Introduction
Neuroblastomas (NBs) are known for their
unpredictable behavior; some spontaneously regress, some mature,
whereas others develop into aggressive forms (1). Moreover, around 7% of all tumors
observed in children are NBs, next only to leukemia and
brain/central nervous system tumors (2). Notably, NB accounts for approximately
15% of childhood cancer-related mortality (2,3).
Though aggressive treatment strategies such as surgery, radiation,
and/or chemotherapy have improved in recent decades, the prognosis
for patients with disseminated NB is grim, with a 5-year survival
rate of ~30% (4,5).
Multi-agent chemotherapy, including cisplatin,
rapamycin, 13-cis-retinoic acid (CRA), and vincristine, is
the conventional therapy for patients with advanced stages of NB
(6–8). However, drug resistance arises in the
majority of stage IV and relapsed NB, often leading to treatment
failure (9,10). Furthermore, aggressive therapy also
causes severe, long-term side effects in patients, including
deafness, cardiac failure, and secondary malignancies (3,11).
Cisplatin is one of the frontline chemotherapeutic drugs for NB and
widely used in clinical therapy (12). Unfortunately, due to acquired
cisplatin resistance of NBs, the prognosis of advanced NB patients
after cisplatin treatment is still poor (13–15).
Thus, the development of novel antitumor strategies is essential to
overcome cisplatin resistance and to prevent tumor progression.
Rearranged during transfection (RET) is a receptor
tyrosine kinase that is expressed in various neurons including NB.
Activation of RET is correlated with poor progression of NB and
associated with promoting cell proliferation and metastasis
(16,17). RET is triggered by anaplastic
lymphoma kinase (ALK) in NB and inhibition of RET impaired tumor
growth in vivo in ALK mutated NB (16). A previous study using cell lines and
primary cancer samples has also demonstrated a correlation between
high C-X-C chemokine receptor type 4 (CXCR4) expression levels in
NB cells and increased occurrence of bone marrow metastases
(18). CXCR4 was also demonstrated
to support the development of NB primary tumors (19). Thus, RET and CXCR4 are the potential
therapeutic targets of NB.
Vandetanib (Caprelsa, AstraZeneca Pharmaceuticals)
is a small-molecule receptor tyrosine kinase inhibitor of VEGF
receptor 2 (VEGFR2), EGF receptor (EGFR), and RET tyrosine kinase
activity as well as mutated RET (20,21).
Vandetanib is widely used as a chemotherapeutic agent in thyroid
carcinoma (22–24), glioblastoma (25), non-small cell lung cancer (26), and pulmonary adenocarcinoma
(27). Vandetanib has been
demonstrated to inhibit NB migration and invasion by reducing CXCR4
expression (28). The combination
of vandetanib with CRA was more effective in reducing tumor growth
than either treatment alone in NB (29). However, whether vandetanib exhibits
antitumor activity in cisplatin-resistant NB is still unclear. In
the present study, we aimed to determine the potential of
vandetanib in cisplatin-resistant NB therapy. The NB cell line
SH-SY5Y, with a strong ability for proliferation and invasion, and
which is established from a metastatic bone tumor, was used in our
study.
Materials and methods
Primary NB tumors
In total, 30 diagnostic primary NB tumor samples
were obtained from the Department of Pediatric Surgical Oncology,
Children's Hospital of Chongqing Medical University. Research was
approved by the Research Ethics Committees of Chongqing Medical
University. Written informed consent was signed by the parents or
guardians of the pediatric patients. The patients were classified
as cisplatin-resistant and -sensitive according to the prognosis of
patients with cisplatin treatment and the expression of ERCC1 gene,
a marker of cisplatin sensitivity. Before surgery, four cisplatin
treatments, combined with vincristine, cyclophosphamide and
etoposide, were performed. During the treatments, the tumor volume
was assessed by B ultrasound examination once a month. Following
surgery, the tumor tissues were collected for ERCC1 mRNA detection.
The patients with reduction of tumor volume and ERCC1 negative
expression were classified as cisplatin-sensitive. The patients
without reduction of tumor volume and ERCC1 positive expression
were classified as cisplatin-resistant.
Cell culture and treatment
The NB cell line SH-SY5Y was purchased from ATCC
(Manassas, VA, USA). The cells were grown at 37°C in 5%
CO2 in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented
with 10% heat-inactivated fetal bovine serum (EMD Millipore,
Billerica, MA, USA), L-glutamine, sodium pyruvate, nonessential
amino acids, and penicillin/streptomycin (Sigma-Aldrich, St. Louis,
MO, USA). The SH-SY5Y cells were maintained at the initial
cisplatin concentration of 10 µM (IC50). The dose of
cisplatin was titrated gradually to a final concentration of 80 µM
after 6 weeks. The selected cisplatin-resistant SH-SY5Y cells were
named SH-SY5Y-R cells, and the cisplatin-sensitive SH-SY5Y cells
were named SH-SY5Y-S cells. SH-SY5Y-R cells were established and
then were maintained in DMEM medium with 10% FBS containing 80 µM
cisplatin.
Cell viability assay
Cells (1,000) were plated in each well of a 96-well
plate. Then cisplatin at different concentrations and vandetanib
(0, 2.5, 5 and 10 µM) were added into the cell and incubated for
24–72 h. Cell viability was evaluated by Cell Counting Kit-8 (CCK8)
assay (Dako; Agilent Technologies, Inc., Dallas, TX, USA). The
relative cell viability was calculated as the OD 450 nm of the
treated group/the OD 450 nm of the blank group. The IC50
was calculated using SPSS (version 21.0; IBM SPSS, Armonk, NY, USA)
according to the guidelines published by Sebaugh (30).
Colony formation assay
Cells (1,000) were plated in each well of a 6-well
plate. Then vandetanib (5 µM) was added into the cells and
incubated for 7–10 days. The plate was fixed with 4%
paraformaldehyde for 15 min and stained with crystal violet
(Beyotime, Beijing, China) for 10 min at room temperatures. The
number of colonies formed were counted and analyzed.
Invasion assay
Following dilution with DMEM medium (1:5), Matrigel
was added into an 8.0-µm Transwell (BD, Franklin Lakes, NJ, USA).
Then, 30 min later, 2×104 SH-SY5Y-S or SH-SY5Y-R cells
were added into the upper well containing serum-free medium, with
or without vandetanib (5 µM) treatment. The lower well was fixed
with DMEM medium containing 10% FBS. Subsequently, 24 h later, the
Transwell was fixed with 4% paraformaldehyde and stained with
crystal violet (Beyotime, Beijing, China). The invaded cells were
counted and analyzed.
Western blotting
Western blotting analysis was performed as
previously described (31).
Briefly, SH-SY5Y-R cells with or without vandetanib treatment were
lysed in RIPA lysis buffer (Beyotime, Beijing, China) containing 1%
protease inhibitor cocktail (EMD Millipore). Following
concentration determination by BCA assay (Beyotime), 10 µg of total
protein was added and separated by 10–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The protein
was transferred to a polyvinylidene fluoride (PVDF) membrane (EMD
Millipore) and blocked with 5% non-fat milk in TBS/T buffer. The
following antibodies were used: p-RET (rabbit monoclonal antibody;
cat no. 3221; 1:800; Cell Signaling Technology, Inc., Danvers, MA,
USA), CXCR4 (rabbit monoclonal antibody; cat no. ab124824; 1:1,000;
Abcam, Cambridge, UK), and GAPDH (mouse monoclonal antibody; cat
no. sc-293335; 1:5,000; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) was used as a loading control. The density of each band
was assessed with ImageJ software (NIH, Bethesda, MA, USA).
Animal study
BALB/c-nu mice (5–6 weeks old, 18–20 g) were
purchased from the Model Animal Center of the Nanjing University
and housed in barrier facilities on a 12-h light/dark cycle. All
experimental procedures were approved by the Institutional Animal
Care and Use Committee of Chongqing Medical University. All mouse
care and experiments were carried out in accordance with
institutional guidelines concerning animal use and care of
Chongqing Medical University. One week after receiving the mice,
5×106 SH-SY5Y-R cells were subcutaneously injected into
the left dorsal flank. When the tumor reached ~100 mm3,
the mice were randomly assigned to four groups (n=4/group). The
mice were injected intratumorally with 100 µl PBS, 20 nmol
cisplatin diluted in 100 µl PBS, 100 nmol cisplatin diluted in 100
µl PBS and 0.6 mg vandetanib diluted in 100 µl PBS every day. The
tumor size was assessed every five days with calipers by the same
investigators and the tumor volume was calculated using the
equation (length × width2 × 0.52). On day 35 post-tumor
cell injection, the animals were euthanized, and the tumors were
excised, weighed, and paraffin-embedded.
Immunostaining and TUNEL assay
Immunostaining was performed as previously described
(32). The following antibodies
were used: Anti-PCNA antibody (mouse monoclonal antibody; cat no.
sc25280; 1:100; Santa Cruz Biotechnology, Inc.), anti-p-RET
antibody (rabbit monoclonal antibody; cat no. 3221; 1:200; Cell
Signaling Technology, Inc.), anti-CXCR4 antibody (rabbit monoclonal
antibody; cat no. ab124824; 1:200; Abcam), anti-CD31 antibody
(mouse monoclonal antibody; cat no. 555444; 1:100; BD Biosciences).
All specimens were evaluated using Olympus BX600 microscope and
Spot Flex camera (Olympus, Tokyo, Japan). The positive and total
cells in 3–5 random fields were counted and analyzed.
Apoptotic DNA fragmentation was examined using an
in situ DeadEnd™ Fluorometric TUNEL System assay kit
(Promega, Madison, WI, USA) according to the manufacturer's
protocol. The localized green fluorescence of apoptotic cells from
the fuorescein-12-dUTP was detected by fluorescence microscopy. The
cell nuclei were stained with DAPI (Beyotime). The apoptotic cells
in 5 random fields were counted and analyzed.
Statistical analysis
All statistical analyses were carried out using SPSS
19.0 statistical software (SPSS Inc., Chicago, IL, USA). The
2-tailed Student's t-test was used to evaluate the significance of
differences between two groups of data and one-way ANOVA was used
for statistics in multiple groups in all pertinent experiments. All
experiments were performed 3–5 times. P<0.05 was considered to
indicate a statistically significant result.
Results
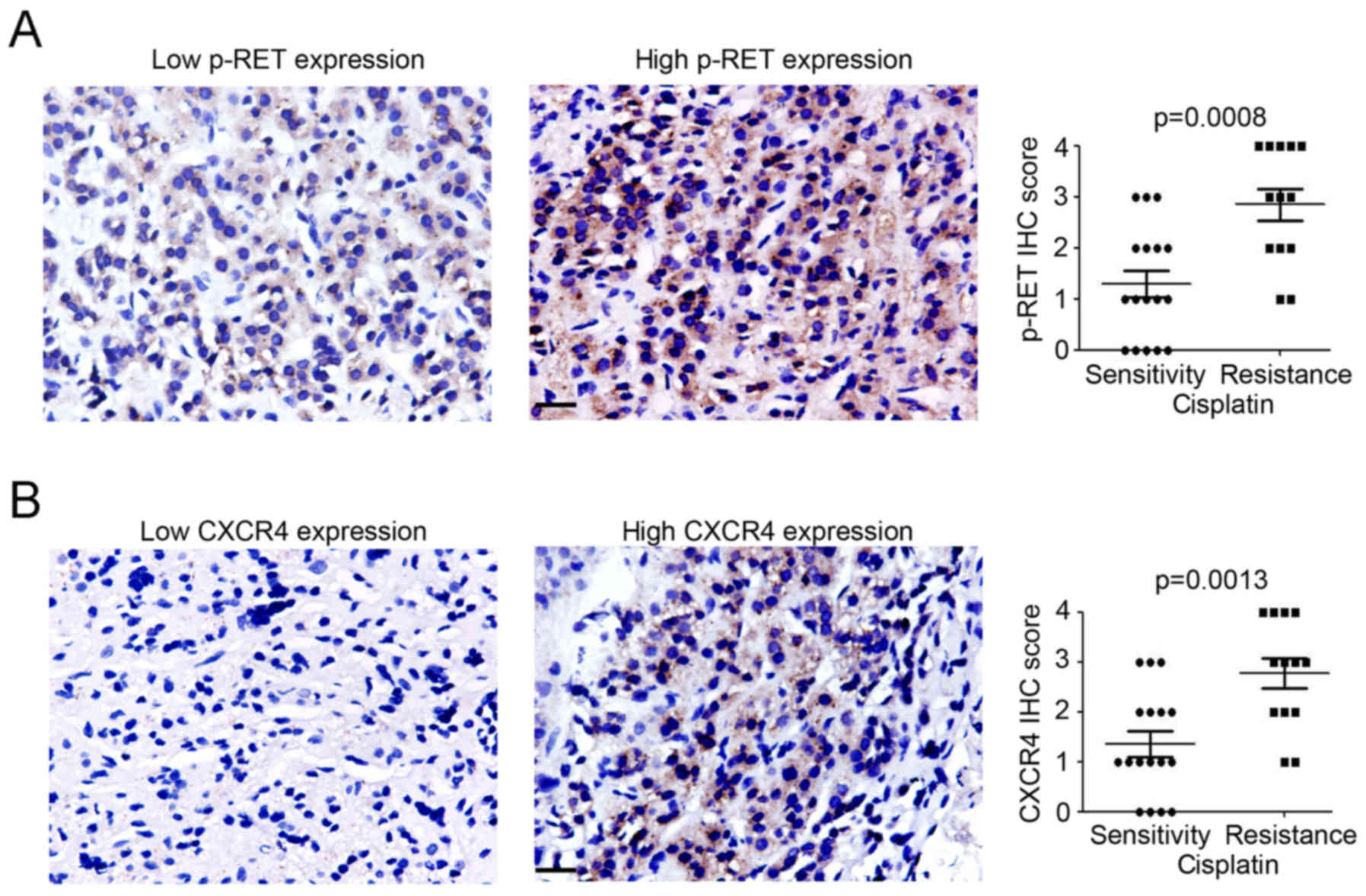
High expression of p-RET and CXCR4 in
cisplatin-resistant NB tissues
To determine the potential utility of vandetanib in
cisplatin-resistant NB patients, IHC staining was employed to
analyze p-RET and CXCR4 expression in 30 NB tissue samples, which
were classified as originating from either cisplatin-sensitive or
-resistant patients. As shown in Fig.
1, increased p-RET- and CXCR4-positive cells were found in the
cisplatin-resistant NB tissues. This suggested that p-RET and CXCR4
may play a crucial role in maintaining the cisplatin resistance of
NB tissues.
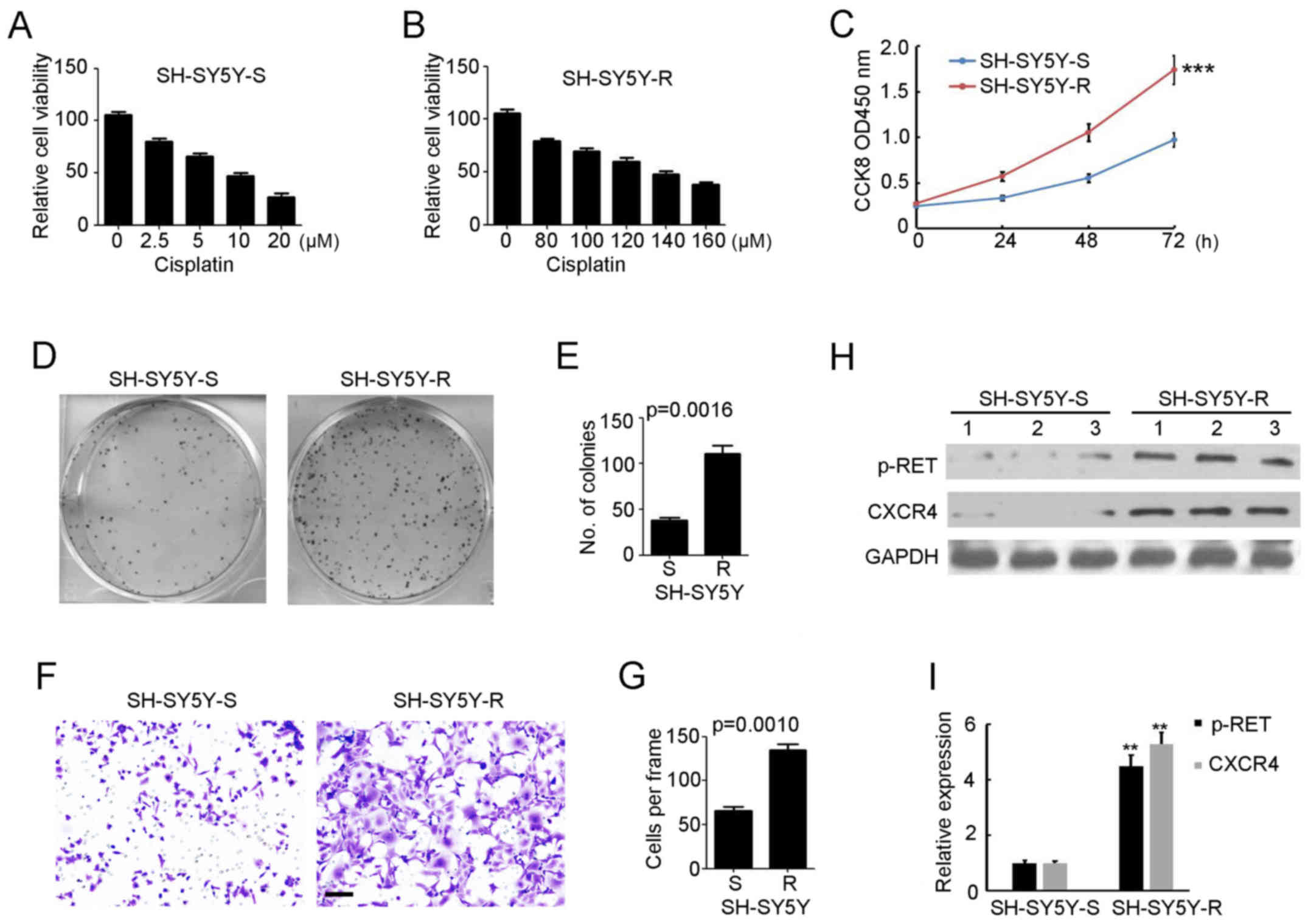
High expression of p-RET and CXCR4 in
cisplatin-resistant NB cells
To further investigate the expression of p-RET and
CXCR4 in cisplatin-sensitive and -resistant NB cells, cisplatin was
used to treat SH-SY5Y cells, and the cisplatin-resistant cells were
selected and named SH-SY5Y-R. As shown in Fig. 2A and B, the IC50 values
of cisplatin for SH-SY5Y-S (cisplatin-sensitive SH-SY5Y cells) and
SH-SY5Y-R were approximately 10 and 130 µM, respectively. These
results were identical to a previous study conducted by our
laboratory (33). A CCK8 assay
demonstrated higher viability of proliferation in SH-SY5Y-R cells
compared with SH-SY5Y-S cells (Fig.
2C). Furthermore, increased colony formation (Fig. 2D and E) and invading cells (Fig. 2F and G) were observed in the
SH-SY5Y-R cells as determined by colony formation assay and
Matrigel invasion assay in vitro, respectively. Western
blotting demonstrated that the expression of p-RET and CXCR4 was
significantly increased in SH-SY5Y-R cells (Fig. 2H and I). Collectively, these results
indicated that cisplatin-resistant NB cells exhibited increased
malignancy and invasive properties, combined with upregulation of
p-RET and CXCR4 expression.
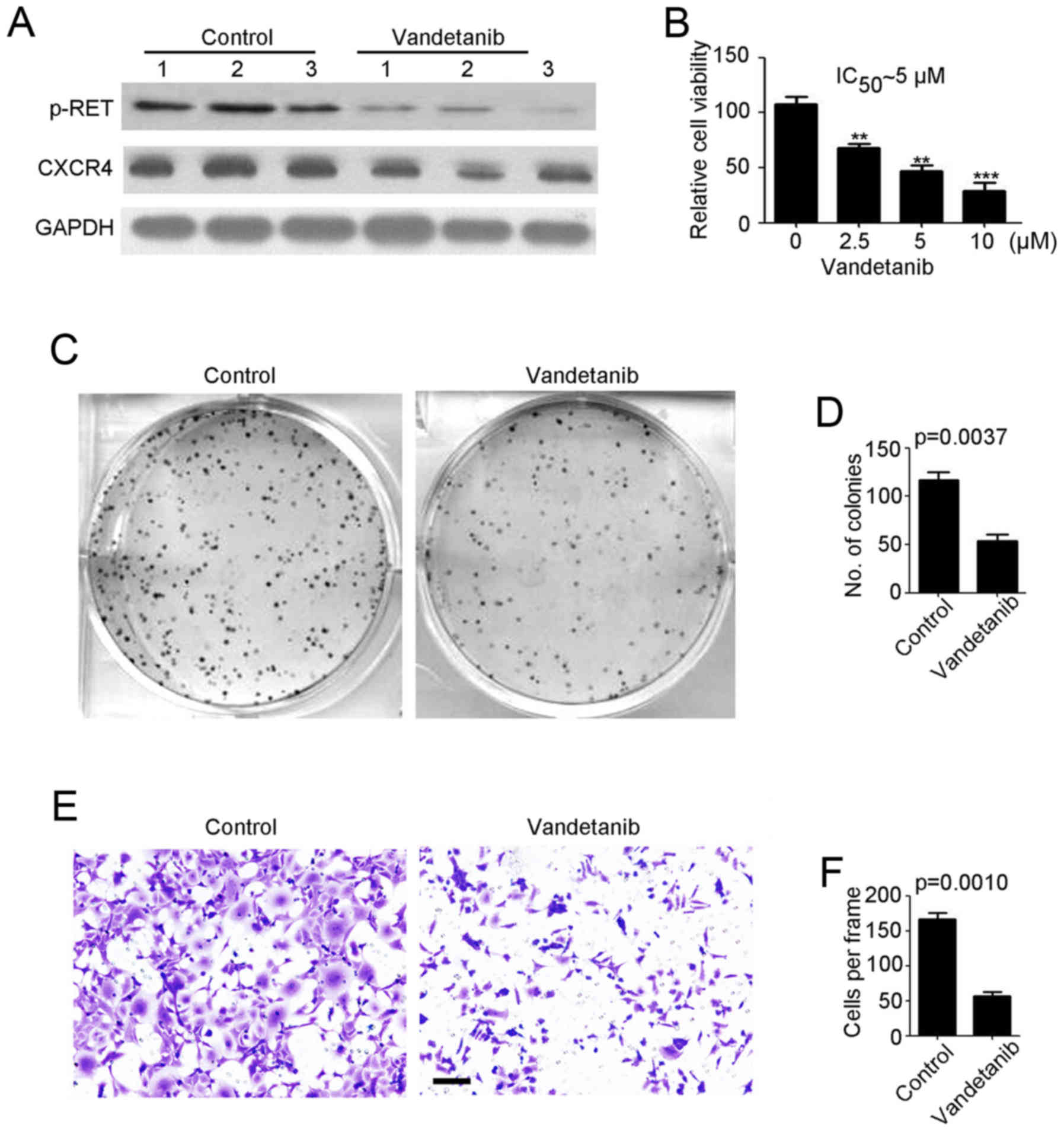
Vandetanib inhibits
cisplatin-resistant NB tumorigenesis and invasion in vitro
We employed vandetanib to treat cisplatin-resistant
NB cells. As shown in Fig. 3A,
vandetanib efficiently reduced p-RET and CXCR4 expression in
SH-SY5Y-R cells. A cell viability assay demonstrated that SH-SY5Y-R
cell proliferation was significantly inhibited by vandetanib in a
concentration-dependent manner (Fig.
3B). In addition, we demonstrated that the IC50 of
vandetanib for SH-SY5Y-R cells was ~5 µM (Fig. 3B). Thus, 5 µM vandetanib was used in
the following experiments. A colony formation assay, revealed that
fewer colonies were formed by the vandetanib-treated SH-SY5Y-R
(Fig. 3C and D) cells. Then, a
Transwell invasion assay was performed to determine the effects of
vandetanib on NB. We determined that vandetanib markedly prevented
SH-SY5Y-R cell invasion (Fig. 3E and
F). In summary, vandetanib may be an effective agent for
cisplatin-resistant NB therapy.
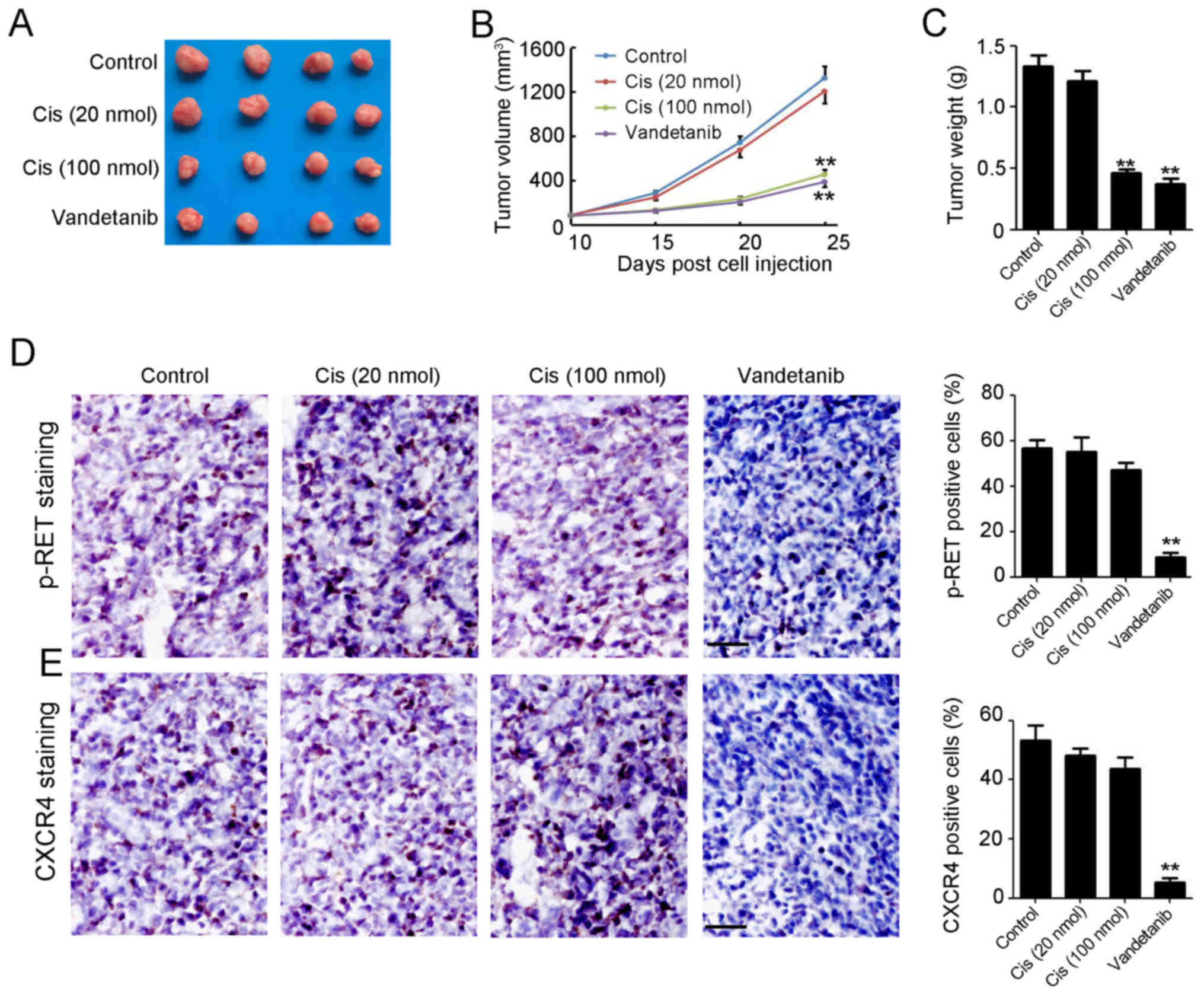
Vandetanib inhibits
cisplatin-resistant NB tumor growth in vivo
To further investigate whether vandetanib inhibited
cisplatin-resistant NB tumor growth and enhanced sensitivity of NB
to cisplatin in vivo, SH-SY5Y-R cells were injected into the
flank of female wild-type (WT) BALB/c nude mice to establish a
subcutaneous tumor model. When the tumor volume reached ~100
mm3, vandetanib and cisplatin were administered to the
mice. As shown in Fig. 4A,
treatment of mice with a high dose of cisplatin (100 nmol/day)
markedly reduced tumor volume (Fig.
4B) and weight (Fig. 4C) by
65.7 and 65.4%, respectively. However, a low-dose of cisplatin had
no observed inhibitory effect on tumor volume (Fig. 4B) or weight (Fig. 4C). In contrast, injection of
vandetanib alone significantly reduced tumor volume (Fig. 4B) and weight (Fig. 4C) by 70.8 and 71.8%, respectively.
IHC staining demonstrated a reduction in p-RET and CXCR4 expression
in vandetanib-treated NB tumors (Fig.
4D and E). The aforementioned data demonstrated that vandetanib
may be an effective agent for cisplatin-resistant NB therapy.
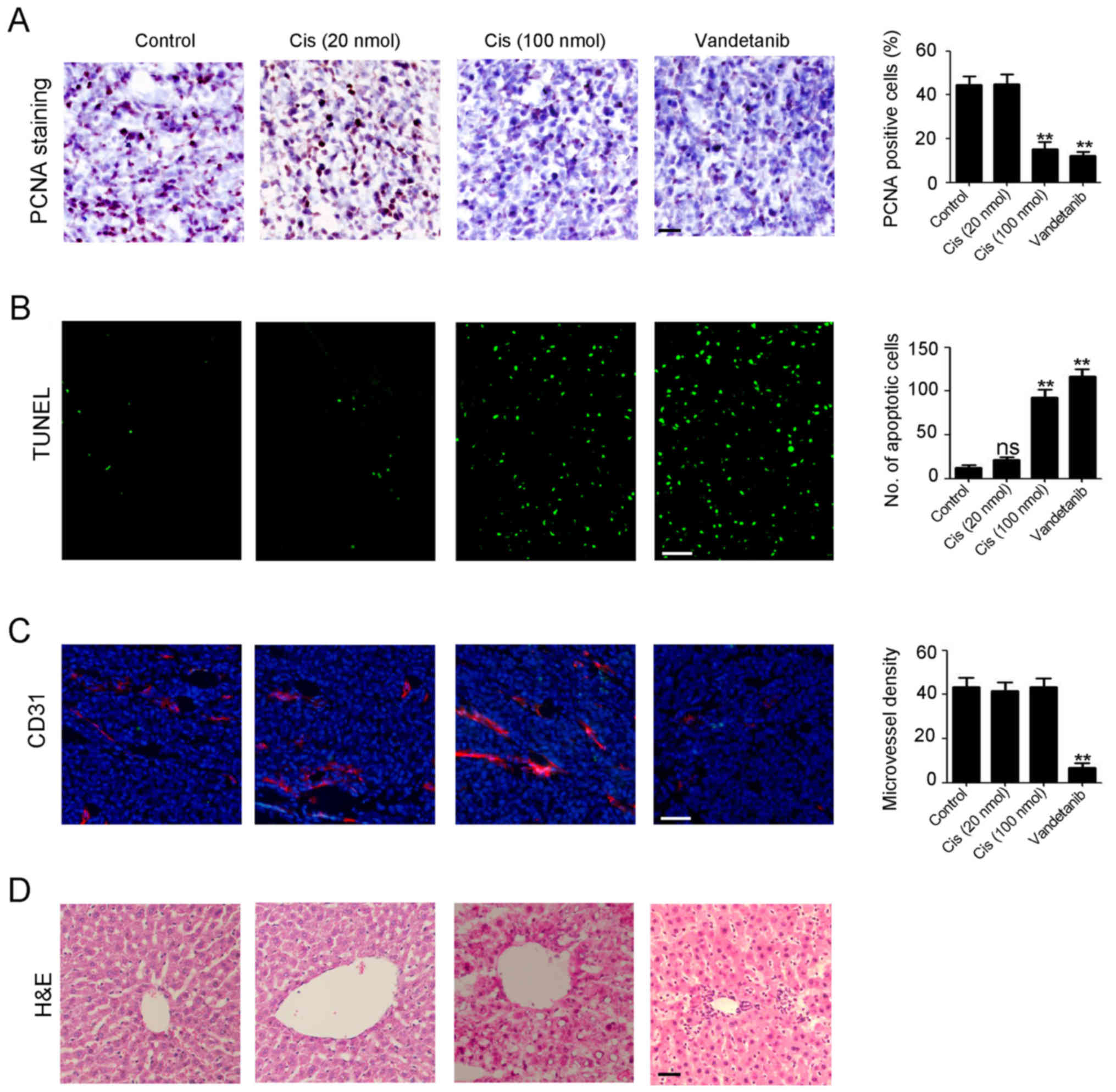
Vandetanib exerts low toxicity in
cisplatin-resistant NB treatment
PCNA staining and TUNEL assay were performed to
detect the proliferative and apoptotic cells in NB tissues. Less
proliferative cells were revealed in both high-dose
cisplatin-treated and vandetanib-treated tumors (Fig. 5A). Additionally, more apoptotic
cells were revealed in the aforementioned tumors (Fig. 5B). Furthermore, decreased
angiogenesis was observed in the vandetanib-treated tumors than in
those exposed to cisplatin alone (Fig.
5C). Notably, severe liver toxicity occurred in the high-dose
cisplatin treatment group, in contrast to the low dose cisplatin
and control groups (Fig. 5D). No
liver toxicity was observed in the vandetanib treatment group
(Fig. 5D). The aforementioned data
demonstrated that vandetanib exhibited low toxicity in
cisplatin-resistant NB treatment.
Discussion
New treatment strategies are clearly needed for
children with recurrent or refractory NBs. In the present study we
demonstrated greater expression of p-RET and CXCR4 in
cisplatin-resistant NB tumors compared with cisplatin-sensitive
tumors. Vandetanib rapidly inhibited cisplatin-resistant NB cell
proliferation, tumorigenesis, and invasion. Vandetanib alone
induced a significant reduction in cisplatin-resistant NB tumor
growth in vivo in a xenograft mouse model. While high-dose
cisplatin treatment yielded similar results, it caused severe liver
toxicity in mice.
Cisplatin is one of the frontline chemotherapeutic
drugs for NB and widely used in clinical therapy (12), however the use of cisplatin is
limited due to the therapy resistance (13–15).
In our study, we determined that cisplatin-resistant cells
possessed more aggressive characteristics. Furthermore, we
determined that p-RET and CXCR4 expression was significantly
upregulated in cisplatin-resistant NB cells and tumor tissues of
patients. This indicated that p-RET and CXCR4 upregulation may be
an adaptation to cisplatin treatment and could play a crucial role
in NB cisplatin resistance. Furthermore, treatment of
chemosensitive NB cells with cisplatin reversibly increased EGFR
expression, whereas cisplatin-resistant cells revealed enhanced
EGFR expression independent of the presence of cisplatin (34). Inhibition of EGFR, using gefitinib,
revealed minor chemosensitizing effects in NB (35), whereas EGFR-targeted antibodies and
growth factor toxins scFv(14E1)-Pseudomonas exotoxin A (ETA) and
TGF-α-ETA exerted anticancer effects in NB cell lines (34). In the present study we revealed that
EGFR expression was upregulated in cisplatin-resistant SH-SY5Y
cells (data not shown), but its expression was not inhibited by
vandetanib at the concentration of 5 µM (data not shown).
Therefore, EGFR may be another adaptation to cisplatin treatment in
NB, but it was not the effector of vandetanib in the inhibition of
cisplatin-resistant NB tumor progression at low concentrations.
Increasing the concentration of cisplatin is the
most common strategy to offset cisplatin resistance. However,
high-dose cisplatin may cause severe liver toxicity, which is the
main side-effect of cisplatin therapy (36,37).
In the present study we demonstrated that vandetanib was as
effective as high-dose cisplatin in impairing cisplatin-resistant
NB subcutaneous tumor growth in vivo. Notably, less liver
toxicity was observed in the vandetanib treatment group than in the
high-dose cisplatin treatment group. These results provide solid
evidence, demonstrating the advantages of vandetanib in the
treatment of cisplatin-resistant NB. Whether combination of
vandetanib with cisplatin produces a better therapeutic effect in
NB will be investigated in a future study. Different concentrations
of vandetanib will be used to treat cisplatin resistance in NB
after combination with different concentrations of cisplatin. The
potential synergy will be analyzed according to previous models
(38,39).
Previously, RET rearrangements have been reported in
NB (17). Activated ALK triggered
RET upregulation in mouse sympathetic ganglia at birth, as well as
in murine and human NB (16). RET
inhibition strongly impaired tumor growth in vivo in both
MYCN/KI AlkR1279Q and MYCN/KI AlkF1178L mice (16). Inhibition of RET phosphorylation by
vandetanib treatment resulted in the induction of apoptosis in the
majority of NB cell lines in vitro, and inhibited tumor
growth in a mouse xenograft model, via both reduction in tumor
vascularity and induction of apoptosis (16). Notably, in the present study we
first demonstrated that inhibition of RET phosphorylation resulted
in the inhibition of proliferation, invasion, and induction of
apoptosis in cisplatin-resistant NB cells. Vandetanib treatment was
an efficient therapy for cisplatin-resistant NB tumor growth,
inducing apoptosis and inhibiting proliferation and
angiogenesis.
CXCR4 has been demonstrated to be one of the most
frequently expressed chemokines, affecting tumor cell
proliferation, survival, and metastasis in various cancers
(40,41). In NB, CXCR4 has been proposed to be
involved in the mechanisms by which cells metastasize to specific
sites from the primary site (18,42).
Greater expression of CXCR4 in NBs was correlated with high-stage
disease and worse clinical outcome than lower expression of CXCR4
(43,44). Functional studies have demonstrated
that inhibition of CXCR4 was an efficient strategy to inhibit NB
cell proliferation and metastasis (45–47). A
previous study by Ding et al demonstrated the inhibitory
role of vandetanib on NB cell migration and invasion through
downregulation of CXCR4 and MMP-14 expression (28). The present study for the first time
also indicated that vandetanib treatment caused a significant
decrease in CXCR4 expression and cisplatin-resistant NB cell
invasion. Ding et al demonstrated that the IC50
of vandetanib for SH-SY5Y cells was ~10 µM. However we demonstrated
that the IC50 of vandetanib for SH-SY5Y-R cells was ~5
µM. This could be attributed to the higher expression of CXCR4 in
cisplatin-resistant SH-SY5Y cells, which enhances the sensitivity
of vandetanib.
In conclusion, the present study indicated that
vandetanib is an efficient therapeutic agent for
cisplatin-resistant NBs, inhibiting p-RET and CXCR4 expression. It
identified vandetanib as a potential therapy for
cisplatin-resistant NBs. In particular, the combination of
vandetanib with cisplatin may represent a novel therapeutic
strategy in NB patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nakazawa A, Haga C, Ohira M, Okita H,
Kamijo T and Nakagawara A: Correlation between the international
neuroblastoma pathology classification and genomic signature in
neuroblastoma. Cancer Sci. 106:766–771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oeffinger KC, Mertens AC, Sklar CA,
Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie
W, Kadan-Lottick NS, et al: Chronic health conditions in adult
survivors of childhood cancer. N Engl J Med. 355:1572–1582. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet (London, England). 369:2106–2120. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tao X: Antibody therapy and neuroblastoma.
N Engl J Med. 364:289–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barone G, Anderson J, Pearson AD, Petrie K
and Chesler L: New strategies in neuroblastoma: Therapeutic
targeting of MYCN and ALK. Clin Cancer Res. 19:5814–5821. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brodeur GM, Iyer R, Croucher JL, Zhuang T,
Higashi M and Kolla V: Therapeutic targets for neuroblastomas.
Expert Opin Ther Targets. 18:277–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morgenstern DA, Baruchel S and Irwin MS:
Current and future strategies for relapsed neuroblastoma:
Challenges on the road to precision therapy. J Pediatr Hematol
Oncol. 35:337–347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alisi A, Cho WC, Locatelli F and Fruci D:
Multidrug resistance and cancer stem cells in neuroblastoma and
hepatoblastoma. Int J Mol Sci. 14:24706–24725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fruci D, Cho WC, Nobili V, Locatelli F and
Alisi A: Drug transporters and multiple drug resistance in
pediatric solid tumors. Curr Drug Metab. 17:308–316. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matthay KK, Reynolds CP, Seeger RC,
Shimada H, Adkins ES, Haas-Kogan D, Gerbing RB, London WB and
Villablanca JG: Long-term results for children with high-risk
neuroblastoma treated on a randomized trial of myeloablative
therapy followed by 13-cis-retinoic acid: A children's oncology
group study. J Clin Oncol. 27:1007–1013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Bernardi B, Carli M, Casale F, Corciulo
P, Cordero di Montezemolo L, De Laurentis C, Bagnulo S, Brisigotti
M, Marchese N, Garaventa A, et al: Standard-dose and high-dose
peptichemio and cisplatin in children with disseminated poor-risk
neuroblastoma: Two studies by the Italian Cooperative Group for
Neuroblastoma. J Clin Oncol. 10:1870–1878. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Landier W, Knight K, Wong FL, Lee J,
Thomas O, Kim H, Kreissman SG, Schmidt ML, Chen L, London WB, et
al: Ototoxicity in children with high-risk neuroblastoma:
Prevalence, risk factors, and concordance of grading scales-a
report from the Children's Oncology Group. J Clin Oncol.
32:527–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vella S, Penna I, Longo L, Pioggia G,
Garbati P, Florio T, Rossi F and Pagano A: Perhexiline maleate
enhances antitumor efficacy of cisplatin in neuroblastoma by
inducing over-expression of NDM29 ncRNA. Sci Rep. 5:181442015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ryan J, Tivnan A, Fay J, Bryan K, Meehan
M, Creevey L, Lynch J, Bray IM, O'Meara A, Tracey L, et al:
MicroRNA-204 increases sensitivity of neuroblastoma cells to
cisplatin and is associated with a favourable clinical outcome. Br
J Cancer. 107:967–976. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cazes A, Lopez-Delisle L, Tsarovina K,
Pierre-Eugene C, De Preter K, Peuchmaur M, Nicolas A, Provost C,
Louis-Brennetot C, Daveau R, et al: Activated Alk triggers
prolonged neurogenesis and Ret upregulation providing a therapeutic
target in ALK-mutated neuroblastoma. Oncotarget. 5:2688–2702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Futami H and Sakai R: RET protein promotes
non-adherent growth of NB-39-nu neuroblastoma cell line. Cancer
Sci. 100:1034–1039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meier R, Mühlethaler-Mottet A, Flahaut M,
Coulon A, Fusco C, Louache F, Auderset K, Bourloud KB, Daudigeos E,
Ruegg C, et al: The chemokine receptor CXCR4 strongly promotes
neuroblastoma primary tumour and metastatic growth, but not
invasion. PLoS One. 2:e10162007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liberman J, Sartelet H, Flahaut M,
Mühlethaler-Mottet A, Coulon A, Nyalendo C, Vassal G, Joseph JM and
Gross N: Involvement of the CXCR7/CXCR4/CXCL12 axis in the
malignant progression of human neuroblastoma. PLoS One.
7:e436652012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wedge SR, Ogilvie DJ, Dukes M, Kendrew J,
Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove
HL, et al: ZD6474 inhibits vascular endothelial growth factor
signaling, angiogenesis, and tumor growth following oral
administration. Cancer Res. 62:4645–4655. 2002.PubMed/NCBI
|
|
21
|
Vidal M, Wells S, Ryan A and Cagan R:
ZD6474 suppresses oncogenic RET isoforms in a Drosophila model for
type 2 multiple endocrine neoplasia syndromes and papillary thyroid
carcinoma. Cancer Res. 65:3538–3541. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wells SA Jr, Gosnell JE, Gagel RF, Moley
J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J and
Schlumberger M: Vandetanib for the treatment of patients with
locally advanced or metastatic hereditary medullary thyroid cancer.
J Clin Oncol. 28:767–772. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fox E, Widemann BC, Chuk MK, Marcus L,
Aikin A, Whitcomb PO, Merino MJ, Lodish M, Dombi E, Steinberg SM,
et al: Vandetanib in children and adolescents with multiple
endocrine neoplasia type 2B associated medullary thyroid carcinoma.
Clin Cancer Res. 19:4239–4248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wells SA Jr, Robinson BG, Gagel RF, Dralle
H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR,
et al: Vandetanib in patients with locally advanced or metastatic
medullary thyroid cancer: A randomized, double-blind phase III
trial. J Clin Oncol. 30:134–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee EQ, Kaley TJ, Duda DG, Schiff D,
Lassman AB, Wong ET, Mikkelsen T, Purow BW, Muzikansky A,
Ancukiewicz M, et al: A multicenter, phase II, randomized,
noncomparative clinical trial of radiation and temozolomide with or
without vandetanib in newly diagnosed glioblastoma patients. Clin
Cancer Res. 21:3610–3618. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siegfried JM, Gubish CT, Rothstein ME,
Henry C and Stabile LP: Combining the multitargeted tyrosine kinase
inhibitor vandetanib with the antiestrogen fulvestrant enhances its
antitumor effect in non-small cell lung cancer. J Thorac Oncol.
7:485–495. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gautschi O, Zander T, Keller FA, Strobel
K, Hirschmann A, Aebi S and Diebold J: A patient with lung
adenocarcinoma and RET fusion treated with vandetanib. J Thorac
Oncol. 8:e43–e44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding X, Xiang L, Wang N, Zhao Z and Jin X,
Sun Y, Duan W, Wang S and Jin X: Vandetanib-induced inhibition of
neuroblastoma cell migration and invasion is associated with
downregulation of the SDF-1/CXCR4 axis and matrix metalloproteinase
14. Oncol Rep. 31:1165–1174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zage PE, Zeng L, Palla S, Fang W, Nilsson
MB, Heymach JV and Zweidler-McKay PA: A novel therapeutic
combination for neuroblastoma: The vascular endothelial growth
factor receptor/epidermal growth factor receptor/rearranged during
transfection inhibitor vandetanib with 13-cis-retinoic acid.
Cancer. 116:2465–2475. 2010.PubMed/NCBI
|
|
30
|
Sebaugh JL: Guidelines for accurate
EC50/IC50 estimation. Pharm Stat. 10:128–134.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dai L, Cui X, Zhang X, Cheng L, Liu Y,
Yang Y, Fan P, Wang Q, Lin Y, Zhang J, et al: SARI inhibits
angiogenesis and tumour growth of human colon cancer through
directly targeting ceruloplasmin. Nat Commun. 7:119962016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai L, Cheng L, Zhang X, Jiang Q, Zhang S,
Wang S, Li Y, Chen X, Du T, Yang Y, et al: Plasmid-based
STAT3-siRNA efficiently inhibits breast tumor growth and metastasis
in mice. Neoplasma. 58:538–547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang C, Tan J, Zhu J, Wang S and Wei G:
YAP promotes tumorigenesis and cisplatin resistance in
neuroblastoma. Oncotarget. 8:37154–37163. 2017.PubMed/NCBI
|
|
34
|
Michaelis M, Bliss J, Arnold SC, Hinsch N,
Rothweiler F, Deubzer HE, Witt O, Langer K, Doerr HW, Wels WS, et
al: Cisplatin-resistant neuroblastoma cells express enhanced levels
of epidermal growth factor receptor (EGFR) and are sensitive to
treatment with EGFR-specific toxins. Clin Cancer Res. 14:6531–6537.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rössler J, Odenthal E, Geoerger B,
Gerstenmeyer A, Lagodny J, Niemeyer CM and Vassal G: EGFR
inhibition using gefitinib is not active in neuroblastoma cell
lines. Anticancer Res. 29:1327–1333. 2009.PubMed/NCBI
|
|
36
|
Bakir S, Yazgan ÜC, Ibiloglu I, Elbey B,
Kizil M and Kelle M: The protective effect of pomegranate extract
against cisplatin toxicity in rat liver and kidney tissue. Arch
Physiol Biochem. 121:152–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Katanić J, Matić S, Pferschy-Wenzig EM,
Kretschmer N, Boroja T, Mihailović V, Stanković V, Stanković N,
Mladenović M, Stanić S, et al: Filipendula ulmaria extracts
attenuate cisplatin-induced liver and kidney oxidative stress in
rats: In vivo investigation and LC-MS analysis. Food Chem Toxicol.
99:86–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Greco WR, Bravo G and Parsons JC: The
search for synergy: A critical review from a response surface
perspective. Pharmacol Rev. 47:331–385. 1995.PubMed/NCBI
|
|
39
|
Minto CF, Schnider TW, Short TG, Gregg KM,
Gentilini A and Shafer SL: Response surface model for anesthetic
drug interactions. Anesthesiology. 92:1603–1616. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Papangeli I, Kim J, Maier I, Park S, Lee
A, Kang Y, Tanaka K, Khan OF, Ju H, Kojima Y, et al: MicroRNA
139-5p coordinates APLNR-CXCR4 crosstalk during vascular
maturation. Nat Commun. 7:112682016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu H, Liu Y, Liu W, Zhang W and Xu J:
EZH2-mediated loss of miR-622 determines CXCR4 activation in
hepatocellular carcinoma. Nat Commun. 6:84942015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Geminder H, Sagi-Assif O, Goldberg L,
Meshel T, Rechavi G, Witz IP and Ben-Baruch A: A possible role for
CXCR4 and its ligand, the CXC chemokine stromal cell-derived
factor-1, in the development of bone marrow metastases in
neuroblastoma. J Immunol. 167:4747–4757. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Airoldi I, Raffaghello L, Piovan E, Cocco
C, Carlini B, Amadori A, Corrias MV and Pistoia V: CXCL12 does not
attract CXCR4+ human metastatic neuroblastoma cells: Clinical
implications. Clin Cancer Res. 12:77–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang L, Yeger H, Das B, Irwin MS and
Baruchel S: Tissue microenvironment modulates CXCR4 expression and
tumor metastasis in neuroblastoma. Neoplasia. 9:36–46. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Catani MV, Corasaniti MT, Navarra M,
Nisticò G, Finazzi-Agrò A and Melino G: gp120 induces cell death in
human neuroblastoma cells through the CXCR4 and CCR5 chemokine
receptors. J Neurochem. 74:2373–2379. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhi Y, Duan Y, Zhou X, Yin X, Guan G,
Zhang H, Dong Q and Yang K: NF-κB signaling pathway confers
neuroblastoma cells migration and invasion ability via the
regulation of CXCR4. Med Sci Monit. 20:2746–2752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Clift IC, Bamidele AO, Rodriguez-Ramirez
C, Kremer KN and Hedin KE: β-Arrestin1 and distinct CXCR4
structures are required for stromal derived factor-1 to
downregulate CXCR4 cell-surface levels in neuroblastoma. Mol
Pharmacol. 85:542–552. 2014. View Article : Google Scholar : PubMed/NCBI
|