Introduction
Cervical carcinoma is the second most prevalent
female cancer and the most common malignancy in terms of both
incidence and mortality worldwide (1). More than 80% of the cervical cancers
occur in developing countries (2).
Several therapies are used to treat the disease, but each of them
has adverse effects (3). Therefore,
the development of a safer and more efficient chemotherapeutic
treatment for cervical carcinoma is very important.
The process of apoptosis or programmed cell death is
tightly controlled and it plays important roles in many biological
processes ranging from fetal development to adult tissue
homeostasis (4). Apoptosis is
characterized by morphological changes, including cell shrinkage,
nuclear reorganization, blebbing of active membrane, and
fragmentation of the cell into membrane-enclosed vesicles (5). As malignant cells suppress this
response to survive, apoptosis has been an important focus of the
current cancer research. Apoptosis can be initiated through the
intrinsic (mitochondrial-mediated) and extrinsic (death
receptor-mediated) pathway (6–8).
Several important events occur in the mitochondria right after
intrinsic apoptotic stimulation, including the release of
pro-apoptotic factors such as cytochrome c from the
mitochondria into the cytoplasm (9,10). In
the cytosol, cytochrome c interacts with apoptotic protease
activating factor-1 (Apaf-1), leading to the activation of the
cysteine-aspartic protease caspase-9, which activates caspase-3
followed by activation of the rest of the caspase cascade, leading
to programmed cell death (11). In
addition, the activation of mitochondria and the release of
regulatory factors from the mitochondrial intermembrane space
control a number of Bcl-2 family of regulatory proteins downstream
(12–14). Some of these proteins such as Bcl-2
are anti-apoptotic and prevent the release of cytochrome c,
whereas others such as Bax promote the release of cytochrome
c (15). The extrinsic
pathway involves the binding of ligands of the tumor necrosis
factor (TNF) superfamily to cell surface death receptors and the
subsequent activation of membrane-proximal caspases (caspase-8 and
−10) (16).
Reactive oxygen species (ROS) [e.g., superoxide
anions (O2·−), hydrogen peroxide
(H2O2), and hydroxyl radicals (OH·)] are
by-products of cellular metabolic pathways and are crucial
secondary messengers in various intracellular signaling pathways
(17,18). Recently, it has become clear that
ROS play a significant role in the cause of apoptotic cell death
under physiological as well as pathological conditions, and that
interestingly, mitochondria are both the source and the target of
ROS (19,20). Several investigators suggested
evidence that intracellular ROS can directly cause the
mitochondrial permeability transition activation, loss of
mitochondrial membrane potential (MMP), and release of cytochrome
c from mitochondria (21,22).
The cell cycle is deregulated in tumors, causing
lack of differentiation and aberrant growth of cells (23–25).
The cell cycle regulates cell division, differentiation, growth and
programmed cell death (26). Many
anticancer agents have been developed to arrest the cell cycle at
specific checkpoints, thereby causing apoptotic cell death
(27). G2 or pre-mitotic phase is
the third and final subphase of interphase in the cell cycle,
directly preceding mitosis and following successful completion DNA
replication during S phase. G2 ends with the onset of prophase, the
first phase of mitosis, during which the chromatin condenses into
chromosomes. A series of chroman analogs previously reported as
potassium channel openers were examined for their in vitro
growth inhibitory effect on human glioma cells (28). We evaluated the cytotoxic effect of
six chroman analogs that were kindly provided by Dr D.S. Shin on
HeLa cells using the WST-8 assay, and found that
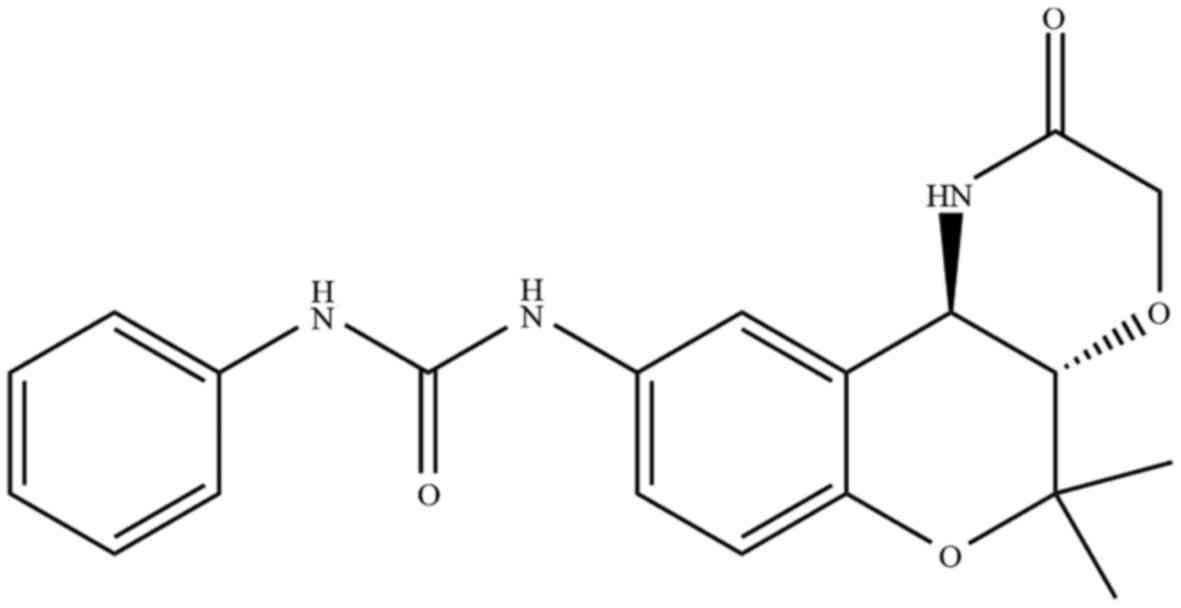
1-[(3S,4R)-2,2-dimethyl-3-oxo-4-(2-piperidonyl)chroman-6-yl]-3-phenylurea
(S32) (Fig. 1) showed the strongest
effect. Notably, we found that chroman derivatives containing a
phenylurea group were more cytotoxic than derivatives without this
group (Table I), showing that this
group is important for cytotoxicity (21). Phenylurea-type 2,2-dimethylchromans
have been identified as a new class of potential antitumor agents
for an innovative therapeutic approach for high-grade glioma
(28). However, the underlying
molecular mechanisms attributed to the growth inhibitory and
cytotoxic effects of S32 are poorly understood. In this study, we
investigated the anticancer effect of S32 derivatives in HeLa cells
to identify appropriate novel candidate antitumor drugs.
 | Table I.Evaluation of the cytotoxic effects
of phenylurea-including compounds and non-including compounds
(mother compound) on HeLa cells. |
Materials and methods
Chemicals
The Annexin V-FITC kit and propidium iodide
(PI)/RNase staining buffer for apoptosis were obtained from BD
Biosciences (Franklin Lakes, NJ, USA). Eagle's minimum essential
medium (EMEM), penicillin-streptomycin and trypsin-EDTA were
obtained from HyClone (HyClone: GE Healthcare Life Sciences, Logan,
UT, USA). Fetal bovine serum (FBS) was purchased from Gibco-BRL
(Gibco-BRL: Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cell
Counting kit-8 (CCK-8) was obtained from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). Dimethyl sulfoxide (DMSO) and
phosphate-buffered saline (PBS, pH 7.4) were purchased from
Sigma-Aldrich Chemical Co. (Sigma-Aldrich Chemical Co.: Merck KGaA,
Darmstadt, Germany). All other chemicals were of analytical reagent
grade.
Cell lines
HeLa cells obtained from the American Type Culture
Collection (ATCC) (Manassas, VA, USA) were cultured in EMEM
supplemented with 10% FBS and 1% penicillin-streptomycin at 37°C in
a humidified atmosphere with 5% CO2.
Preparation of chroman analog
sample
1-[(3S,4R)-2,2-dimethyl-3-oxo-4-(2-piperidonyl)chroman-6-yl]-3-phenylurea
(S32) and other chroman analogs (S10, S16, S18, S24, and S26) were
obtained from the laboratory of Dr D.S. Shin (Changwon National
University). The stock solutions of all chroman analogs were
prepared in DMSO as 100 mM and maintained at 4°C. Further dilutions
were made immediately prior to each experiment.
Cell viability and proliferation
assay
HeLa cells were seeded at 5×103 cells
into each well of a 96-well microplate. After 24 h, media were
replaced with fresh media containing the various concentrations
(20, 40 and 80 µM) of S32. The plate was incubated for a further 48
h. Then, CCK-8 reagent (10 µl) was added to each well of the plate
and incubated for 2 h. The cell viability was assessed using WST-8
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium]
according to the manufacturer's recommendations (29). The optical density for living cells
was read at 450 nm using a multi-microplate reader (Synergy HT;
BioTek Instruments, Inc., Winooski, VT, USA). For determination of
cell proliferation, cells were seeded at 5×103 cells/ml
media in 96-well microplates and treated with or without S32 (70
µM) for various times (0, 12, 24, 36, 48 and 60 h). Each experiment
was repeated at least three times.
Measurement of apoptotic cell
morphology
HeLa cells were distributed (1×105
cells/well) into a 6-well plate and allowed to stand overnight. The
cells were treated with S32 (70 µM) for 24 and 48 h. Wells that
were not treated with S32 received an equivalent volume of DMSO
(<0.1%) used as a control. Phase-contrast images were captured
with a Nikon Phase Contrast-2, ELWD 0.3 inverted microscope.
Measurement of ROS
Production of ROS was assessed using the fluorescent
indicator 2′,7′-dichlorodihydrofluorescein diacetate
(H2DCF-DA), a cell-permeable indicator for ROS, shown to
react with H2O2 (30). H2DCF-DA is oxidized to
highly green fluorescent 2′,7′-dichlorofluorescein (DCF) via the
generation of ROS. HeLa cells (3×105 cells in a 60-mm
dish) treated with (70 µM) or without S32 were collected by
trypsinization and centrifugation at 300 × g for 5 min. The pellets
were washed with cold PBS and stained with 2 µl of
H2DCF-DA for 30 min at 37°C in a dark room. Relative
fluorescence intensities were observed using the FACSCalibur flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed by
CellQuest Pro software (Becton-Dickinson: BD Biosciences, Franklin
Lakes, NJ, USA) with the FL-1 channel (green) set to 530 nm.
Measurement of MMP (ΔΨm)
Changes in MMP were detected by using a fluorescent
probe, rhodamine 123 (RH-123; Molecular Probes, Inc.; Thermo Fisher
Scientific, Inc., Eugene, OR, USA). HeLa cells (3×105
cells in a 60-mm dish) treated with (70 µM) or without S32 were
collected by trypsinization and centrifugation at 300 × g for 5
min. The pellets were washed with cold PBS and stained with 5 µl of
rhodamine and intensities were observed by the FACSCalibur flow
cytometer (BD Biosciences) and analyzed by CellQuest software
(Becton-Dickinson: BD Biosciences) with the FL-1 channel.
[3H]-thymidine
incorporation assay
The [3H]-thymidine incorporation assay
was performed as described in a previously study (31). HeLa cells were cultured in 6-well
plates in growth media (EMEM + 10% FBS + 1%
penicillin-streptomycin). After the cells were grown to 70–80%
confluence, they were rendered quiescent by incubation for 24 h in
EMEM containing 2% FBS. Cells were then treated with (70 µM) or
without S32 in EMEM supplemented with 10% FBS. After incubation for
21 or 45 h, [3H]-thymidine was added at 1 µCi/ml (1 µCi
= 37 kBq) and further incubated for 3 h. Incorporated
[3H]-thymidine was measured by using a Liquid
Scintillation Analyzer (Tris-Carb 2910TR; PerkinElmer, Inc.,
Waltham, MA, USA).
Cell cycle arrest analysis
HeLa cells (3×105 cells in a 60-mm Petri
dish) treated with (70 µM) or without S32 were collected by
trypsinization and washed with cold PBS by centrifugation at 412 ×
g for 6 min. After suspension in PBS and fixation with 70% ethanol
(v/v), samples were washed with cold PBS and stained with PI/RNase
staining buffer for 15 min at room temperature. The number of cells
in the different cell cycle phases was analyzed using a FACSCalibur
flow cytometer analysis system (BD Biosciences) and 20,000 events
were analyzed for each sample. The percentage of cells in the
different phases was determined using ModFit software (Verity
Software House, Inc., Topsham, ME, USA).
Annexin V-FITC/PI apoptotic
analysis
HeLa cells (3×105 cells in a 60-mm dish)
treated with (70 µM) or without S32 were collected by
trypsinization and washed with ice-cold PBS via centrifugation at
2,500 × g for 3 min. Subsequently, 1×105 cells were
resuspended in 100 µl of binding buffer and stained with 5 µl of
Annexin V-FITC and 10 µl of PI (50 µg/ml) for 15 min at room
temperature in the dark. Analysis was performed using FACSCalibur
flow cytometer (BD Biosciences) with 10,000 events each time. The
data were analyzed by CellQuest Pro software (Becton-Dickinson: BD
Biosciences).
Protein extraction and western blot
analysis
After the treatment of HeLa cells (1×105
cells in a 150-mm dish) with (70 µM) or without S32, total cell
lysates and cytosolic fractions were prepared as described in a
previous study (32). Protein
contents of the lysates were determined by the Bradford protein
assay. Proteins (20 µg) were separated by SDS-PAGE and transferred
onto nitrocellulose membranes by western blot analysis. The
following primary polyclonal antibodies were used: β-actin (cat.
no. 4967), pro-caspase-8 (cat. no. 4790), pro-caspase-9 (cat. no.
9502), Bcl-2 (cat. no. 2872) (1:1,000 dilution; rabbit; Cell
Signaling Technology, Inc., Danvers, MA, USA), pro-caspase-3
(1:300; mouse; cat. no. sc-7272; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) and Bax (1:1,000; mouse; cat. no. 556467; BD
Biosciences, San Diego, CA, USA). The results were quantified using
ImageJ v.1.43 software (National Institutes of Health, Bethesda,
MD, USA).
Statistical analysis
Each experiment was repeated at least three times.
The results are expressed as the mean ± standard deviation (SD)
values of three independent experiments. Statistical analysis was
performed by one-way analysis of variation (ANOVA). The criterion
for significance was set at P<0.05. For the statistical and
graphical evaluations, Microsoft Excel 2007 was used.
Results
S32 inhibits the viability and
proliferation of HeLa cells
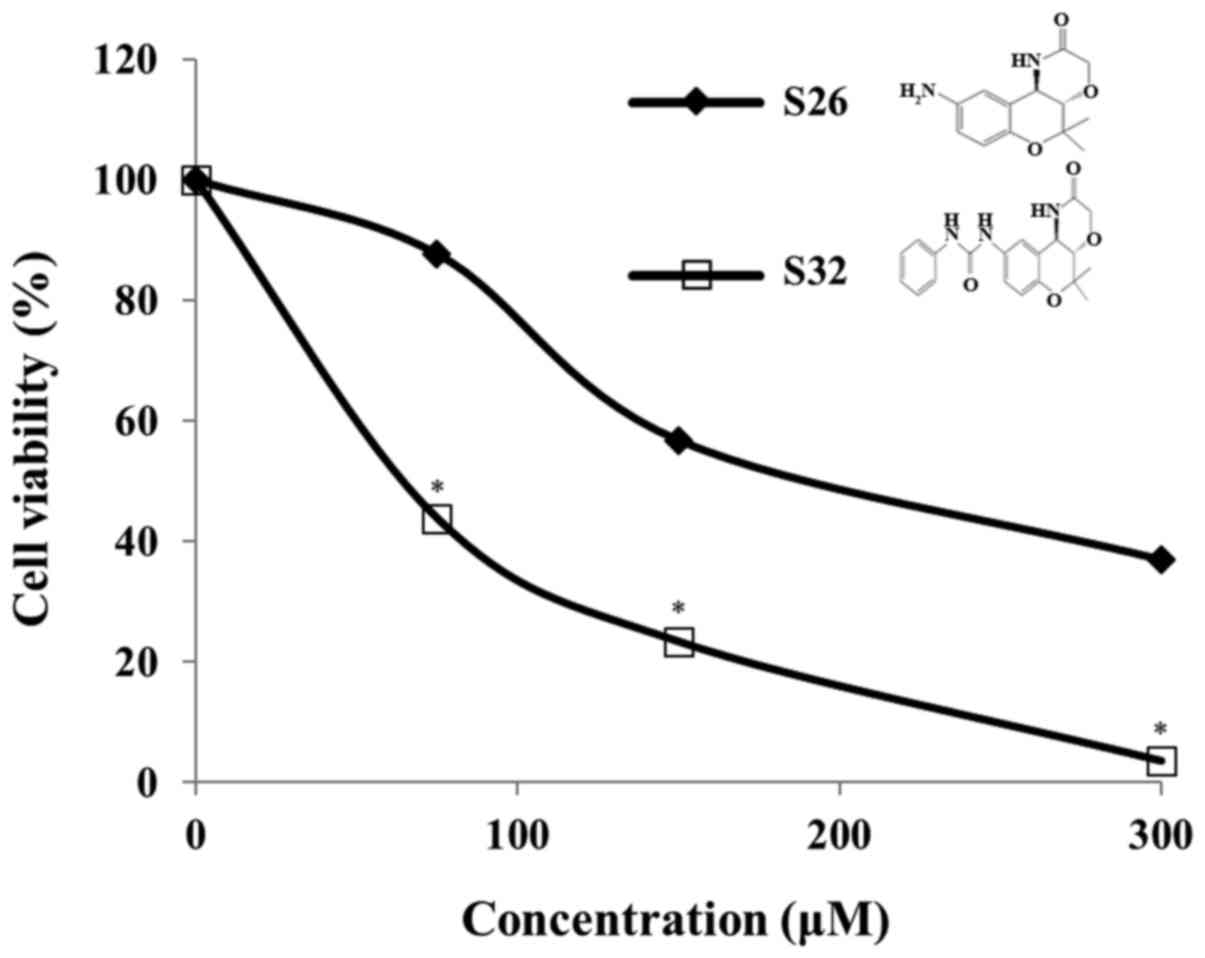
In the present study, we evaluated the cytotoxic
effect of chroman compounds, S26 and S32, at different
concentrations (75, 150, and 300 µM) on HeLa cells. After
incubation with the chroman compounds for 48 h, we observed a
significant (P<0.05) concentration-dependent cytotoxicity of S32
compared to that observed with the control. In addition, we found
that S32 inhibited cell viability to a greater extent than the
mother compound S26 (Fig. 2).
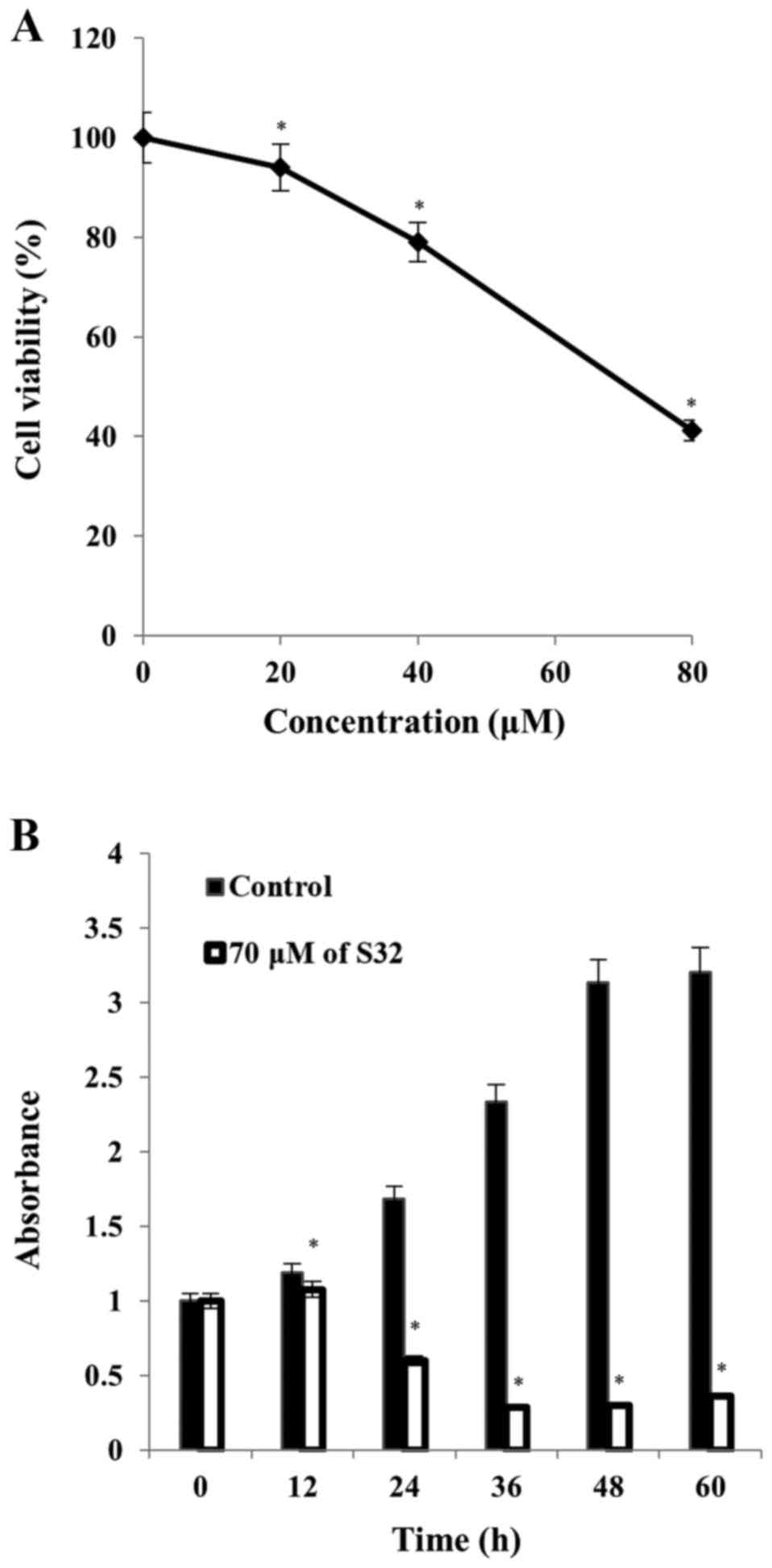
Therefore, we selected S32 for further study. As shown in Fig. 3A, the IC50 value of S32
was 70 µM. Treatment of HeLa cells with 70 µM of S32 for various
time periods (0, 12, 24, 36, 48 and 60 h) showed that their growth
gradually increased until 12 h and started to decrease at 24 h,
whereas untreated cells maintained exponential proliferation
(Fig. 3B). These data demonstrated
that S32 decreased HeLa cell viability in a concentration- and
time-dependent manner.
S32 induces apoptosis-related cell
morphology
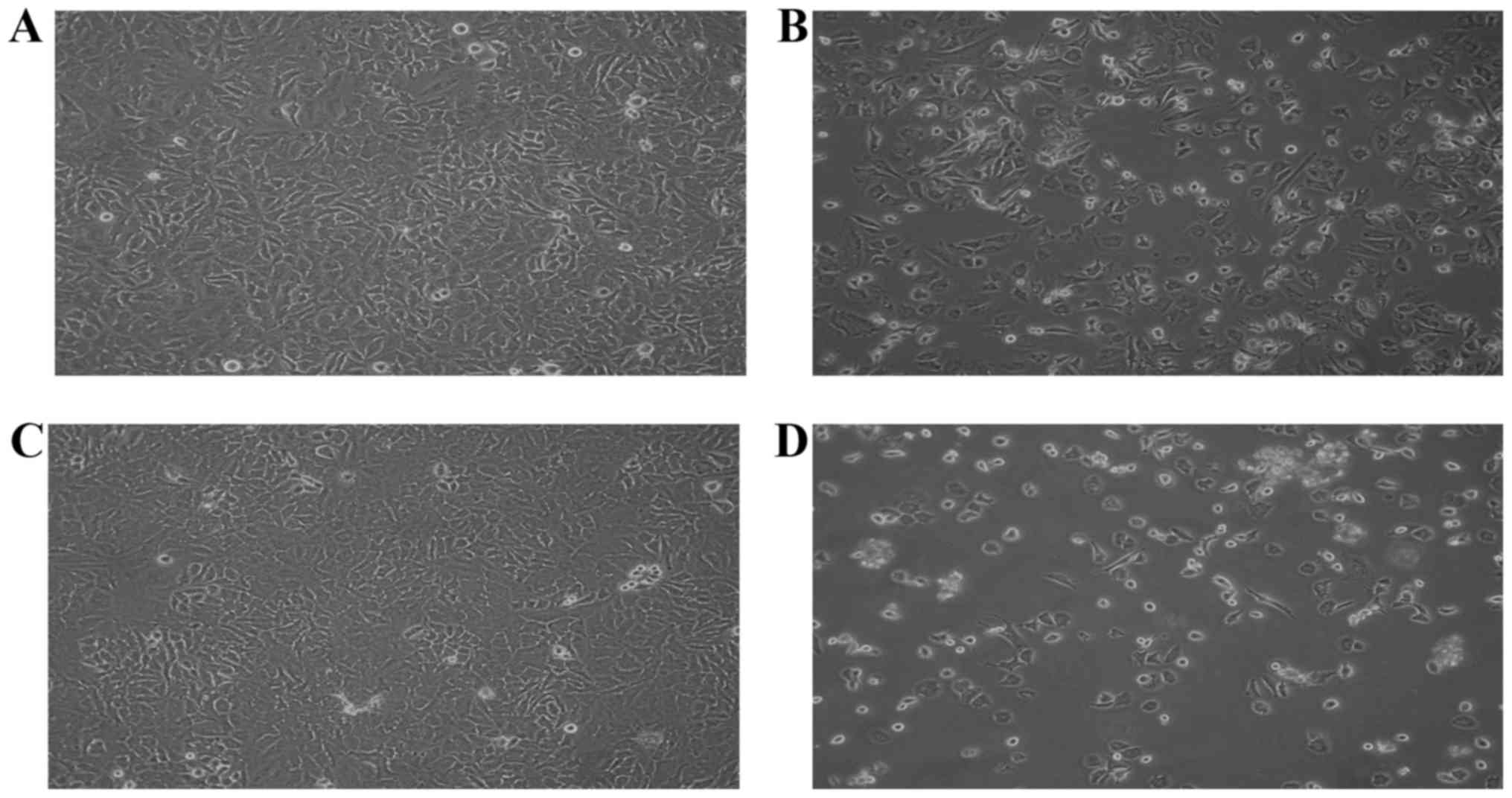
Morphological changes are important characteristics
of apoptotic cells. Microscopic analysis revealed the occurrence of
apoptosis in cells treated with 70 µM of S32 for various durations
(Fig. 4). As shown in Fig. 4A and C, non-treated HeLa cells
proliferated regularly throughout the culture plate and grew to
near confluence. After 24 h of treatment with S32, some cells were
detached from the plate but the majority of the attached cells
maintained a normal shape (Fig.
4B). After 48 h of treatment, the number of floating cells
increased and the attached cells showed cell shrinkage and
disruption, indicating apoptosis (Fig.
4D).
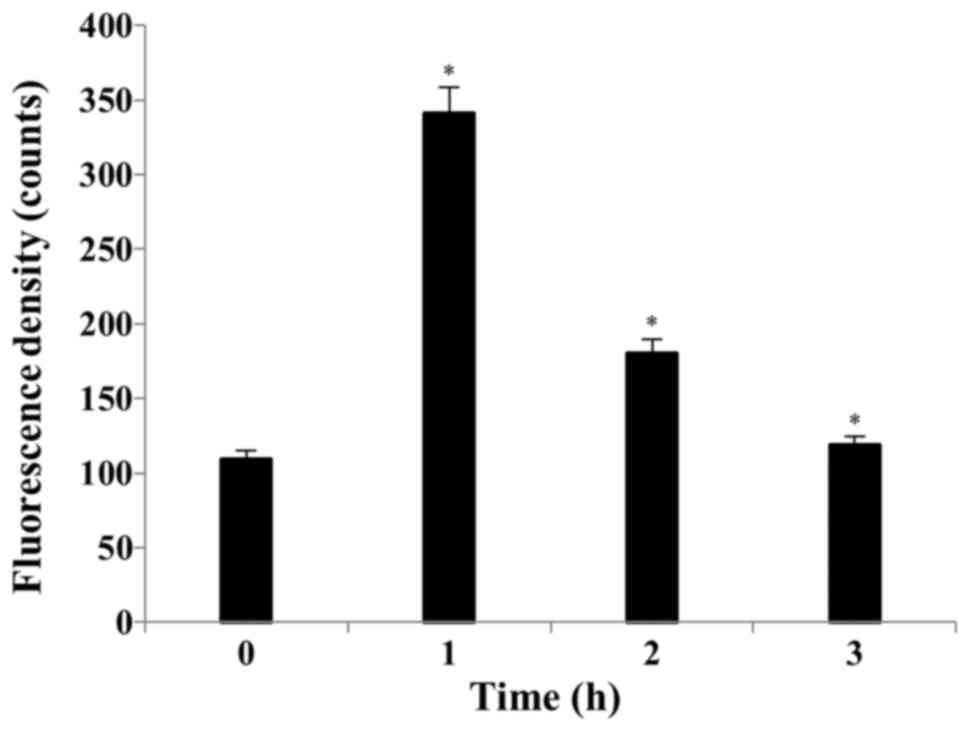
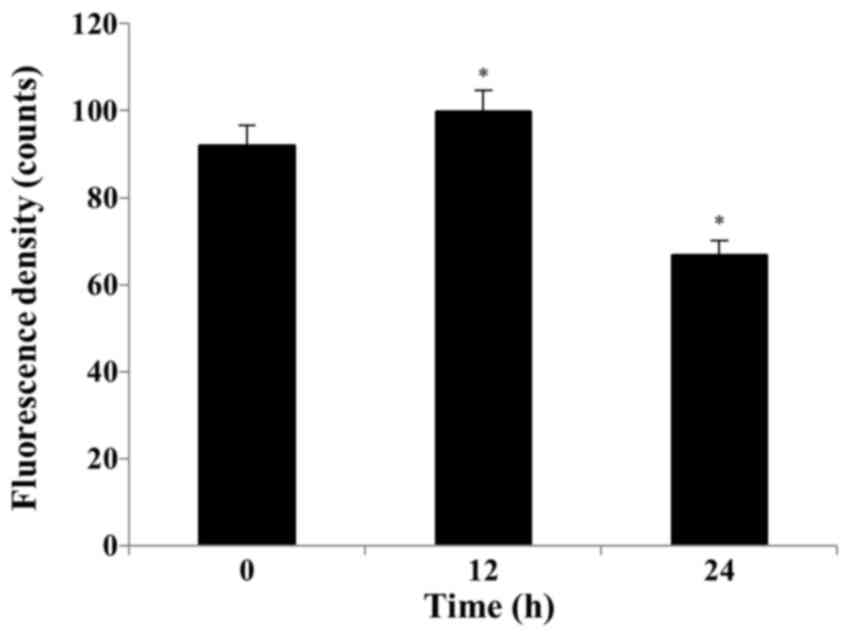
S32 induces ROS production and
depolarization of the MMP
Several studies have reported that ROS can activate
the mitochondrial permeability transition and loss of MMP (33). By using the cell permeable dye, we
showed that S32 has the capacity to induce the generation of
intracellular ROS. Treatment of the HeLa cells with 70 µM of S32
for 1 h induced ROS generation compared to that observed with the
control. As shown in Fig. 5, the
mean H2DCF-DA fluorescence increased from 109.26 to
341.67 after treatment with S32 for 1 h. Apoptosis induces
mitochondrial membrane depolarization. A decrease in
H2DCF-DA fluorescence suggests the loss of MMP. We
examined S32-induced MMP loss in HeLa cells. As shown in Fig. 6, the mean RH-123 fluorescence
significantly (P<0.05) increased from 91.93 (0 h) to 99.73 (12
h) and then significantly (P<0.05) decreased to 66.75 after
treatment with S32 for 24 h, respectively, suggesting that S32
induced apoptosis.
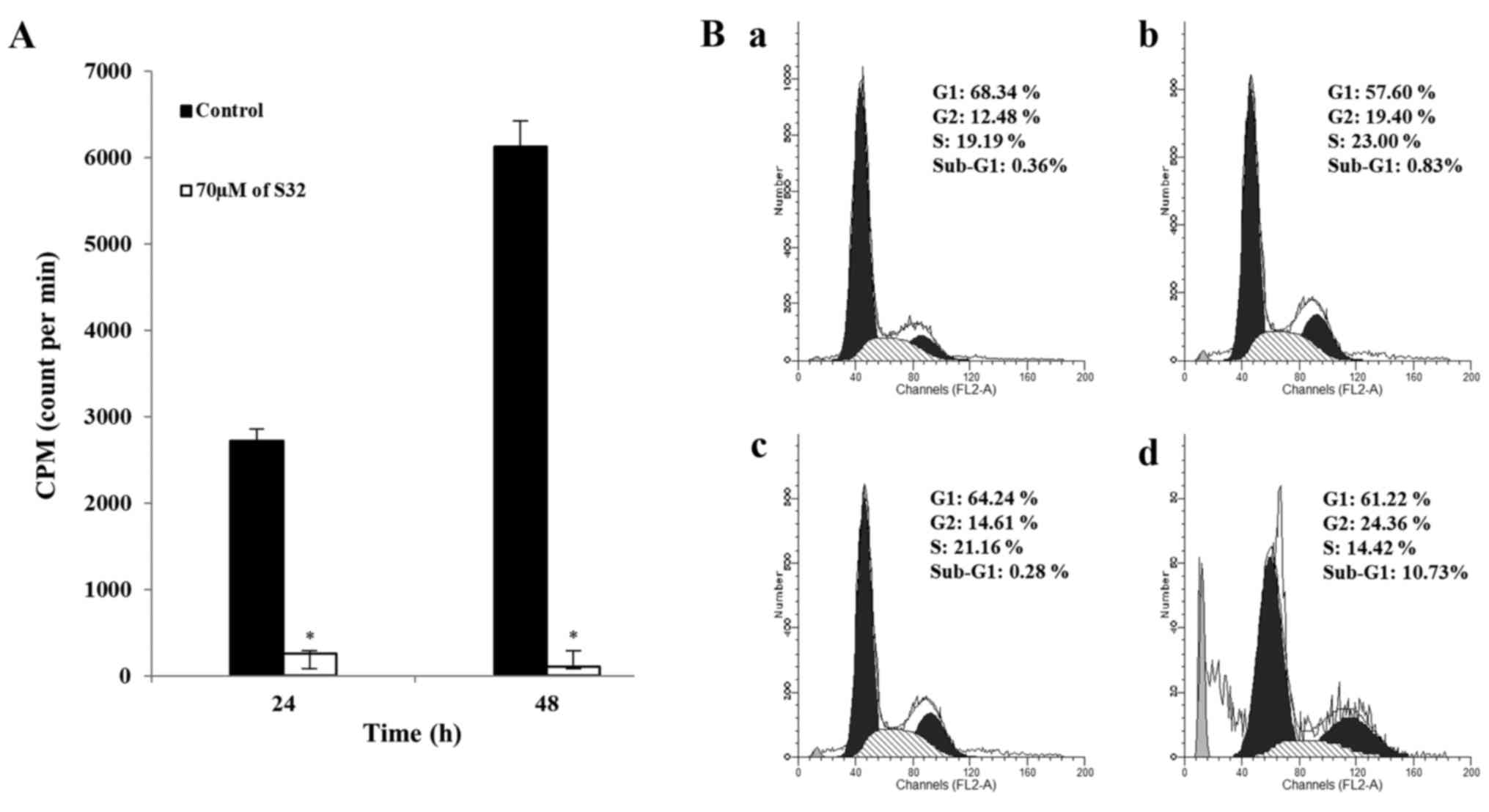
S32 promotes inhibition of DNA
replication and induction of G2 phase cell cycle arrest in HeLa
cells
To identify that S32 affects cells at the DNA level,
we analyzed DNA replication in cells treated with 70 µM of S32 by
using a [3H]-thymidine incorporation assay. As shown in
Fig. 7A, [3H]-thymidine
incorporation was significantly (P<0.05) reduced in HeLa cells
treated with S32, suggesting that DNA replication was inhibited in
a time-dependent manner.
Cell proliferation and apoptosis are controlled by
regulators of cell cycle progression and apoptotic impulses
(34,35). The appearance of a
sub-G0/G1 peak, also termed apoptotic peak,
on flow cytometric DNA content histograms is thought to be one of
the features of cells undergoing apoptosis (36). To examine the effect of S32 on cell
cycle progression, HeLa cells were treated with 70 µM of S32 for 24
or 48 h, and analyzed by using flow cytometry. Treatment with S32
(Fig. 7Bb and d) increased the
fraction of G2-phase cells compared to the untreated cells
(Fig. 7Ba and d). In addition,
treatment of S32 increased the percentage of sub-G1 phase
(apoptotic) cells in a time-dependent manner. These data
demonstrated that S32 induced G2 phase cell cycle arrest in HeLa
cells.
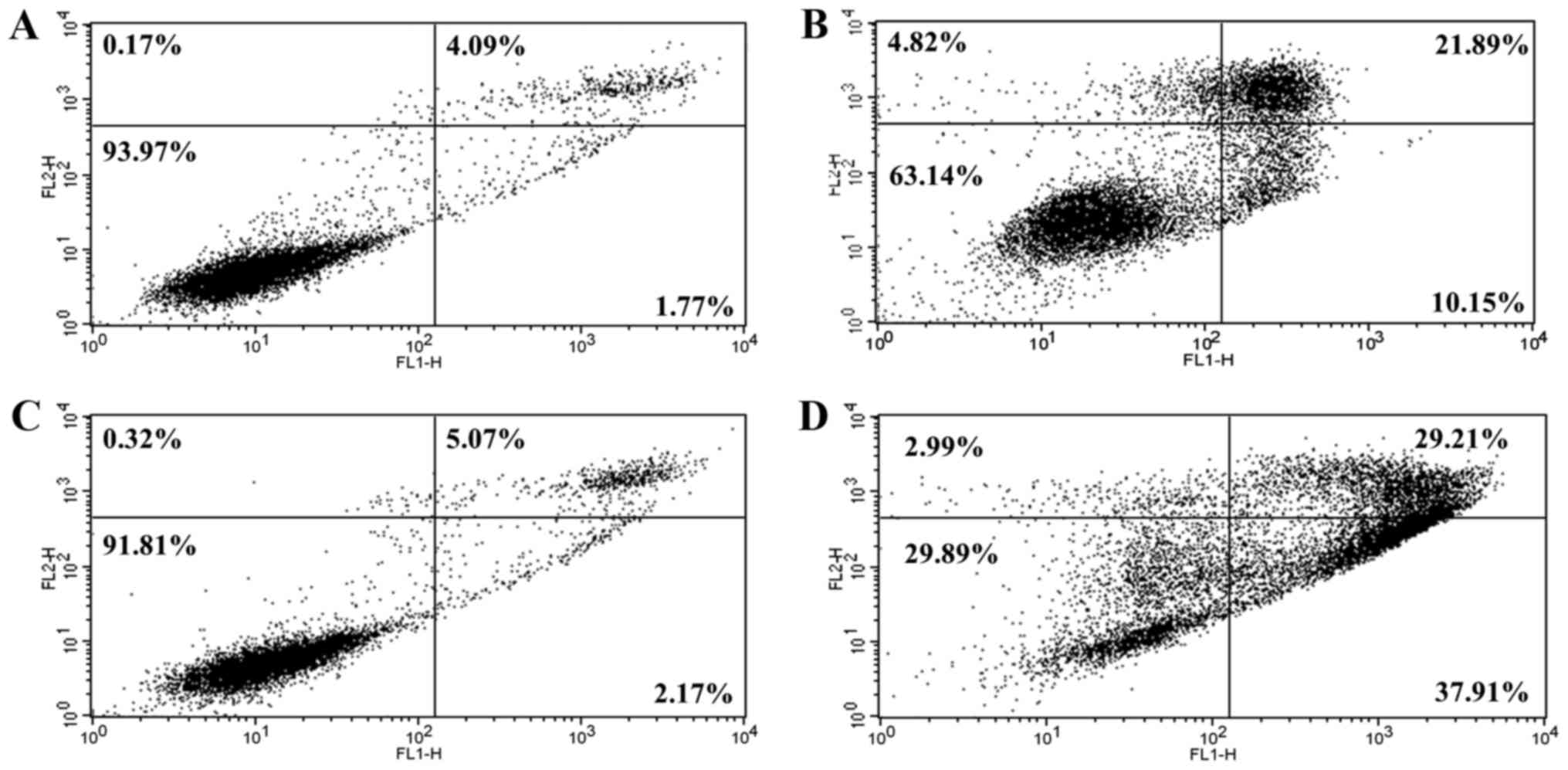
S32 induces apoptosis in HeLa
cells
To confirm that apoptosis was induced by S32, HeLa
cells were treated with 70 µM of S32 for 24 or 48 h and were then
analyzed using flow cytometry after staining with Annexin V-FITC
and PI. The staining of cells with Annexin V-FITC and PI is used to
distinguish and quantify non-apoptotic (Annexin
V-FITC−/PI−), early apoptotic (Annexin
V-FITC+/PI−), and late apoptotic (or
necrotic) (Annexin V-FITC+/PI+ and Annexin
V-FITC−/PI+) cells (37). Treatment with S32 increased the
fraction of apoptotic cells (Fig. 8B
and D) compared to the non-treated cells (Fig. 8A and C), confirming that S32 induced
apoptosis in HeLa cells in a time-dependent manner.
S32 induces mitochondrial-mediated
apoptosis
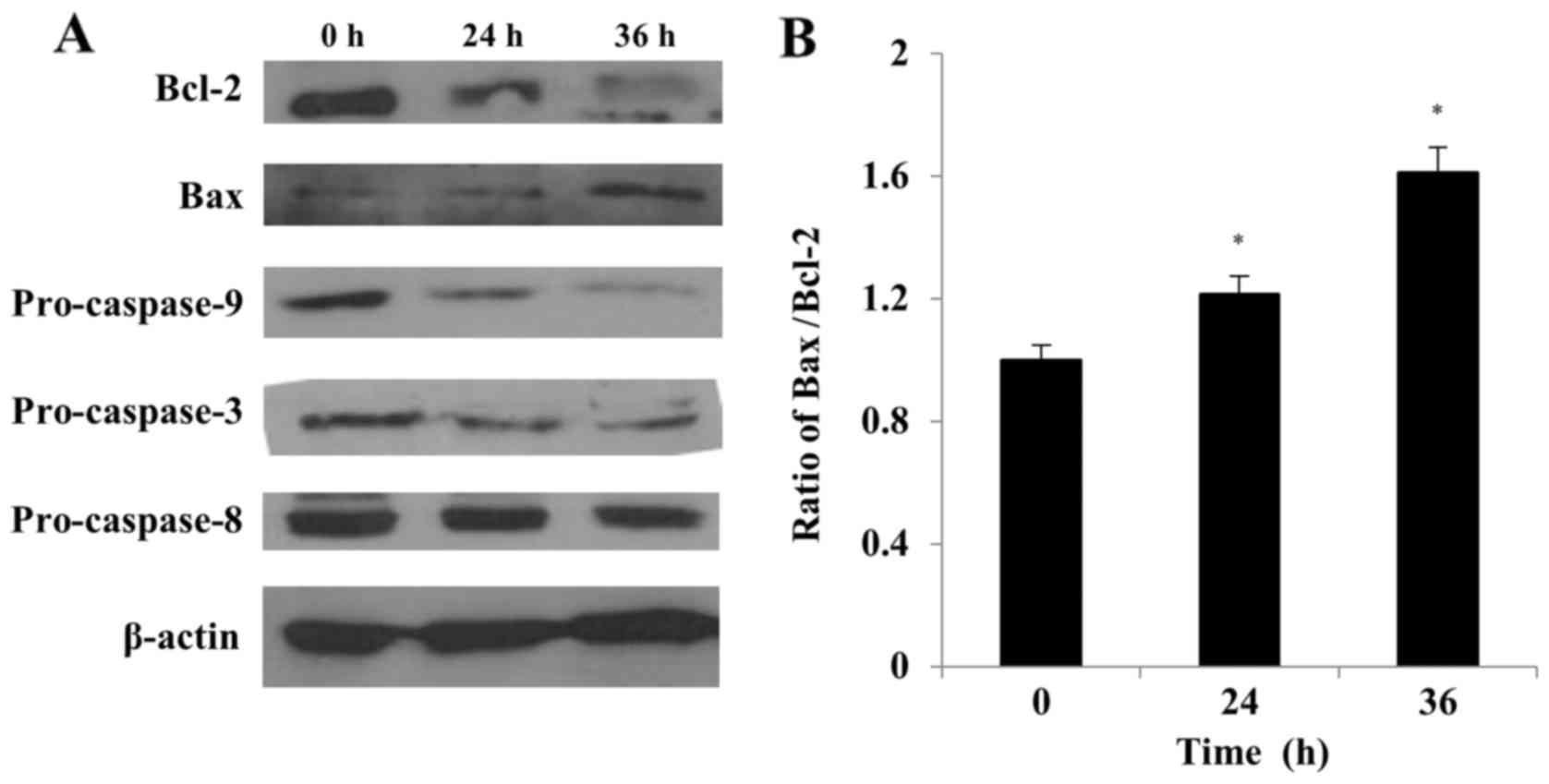
The Bcl-2 family of proteins plays a crucial role in
the regulation of cell life and death (38). The ratio between pro- (e.g., Bax)
and anti-apoptotic (e.g., Bcl-2) proteins determines, in part, the
susceptibility of cells to a death signal (39). We evaluated the effect of S32
treatment on the Bax/Bcl-2 ratio by using western blot analysis. As
shown in Fig. 9, the Bcl-2 level
decreased while the Bax level increased with time in cells treated
with S32, indicating that the Bax/Bcl-2 ratio significantly
(P<0.05) increased in a time-dependent manner (Fig. 9B).
In response to apoptotic stimuli, the outer
mitochondrial membrane becomes permeable, resulting in the release
of cytochrome c and other caspase activators (11). We evaluated whether the
caspase-dependent mitochondrial-mediated pathway is involved in
S32-induced apoptosis to determine the underlying molecular
mechanism of this process. The pro-caspase-9 and −3 levels were
markedly decreased in cells treated with S32, while the
pro-caspase-8 level decreased slightly in a time-dependent manner
(Fig. 9), demonstrating the
activation of the caspase cascade. These results suggested that S32
induces apoptosis in HeLa cells primarily via the
mitochondrial-mediated pathway.
Discussion
Cromakalim, a potassium channel opener, has been
shown to have antitumor potential in human neuroblastoma and
astrocytoma cell lines (15). The
mechanism of this antitumor effect was suggested to involve the
activation of ATP-sensitive K+ channels, leading to the
inhibition of intracellular Ca2+ signaling (40). We previously reported that chroman
analogs have a cytotoxic effect on HeLa cells and that phenylurea
derivatives show a stronger effect than other chroman compounds
(15). Among the
phenylurea-including compounds, S32 showed the strongest
cytotoxicity at a low concentration with an IC50 of
72.46 µM. In this study, we investigated the underlying mechanism
attributed to the cell death induced by chroman analogs in human
cervical carcinoma HeLa cells.
Various methods have been developed to monitor the
different stages of the apoptotic pathway (41,42).
First, we assessed the morphological changes in the HeLa cells
treated with S32. The apoptotic cell population increased with time
after S32 treatment, and marked morphological changes indicated
apoptosis. The results of the Annexin V-FITC/PI dual staining assay
indicated that S32 induced early apoptosis in the HeLa cells in a
time-dependent manner.
The cell cycle machinery tightly controls cell
growth and the inhibition thereof (43), and dysregulation of cell cycle
progression has been shown to be involved in the inhibition of
apoptosis (34,44). Flow cytometric analysis of the DNA
content indicated that S32 induced G2-phase cell cycle arrest in
HeLa cells, subsequently leading to an increase in the fraction of
apoptotic cells. When DNA replication is blocked or if the template
is damaged by radiation or other factors, signals are generated
that can result in cell cycle arrest or apoptosis (45). Any cell that is damaged beyond the
capacity of the DNA repair system is eliminated. The
[3H]-thymidine incorporation assay suggested that S32
inhibited DNA replication and cell proliferation in the HeLa
cells.
ROS generation induces the depolarization of the
MMP, thereby triggering a series of mitochondrial-mediated events
including apoptosis (33). We found
that S32-induced apoptosis in HeLa cells was associated with an
early increase in intracellular ROS generation and depolarization
of the MMP. These results prompted us to investigate the apoptotic
pathway in S32-treated cells further by using western blot
analysis. As ROS generation causes dimerization of Bax in the
cytosol, ROS might be directly associated with apoptosis (46). The Bcl-2 family proteins Bcl-2 and
Bax play crucial roles in the initiation of the
mitochondrial-mediated apoptotic pathway (14). Bax activates cytochrome c
release into the cytosol, while Bcl-2 prevents this by preserving
mitochondrial integrity (46). The
ratio of Bax to Bcl-2 was shown to be a determining factor in the
induction of mitochondrial-mediated apoptosis in drug-induced
apoptosis in hepatocellular carcinoma cells (47). We showed that S32 increased the
ratio of Bax/Bcl-2, which may be involved in cell death initiation.
The mitochondrial cytochrome c release induces the formation
of the apoptosome complex composed of Apaf-1 and caspase-9, which
subsequently activates downstream caspases (11). In the present study, both caspase-9
and −3 were found to be activated by treatment with S32, confirming
that S32 induced apoptosis via the mitochondrial-mediated pathway.
Based on the slight decrease in pro-caspase-8, we hypothesized that
S32 additionally induced the extrinsic apoptotic pathway.
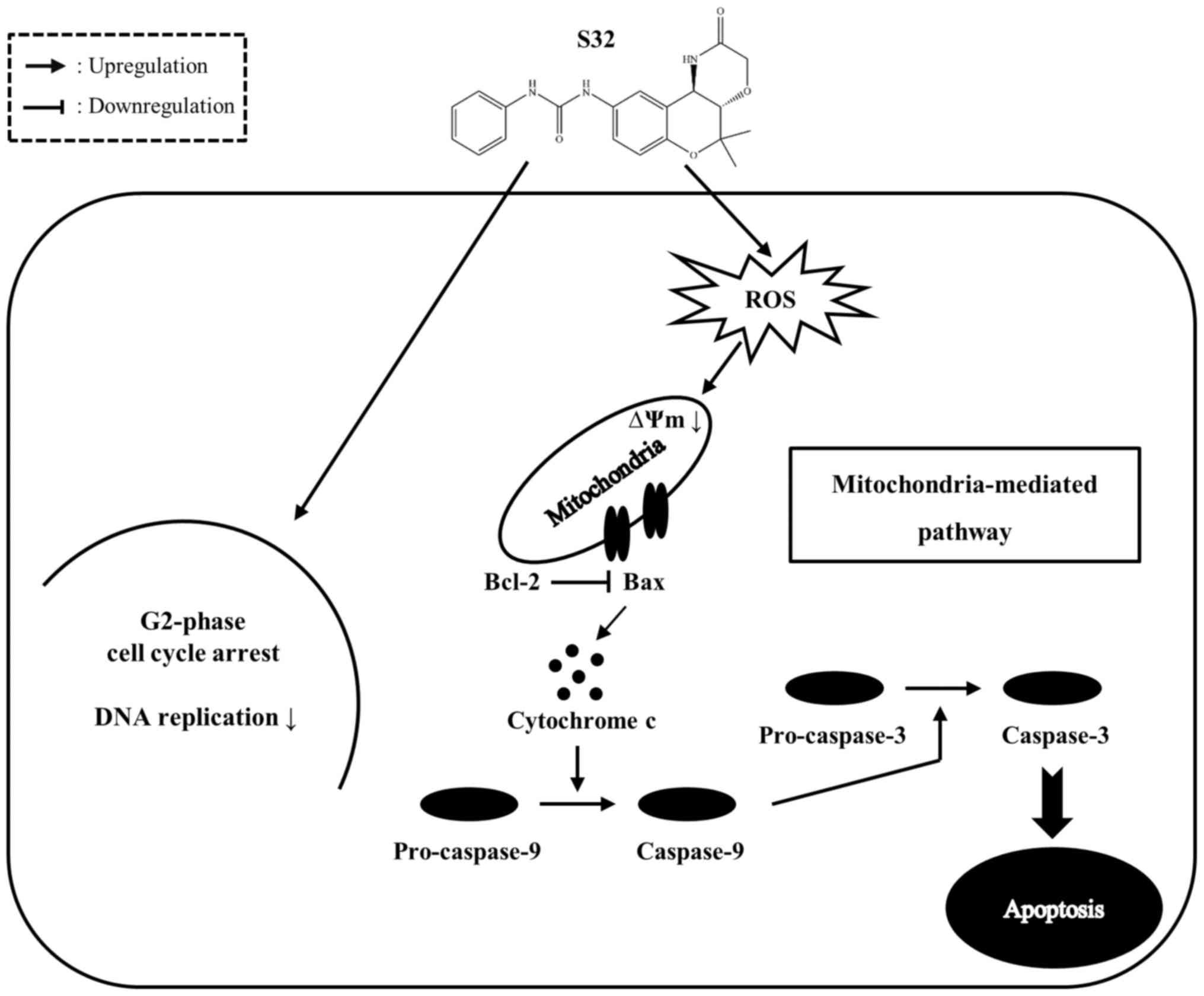
In the present study, we demonstrated that S32
inhibits proliferation of HeLa cells by inducing G2-phase cell
cycle arrest and inhibiting DNA replication. In addition, S32
induces apoptosis by promoting ROS generation and MMP disruption
(Fig. 10). Taken together, our
results suggest that S32 triggers apoptosis mainly via the
mitochondrial-mediated pathway, which can be further investigated
in future studies, and our findings indicate that S32 has potential
as an anticancer agent.
Acknowledgements
The authors would like to thank Dr Dong-Soo Shin
(Changwon National University) for providing the chroman
analogs.
Funding
This research study was supported by the 2016 Inje
University research grant.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
DKK and HJ conceptualized the study. DSS (Changwon
National University) provided chroman analog samples. HJ performed
the all of experiments. YS assisted the all of the experiments. All
authors contributed to the data and analyses. HJ wrote the report.
DKK and HJ reviewed and edited the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the study are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
References
|
1
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization, . Control of
cancer of the cervix uteri: Review article based on a report of a
WHO meeting, November 1985, Geneva. Bull World Health Organ.
64:607–618. 1986.PubMed/NCBI
|
|
3
|
Yaoxian W, Hui Y, Yunyan Z, Yanqin L, Xin
G and Xiaoke W: Emodin induces apoptosis of human cervical cancer
HeLa cells via intrinsic mitochondrial and extrinsic death receptor
pathway. Cancer Cell Int. 13:712013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reed JC: Apoptosis-regulating proteins as
targets for drug discovery. Trends Mol Med. 7:314–319. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Earnshaw WC: Nuclear changes in apoptosis.
Curr Opin Cell Biol. 7:337–343. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Degterev A, Boyce M and Yuan J: A decade
of caspases. Oncogene. 22:8543–8567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ziegler DS and Kung AL: Therapeutic
targeting of apoptosis pathways in cancer. Curr Opin Oncol.
20:97–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ehrlich E: Regulation of BAX mediated
apoptosis by BCL2 family members. SABiosciences. 2011.https://www.qiagen.com/fi/spotlight-pages/newsletters-and-magazines/articles/reviews-online-apoptosis/
|
|
9
|
Reed JC and Green DR: Remodeling for
demolition: Changes in mitochondrial ultrastructure during
apoptosis. Mol Cell. 9:1–3. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang C and Youle RJ: The role of
mitochondria in apoptosis. Annu Rev Genet. 43:95–118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marzo I, Brenner C, Zamzami N, Susin SA,
Beutner G, Brdiczka D, Rémy R, Xie ZH, Reed JC and Kroemer G: The
permeability transition pore complex: A target for apoptosis
regulation by caspases and bcl-2-related proteins. J Exp Med.
187:1261–1271. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuwana T and Newmeyer DD: Bcl-2-family
proteins and the role of mitochondria in apoptosis. Curr Opin Cell
Biol. 15:691–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Zhao J, Kang S, Yi M, You S, Shin
DS and Kim DK: A novel cromakalim analogue induces cell cycle
arrest and apoptosis in human cervical carcinoma HeLa cells through
the caspase- and mitochondria-dependent pathway. Int J Oncol.
39:1609–1617. 2011.PubMed/NCBI
|
|
16
|
LeBlanc H, Lawrence D, Varfolomeev E,
Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D and
Ashkenazi A: Tumor-cell resistance to death receptor - induced
apoptosis through mutational inactivation of the proapoptotic Bcl-2
homolog Bax. Nat Med. 8:274–281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inal ME, Kanbak G and Sunal E: Antioxidant
enzyme activities and malondialdehyde levels related to aging. Clin
Chim Acta. 305:75–80. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Le Bras M, Clément MV, Pervaiz S and
Brenner C: Reactive oxygen species and the mitochondrial signaling
pathway of cell death. Histol Histopathol. 20:205–219.
2005.PubMed/NCBI
|
|
19
|
Fleury C, Mignotte B and Vayssière JL:
Mitochondrial reactive oxygen species in cell death signaling.
Biochimie. 84:131–141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan WH, Wu CC and Yu JS: Curcumin
inhibits UV irradiation-induced oxidative stress and apoptotic
biochemical changes in human epidermoid carcinoma A431 cells. J
Cell Biochem. 90:327–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang CP, Ding H, Shi DH, Wang YR, Li EG
and Wu JH: Pro-apoptotic effects of tectorigenin on human
hepatocellular carcinoma HepG2 cells. World J Gastroenterol.
18:1753–1764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hartwell LH and Kastan MB: Cell cycle
control and cancer. Science. 266:1821–1828. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dictor M, Ehinger M, Mertens F, Akervall J
and Wennerberg J: Abnormal cell cycle regulation in malignancy. Am
J Clin Pathol. 112 Suppl 1:S40–S52. 1999.PubMed/NCBI
|
|
26
|
Bonelli P, Tuccillo FM, Borrelli A,
Schiattarella A and Buonaguro FM: CDK/CCN and CDKI alterations for
cancer prognosis and therapeutic predictivity. BioMed Res Int.
2014:3610202014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu YJ, Yang SH, Chien CM, Lin YH, Hu XW,
Wu ZZ, Wu MJ and Lin SR: Induction of G2/M phase arrest and
apoptosis by a novel enediyne derivative, THDB, in chronic myeloid
leukemia (HL-60) cells. Toxicol In Vitro. 21:90–98. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goffin E, Lamoral-Theys D, Tajeddine N, de
Tullio P, Mondin L, Lefranc F, Gailly P, Rogister B, Kiss R and
Pirotte B: N-Aryl-N'-(chroman-4-yl)ureas and thioureas display in
vitro anticancer activity and selectivity on apoptosis-resistant
glioblastoma cells: Screening, synthesis of simplified derivatives,
and structure-activity relationship analysis. Eur J Med Chem.
54:834–844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tominaga H, Ishiyama M, Ohseto F, Sasamoto
K, Hamamoto T, Suzuki K and Watanabe M: A water-soluble tetrazolium
salt useful for colorimetric cell viability assay. Anal Commun.
36:47–50. 1999. View Article : Google Scholar
|
|
30
|
Bobyleva V, Pazienza TL, Maseroli R,
Tomasi A, Salvioli S, Cossarizza A, Franceschi C and Skulachev VP:
Decrease in mitochondrial energy coupling by thyroid hormones: A
physiological effect rather than a pathological hyperthyroidism
consequence. FEBS Lett. 430:409–413. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin SY, Liu JD, Chang HC, Yeh SD, Lin CH
and Lee WS: Magnolol suppresses proliferation of cultured human
colon and liver cancer cells by inhibiting DNA synthesis and
activating apoptosis. J Cell Biochem. 84:532–544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qi F, Li A, Zhao L, Xu H, Inagaki Y, Wang
D, Cui X, Gao B, Kokudo N, Nakata M, et al: Cinobufacini, an
aqueous extract from Bufo bufo gargarizans Cantor, induces
apoptosis through a mitochondria-mediated pathway in human
hepatocellular carcinoma cells. J Ethnopharmacol. 128:654–661.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park MT, Kim MJ, Kang YH, Choi SY, Lee JH,
Choi JA, Kang CM, Cho CK, Kang S, Bae S, et al: Phytosphingosine in
combination with ionizing radiation enhances apoptotic cell death
in radiation-resistant cancer cells through ROS-dependent and
-independent AIF release. Blood. 105:1724–1733. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee S, Christakos S and Small MB:
Apoptosis and signal transduction: Clues to a molecular mechanism.
Curr Opin Cell Biol. 5:286–291. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alenzi FQ: Links between apoptosis,
proliferation and the cell cycle. Br J Biomed Sci. 61:99–102. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao W, Li XQ, Wang X, Fan HT, Zhang XN,
Hou Y, Liu SB and Mei QB: A novel polysaccharide, isolated from
Angelica sinensis (Oliv.) Diels induces the apoptosis of
cervical cancer HeLa cells through an intrinsic apoptotic pathway.
Phytomedicine. 17:598–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen S, Cheng AC, Wang MS and Peng X:
Detection of apoptosis induced by new type gosling viral enteritis
virus in vitro through fluorescein annexin V-FITC/PI double
labeling. World J Gastroenterol. 14:2174–2178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reed JC, Jurgensmeier JM and Matsuyama S:
Bcl-2 family proteins and mitochondria. Biochim Biophys Acta.
1366:127–137. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee YS, Sayeed MM and Wurster RD: In vitro
antitumor activity of cromakalim in human brain tumor cells.
Pharmacology. 49:69–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kiechle FL and Zhang X: Apoptosis:
Biochemical aspects and clinical implications. Clin Chim Acta.
326:27–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Otsuki Y, Li Z and Shibata MA: Apoptotic
detection methods - from morphology to gene. Prog Histochem
Cytochem. 38:275–339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sánchez I and Dynlacht BD: New insights
into cyclins, CDKs, and cell cycle control. Semin Cell Dev Biol.
16:311–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Smith DM, Gao G, Zhang X, Wang G and Dou
QP: Regulation of tumor cell apoptotic sensitivity during the cell
cycle (Review). Int J Mol Med. 6:503–507. 2000.PubMed/NCBI
|
|
45
|
Zhang Y, Ahn EY, Jiang Y, Kim DK, Kang SG,
Wu C, Kang SW, Park JS, Son BW and Jung JH:
3-Chloro-2,5-dihydroxybenzyl alcohol activates human cervical
carcinoma HeLa cell apoptosis by inducing DNA damage. Int J Oncol.
31:1317–1323. 2007.PubMed/NCBI
|
|
46
|
Hwang J, Yi M, Zhang X, Xu Y, Jung JH and
Kim DK: Cytochalasin B induces apoptosis through the mitochondrial
apoptotic pathway in HeLa human cervical carcinoma cells. Oncol
Rep. 30:1929–1935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qi F, Inagaki Y, Gao B, Cui X, Xu H,
Kokudo N, Li A and Tang W: Bufalin and cinobufagin induce apoptosis
of human hepatocellular carcinoma cells via Fas- and
mitochondria-mediated pathways. Cancer Sci. 102:951–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|