Introduction
Fucoxanthin (Fx) is a non-provitamin A of high polar
xanthophyll that has an unusual allenic bond, an epoxide group and
a conjugated carbonyl group in a polyene chain. Fx occurs
dominantly in marine brown algae and diatoms and is responsible for
photosynthesis and photoprotection. Undaria pinnatifida
(wakame), Hizikia fusiforme (hiziki) and Sargassum
horneri (akamoku) are particularly excellent sources of Fx
among Japanese algal foods (1,2). It
has been demonstrated that Fx is extremely safe in terms of
toxicity, showing no adverse effects in animal experiments
(3,4). Several researchers have conclusively
reported the anticancer (5–7), anti-inflammatory (8), anti-diabetic (9) and anti-obesity effects of Fx in
animals and humans (10,11). Moreover, Fx possesses strong
potential for cell growth inhibition and the induction of apoptosis
in human neuroblastoma, gastric cancer, hepatoma, colorectal cancer
(CRC) and promyelocytic leukemia cells (12–16).
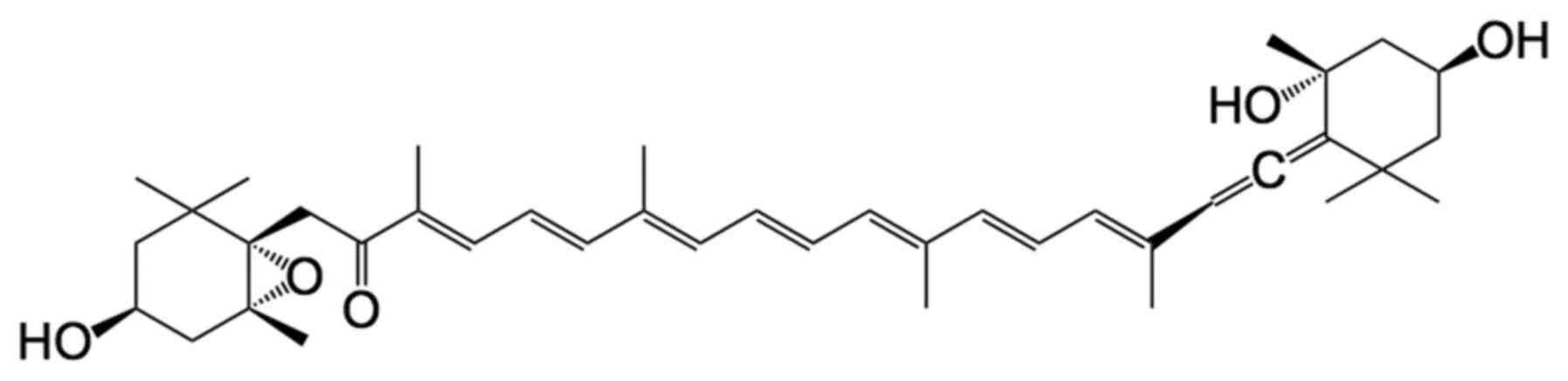
Fx is metabolically converted to fucoxanthinol (FxOH) (Fig. 1) and amarouciaxanthin A (Amx A) in
the mouse intestine and liver (17). After the oral administration of
wakame or Fx concentrate in humans, higher levels of FxOH and lower
levels of cis-FxOH have been detected in human plasma
(18,19). Thus, FxOH is an important indicator
of Fx function and may be a promising candidate for human cancer
chemoprevention. FxOH significantly attenuated the proliferation of
cancer cells derived and cultured from human CRC tissue (20). However, despite the strong
anticancer effects of FxOH, its underlying mechanisms are not well
known.
CRC is the third most common cause of cancer-related
death worldwide, and therefore it is urgent to reduce CRC
prevalence (21). Although non- or
less polar carotenoids such as β-carotene and lutein exhibit cancer
preventive effects, as shown in many epidemiological studies, their
utility has ‘insufficient evidence’. In the case of Fx or brown
algae, prospective clinical trials, cohorts or follow-back studies
for cancer prevention have not been attempted.
CRC stem cells (CCSCs) occupy only a small subset of
CRC tissue, but they are thought to play a central role in cancer
development. Self-renewal, differentiation, sphere formation, and
tumorigenicity in immunodeficient animals have been characterized
for CCSCs (22,23). CCSCs often acquire an
epithelial-mesenchymal transition (EMT) phenotype accompanied by
the activation of related proteins, and EMT not only promotes their
migration and invasion, but is also believed to be a leading cause
of CRC recurrence and distant metastasis (24). Therefore, attenuation of the EMT
phenotype of CCSCs may represent a promising approach for cancer
prevention, cancer recurrence/metastasis prevention and survival
rate elongation. It is well known that spheroids formed from CRC
cells, called colonospheres (Csps), are considered a representative
CCSC model phenotype since they contain a high abundance of CCSCs
and possess sphere reconstruction and tumorigenic capacities
(22,23,25).
We recently revealed that FxOH strongly induces the apoptosis of
HT-29 Csps and attenuates tumorigenicity in a xenograft mouse model
(26). However, little information
regarding the EMT-suppressing effects of FxOH on CCSCs or Csps is
available to date. Moreover, there are no studies reports regarding
the anti-metastatic effects of FxOH in in vivo setting.
Thus, we aimed to clarify the inhibitory activity of FxOH on EMT in
the present study.
Intracellular low-molecular-weight metabolites that
are essential for regulating energy systems such as glycolysis and
the TCA cycle are suggested to be prognostic indicators
representing cellular genomic and proteomic alterations after
encounters with various endogenous and exogenous stimuli.
Comprehensive metabolite analyses have revealed many marker
metabolite candidates in serum, plasma, urine and cancer tissue in
CRC patients and an animal model (27–29).
Therefore, intracellular low-molecular-weight metabolites likely
represent a convenient approach to promptly diagnose the cellular
conditions of CCSCs with complicated genetic and protein
backgrounds. However, the marker metabolites representing the EMT
phenotype in CCSCs remain elusive.
In the present study, we investigated the
EMT-suppressive effects of FxOH on Csps formed from human CRC HT-29
and HCT116 cells. The molecules through which FxOH exerted EMT
suppression were investigated. In addition, we examined the
alterations exerted by FxOH on the metabolite profiles of Csps and
identified marker metabolites with EMT potential in Csps.
Materials and methods
Chemicals and cell culture
All-trans-FxOH (purity, ≥98%) was provided by
Dr Hayato Maeda (Hirosaki University, Japan) (Fig. 1). EGF, bFGF and DMEM/F12 medium were
purchased from Wako Pure Chemicals (Osaka, Japan). B27 was obtained
from Miltenyi Biotec, Inc. (Auburn, CA, USA). HT-29 and HCT116
human CRC cells were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA). These cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
heat-inactivated fetal bovine serum (FBS), 4 mM L-glutamine, 40,000
U/l penicillin and 40 mg/l streptomycin. All other chemicals and
solvents were of analytical grade.
Colonosphere formation
HT-29 and HCT116 parental cells (PCs) were
trypsinized from culture plates, washed twice with PBS, suspended
in stem cell medium (SCM) composed of DMEM/F12 medium, 20 ng/ml
EGF, 10 ng/ml bFGF, 0.2% B27 and an antibiotic-anti-mycotic agent,
plated at a density of 3×104 cells/ml SCM in 10-cm
dishes or 24-well ultra-low attachment plates (Corning Inc.,
Corning, NY, USA) and incubated for 2 days at 37°C in a humidified
atmosphere containing 5% CO2. All experiments utilizing
colonospheres (Csps) described below were performed using Csps
grown for 2 days.
Analysis of the suppression of
colonosphere formation
Csps derived from HT-29 and HCT116 PCs were formed
in a 24-well ultra-low attachment plate for 2 days. After the Csps
formed, a total of 2–10 mM FxOH reconstituted in dimethyl sulfoxide
(DMSO) was applied to the cell medium at a final concentration of
10–50 µM (0.5 v/v%), or vehicle alone (DMSO) was applied. The cells
were harvested and trypsinized after incubation for 24 h. Viable
cells in Csps were counted using a trypan blue exclusion method.
The Csps treated with FxOH were treated with ribonuclease A,
stained with propidium iodide (PI) and subjected to flow cytometry.
The percentage of apoptotic cells (sub-G1, hypodiploid cells) was
estimated by a FACSaria-III flow cytometer (BD Biosciences, San
Jose, CA, USA).
Western blot analysis
pAkt (Ser473) (cat. no. 4060), β-catenin
(cat. no. 9582), pβ-catenin
(Ser31/37/Thr42) (cat. no. 9561),
cyclin D1 (cat. no. 2922) and PPARγ (cat. no. 2435) antibodies and
phospho-ERK1/2 pathway (cat. no. 9911) and phospho-Stat antibody
sampler (cat. no. 9914) kits were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). β-actin (cat. no. GTX109639),
E-cadherin (cat. no. GTX100443), N-cadherin (cat. no. GTX127345),
caspase-3 (cat. no. GTX110543), pFAK (Tyr397) (cat. no.
GTX24803), LGR5 (cat. no. GTX129862) and vimentin (cat. no.
GTX132610) antibodies were obtained from GeneTex (Irvine, CA, USA).
The paxillin (Tyr31) antibody (MAB61641) was obtained
from R&D Systems (Minneapolis, MN, USA). CD44 (cat. no.
MS-668-P0), MMP-9 (cat. no. MS-817-P0) and p53 (cat. no. MS-105-P0)
antibodies were obtained from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). EpCAM (cat. no. 11-581-C025) and integrin β1
(cat. no. 11-219-C100) antibodies were obtained from Exbio (Prague,
Czech Republic). Epithelial type cells obtained immediately before
preparing Csps were used as the parental cells (PCs) of the Csps.
Csps derived from HT-29 and HCT116 PCs formed in 10-cm ultra-low
attachment plates for 2 days and were then treated with 50 µM FxOH
and vehicle (DMSO) for 4–24 h. The cells were harvested, washed
twice with phosphate-buffered saline (PBS) and then lysed in a
lysis buffer to obtain whole-cell lysates. The protein
concentrations were photometrically measured using the Bradford
assay (Bio-Rad, Hercules, CA, USA). Fifty micrograms of whole-cell
proteins were separated on SDS-polyacrylamide minigels. The gels
were then electroblotted onto polyvinylidene fluoride (PVDF)
membranes. The PVDF membranes were incubated in 1% BSA blocking
buffer at room temperature and probed with each of the primary
antibodies (1:1,000 dilution) in blocking buffer overnight at 4°C
following the manufacturer's instructions. The membranes were
washed and incubated with HRP-conjugated anti-mouse or anti-rabbit
secondary antibodies. The membranes were again washed and
subsequently subjected to chemiluminescence reagents. The stripping
process to avoid the detection of previous bands may induce unclear
blot when we use a new antibody on the same membrane. Thus, we
repeated western blotting, loading the same amount of sample, to
obtain the new membrane for several times.
Migration and invasion analyses
Migration and invasion assays were performed using a
24-well Transwell chamber with an 8-µm pore size and a 24-well
Matrigel invasion chamber (pore size, 8 µm) (Corning Costar,
Cambridge, MA, USA). Csps derived from HT-29 and HCT116 PCs formed
in 10-cm ultra-low attachment plates for the assays, were
trypsinized and were washed twice with PBS. Then, 3×104
suspended cells were seeded in 500 µl of SCM in a 15-ml centrifuge
tube and treated with FxOH (20 and 50 µM) or vehicle (DMSO). One
hundred microliters of the cell suspension containing FxOH was
applied to the upper compartment of each chamber. DMEM containing
10% FBS was loaded into the lower compartment in both chambers. The
migration and invasion chambers were then incubated for 6 and 24 h,
respectively, at 37°C. Cells that migrated or invaded to the
underside of the upper compartment were fixed with formalin and
stained with Giemsa solution/(1:00 dilution). Migrated or invaded
cells were visualized at ×40 magnification and counted as the
numbers of migrated or invaded cells/field in three random
fields.
GC-MS analysis
2-Isopropylmalic acid (2-IPMA), methoxyamine
hydrochloride and
N-methyl-N(trimethylsilyl)-trifluoroacetate (MSTFA)
were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany),
MP Biomedicals (Solon, OH, USA) and GL Sciences (Tokyo, Japan),
respectively. Csps treated with FxOH were harvested from culture
plates, trypsinized, and washed twice with PBS. The pelleted cells
were re-suspended in 50 µl of cold PBS, to which 2 µl of 0.1 mg/ml
2-IPMA was added as an internal standard, and then the suspensions
were disrupted by sonication for 5 sec on ice. Total protein
contents were determined using the Bradford method with 1 µl of
each suspension. Metabolites were extracted with 250 µl of
CH3OH/CHCl3/DW (2.5:1:1, v/v/v), centrifuged
at 16,000 × g for 5 min and the upper phase was washed with 200 µl
of DW. The extracts obtained were evaporated to dryness. The
residues were oxymated with 30 µl of 20 mg/ml methoxyamine
hydrochloride in dry pyridine at 30°C for 90 min, followed by the
application of 15 µl of MSTFA at 37°C for 30 min. All GC-MS
analyses were carried out using a GCMS-QP5000 system (Shimadzu,
Kyoto, Japan) equipped with a non-polar capillary column [Rxi-5ms,
30 m × 0.25 mm i.d., film thickness, 0.25 µm; Restek, Co. Ltd.,
GmbH (Bad Homburg, Germany)] and online analysis software (CLASS
5000). The carrier gas (He) flow rate was at 0.5 ml/min (15.7 kPa),
and injections were 1 µl in split-ratio mode (split ratio, 33%).
The column temperature was initially 80°C for 2 min, increased to
330°C at 4°C/min and then maintained at 330°C for 8 min. The
interface and source temperatures were 250 and 230°C, respectively.
Identification was confirmed by comparing the spectra of single
components with those stored in the acquisition system library. All
metabolite contents were expressed as pmol metabolite/µg of total
protein content.
Statistical analysis
All experiments were performed at least twice and
are presented as representative data. Significant differences for
multiple comparisons were determined by one-way ANOVA followed by
Tukey-Kramer post hoc test. Differences were considered
statistically significant at P<0.05 as indicated with the
relevant symbols in the figures.
Results
Stemness and metabolite
characteristics of colonospheres
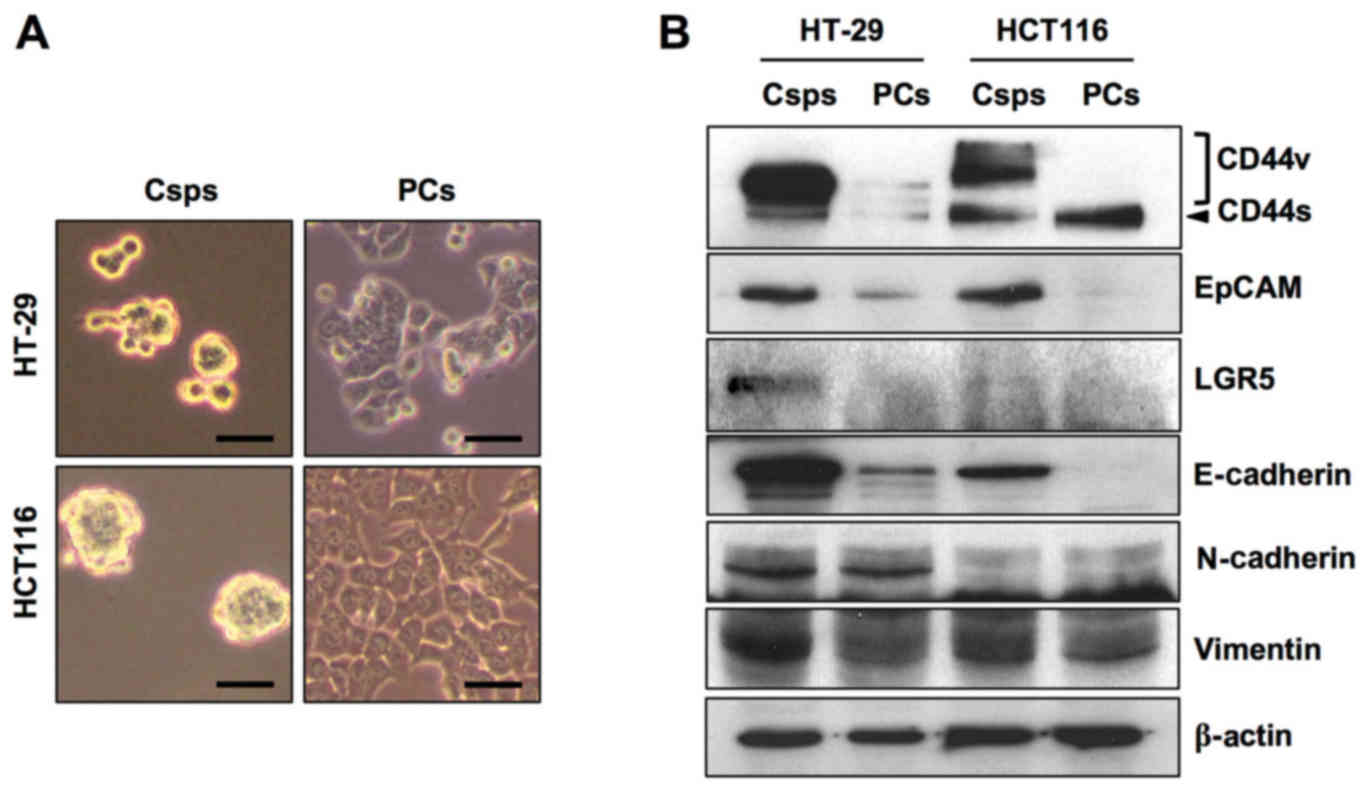
Among the three CCSC surface markers (CD44, EpCAM
and LGR5), CD44 variant forms (CD44v) and EpCAM were overexpressed
in both HT-29 and HCT116 Csps compared with the PCs (Fig. 2). CD44 standard form (CD44s) and
LGR5 were strongly increased in HT-29 Csps, while LGR5 was weakly
increased in HCT116 Csps. Among the three key proteins representing
the EMT phenotype (E-cadherin, N-cadherin and vimentin), the
expression of E-cadherin and vimentin was elevated in both Csps
compared with the expression in the PCs. N-cadherin expression did
not discriminate between Csps and PCs for both cell types.
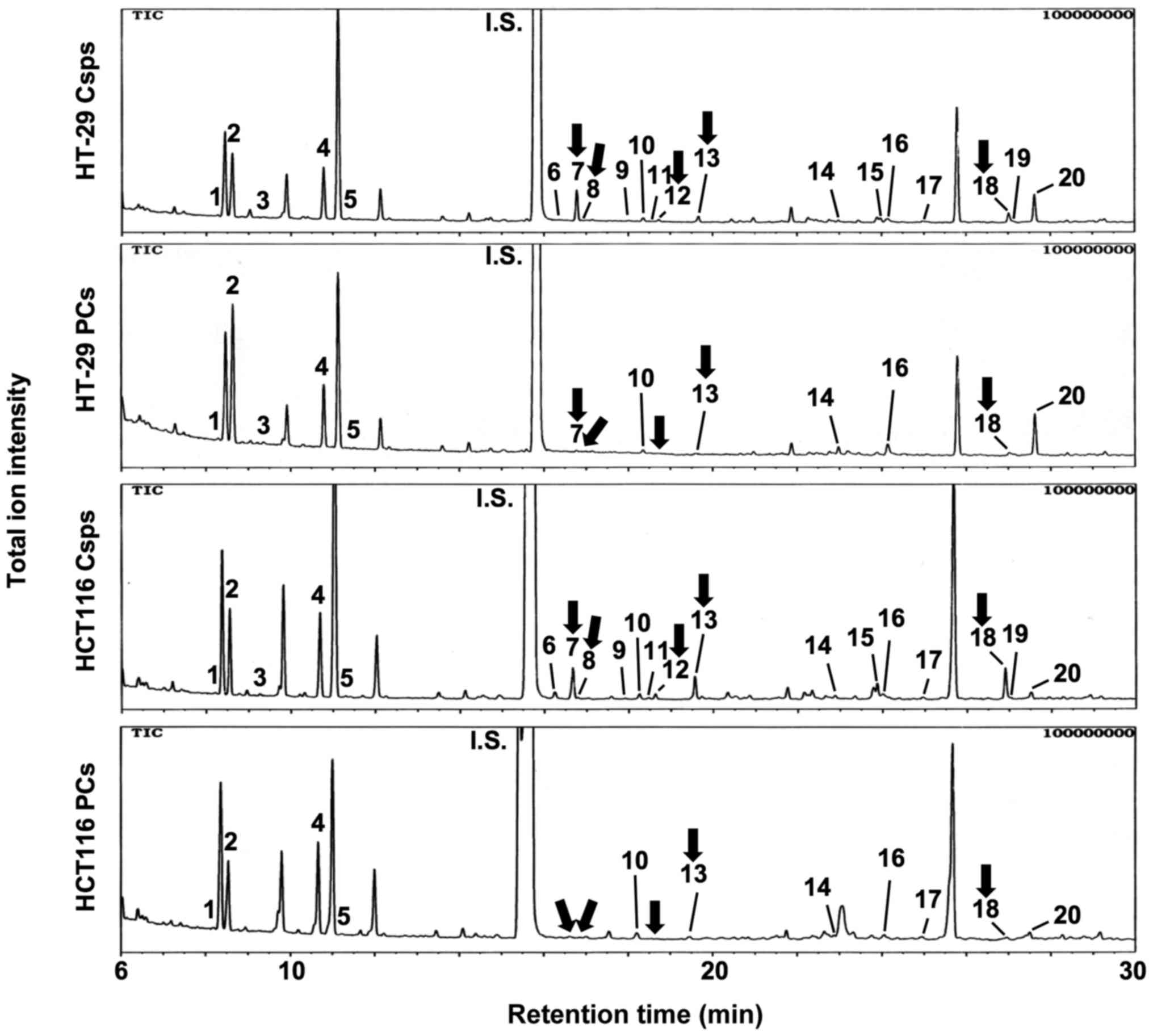
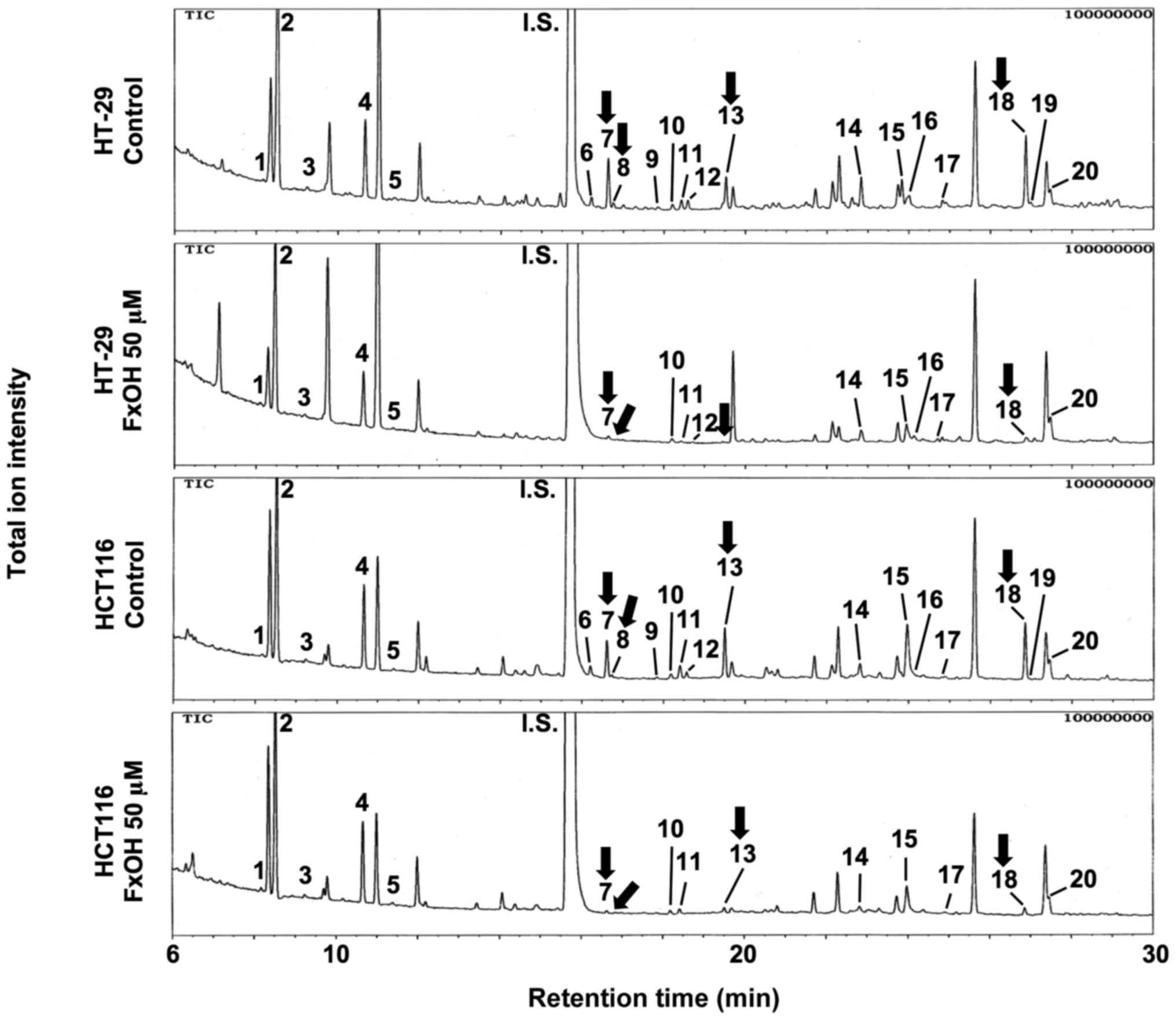
In the next experiment, metabolite profiles were
constructed using GC-MS (Fig. 3).
The quantitative data obtained for the Csps and PCs derived from
both cell types for all 20 metabolites analyzed are presented in
Table I. Four amino acids,
specifically glycine, serine, threonine and glutamic acid, as well
as succinic acid, a TCA cycle metabolite, were significantly
increased in the HCT116 Csps compared with the PCs. Although no
significant changes were observed between HT-29 Csps and PCs, there
was a tendency toward a changing pattern for these five metabolites
similar to that of HCT116 cells. Overall, metabolites were weakly
increased in Csps compared with the PCs, but no significant change
was observed for other metabolites from Csps or PCs derived from
both cell lines.
 | Table I.Metabolite profiles of the
colonospheres from HT-29 and HCT116 parental cells. |
Table I.
Metabolite profiles of the
colonospheres from HT-29 and HCT116 parental cells.
|
|
| pmol metabolite/µg
total protein content |
|---|
|
|
|
|
|---|
|
|
| HT-29 cells | HCT116 cells |
|---|
|
|
|
|
|
|---|
| Peak
no.a | Group -
compound | Csps | PCs | Csps | PCs |
|---|
|
| Amino acid |
| 3 |
Valine | 2.9±2.1 | 0.6±0.6 | 0.1±0.1 | 2.7±2.7 |
| 5 |
Leucine | 1.4±0.8 | 1.6±1.6 | 1.6±0.7 | ND |
| 6 |
Proline | 6.6±2.3 | ND | ND | ND |
| 7 |
Glycine | 19.3±3.9 | 6.9±3.1 |
16.6±2.2b | 1.0±0.5 |
| 11 |
Alanine | ND | ND | 0.6±0.6 | ND |
| 12 |
Serine | 2.2±0.5 | 0.6±0.6 |
2.6±0.2b | ND |
| 13 |
Threonine | 6.2±0.7 | 2.4±1.6 |
10.6±1.1b | 0.5±0.5 |
| 15 |
Aspartic acid | 3.8±0.8 | 1.8±1.1 | 3.7±3.7 | ND |
| 18 |
Glutamic acid | 9.0±2.3 | 2.6±1.1 |
13.2±1.4b | 0.9±0.5 |
| 19 |
Phenylalanine | 0.5±0.4 | 0.7±0.4 | 0.1±0.1 | ND |
|
| Dicarboxylic acid
(TCA cycle) |
| 8 |
Succinic acid | 2.8±1.8 | 0.5±0.5 |
0.9±0.3b | ND |
| 9 | Fumaric
acid | 0.5±0.5 | ND | 0.1±0.1 | ND |
| 14 | Malic
acid | 3.2±1.2 | 5.6±1.1 | 1.5±1.5 | 0.5±0.5 |
|
| Carboxylic
acid |
| 1 | Pyruvic
acid | 1.4±0.8 | 1.1±1.1 | 2.0±0.3 | 1.7±0.4 |
| 2 |
Propionic acid | 74.2±10.0 | 97.1±23.9 | 54.5±6.2 | 59.4±19.4 |
| 4 | Butyric
acid | 34.3±5.1 | 41.5±6.9 | 63.7±10.3 | 51.6±4.6 |
| 10 |
Pelargonic acid | 3.8±0.8 | 3.1±0.5 | 4.9±0.3 | 3.2±0.8 |
| 16 |
γ-aminobutyric acid | 5.6±2.0 | 9.1±1.1 | 6.7±1.7 | 2.3±0.6 |
| 17 |
2,3,4-trihydroxybutyric
acid | ND | ND | 0.9±0.9 | 0.8±0.4 |
| 20 | Lauric
acid | 10.1±4.5 | 12.9±11.4 | 8.8±4.0 | 8.6±3.4 |
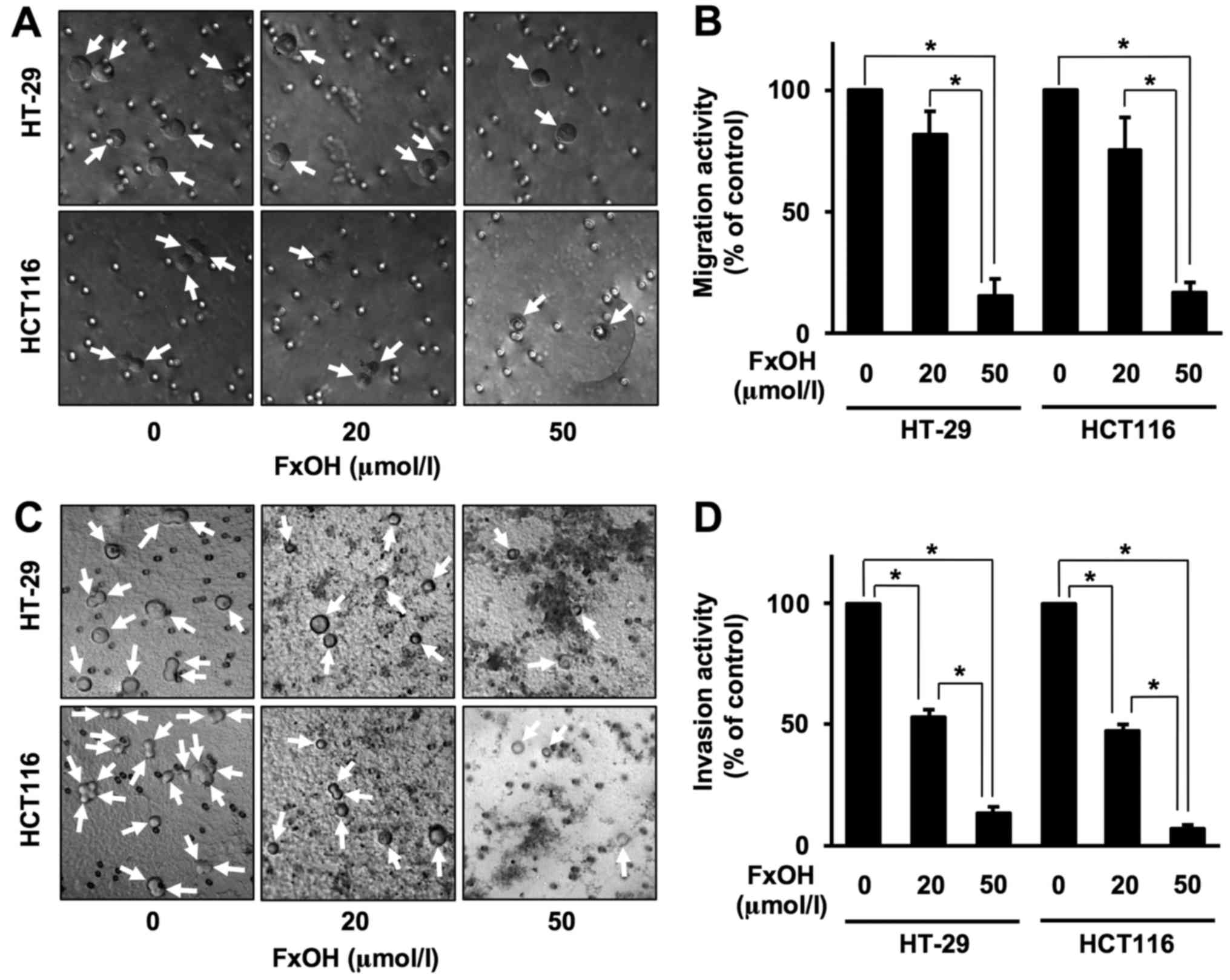
Antiproliferative effects of FxOH in
colonospheres
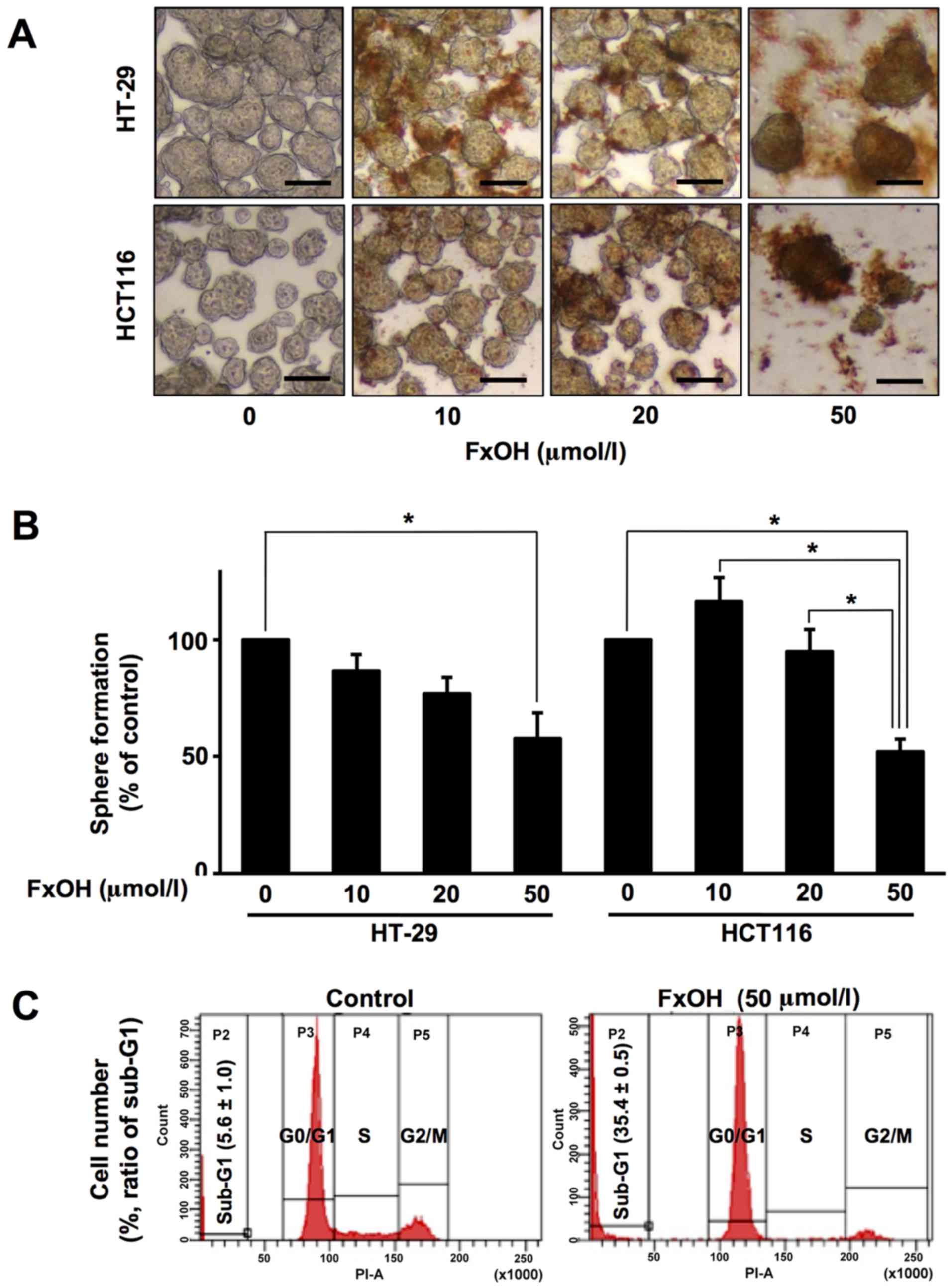
Treatment with 10, 20 and 50 µM FxOH inhibited the
growth of Csps from the HT-29 and HCT116 cells in a dose-dependent
manner (Fig. 4A and B). Sphere
formation was as follows for Csps from HT-20 and HCT116 cells: 10
µM FxOH, 86.7±7.1 and 116.3±10.4%, respectively; 20 µM, 77.0±7.0
and 95.0±9.4%, respectively; and 50 µM, 57.7±11.0 and 52.1±5.4%,
respectively. Vehicle (DMSO) alone exerted no effects on cell
proliferation. Flow-cytometric analyses exhibited that the
percentage of sub-G1 phase cells (apoptosis-induced cells) in the
FxOH-treated Csps (35.4±0.5%) was higher than that noted in the
control Csps (5.6±1.0%) (Fig. 4C).
In addition, FxOH abrogated p53 expression and increased the p17
and p19 active subunits of caspase-3, suggesting that this growth
inhibition is linked to apoptosis (Fig.
7).
Suppressive effects of FxOH on the
migration and invasion of colonospheres
Treatment with 20 and 50 µM FxOH inhibited the
migration and invasion of both HT-29 and HCT116 Csps in a
dose-dependent manner (Fig. 5).
Migration activities were as follows for Csps from HT-29 and HCT116
cells: 20 µM FxOH, 82.1±9.3 and 75.7±13.2%, respectively; and 50
µM, 15.0±7.4 and 16.7±4.2%, respectively. Invasion activities were
as follows for HT-29 and HCT116 Csps: 20 µM FxOH, 52.8±3.3 and
47.4±2.7%, respectively; and 50 µM, 13.4±2.7 and 7.4±1.3%,
respectively. Vehicle (DMSO) alone exerted no effects on both
migration and invasion capacities.
Changes in the metabolite profiles of
colonospheres with and without FxOH
Csps derived from both cell types were treated with
50 µM FxOH, and a total of 20 metabolites were analyzed using
GC-MS. The quantitative data are presented in Table II and Fig. 6. Among them, three amino acids,
specifically glycine, threonine and glutamic acid, were
significantly decreased in both or either Csps compared with
control Csps. Succinic acid, a carboxylic acid involved in the TCA
cycle, was significantly decreased in both HT-29 and HCT116 Csps
compared with control Csps. Although no significant change was
observed in other metabolites, the majority of metabolites were
weakly decreased in both HT-29 and HCT116 Csps treated with FxOH
compared with control Csps.
 | Table II.Metabolite profiles of colonospheres
from the HT-29 and HCT116 cells following FxOH treatment at 24
h. |
Table II.
Metabolite profiles of colonospheres
from the HT-29 and HCT116 cells following FxOH treatment at 24
h.
|
|
| pmol metabolite/µg
total protein content |
|---|
|
|
|
|
|---|
|
|
| HT-29 cells | HCT116 cells |
|---|
|
|
|
|
|
|---|
| Peak
no.a | Group -
compound | Csps | PCs | Csps | PCs |
|---|
|
| Amino acid |
| 3 |
Valine | 2.5±1.5 | 1.0±1.0 | 1.8±1.8 | 1.5±0.7 |
| 5 |
Leucine | 3.7±2.1 | 0.9±0.9 | 1.6±0.5 | 0.7±0.7 |
| 6 |
Proline | 8.6±4.4 | 21.0±11.0 | 4.3±4.3 | ND |
| 7 |
Glycine | 35.9±0.7 |
14.1±6.4b | 29.6±5.7 |
6.5±2.5b |
| 11 |
Aranine | 2.7±2.7 | 0.3±0.3 | 4.5±4.5 | 1.9±1.0 |
| 12 |
Serine | 3.8±2.1 | 2.4±1.5 | 5.0±1.8 | 0.6±0.6 |
| 13 |
Threonine | 15.3±5.1 | 6.7±3.8 | 22.7±3.9 |
3.3±0.8b |
| 15 |
Aspartic acid | 10.1±4.8 | 2.2±1.1 | 5.9±3.4 | 0.8±0.8 |
| 18 |
Glutamic acid | 29.1±8.2 | 11.3±4.9 | 31.0±2.1 |
7.8±3.0b |
| 19 |
Phenylalanine | 2.6±0.3 | 0.7±0.7 | 0.8±0.8 | 0.9±0.9 |
|
| Dicarboxylic acid
(TCA cycle) |
| 8 |
Succinic acid | 6.7±1.4 |
0.6±0.6b | 1.6±0.3 | NDb |
| 9 | Fumaric
acid | 1.5±1.2 | 0.6±0.6 | 0.9±0.9 | 0.3±0.3 |
| 14 | Malic
acid | 13.9±4.0 | 4.2±2.1 | 6.2±2.3 | 2.1±1.4 |
|
| Carboxylic
acid |
| 1 | Pyruvic
acid | 1.0±1.0 | 1.1±1.1 | 2.7±0.5 | 2.4±0.3 |
| 2 |
Propionic acid | 188.5±42.8 | 110.2±31.5 | 121.2±32.0 | 91.0±27.9 |
| 4 | Butyric
acid | 44.9±11.6 | 31.2±3.9 | 42.6±14.5 | 21.4±13.2 |
| 10 |
Pelargonic acid | 3.0±1.7 | 3.1±0.2 | 3.6±1.2 | 1.6±1.0 |
| 16 |
γ-aminobutyric acid | 16.0±2.7 | 5.4±2.8 | 30.7±18.1 | 8.2±6.9 |
| 17 |
2,3,4-Trihydroxybutyric
acid | 2.7±0.8 | 1.7±1.0 | ND | 0.3±0.3 |
| 20 | Lauric
acid | 9.7±2.7 | 10.1±3.0 | 15.3±1.6 | 14.7±4.3 |
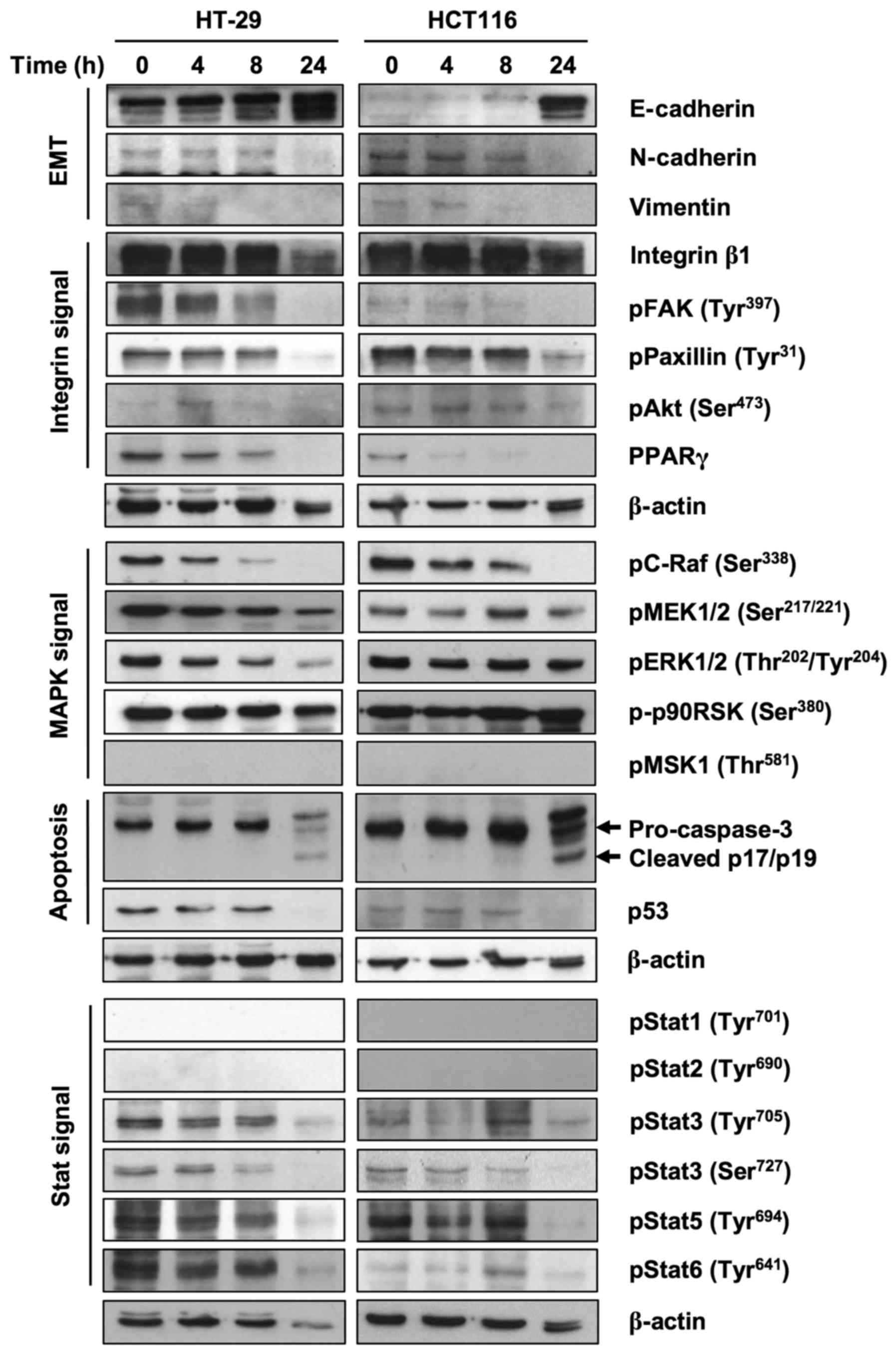
Expression of molecules related to
FxOH treatment
To clarify the proteins involved in the inhibition
of EMT, invasion and migration, we performed a western blot assay.
FxOH treatment increased E-cadherin at 24 h and decreased
N-cadherin at 24 h and vimentin at 8 h in Csps in both cell lines
(Fig. 7). FxOH also decreased the
activation of integrin, MAPK and Stat signaling by inhibiting
phosphorylation of their key proteins in Csps in both cell lines.
Regarding integrin signaling, protein levels of Integrin β1 and
phosphorylation levels of paxillin and Akt are clearly reduced at
24 h. The phosphorylation levels of pFAK and protein levels of
PPARγ were reduced in a time-dependent manner. Regarding MAPK
signaling, the phosphorylation levels of C-Raf decreased in a
time-dependent manner. The phosphorylation levels of MEK were
reduced at 24 h. The phosphorylation levels of ERK were
downregulated only in HT-29 Csps. However, phosphorylation levels
of p90RSK did not changed during the experiment. Regarding Stat
signaling, phosphorylation levels of Stat3, Stat5 and Stat6 were
reduced clearly at 24 h. We also observed reduced pro-caspase-3 at
24 h and cleaved p17/p19 (active forms of caspase-3) at 24 h and
reduced p53 levels. As shown in the figure, phosphorylated MSK1,
Stat1 and Stat2 were not detected in this experiment.
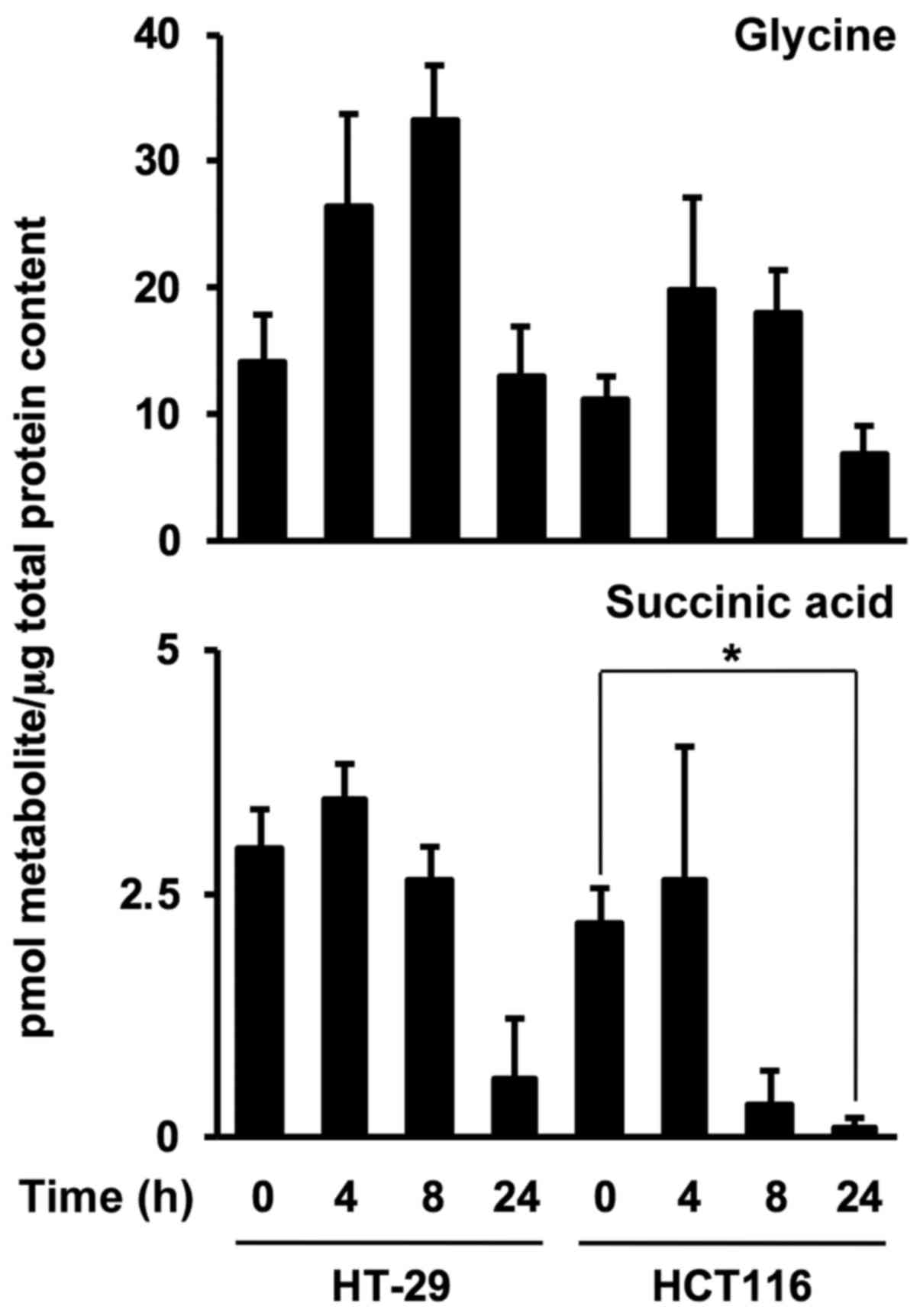
Time-dependent metabolite levels in
colonospheres after FxOH treatment
To investigate FxOH-induced metabolite alterations
in Csps, levels of the two metabolites were evaluated in HT-29 and
HCT116 Csps treated with 50 µM FxOH for 4, 8 and 24 h. FxOH
significantly increased glycine levels from 0 until 8 h and
decreased glycine levels from 8 until 24 h in HT-29 Csps. Succinic
acid levels in both Csps treated with FxOH were drastically
decreased at 8 or 24 h compared with levels in each Csps at 0 h
(Fig. 8).
Discussion
The results of the present study suggest that
fucoxanthinol (FxOH) suppressed EMT. Conversely, glycine and
succinic acid were found to be prognostic indicators of
physiological changes in Csps treated with FxOH. This is the first
study demonstrating EMT inhibition by FxOH treatment accompanied by
detectable metabolite alterations. In addition, FxOH induced
apoptosis by inhibiting integrin, MAPK and Stat signaling
activations. Moreover, the decrease in p53 expression and
activation of caspase-3 were suggested to be involved in
FxOH-induced apoptosis in HT-29 and HCT116 Csps.
We first confirmed the existence of CCSC surface
protein markers and EMT phenotype protein markers in HT-29 and
HCT116 Csps (Fig. 2). The
enhancement of CD44v, EpCAM, LGR5 and vimentin, an EMT marker,
suggested that both Csps possessed CCSC and EMT properties. In
general, the suppression of E-cadherin expression in cancer cells
indicated their transformation into the EMT phenotype. Thus, the
upregulation of E-cadherin and vimentin observed in this study
represented a lack of consistency. Indeed, other researchers
observed decreased E-cadherin and increased vimentin in Csps formed
from HT-29 and HCT116 PCs (30).
Although we have no idea why E-cadherin was increased in the Csps
compared with PCs in this experiment, we used these Csps as a
near-Csp model. Regarding N-cadherin, it is generally acknowledged
that this marker as well as vimentin may be elevated upon
transformation into the EMT phenotype.
We also found that incubation with SCM for both
HT-29 and HCT116 Csps resulted in metabolic reprogramming during
the PC to Csp steps, in which glycine, serine, threonine, glutamic
acid and succinic acid levels were significantly elevated. In
addition, these metabolites showed a similar increase in Csps
formed from HT-29 PCs, although without significance (Fig. 3 and Table I). It is speculated that the
activation of cytosol glycolysis and serine metabolism and the
promotion of the mitochondrial GSH/GSSG redox system and TCA cycle
are essential metabolic pathways in Csps derived from HT-29 and
HCT116 PCs. Positive aerobic glycolysis and subsequent metabolic
reprogramming in cancer cells are collectively known as the Warburg
effect (31). Various researchers
have demonstrated the impact of the Warburg effect on the
metabolite profiles of CSCs or CSC-like spheroids reconstructed
from original breast, colorectal, hepatic and ovarian cancer PCs
(32–36). Among the amino acids, aspartate,
serine, glutamic acid and glutamine are assumed to be particularly
good targets for cancer therapeutics in CSCs. Furthermore, several
pathways, such as amino acid metabolism, the redox system, the TCA
cycle and fatty acid biosynthesis, are also targets of CSCs.
However, the precise mechanisms and physiological significance
underlying the metabolite contents of CSCs remain unclear.
Properties such as gene expression, morphology and
chemoresistance differ between HT-29 and HCT116 cells. For example,
HT-29 is p53-mutant and HCT116 is p53-wild-type (37). Although HCT116 cells possess a
phenotype that more closely resembles EMT compared with that of
HT-29 cells, both cell types demonstrated similar capacity in terms
of invasion, sphere formation and tumorigenicity (30,38).
HCT116 cells are more sensitive to 5-fluorouracil treatment than
HT-29 cells (39). In the present
study, FxOH inhibited sphere formation, migration and invasion to
the same degree in both Csp types and induced apoptosis through the
same molecular regulations with similar temporal expression
patterns (Figs. 4, 5 and 7).
We previously reported that FxOH induced apoptosis along with the
downregulation of pAkt (Ser473), peroxisome
proliferator-activated receptor (PPAR)β/δ and PPARγ in HT-29 Csps
(26). In the present study, we
further showed that FxOH treatment began to suppress PPARγ and
inhibit pC-Raf (Ser338) starting at 4 h, and decreased
amounts of vimentin or increased E-cadherin levels were observed at
8 h, followed by caspase-3 activation and p53 depression at 24 h in
both Csps. A highly polar xanthophyll, astaxanthin, as well as FxOH
both inhibit EMT accompanied by the attenuation of reactive oxygen
species production, inflammatory cytokine production and NF-κB
activation in rat peritoneal mesothelial cells (40). The apocarotenoids crocetin and
crocin promote EMT attenuation by inhibiting N-cadherin and
β-catenin expression and increasing E-cadherin expression in
aggressive prostate cancer PC3 and 22rvl cells (41).
In addition to regulating proteins, the metabolite
changes in Csps treated with FxOH were very similar between each
cell type (Figs. 6 and 8, and Table
II). FxOH treatment markedly decreased glycine and succinic
acid in both Csps at 8 or 24 h. Therefore, FxOH may attenuate the
mitochondrial GSH/GSSG redox system and TCA cycle in Csps.
Previously, Fx was shown to rapidly elicit the mitochondrial
membrane potential in human promyelocytic leukemia HL-60 and
HP100-1 cells (16). Our findings
indicate that FxOH treatment may accompany the mitochondrial
disruption of spheroids, regardless of the different phenotypes of
cancer cells. Little is known about changes to anti-metabolism
capacity by carotenoids in CSCs or CSC-like spheroids. A
randomized, double-blind, placebo-controlled study, the
Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study,
demonstrated that β-carotene significantly increased 17 metabolites
in the sera of male smokers (42).
In a diethylnitrosamine (DEN)-induced mouse hepatic tumor model,
acyclic retinoid (0.06%) administration resulted in significant
changes in 88 metabolites in liver tumor tissue compared with mice
that did not receive DEN treatment (43). In contrast, a colorimetric lipid,
curcumin, induced apoptosis accompanied by glutamine reduction in
CD44-positive CSC-like cells derived from HT-29 cells (44). Some anticancer drugs such as
5-fluorouracil and gemcitabine alter cellular metabolism pathways
(45). In the present study, we
revealed that FxOH exerts anti-metabolism activity in cancer cells
similar to that observed for other carotenoid or carotenoid-derived
compounds and anticancer drugs.
In summary, FxOH attenuated EMT, inhibited the
activation of integrin, MAPK, and Stat signaling and altered
metabolite profiles in CSC-like cells of Csps derived from human
CRC HT-29 and HCT116 cells. Glycine and succinic acid were
suggested to be metabolite markers of EMT suppression induction in
Csps. Further studies may reveal that these two metabolites are
helpful in understanding the cellular conditions of CCSCs in human
or animal colorectal mucosal tissue following Fx or FxOH
administration.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a grant
from Japan Society for the Promotion of Science KAKENHI (no.
16K07880).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MT and MM conceived and designed the study. MT, MM
and SK performed the experiments. MT and MM wrote the paper. TE,
HM, JH, KO and KM performed interepretation of data, reviewed and
edited the manuscript. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CCSCs
|
colorectal cancer stem cells
|
|
CD44s
|
CD44 standard form
|
|
CD44v
|
CD44 variant forms
|
|
Csps
|
colonospheres
|
|
DEN
|
diethylnitrosamine
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
DW
|
distilled water
|
|
EMT
|
epithelial-mesenchymal transition
|
|
FBS
|
fetal bovine serum
|
|
FxOH
|
fucoxanthinol
|
|
Fx
|
fucoxanthin
|
|
2-IPMA
|
2-isopropylmalic acid
|
|
MSTFA
|
N-methyl-N(trimethylsilyl)-trifluoroacetate
|
|
PCs
|
parental cells
|
|
PPAR
|
peroxisome proliferator-activated
receptor
|
|
SCM
|
stem cell medium
|
References
|
1
|
Terasaki M, Hirose A, Narayan B, Baba Y,
Kawagoe C, Yasui H, Saga N, Hosokawa M and Miyashita K: Evaluation
of recoverable functional lipid components of several brown
seaweeds (phaeophyta) from Japan with special reference to
fucoxanthin and fucosterol contents. J Phycol. 45:974–980. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Terasaki M, Narayan B, Kamogawa H, Nomura
M, Stephen NM, Kawagoe C, Hosokawa M and Miyashita K: Carotenoid
profile of edible Japanese seaweeds: An improved HPLC method for
separation of major carotenoids. J Aquat Food Prod Technol.
21:468–479. 2012. View Article : Google Scholar
|
|
3
|
Beppu F, Niwano Y, Tsukui T, Hosokawa M
and Miyashita K: Single and repeated oral dose toxicity study of
fucoxanthin (FX), a marine carotenoid, in mice. J Toxicol Sci.
34:501–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iio K, Okada Y and Ishikura M: Single and
13-week oral toxicity study of fucoxanthin oil from microalgae in
rats. Shokuhin Eiseigaku Zasshi. 52:183–189. 2011.(In Japanese).
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okuzumi J, Takahashi T, Yamane T, Kitao Y,
Inagake M, Ohya K, Nishino H and Tanaka Y: Inhibitory effects of
fucoxanthin, a natural carotenoid, on
N-ethyl-N'-nitro-N-nitrosoguanidine-induced
mouse duodenal carcinogenesis. Cancer Lett. 68:159–168. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JM, Araki S, Kim DJ, Park CB, Takasuka
N, Baba-Toriyama H, Ota T, Nir Z, Khachik F, Shimidzu N, et al:
Chemopreventive effects of carotenoids and curcumins on mouse colon
carcinogenesis after 1,2-dimethylhydrazine initiation.
Carcinogenesis. 19:81–85. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishino H: Cancer prevention by
carotenoids. Mutat Res. 402:159–163. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shiratori K, Ohgami K, Ilieva I, Jin XH,
Koyama Y, Miyashita K, Yoshida K, Kase S and Ohno S: Effects of
fucoxanthin on lipopolysaccharide-induced inflammation in vitro and
in vivo. Exp Eye Res. 81:422–428. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishikawa S, Hosokawa M and Miyashita K:
Fucoxanthin promotes translocation and induction of glucose
transporter 4 in skeletal muscles of diabetic/obese
KK-Ay mice. Phytomedicine. 19:389–394. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maeda H, Hosokawa M, Sashima T, Funayama K
and Miyashita K: Fucoxanthin from edible seaweed, Undaria
pinnatifida, shows antiobesity effect through UCP1 expression
in white adipose tissues. Biochem Biophys Res Commun. 332:392–397.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hitoe S and Shimoda H: Seaweed fucoxanthin
supplementation improves obesity parameters in mildly obese
Japanese subjects. Funct Food Health Dis. 7:246–262. 2017.
|
|
12
|
Hosokawa M, Kudo M, Maeda H, Kohno H,
Tanaka T and Miyashita K: Fucoxanthin induces apoptosis and
enhances the antiproliferative effect of the PPARgamma ligand,
troglitazone, on colon cancer cells. Biochim Biophys Acta.
1675:113–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Das SK, Hashimoto T and Kanazawa K: Growth
inhibition of human hepatic carcinoma HepG2 cells by fucoxanthin is
associated with down-regulation of cyclin D. Biochim Biophys Acta.
1780:743–749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murakami C, Takemura M, Sugiyama Y,
Kamisuki S, Asahara H, Kawasaki M, Ishidoh T, Linn S, Yoshida S,
Sugawara F, et al: Vitamin A-related compounds, all-trans retinal
and retinoic acids, selectively inhibit activities of mammalian
replicative DNA polymerases. Biochim Biophys Acta. 1574:85–92.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okuzumi J, Nishino H, Murakoshi M,
Iwashima A, Tanaka Y, Yamane T, Fujita Y and Takahashi T:
Inhibitory effects of fucoxanthin, a natural carotenoid, on
N-myc expression and cell cycle progression in human
malignant tumor cells. Cancer Lett. 55:75–81. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kotake-Nara E, Terasaki M and Nagao A:
Characterization of apoptosis induced by fucoxanthin in human
promyelocytic leukemia cells. Biosci Biotechnol Biochem.
69:224–227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asai A, Sugawara T, Ono H and Nagao A:
Biotransformation of fucoxanthinol into amarouciaxanthin A in mice
and HepG2 cells: Formation and cytotoxicity of fucoxanthin
metabolites. Drug Metab Dispos. 32:205–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asai A, Yonekura L and Nagao A: Low
bioavailability of dietary epoxyxanthophylls in humans. Br J Nutr.
100:273–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hashimoto T, Ozaki Y, Mizuno M, Yoshida M,
Nishitani Y, Azuma T, Komoto A, Maoka T, Tanino Y and Kanazawa K:
Pharmacokinetics of fucoxanthinol in human plasma after the oral
administration of kombu extract. Br J Nutr. 107:1566–1569. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi K, Hosokawa M, Kasajima H,
Hatanaka K, Kudo K, Shimoyama N and Miyashita K: Anticancer effects
of fucoxanthin and fucoxanthinol on colorectal cancer cell lines
and colorectal cancer tissues. Oncol Lett. 10:1463–1467. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dalerba P, Dylla SJ, Park IK, Liu R, Wang
X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vermeulen L, Todaro M, de Sousa Mello F,
Sprick MR, Kemper K, Alea Perez M, Richel DJ, Stassi G and Medema
JP: Single-cell cloning of colon cancer stem cells reveals a
multi-lineage differentiation capacity. Proc Natl Acad Sci USA.
105:13427–13432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Findlay VJ, Wang C, Watson DK and Camp ER:
Epithelial-to-mesenchymal transition and the cancer stem cell
phenotype: Insights from cancer biology with therapeutic
implications for colorectal cancer. Cancer Gene Ther. 21:181–187.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanwar SS, Yu Y, Nautiyal J, Patel BB and
Majumdar AP: The Wnt/beta-catenin pathway regulates growth and
maintenance of colonospheres. Mol Cancer. 9:212–225. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Terasaki M, Maeda H, Miyashita K, Tanaka
T, Miyamoto S and Mutoh M: A marine bio-functional lipid,
fucoxanthinol, attenuates human colorectal cancer stem-like cell
tumorigenicity and sphere formation. J Clin Biochem Nutr. 61:25–32.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshida M, Hatano N, Nishiumi S, Irino Y,
Izumi Y, Takenawa T and Azuma T: Diagnosis of gastroenterological
diseases by metabolome analysis using gas chromatography-mass
spectrometry. J Gastroenterol. 47:9–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dazard JE, Sandlers Y, Doerner SK, Berger
NA and Brunengraber H: Metabolomics of ApcMin/+ mice
genetically susceptible to intestinal cancer. BMC Syst Biol.
8:722014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshie T, Nishiumi S, Izumi Y, Sakai A,
Inoue J, Azuma T and Yoshida M: Regulation of the metabolite
profile by an APC gene mutation in colorectal cancer. Cancer Sci.
103:1010–1021. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han XY, Wei B, Fang JF, Zhang S, Zhang FC,
Zhang HB, Lan TY, Lu HQ and Wei HB: Epithelial-mesenchymal
transition associates with maintenance of stemness in
spheroid-derived stem-like colon cancer cells. PLoS One.
8:e733412013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sato M, Kawana K, Adachi K, Fujimoto A,
Yoshida M, Nakamura H, Nishida H, Inoue T, Taguchi A, Takahashi J,
et al: Spheroid cancer stem cells display reprogrammed metabolism
and obtain energy by actively running the tricarboxylic acid (TCA)
cycle. Oncotarget. 7:33297–33305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vermeersch KA, Wang L, Mezencev R,
McDonald JF and Styczynski MP: OVCAR-3 spheroid-derived cells
display distinct metabolic profiles. PLoS One. 10:e01182622015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Penkert J, Ripperger T, Schieck M,
Schlegelberger B, Steinemann D and Illig T: On metabolic
reprogramming and tumor biology: A comprehensive survey of
metabolism in breast cancer. Oncotarget. 7:67626–67649. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin SH, Liu T, Ming X, Tang Z, Fu L,
Schmitt-Kopplin P, Kanawati B, Guan XY and Cai Z: Regulatory role
of hexosamine biosynthetic pathway on hepatic cancer stem cell
marker CD133 under low glucose conditions. Sci Rep. 6:211842016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen KY, Liu X, Bu P, Lin CS, Rakhilin N,
Locasale JW and Shen X: A metabolic signature of colon cancer
initiating cells. Conf Proc IEEE Eng Med Biol Soc. 2014:4759–4762.
2014.PubMed/NCBI
|
|
37
|
Küntzer J, Eggle D, Lenhof HP, Burtscher H
and Klostermann S: The roche cancer genome database (RCGDB). Hum
Mutat. 31:407–413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matsuda Y, Miura K, Yamane J, Shima H,
Fujibuchi W, Ishida K, Fujishima F, Ohnuma S, Sasaki H, Nagao M, et
al: SERPINI1 regulates epithelial-mesenchymal transition in an
orthotopic implantation model of colorectal cancer. Cancer Sci.
107:619–628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Russo P, Malacarne D, Falugi C, Trombino S
and O'Connor PM: RPR-115135, a farnesyltransferase inhibitor,
increases 5-FU-cytotoxicity in ten human colon cancer cell lines:
Role of p53. Int J Cancer. 100:266–275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hara K, Hamada C, Wakabayashi K, Kanda R,
Kaneko K, Horikoshi S, Tomino Y and Suzuki Y: Scavenging of
reactive oxygen species by astaxanthin inhibits
epithelial-mesenchymal transition in high glucose-stimulated
mesothelial cells. PLoS One. 12:e01843322017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Festuccia C, Mancini A, Gravina GL,
Scarsella L, Llorens S, Alonso GL, Tatone C, Di Cesare E, Jannini
EA, Lenzi A, et al: Antitumor effects of saffron-derived
carotenoids in prostate cancer cell models. BioMed Res Int.
2014:1350482014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mondul AM, Sampson JN, Moore SC, Weinstein
SJ, Evans AM, Karoly ED, Virtamo J and Albanes D: Metabolomic
profile of response to supplementation with β-carotene in the
Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin
Nutr. 98:488–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qin XY, Tatsukawa H, Hitomi K, Shirakami
Y, Ishibashi N, Shimizu M, Moriwaki H and Kojima S: Metabolome
analyses uncovered a novel inhibitory effect of acyclic retinoid on
aberrant lipogenesis in a mouse diethylnitrosamine-induced hepatic
tumorigenesis model. Cancer Prev Res. 9:205–214. 2016. View Article : Google Scholar
|
|
44
|
Huang YT, Lin YW, Chiu HM and Chiang BH:
Curcumin induces apoptosis of colorectal cancer stem cells by
coupling with CD44 marker. J Agric Food Chem. 64:2247–2253. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Amelio I, Cutruzzolá F, Antonov A,
Agostini M and Melino G: Serine and glycine metabolism in cancer.
Trends Biochem Sci. 39:191–198. 2014. View Article : Google Scholar : PubMed/NCBI
|