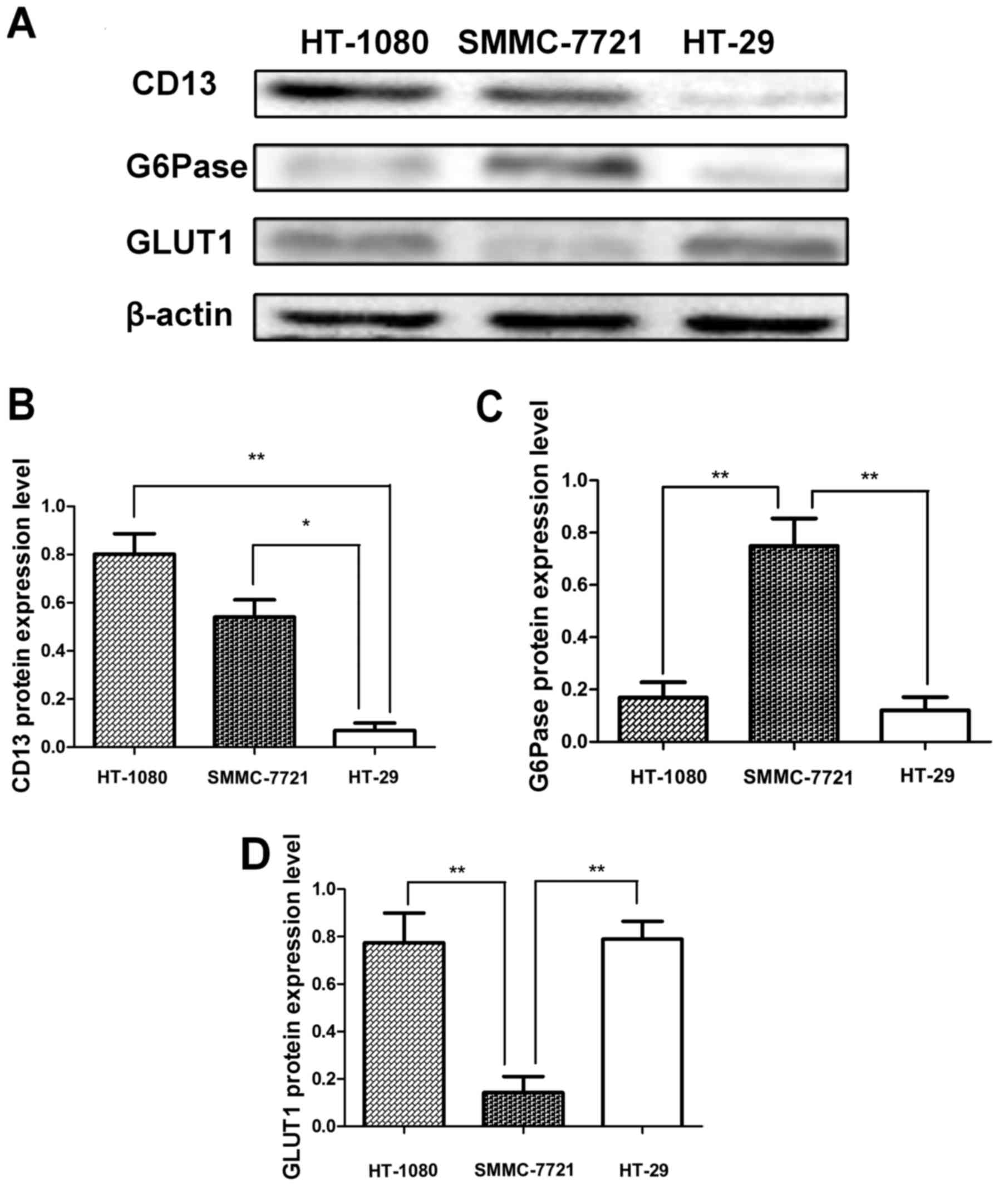
Western blot analysis
The western blot analysis of CD13 expression in
HT-1080 and SMMC-7721 cells showed positive bands correlated to the
molecular size of CD13, whereas a low signal band was observed for
HT-29 cells (Fig. 2A). In addition,
the western blotting analysis of G6Pase indicated that two weak
bands were observed for HT-1080 and HT-29 cells, and G6Pase was
expressed in the SMMC-7721 cells. The semi-quantitative analysis
[the semi-quantitative analysis method based on a previously
reported method (31)] results of
the expression levels of CD13 (Fig.
2B), G6Pase (Fig. 2C) and GLUT1
(Fig. 2D) corresponded to our
observed results. The CD13 expression levels in the HT-1080 and
SMMC-7721 cells were notably higher compared to that of HT-29
(0.80±0.12 vs. 0.07±0.04, P=0.0097<0.01; and 0.54±0.10 vs.
0.07±0.04, P=0.02<0.05). The G6Pase expression level in the
SMMC-7721 cells exhibited an obviously greater value in comparison
with HT-1080 and HT-29 cells (0.75±0.15 vs. 0.17±0.08,
P=0.0083<0.01; and 0.75±0.15 vs. 0.12±0.07, P=0.0085<0.01).
In addition, the GLUT1 expression level in SMMC-7721 cells was
significantly lower than that of HT-1080 and HT-29 cells (0.14±0.06
vs. 0.77±0.10, P=0.0098<0.01 and 0.14±0.06 vs. 0.79±0.06,
P=0.0094<0.01).
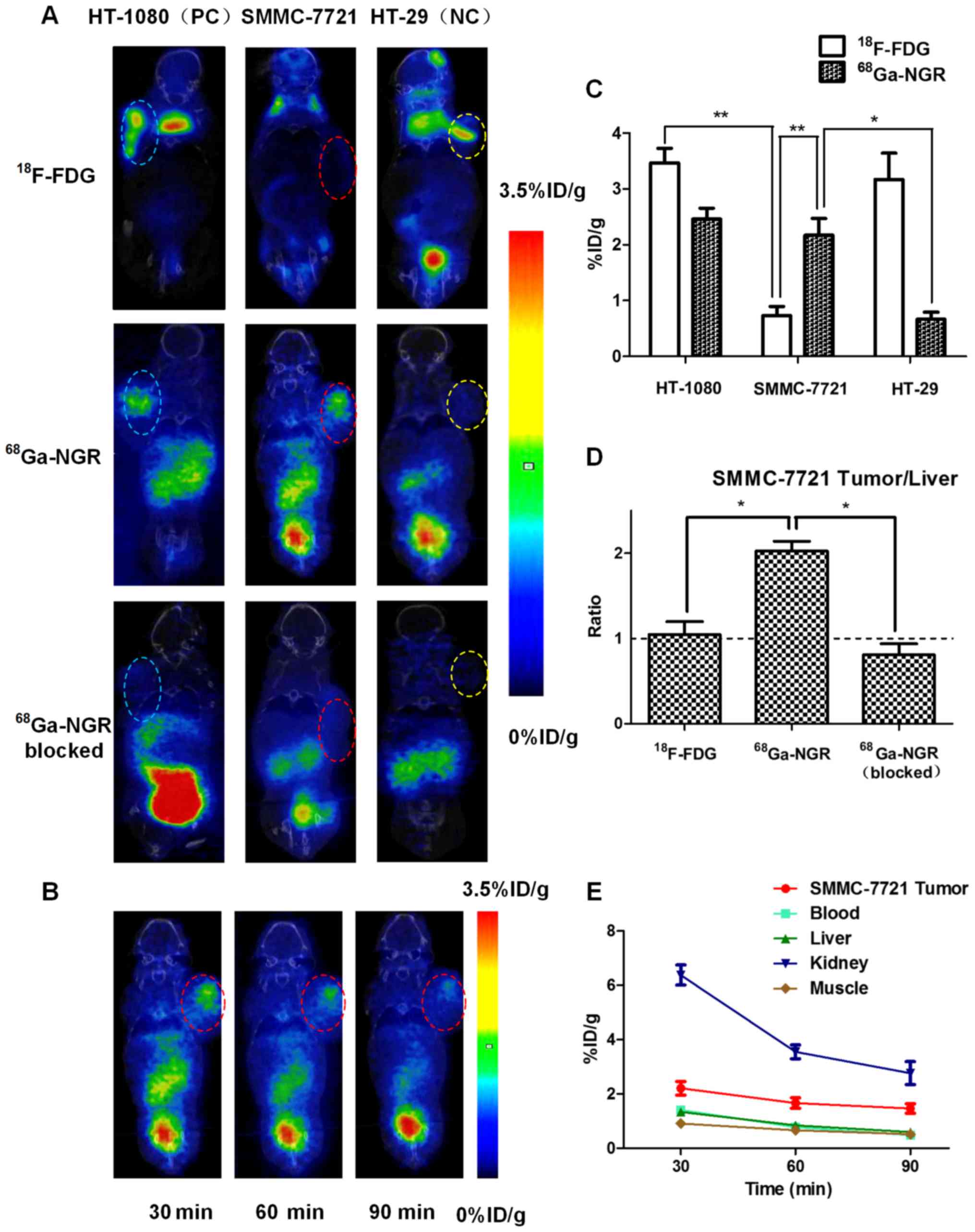
In vivo micro-PET/CT imaging
studies
The imaging efficacies of 18F-FDG and
68Ga-NGR were evaluated and compared in nude mice
bearing HT-1080, SMMC-7721 and HT-29 xenografts by micro-PET/CT
imaging (Fig. 3A). The results
revealed that 18F-FDG uptake in SMMC-7721 tumors was
significantly lower than that in HT-1080 and HT-29 tumors
(0.73±0.26% ID/g vs. 3.47±0.31% ID/g, P=0.0031<0.01 and
0.73±0.26% ID/g vs. 3.17±0.29% ID/g, P=0.0197<0.05) at 30 min
post-injection. Regarding the uptake of 68Ga-NGR in
SMMC-7721 tumors, it was similar to that in the CD13-positive
control HT-1080 tumors (2.46±0.23% ID/g vs. 2.17±0.21% ID/g,
P=0.18>0.05), and was significantly higher than that in the
CD13-negative control HT-29 malignancy (2.17±0.21% ID/g vs.
0.67±0.20% ID/g, P=0.0148<0.05). For SMMC-7721 tumors, the
uptake of 68Ga-NGR was 2.97-fold higher compared to that
of 18F-FDG (2.17±0.21% ID/g vs. 0.73±0.26% ID/g,
P=0.0030<0.01). The corresponding quantitative analyses are
showed in Fig. 3C. In addition,
68Ga-NGR in SMMC-7721 tumors exhibited a higher imaging
efficiency than that in CD13 blocked SMMC-7721 tumors (2.17±0.21%
ID/g vs. 0.55±0.12% ID/g, P=0.0078<0.01), which implies that
68Ga-NGR selectively targets CD13.
The tumor/liver (T/L) ratios of 18F-FDG
and 68Ga-NGR in SMMC-7721 xenografts at 30 min
post-injection were calculated and are compared in Fig. 3D, and the quantitative comparison
results revealed that the T/L ratio of 68Ga-NGR was
2.05±0.16, which was significantly higher than that of
18F-FDG (1.01±0.25, P=0.0290<0.05) and
68Ga-NGR (CD13 blocked) (0.81±0.18, P=0.0294<0.05).
In addition, the tumor/background (T/B) ratios of
68Ga-NGR in HT-1080 and HT-29 xenografts were 2.14±0.43
and 1.18±0.25 respectively, which indicated that
68Ga-NGR could image CD13+ tumors.
Multiple time-point (30, 60 and 90 min) micro-PET/CT
scans were performed in SMMC-7721 xenograft (Fig. 3B). The tumor uptakes of
68Ga-NGR were 2.17±0.21 % ID/g, 1.66±0.17% ID/g and
1.47±0.15 % ID/g, respectively. The dynamic uptake tendencies of
68Ga-NGR in major organs (blood, liver, kidneys and
muscle) are shown in Fig. 3E.
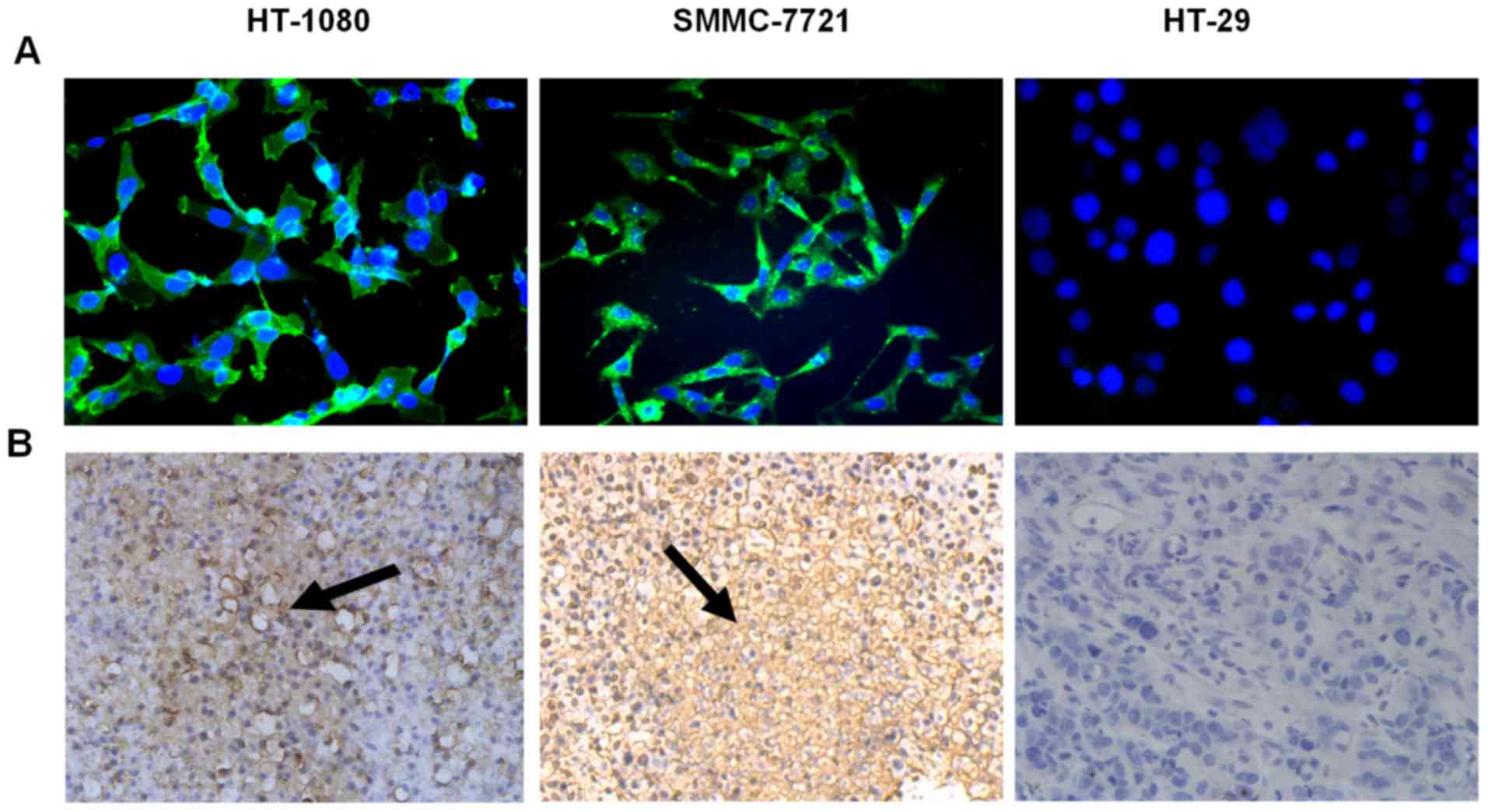
Immunofluorescence staining and
immunohistostaining studies
The immunofluorescence staining study indicated that
CD13 was selectively expressed at the level of the cell surface of
HT-1080 and SMMC-7721 tumors, but not in HT-29 cells (Fig. 4A).
The results obtained from immunohistostaining assays
further indicated that CD13 was expressed on the cell surface of
HT-1080 and SMMC-7721 tumor cells as well as the neovascular
endothelial cells, but not in HT-29 tumor cells, which was
consistent with the results obtained by western blotting analysis
and immunofluorescence staining (Fig.
4B).
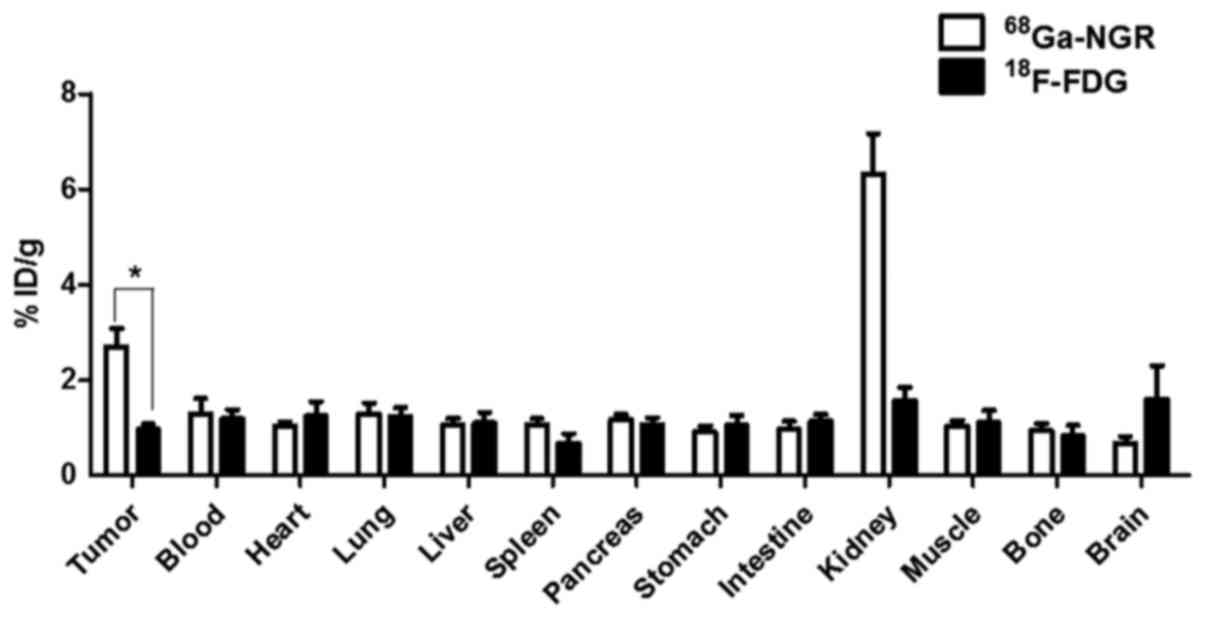
Biodistribution studies
The in vivo biodistribution analysis showed
that, except for the kidneys, the highest accumulation of
68Ga-NGR was detected in SMMC-7721 HCC tumors (Fig. 5). The accumulation of
68Ga-NGR in SMMC-7721 HCC tumors was 2.81-fold higher
compared to that of 18F-FDG (2.70±0.65% ID/g vs.
0.96±0.19% ID/g, P=0.0298<0.05).
Discussion
In the present study, we found that the uptake of
68Ga-NGR was 2.97-fold higher than that of
18F-FDG by SMMC-7721 tumors, based on micro-PET/CT
imaging studies, and 2.81-fold higher, based on biodistribution
studies. However, the T/L ratio of 68Ga-NGR was
2.03-fold higher than that of 18F-FDG in the
well-differentiated HCC models. These findings strongly reflected
that 68Ga-NGR was potentially superior to
18F-FDG for imaging well-differentiated HCC.
The development of radio-labeled peptide probes,
such as RGD and NGR, for diagnostic and therapeutic applications,
has rapidly expanded in the last decades (34–37).
On the aspect of clinical practice, the safety and the
applicability of these probes have been well established in lung
cancer (38–40), brain metastases (41), breast cancer (42) and rheumatoid arthritis (43). Even though HCCs are worldwide
malignant tumors with high incidence of morbidity, there has been
little progress in their detection by radio-labeled probes. Roland
et al utilized [68Ga]NODAGA-RGD to image patients
with HCCs, but the probe uptake by the HCC tumors and the
tumor/liver ratio were both insufficient for HCC detection
(44). This may be attributed to
the low receptor expression and the higher background radioactivity
in the cirrhotic liver leading to poor detection. As the high
expression of CD13 in well-differentiated HCC has been confirmed in
previous studies (23–25), utilizing NGR-based probes for HCC
detection is an attractive prospect. In the present study, the
positive expression of CD13 was confirmed in SMMC-7721 and HT-1080
cell-derived tumors and neovascular endothelial cells by western
blot or immunohistochemical analyses. Furthermore, the high uptake
and T/L ratio of 68Ga-NGR in the well-differentiated HCC
xenografts indicated the ability of 68Ga-NGR to image
well-differentiated HCC. However, the high expression rate of CD13
has already been confirmed not only in a variety of HCC cell lines,
but also clinical samples (23,24),
which suggests that 68Ga-NGR may have high specificity
and sensitivity for further clinical diagnosis of
well-differentiated HCCs. Poor-differentiated HCCs have relatively
low expression of G6Pase and high expression of GLUT1 (17), which may contribute to the low
uptake of 18F-FDG by poor-differentiated HCC. The uptake
performance of 68Ga-NGR, in poor-differentiated HCC,
warrants further investigation.
Chronic hepatitis is the leading cause of cirrhosis
and hepatocellular carcinoma (HCC) (45), and early discrimination of liver
cirrhosis from chronic hepatitis is critical for effective
treatment and optimal prognosis (46). The probe based on the RGD peptide
was reported to specifically bind to integrin αvβ3 receptors in
activated hepatic stellate cells (HSCs). The hepatic deposition
amount of RGD probe was markedly increased in parallel with the
development and progression of liver fibrosis, which indicate that
we could quantitatively assess the extent of liver fibrosis with
probes based on RGD (47). Since
CD13 has also been reported to be a highly specific marker of
hepatocyte differentiation (23,25),
it is possible for 68Ga-NGR to differentiate cirrhosis
from chronic hepatitis and HCCs from non-HCCs. However, how
68Ga-NGR specifically performs in cirrhosis or non-HCCs,
such as cholangiocellular carcinoma (CCC) or focal nodular
hyperplasia (FNH), warrants further investigation.
The additional advantages of peptide-based
radiopharmaceuticals, beyond 18F-FDG, require further
exploration. In the present study, we found that for the imaging of
well-differentiated HCC, the 68Ga-NGR probe was more
efficient, compared to 18F-FDG, in three distinct
aspects, as discussed below. Firstly, the uptake of
68Ga-NGR was substantially higher compared to that of
18F-FDG in well-differentiated HCC tumors, which
indicated its potential to be used as an improved alternative to
18F-FDG for the diagnosis of well-differentiated HCCs.
Secondly, compared with 64Cu or 99mTc-labeled
NGR, reported in previous studies (27,48),
68Ga-NGR displayed a low uptake in liver tissue; and the
T/L ratio of 68Ga-NGR was 2.05±0.16, which was
significantly higher than that of 18F-FDG (1.01±0.25),
64Cu-labeled NGR (0.35) (27), and 99mTc-labeled NGR
(0.34) (48). Lastly, as
68Ga is a cost-effective radioisotope that could be
easily obtained from a 68Ge/68Ga generator,
68Ga-NGR peptide-based radiopharmaceuticals could be
effortlessly and inexpensively produced. Altogether, these
68Ga-NGR properties indicate its excellent potential for
clinical translation in the future.
Generally, malignant tumors are highly
phenotypically and genetically heterogeneous, which is an important
contributing factor for clinical diagnosis and treatment decision
(49). The molecular imaging
approach provides the opportunity to non-invasively visualize the
expression level of molecular markers in vivo, and hence, it
is superior to traditional histopathological examination (50). Based on the present study,
68Ga-NGR could image CD13-positive tumors (SMMC-7721 and
HT-1080 tumors) and 18F-FDG could image G6Pase-negative
tumors (HT-1080 and HT-29 tumors), which indicates that the
different uptake rates of the tracers (68Ga-NGR and
18F-FDG) are closely associated with the expression
levels of the corresponding molecular markers (CD13 and G6Pase,
respectively). These results imply the possibility of a
non-invasive molecular imaging method to evaluate the expression of
corresponding biomarkers.
Despite these promising findings, the advantages of
68Ga-NGR in imaging efficiency have only been
demonstrated in in vitro and xenograft models. More HCC cell
lines and orthotopic HCC models may be explored in our future
studies. However, it is vital for clinical translation studies to
validate the actual effect of 68Ga-NGR in order to
determine the specificity and sensitivity of 68Ga-NGR
for the diagnosis of well-differentiated HCCs in the clinic.
In summary, the present study demonstrated that the
uptake of 68Ga-NGR was significantly higher than that of
18F-FDG in well-differentiated HCC xenografts and
therefore 68Ga-NGR was more suitable for the imaging of
well-differentiated HCC, which suggests its future potential for
clinical translation in the PET/CT diagnosis of HCC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81230033, 81401442,
81227901 and 81601521), the National Key Research and Development
Program of China (grant no. 2016YFC0103804), the Xijing Hospital
Subject Promoting Plan (grant nos. XJZT15G01, XJZT15M07), the
Shaanxi Science & Technology Co-ordination & Innovation
Project (grant no. S2016TQSF0021) and the Shaanxi Science &
Technology Co-ordination & Innovation Project (grant no.
2016KTCQ03-09). We would like to thank Professor Fan Wang from the
Medical Isotopes Research Center of Peking University (Beijing,
China) for her generous support, and Guiyu Li, Mingru Zhang and Shu
Zong for their technical assistance in conducting the present
study.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benson AB III, Abrams TA, Ben-Josef E,
Bloomston PM, Botha JF, Clary BM, Covey A, Curley SA, D'Angelica
MI, Davila R, et al: NCCN clinical practice guidelines in oncology:
Hepatobiliary cancers. J Natl Compr Canc Netw. 7:350–391. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwon HJ, Byun JH, Kim JY, Hong GS, Won HJ,
Shin YM and Kim PN: Differentiation of small (≤2 cm) hepatocellular
carcinomas from small benign nodules in cirrhotic liver on
gadoxetic acid-enhanced and diffusion-weighted magnetic resonance
images. Abdom Imaging. 40:64–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu NC, Chaudhari V, Raman SS, Lassman C,
Tong MJ, Busuttil RW and Lu DS: CT and MRI improve detection of
hepatocellular carcinoma, compared with ultrasound alone, in
patients with cirrhosis. Clin Gastroenterol Hepatol. 9:161–167.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhan HW, Xu W, Ye XJ, Zhao CL, Zhang H, Li
J, Yao Q and Zhang LJ: Application of FDG-PET for detection of
malignant lesions in patients with elevated blood tumor markers but
without a history of malignancy. Mol Med Rep. 2:837–842. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Delbeke D, Martin WH, Sandler MP, Chapman
WC, Wright JK Jr and Pinson CW: Evaluation of benign vs malignant
hepatic lesions with positron emission tomography. Arch Surg.
133:510–515. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iwata Y, Shiomi S, Sasaki N, Jomura H,
Nishiguchi S, Seki S, Kawabe J and Ochi H: Clinical usefulness of
positron emission tomography with fluorine-18-fluorodeoxyglucose in
the diagnosis of liver tumors. Ann Nucl Med. 14:121–126. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayakawa N, Nakamoto Y, Nakatani K, Hatano
E, Seo S, Higashi T, Saga T, Uemoto S and Togashi K: Clinical
utility and limitations of FDG PET in detecting recurrent
hepatocellular carcinoma in postoperative patients. Int J Clin
Oncol. 19:1020–1028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YK, Hsieh DS, Liao CS, Bai CH, Su CT,
Shen YY, Hsieh JF, Liao AC and Kao CH: Utility of FDG-PET for
investigating unexplained serum AFP elevation in patients with
suspected hepatocellular carcinoma recurrence. Anticancer Res.
25:4719–4725. 2005.PubMed/NCBI
|
|
10
|
Cheng G, Torigian DA, Zhuang H and Alavi
A: When should we recommend use of dual time-point and delayed
time-point imaging techniques in FDG PET? Eur J Nucl Med Mol
Imaging. 40:779–787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JW, Kim JH, Kim SK, Kang KW, Park KW,
Choi JI, Lee WJ, Kim CM and Nam BH: A prospective evaluation of
18F-FDG and 11C-acetate PET/CT for detection
of primary and metastatic hepatocellular carcinoma. J Nucl Med.
49:1912–1921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ho CL, Yu SC and Yeung DW:
11C-acetate PET imaging in hepatocellular carcinoma and
other liver masses. J Nucl Med. 44:213–221. 2003.PubMed/NCBI
|
|
13
|
Lam MG, Kwee TC, Basu S and Alavi A:
Underestimated role of 18F-FDG PET for HCC evaluation
and promise of 18F-FDG PET/MR imaging in this setting. J
Nucl Med. 54:1510–1511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheung TT, Ho CL, Chen S, Chan SC, Poon
RT, Fan ST and Lo CM: Reply: Underestimated role of
18F-FDG PET for HCC evaluation and promise of
18F-FDG PET/MR imaging in this setting. J Nucl Med.
54:1511–1512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tichauer KM, Wang Y, Pogue BW and Liu JT:
Quantitative in vivo cell-surface receptor imaging in oncology:
Kinetic modeling and paired-agent principles from nuclear medicine
and optical imaging. Phys Med Biol. 60:R239–R269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma B, Martin A and Zerizer I: Positron
emission tomography-computed tomography in liver imaging. Semin
Ultrasound CT MR:. 34:66–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Izuishi K, Yamamoto Y, Mori H, Kameyama R,
Fujihara S, Masaki T and Suzuki Y: Molecular mechanisms of
[18F]fluorodeoxyglucose accumulation in liver cancer.
Oncol Rep. 31:701–706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Q, Wang J, Zhang H, Zhao D, Zhang Z
and Zhang S: Expression and clinical significance of aminopeptidase
N/CD13 in non-small cell lung cancer. J Cancer Res Ther.
11:223–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wickström MI, Larsson R, Nygren P and
Gullbo J: Aminopeptidase N (CD13) as a target for cancer
chemotherapy. Cancer Sci. 102:501–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ikeda N, Nakajima Y, Tokuhara T, Hattori
N, Sho M, Kanehiro H and Miyake M: Clinical significance of
aminopeptidase N/CD13 expression in human pancreatic carcinoma.
Clin Cancer Res. 9:1503–1508. 2003.PubMed/NCBI
|
|
21
|
Fukasawa K, Fujii H, Saitoh Y, Koizumi K,
Aozuka Y, Sekine K, Yamada M, Saiki I and Nishikawa K:
Aminopeptidase N (APN/CD13) is selectively expressed in vascular
endothelial cells and plays multiple roles in angiogenesis. Cancer
Lett. 243:135–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su L, Cao J, Jia Y, Zhang X, Fang H and Xu
W: Development of synthetic aminopeptidase N/CD13 inhibitors to
overcome cancer metastasis and angiogenesis. ACS Med Chem Lett.
3:959–964. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rocken C, Licht J, Roessner A and
Carl-McGrath S: Canalicular immunostaining of aminopeptidase N
(CD13) as a diagnostic marker for hepatocellular carcinoma. J Clin
Pathol. 58:1069–1075. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagano H, Ishii H, Marubashi S, Haraguchi
N, Eguchi H, Doki Y and Mori M: Novel therapeutic target for cancer
stem cells in hepatocellular carcinoma. J Hepatobiliary Pancreat
Sci. 19:600–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Röcken C, Carl-McGrath S, Gräntzdörffer I,
Mantke R, Roessner A and Lendeckel U: Ectopeptidases are
differentially expressed in hepatocellular carcinomas. Int J Oncol.
24:487–495. 2004.PubMed/NCBI
|
|
26
|
Corti A, Curnis F, Arap W and Pasqualini
R: The neovasculature homing motif NGR: More than meets the eye.
Blood. 112:2628–2635. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen K, Ma W, Li G, Wang J, Yang W, Yap
LP, Hughes LD, Park R and Conti PS: Synthesis and evaluation of
64Cu-labeled monomeric and dimeric NGR peptides for
MicroPET imaging of CD13 receptor expression. Mol Pharm.
10:417–427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang J, Lu X, Wan N, Hua Z, Wang Z, Huang
H, Yang M and Wang F: 68Ga-DOTA-NGR as a novel molecular
probe for APN-positive tumor imaging using MicroPET. Nucl Med Biol.
41:268–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mate G, Kertesz I, Enyedi KN, Mező G,
Angyal J, Vasas N, Kis A, Szabó É, Emri M, Bíró T, et al: In vivo
imaging of Aminopeptidase N (CD13) receptors in experimental renal
tumors using the novel radiotracer 68Ga-NOTA-c(NGR). Eur
J Pharm Sci. 69:61–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shao Y, Liang W, Kang F, Yang W, Ma X, Li
G, Zong S, Chen K and Wang J: A direct comparison of tumor
angiogenesis with 68Ga-labeled NGR and RGD peptides in
HT-1080 tumor xenografts using microPET imaging. Amino Acids.
46:2355–2364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang F, Ma W, Ma X, Shao Y, Yang W, Chen
X, Li L and Wang J: Propranolol inhibits glucose metabolism and
18F-FDG uptake of breast cancer through
posttranscriptional downregulation of hexokinase-2. J Nucl Med.
55:439–445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao M, Yang W, Zhang M, Li G, Wang S,
Wang Z, Ma X, Kang F and Wang J: Evaluation of
68Ga-labeled iNGR peptide with tumor-penetrating motif
for microPET imaging of CD13-positive tumor xenografts. Tumour
Biol. 37:12123–12131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alberici L, Roth L, Sugahara KN, Agemy L,
Kotamraju VR, Teesalu T, Bordignon C, Traversari C, Rizzardi GP and
Ruoslahti E: De novo design of a tumor-penetrating peptide. Cancer
Res. 73:804–812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen H, Niu G, Wu H and Chen X: Clinical
application of radiolabeled RGD peptides for PET imaging of
integrin αvβ3. Theranostics. 6:78–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma W, Shao Y, Yang W, Li G, Zhang Y, Zhang
M, Zuo C, Chen K and Wang J: Evaluation of 188Re-labeled
NGR-VEGI protein for radioimaging and radiotherapy in mice bearing
human fibrosarcoma HT-1080 xenografts. Tumour Biol. 37:9121–9129.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tillmanns J, Schneider M, Fraccarollo D,
Schmitto JD, Länger F, Richter D, Bauersachs J and Samnick S: PET
imaging of cardiac wound healing using a novel
[68Ga]-labeled NGR probe in rat myocardial infarction.
Mol Imaging Biol. 17:76–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hendrikx G, De Saint-Hubert M, Dijkgraaf
I, Bauwens M, Douma K, Wierts R, Pooters I, Van den Akker NM,
Hackeng TM, Post MJ, et al: Molecular imaging of angiogenesis after
myocardial infarction by 111In-DTPA-cNGR and
99mTc-sestamibi dual-isotope myocardial SPECT. EJNMMI
Res. 5:22015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng K, Liang N, Zhang J, Lang L, Zhang
W, Li S, Zhao J, Niu G, Li F, Zhu Z, et al:
68Ga-NOTA-PRGD2 PET/CT for integrin imaging in patients
with lung cancer. J Nucl Med. 56:1823–1827. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wan W, Guo N, Pan D, Yu C, Weng Y, Luo S,
Ding H, Xu Y, Wang L, Lang L, et al: First experience of
18F-alfatide in lung cancer patients using a new
lyophilized kit for rapid radiofluorination. J Nucl Med.
54:691–698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang F, Wang S, Tian F, Zhao M, Zhang M,
Wang Z, Li G, Liu C, Yang W, Li X, et al: Comparing the diagnostic
potential of 68Ga-Alfatide II and 18F-FDG in
differentiating between non-small cell lung cancer and
tuberculosis. J Nucl Med. 57:672–677. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu C, Pan D, Mi B, Xu Y, Lang L, Niu G,
Yang M, Wan W and Chen X: 18F-Alfatide II PET/CT in
healthy human volunteers and patients with brain metastases. Eur J
Nucl Med Mol Imaging. 42:2021–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iagaru A, Mosci C, Shen B, Chin FT, Mittra
E, Telli ML and Gambhir SS: 18F-FPPRGD2 PET/CT: Pilot
phase evaluation of breast cancer patients. Radiology. 273:549–559.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu Z, Yin Y, Zheng K, Li F, Chen X, Zhang
F and Zhang X: Evaluation of synovial angiogenesis in patients with
rheumatoid arthritis using 68Ga-PRGD2 PET/CT: A
prospective proof-of-concept cohort study. Ann Rheum Dis.
73:1269–1272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Haubner R, Finkenstedt A, Stegmayr A,
Rangger C, Decristoforo C, Zoller H and Virgolini IJ:
[68Ga]NODAGA-RGD - Metabolic stability, biodistribution,
and dosimetry data from patients with hepatocellular carcinoma and
liver cirrhosis. Eur J Nucl Med Mol Imaging. 43:2005–2013. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sherigar JM, Gayam V, Khan A, Mukhtar O,
Arefiev Y, Khalid M, Siddiqui I, Rangaraju AM, Budhathoki N,
Mansour M, et al: Clinical efficacy and tolerability of
direct-acting antivirals in elderly patients with chronic hepatitis
C. Eur J Gastroenterol Hepatol. 29:767–776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He X, Hong Y, Wang X, Zhang X, Long J, Li
H, Zhang B, Chen S, Liu Q, Li H, et al: Identification and clinical
significance of an elevated level of serum aminoacylase-1
autoantibody in patients with hepatitis B virus-related liver
cirrhosis. Mol Med Rep. 14:4255–4262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li F, Yan H, Wang J, Li C, Wu J, Wu S, Rao
S, Gao X and Jin Q: Non-invasively differentiating extent of liver
fibrosis by visualizing hepatic integrin αvβ3 expression with an
MRI modality in mice. Biomaterials. 102:162–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma W, Kang F, Wang Z, Yang W, Li G, Ma X,
Li G, Chen K, Zhang Y and Wang J: 99mTc-labeled
monomeric and dimeric NGR peptides for SPECT imaging of CD13
receptor in tumor-bearing mice. Amino Acids. 44:1337–1345. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Berman JJ: Tumor classification: Molecular
analysis meets Aristotle. BMC Cancer. 4:102004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pantel AR and Mankoff DA: Molecular
imaging to guide systemic cancer therapy: Illustrative examples of
PET imaging cancer biomarkers. Cancer Lett. 387:25–31. 2017.
View Article : Google Scholar : PubMed/NCBI
|