Introduction
Malignant pleural mesothelioma (MPM) is a highly
aggressive malignancy associated with environmental exposure to
asbestos (1). The worldwide
incidence of this fatal disease is expected to increase in the near
future (2). Despite recent advances
in clinical treatments, including surgery, chemotherapy,
radiotherapy and trimodality therapy, these methods do not have
good curative effects and have many undesirable side effects. MPM
remains difficult to treat and standard therapies are still in
flux. Therefore, novel and more effective treatment strategies are
urgently required.
Berberine is an isoquinoline alkaloid exacted from a
number of medicinal plant species such as Berberis
aquifolium and Berberis aristata, with active
antibacterial effects. Berberine has extensive pharmacological
effects, including anti-hypertensive, anti-diabetic,
anti-arrhythmia and anti-hyperlipidemic (3–6).
Recent studies have shown that berberine exerts antitumor effects
in a variety of human cancer cells, such as breast cancer,
hepatocellular carcinoma, leukemia, and esophageal cancer, both
in vitro and in vivo, by suppressing proliferation,
inducing apoptosis, arresting the cell cycle, and inhibiting
invasion and metastasis (7–12). In addition, it has been recently
reported that berberine-induced autophagy has been observed in
hepatic and colon cancer cells (13,14).
These findings indicated that berberine is a promising agent for
clinical application in cancer treatment.
However, to the best of our knowledge, the antitumor
effects of berberine have not been investigated in MPM cells.
Therefore, we aimed to investigate the antitumor effects of
berberine in human MPM NCI-H2452 cells, and to characterize the
roles of berberine-induced apoptosis and autophagy, and the
underlying molecular mechanisms, in the antitumor activity of the
drug.
Materials and methods
Reagents
Berberine was obtained commercially from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). It was dissolved in
dimethyl sulfoxide (DMSO; Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China), and then diluted in HyClone™ RPMI-1640
medium (GE Healthcare Life Sciences, Logan, UT, USA) to the desired
concentrations. All concentrations contained a final DMSO
concentration of <0.1%, not enough to affect cell growth. MTT
was also purchased from Sigma-Aldrich (Merck KGaA). The PE Annexin
V Apoptosis Detection kit was purchased from BD Biosciences (San
Diego, CA, USA). Rapamycin, z-VAD-fmk and 3-methyladenine (3-MA)
were purchased from Cayman Chemical Company (Ann Arbor, MI,
USA).
Cell culture
The human MPM NCI-H2452 cell line was purchased from
the Shanghai Institute of Cell Biology, the Chinese Academy of
Sciences (Shanghai, China) and cultured in RPMI-1640 medium
(HyClone Laboratories; GE Healthcare Life Sciences) supplemented
with 10% fetal bovine serum (FBS) (Shanghai ExCell Biology, Inc.,
Shanghai, China), 100 mg/ml streptomycin and 100 U/ml penicillin at
37°C in a humidified atmosphere containing 5% CO2.
Cell viability assay
The effect of berberine on NCI-H2452 cells was
assessed by an MTT assay, as previously described (15). Briefly, cells were seeded onto
96-well culture plates at a density of 5,000 cells/well. After 24
h, cells were treated with berberine at a concentration of 25, 50,
100 or 200 µM, or with DMSO alone (control group) for 24, 48 and 72
h. After the designated period, 20 µl MTT (5 mg/ml) was added to
each well and the plates were cultured at 37°C for additional 4 h.
Formazan crystals that formed in the wells were dissolved in 150 µl
DMSO, and the absorbance value of each well was measured at 490 nm
using a Spectra Max M2 spectrophotometer (Molecular Devices,
Sunnyvale, CA, USA). All experiments were performed in
triplicate.
Detection of apoptosis
To investigate whether the inhibition of cell
proliferation by berberine was mediated by apoptosis, we used
Hoechst 33258 staining to detect apoptosis. After treatment with
berberine at a concentration of 0 (DAMO alone; control group) or
100 µM, chromatin morphologic changes were observed by fluorescence
microscopy after DNA staining with 10 µg/ml Hoechst 33258 (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China).
Reduced nuclear size, chromatin condensation, intense fluorescence,
and nuclear fragmentation were considered to indicate apoptotic
cells. Then, we further detected the apoptosis rate using Annexin
V-PE/7-AAD double staining assay by flow cytometry. The cells were
treated with berberine at the concentration of 0 (DMSO alone;
control group), or 100 µM; or the cells were treated with 100 µM
berberine in the absence or presence of 3-MA, respectively, for 48
h and then harvested and resuspended in Annexin V binding buffer.
The suspension was incubated with 5 µl of Annexin V-PE and 5 µl of
7-AAD for 15 min at room temperature in the dark followed by
addition of 400 µl of binding buffer. Then the samples were
detected immediately by flow cytometry (FACSAria III; BD
Biosciences, San Diego, CA, USA) analysis. All experiments were
carried out in triplicate.
Immunofluorescence analysis of LC3
distribution
NCI-H2452 cells were treated with berberine at a
concentration of 0 (DMSO alone; control group) or 100 µM for 48 h.
Then, the treated cells were fixed in 4% paraformaldehyde for 15
min. After blocking with 5% normal goat serum and 0.3% Triton X-100
in phosphate-buffered saline (PBS), the cells were incubated with
an anti-LC3B antibody (1:50; cat. no. CST-2775s; Cell Signaling
Technology, Inc., Danvers, MA, USA) at 4°C overnight, followed by
incubation with a TRITC-conjugated goat anti-rabbit IgG secondary
antibody (1:200; cat. no. BA1090; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) for 1 h at room temperature. Nuclei
were stained with DAPI (Beyotime Institute of Biotechnology,
Shanghai, China) for 10 min. Coverslips were mounted in antifade
mounting medium and images were obtained using a Leica™
fluorescence microscope equipped with a Leica™ camera (Leica Camera
AG, Wetzlar, Germany).
Western blot analysis
Expression levels of LC3, p62 and proteins
associated with the mitochondrial pathway were measured in
NCI-H2452 cells via immunoblot analysis. The cells were treated as
described in the Figure legends. Whole cell extracts were prepared
in ice-cold lysis buffer. To detect the expression of cytochrome
c, cytosolic and mitochondrial fractions were prepared using
a Mitochondria Extraction kit (Nanjing KeyGen Biotech., Co., Ltd.,
Nanjing, China). After removal of the insoluble fraction by
centrifuging at 20,000 × g for 30 min at 4°C, the protein content
of the supernatant was determined via the bicinchoninic acid (BCA)
method with a commercial protein assay reagent (CoWin Bioscience
Co., Ltd., Beijing, China). Protein samples (30 µg) were subjected
to precast 8–12% SDS-PAGE. After electrophoresis, the proteins were
transferred to PVDF membranes (EMD Millipore, Billerica, MA, USA).
The membranes were then incubated with primary antibodies against
LC3B (1:750), p62 (1:1,000), cytochrome c (1:1,000),
caspase-9 (1:1,000), caspase-3 (1:1,000), PARP [poly(ADP-ribose)
polymerase] (1:1,000), and Cox 4 (1:5,000) in 5% BSA in TBS-T (Cell
Signaling Technology, Inc.) and β-actin (1:1,000; Zhongshan Bio.,
Beijing, China) overnight at 4°C. After incubation with horseradish
peroxidase (HRP)-conjugated secondary antibodies (Zhongshan Bio.)
at a dilution of 1:5,000 in TBS-T for 1 h at room temperature, the
immunoreactive bands were detected using electrochemiluminescence
(ECL; EMD Millipore) and quantified using Multi Gauge V3.2 analysis
software (Fuji Film, Tokyo Japan).
Trypan blue exclusion assay
Cell death was evaluated using a trypan blue
exclusion assay as described previously (13). Briefly, NCI-H2452 cells were
cultured for 48 h with various concentrations of berberine, or with
200 µM berberine in the absence or presence of pan-caspase
inhibitor z-VAD-fmk (20 µM), autophagy inhibitor 3-MA (5 mM), or
autophagy inducer rapamycin (100 nM). Then, both adherent and
non-adherent cells were collected, washed three times with PBS, and
resuspended in PBS at a concentration of 1×106 cells/ml.
After mixing with 0.4% trypan blue (ratio of cells:trypan blue,
9:1), the cells were counted using a hemocytometer. The number of
dead cells with disrupted membranes (blue cells) was counted. The
cell death rate was calculated as the mean percentage of blue
cells/total cells. All experiments were conducted in
triplicate.
Statistical analysis
All data in this study are expressed as the means ±
SD. A one-way analysis of variance (ANOVA) or t-test was performed
to assess differences between groups under different conditions.
All statistical analyses were performed using SPSS 19.0 software
(IBM Corp., Armonk, NY, USA). P<0.05 was considered
statistically significant.
Results
Berberine inhibits the proliferation
of NCI-H2452 cells in a dose- and time-dependent manner
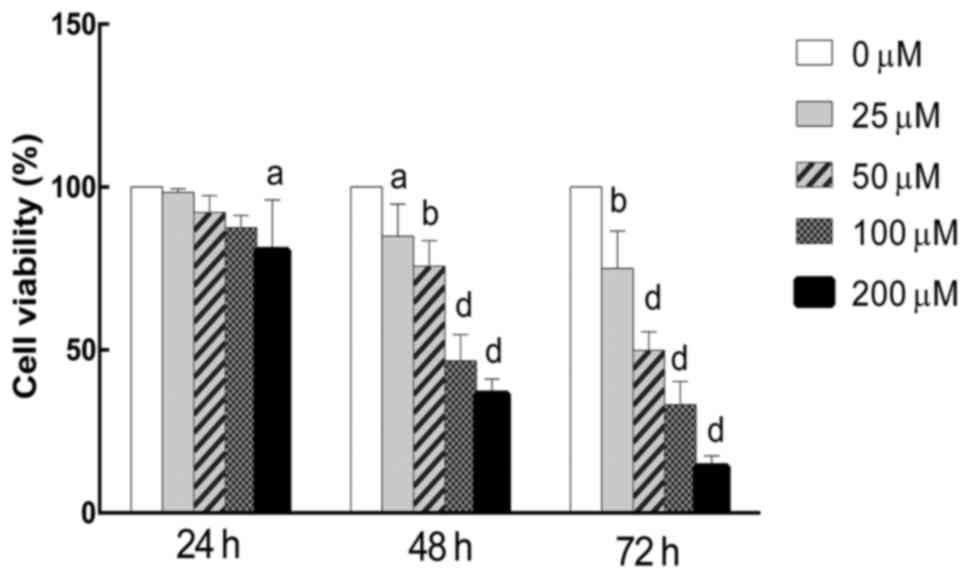
The antiproliferation effect of berberine against
MPM NCI-H2452 cells was evaluated by an MTT assay. The results
showed that berberine inhibited the growth of cells in a dose- and
time-dependent manner. The proliferation rate of berberine-treated
NCI-H2452 cells was 87.58±3.72, 46.68±8.08 and 33.24±7.17%,
respectively, following treatment with berberine at a concentration
of 100 mM for 24, 48 and 72 h (Fig.
1). Based on the MTT assay results, the 100 µM berberine
concentration for 48 h was used in subsequent experiments.
Berberine induces the apoptosis of
NCI-H2452 cells
Previous studies have reported that berberine can
induce apoptosis in some cancer cells (9,10);
therefore, we investigated whether it could induce apoptosis in
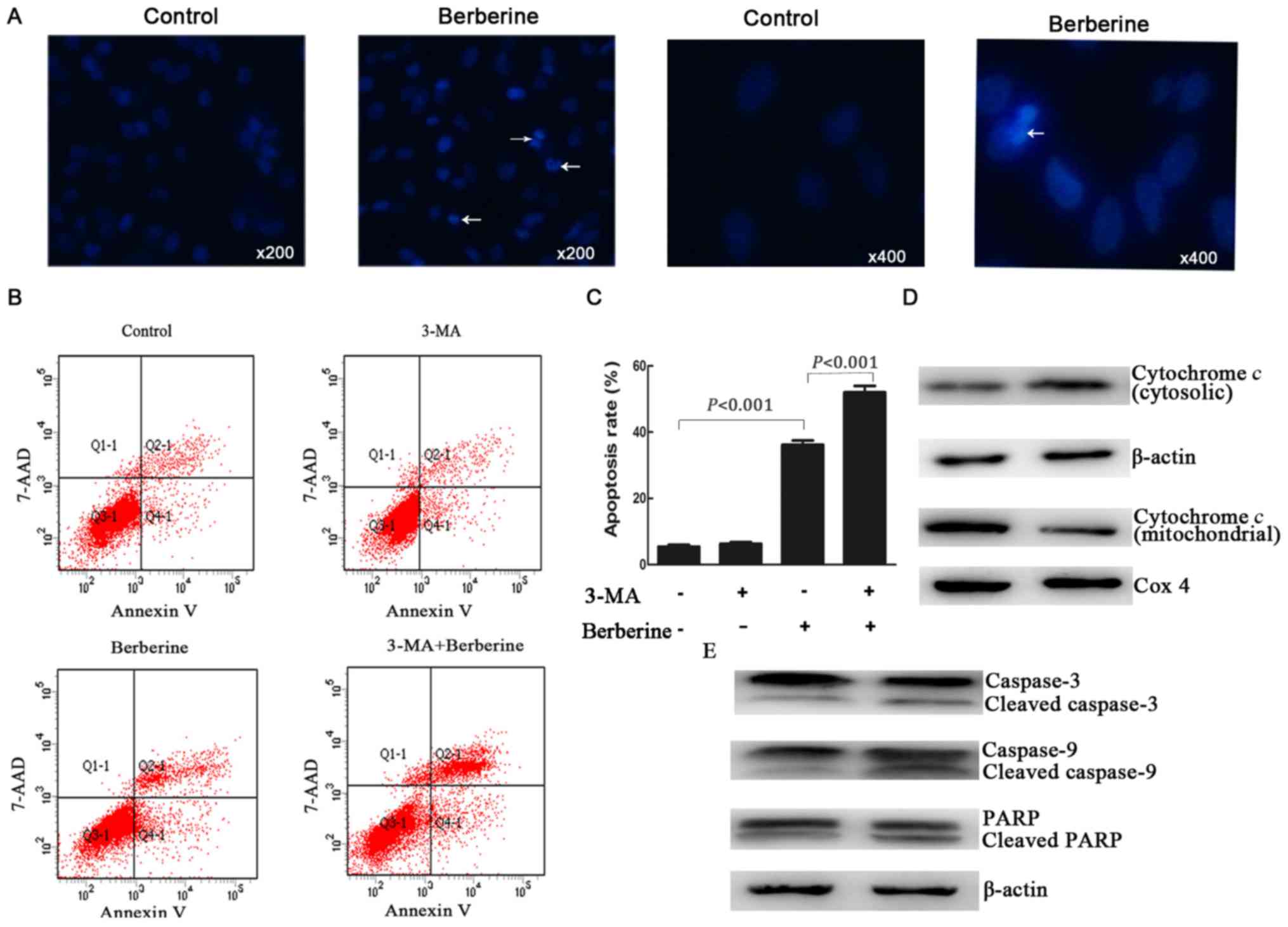
NCI-H2452 cells using Hoechst 33258 staining. As shown in Fig. 2A, the treatment of NCI-H2452 cells
with berberine at 100 µM for 48 h resulted in apoptosis, which was
characterized by unique morphological nuclear changes such as
nuclear condensation and fragmentation.
We further examined the apoptosis rate by Annexin
V-PE/7-AAD double staining assay by flow cytometry. The results
showed that after treatment with berberine at the concentration of
100 µM for 48 h, the apoptosis rate of NCI-H2452 cells increased to
32.23±1.06%, while the apoptosis rate was 3.55±0.86% in untreated
cells (P<0.001; Fig. 2B and
C).
Berberine-induced apoptosis is
mediated by a mitochondrial pathway involving caspase-9
Many antitumor agents induce cancer cells apoptosis
through mitochondrial apoptotic pathways (16,17);
therefore, we further investigated the expression of key proteins
in the mitochondrial pathway. As shown in Fig. 2D and E, treatment with berberine
resulted in the release of cytochrome c into the cytoplasm,
as well as a significant increase in the active forms of caspase-9
and caspase-3, and the proteolytic cleavage of poly(ADP-ribose)
polymerase (PARP).
Berberine induces autophagy in
NCI-H2452 cells
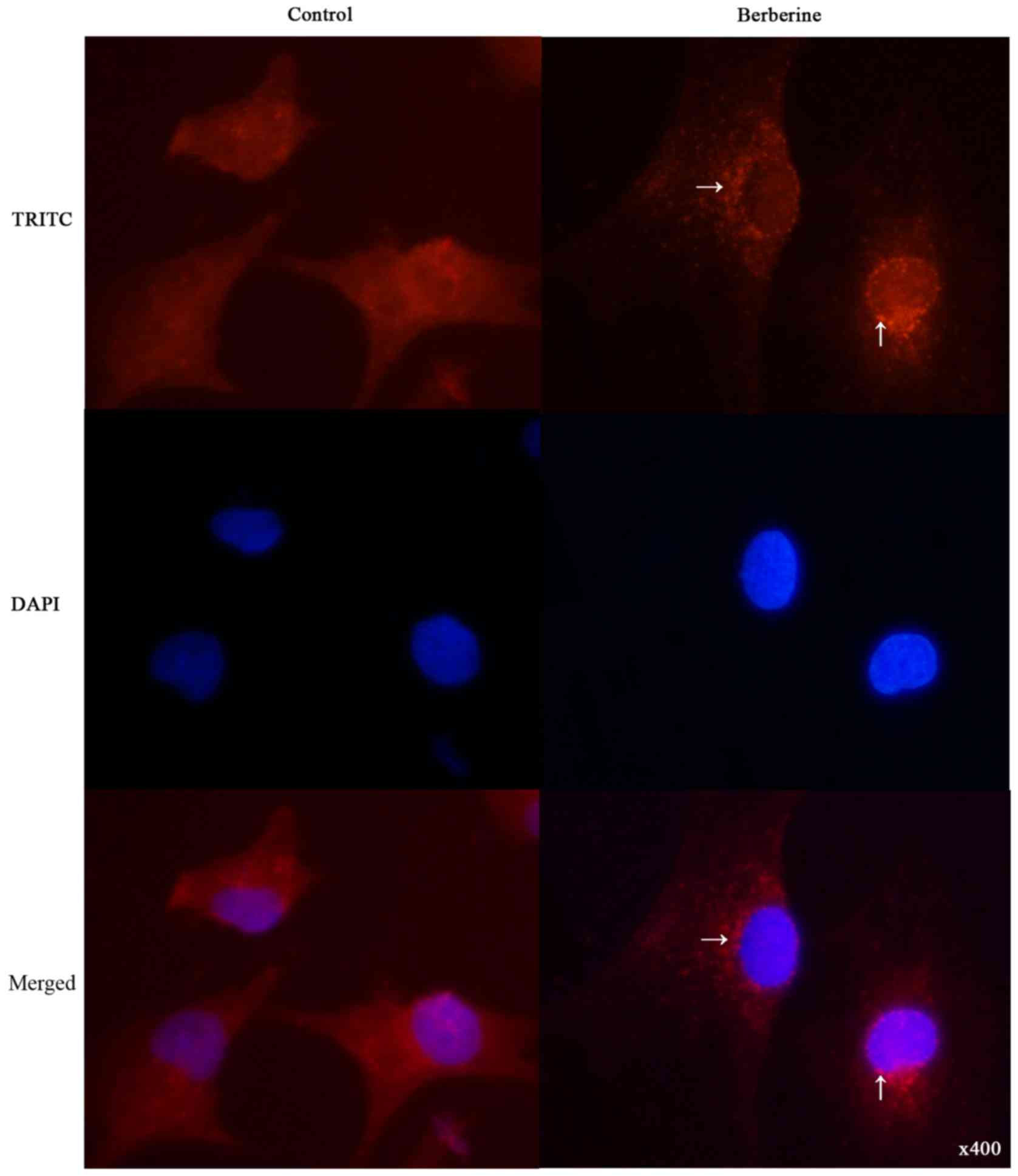
To determine whether berberine induces autophagy in
NCI-H2452 cells, we examined the intracellular distribution of LC3,
an autophagy marker, upon berberine treatment under
immunofluorescence. As shown in Fig.
3, the distribution of LC3 fluorescence changed from diffuse
cytosolic in untreated cells to punctate upon berberine
treatment.
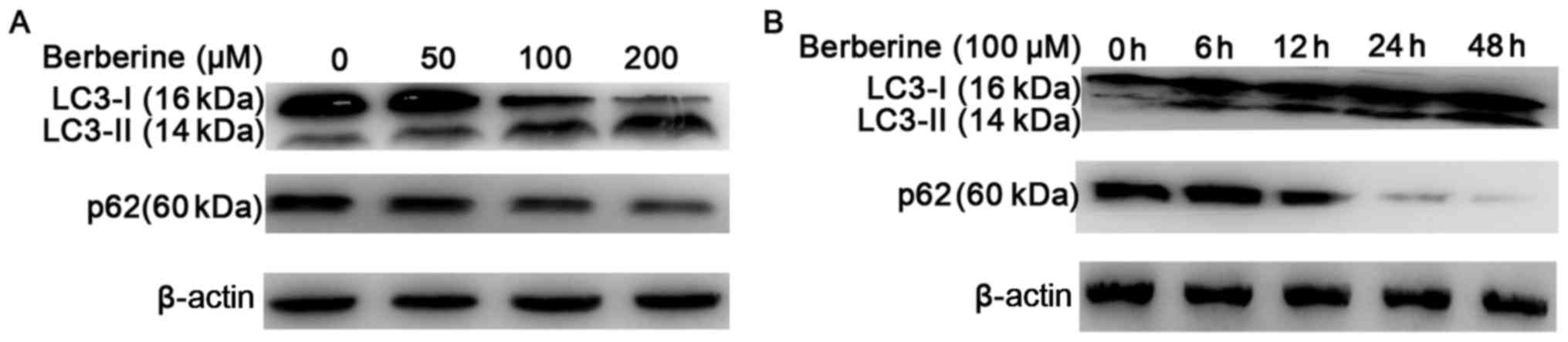
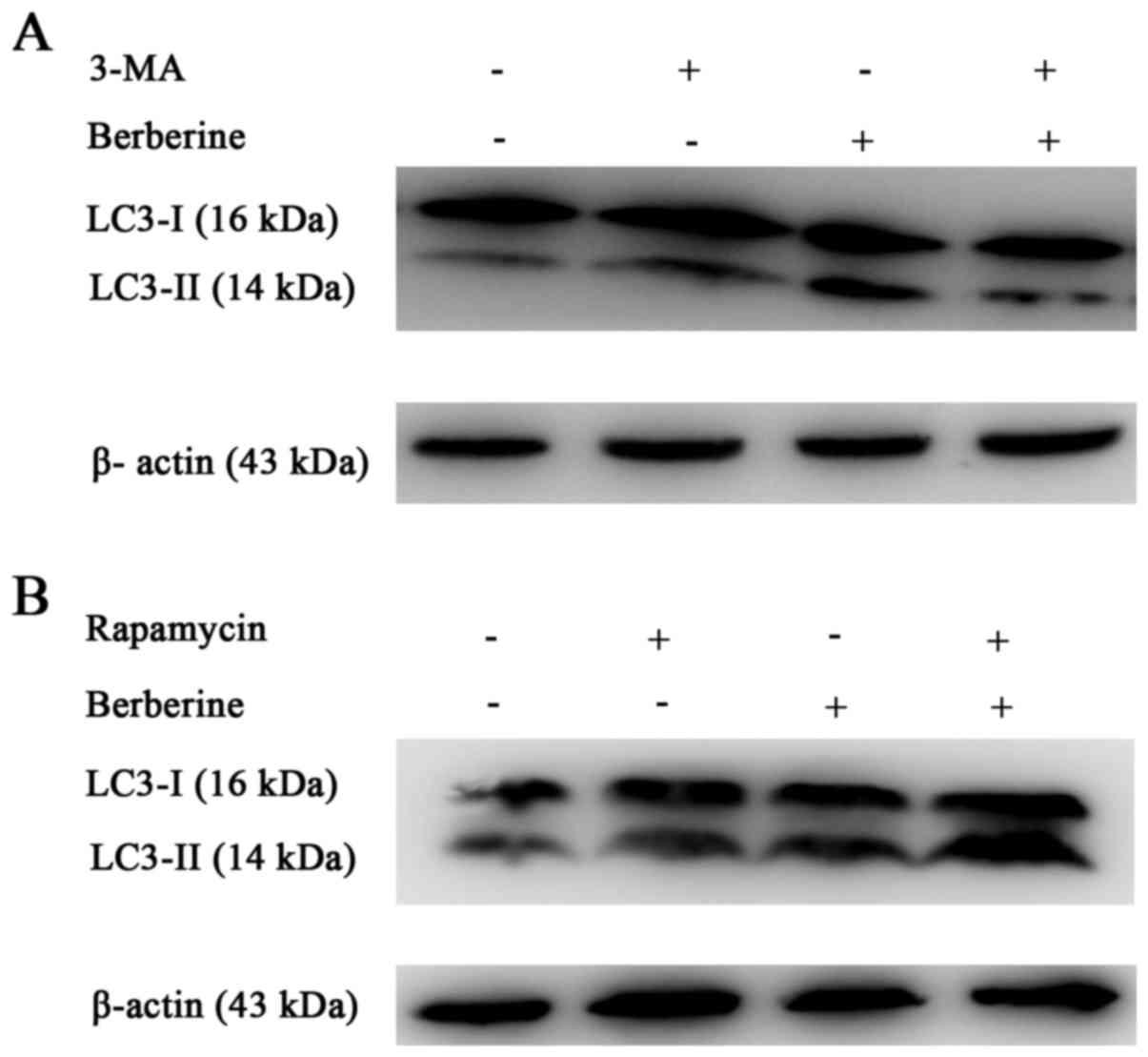
We further analyzed the expression of LC3-II using
western blotting (Fig. 4A and B).
The results showed an increased conversion of LC3-I to the
autophagic LC3-II isoform in NCI-H2452 cells treated with
berberine, in a dose- and time-dependent manner. The increased
conversion was attenuated by pre-treatment with the autophagic
inhibitor 3-MA, and enhanced by the autophagic inducer rapamycin
(Fig. 5A and B). In addition,
treatment with berberine decreased the expression of p62 in a dose-
and time-dependent manner (Fig. 4A and
B). These results suggest that autophagy is induced by
berberine in NCI-H2452 cells.
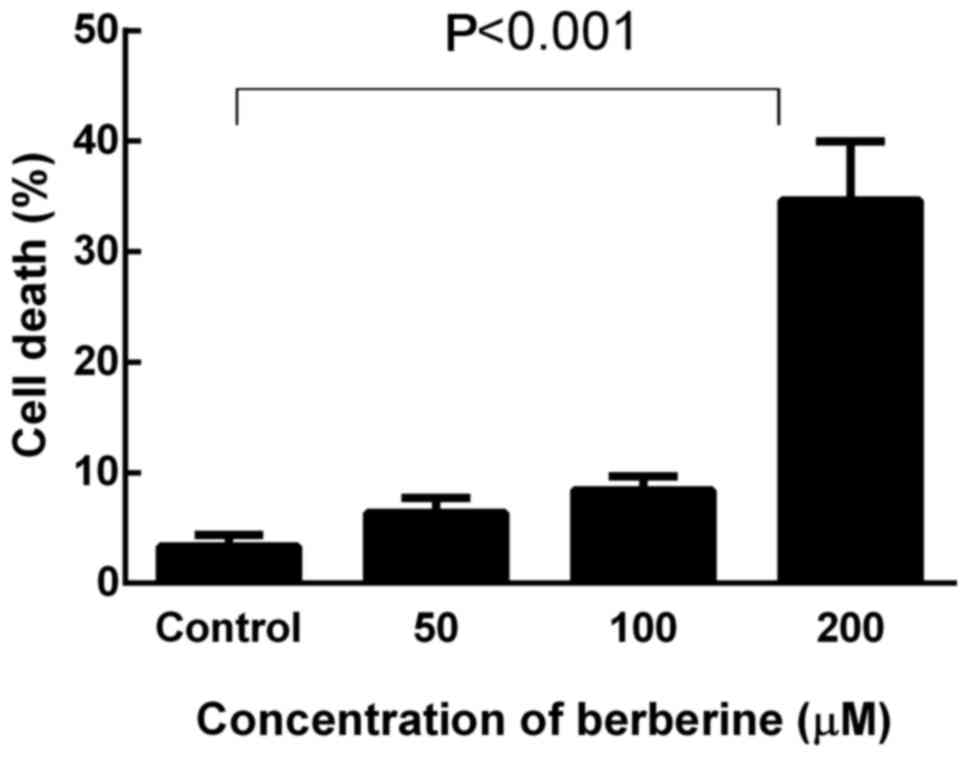
Berberine induces cell death in a
dose-dependent manner
Cell death in response to berberine was quantified
by a trypan blue exclusion assay, and the cells with disrupted
membranes (blue cells) were counted as dead cells. NCI-H2452 cells
were treated with berberine at concentrations of 0, 50, 100 and 200
µM for 48 h. As shown in Fig. 6,
berberine killed MPM cells in a dose-dependent manner. After
treatment with berberine at the concentration of 200 µM for 48 h,
the death rate of NCI-H2452 cells increased to 34.66±5.32%
(P<0.001).
Our western blot results showed that autophagy was
induced in NCI-H2452 cells by treatment with berberine in a
dose-dependent manner. Based on the western blotting results,
autophagy was induced at a concentration of 200 µM. As cell death
was clearly induced with a concentration of 200 µM berberine in the
trypan blue exclusion assay, this concentration was used in
subsequent experiments.
Protective autophagy during berberine
treatment
The results showed that MPM cells treated with
berberine underwent autophagy in a dose- and time-dependent manner,
which was positively correlated with the cytotoxic activity of
berberine; therefore, we further assessed whether berberine-induced
NCI-H2452 cell death was mediated by autophagy. In addition, both
apoptosis and autophagy were induced by berberine treatment in this
study. Therefore, it was necessary to further investigate the
interactions between berberine-induced apoptosis and autophagy. We
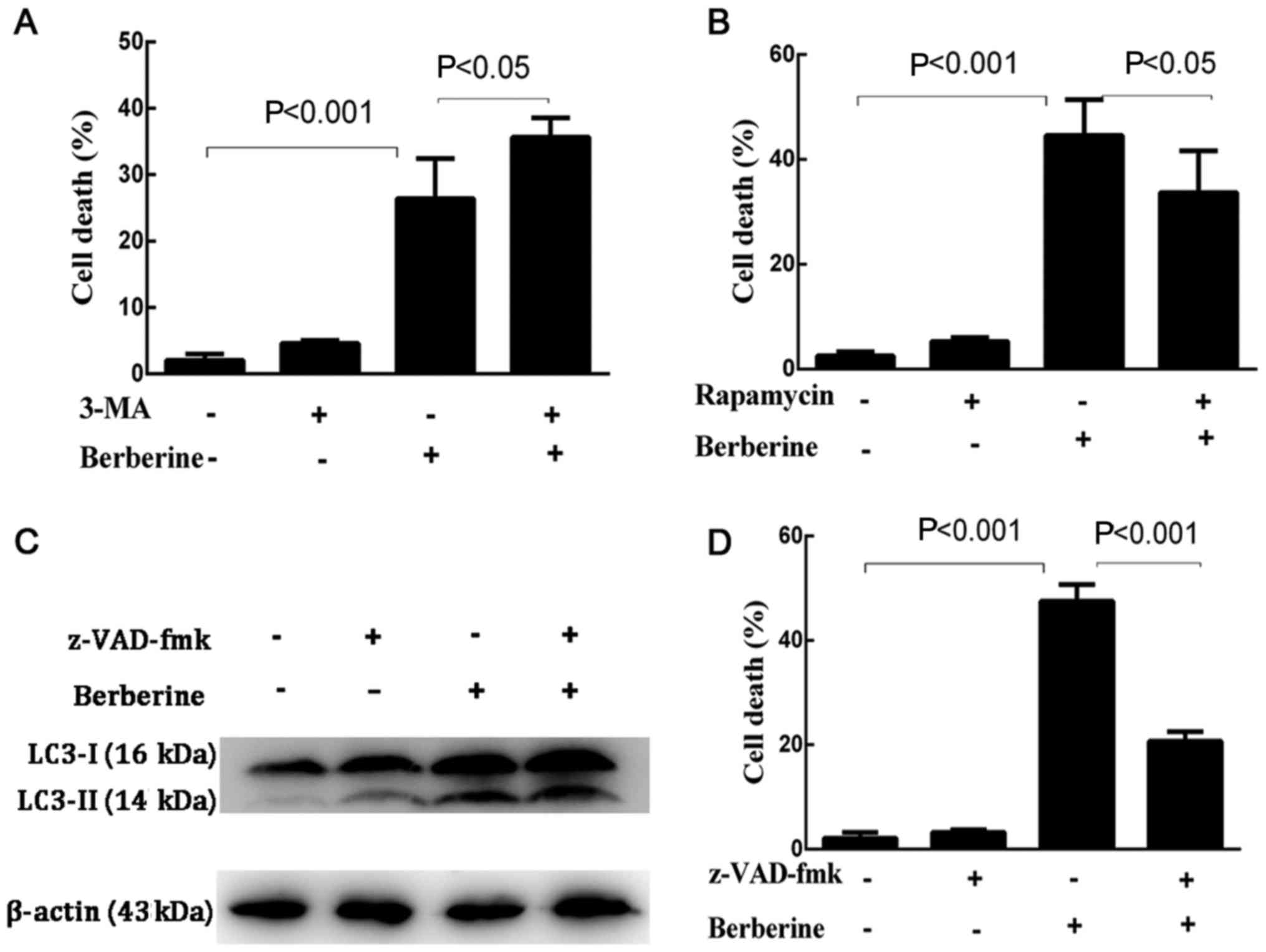
first explored whether the inhibition of autophagy by 3-MA or the
induction of autophagy by rapamycin affected the rate of cell death
induced by berberine (Fig. 7A and
B). The results showed that the death rate of NCI-H2452 cells
treated with 3-MA plus berberine increased significantly compared
with the cells treated with berberine alone, while pre-treatment
with rapamycin decreased the death rate of berberine-treated
cells.
The apoptosis rate of NCI-H2452 cells was assessed
using Annexin V-PE/7-AAD double staining assay by flow cytometry.
Compared with the cells treated with berberine alone, 3-MA-treated
cells underwent extensive apoptosis after berberine treatment
(Fig. 2B and C). However, the
expression level of LC3-II in NCI-H2452 cells treated with
berberine in the presence of z-VAD-fmk was similar to that in the
cells treated with berberine alone (Fig. 7C).
Subsequently, we further investigated if
berberine-induced NCI-H2452 cell death occurs via apoptosis. Cells
were pretreated with z-VAD-fmk, a pan-caspase inhibitor, which only
inhibits apoptosis-mediated cell death, for 1 h, followed by
berberine for 48 h. As shown in Fig.
7D, berberine-induced NCI-H2452 cell death was attenuated by
z-VAD-fmk.
Collectively, the results show that apoptosis is the
main route by which berberine induces NCI-H2452 cell death.
Contrary to previous studies, our results supported a
cytoprotective autophagic activity induced by berberine, preventing
cells from undergoing apoptosis; the inhibition of autophagy may
enhance the antitumor potential of berberine in MPM NCI-H2452
cells.
Discussion
It has been shown that berberine has potent
antitumor activity in many types of tumor cells both in
vitro and in vivo, while having no significant
inhibitory effect on normal cells (18). In the present study, we investigated
the underlying molecular mechanisms of berberine-induced MPM cell
death. Our results suggest that berberine possesses cytotoxic
effects against MPM in vitro. Berberine clearly suppressed
proliferation and induced apoptosis in NCI-H2452 cells.
Malignant cells are frequently resistant to
apoptotic stimuli, and a blockade of apoptosis may promote cancer
progression. The intrinsic mitochondrial pathway is key to
apoptosis. Cytochrome c/caspase-9 activation is an initial
event in the mitochondrial pathway. Cytochrome c is an
apoprotein that is nearly undetectable in the cytosol of normal
cells (19). However, release of
cytochrome c from mitochondria into the cytosol can be
induced when the mitochondrial pathway is activated. Apoptotic
protease-activating factor-1 (Apaf1) and cytochrome c are
both involved in the activation of caspase-9 (20). In turn, activated caspase-9 induces
procaspase-3 to form activated caspase-3, which results in cleavage
and inactivation of the DNA repair enzyme poly(ADP-ribose
polymerase) (21). In the present
study, we found that cytosolic cytochrome c was upregulated
by treatment with berberine. Activated caspase-9, activated
caspase-3 and cleaved PARP were observed in berberine-treated
cells. These results suggest that berberine-induced apoptosis may
occur via cytochrome c/caspase-9/caspase-3 signaling in MPM
NCI-H2452 cells.
Recently, studies report that berberine can exert
antitumor effects not only through apoptosis, but also through
autophagy in hepatic and colon cancer cell lines. Autophagy is a
highly evolutionarily conserved process common in eukaryotes. It is
a lysosomal degradation process in which long-lived proteins and
damaged or aged cellular organelles are encapsulated within
autophagosomes and degraded by lysosomal hydrolases to maintain
cell homeostasis under physiological conditions. While under
pathological conditions, autophagy is generally recognized to serve
protective effects. However, excessive autophagy may lead to
autophagic cell death, also called type II programmed cell death.
Recently, increasing evidence has shown that autophagy has an
important role in tumorigenesis, development and tumor suppression.
Autophagy has been observed in response to antitumor drugs such as
arsenic trioxide, matrine, bufarin and resveratrol, suggesting that
autophagy can be used as a tumor treatment strategy.
However, the role of autophagy in tumor treatment is
still controversial. Some studies have shown that autophagy induced
by antitumor agents has pro-survival effects and decreases drug
efficacy. Autophagy is recognized as one of the mechanisms involved
in the drug resistance of cancer cells. Matrine, resveratrol and
quercetin (22) induce protective
autophagy in many cancer cell types, and acquired
cisplatin-resistance in human lung adenocarcinoma cells is
associated with enhanced autophagy (23).
Various studies have demonstrated that certain
antitumor agents induce cell death through autophagy; thus,
suppression of autophagy can decrease drug cytotoxicity. In
addition, previous studies report that autophagy induced by the
same agent in different cancer cells led to different effects.
Therefore, when we evaluate the activity of one antitumor agent, we
need to investigate whether autophagy is activated during treatment
and further explore the role of autophagy in drug-induced
cytotoxicity.
In the present study, we found that berberine is a
strong inducer of apoptosis and autophagy. The expression of
LC3-II, an autophagic marker, increased in a dose- and
time-dependent manner after treatment with berberine. The cell
death rate also increased in a dose-dependent manner. Based on
these results, we proposed two hypotheses: Berberine-induced
autophagy may be a mechanism of antitumor activity, leading to
autophagic cell death; or it may be a mechanism by which protective
effects are conferred onto MPM cells during berberine treatment.
Therefore, we used inhibitors of apoptosis or autophagy, and an
inducer of autophagy, to evaluate the significance of autophagy in
berberine-induced cell death. Our data indicated that the presence
of autophagy inhibitor 3-MA augmented berberine-induced NCI-H2452
cell death, while the autophagy inducer rapamycin and apoptosis
inhibitor z-VAD-fmk attenuated berberine-induced NCI-H2452 cell
death. An Annexin V-PE/7-AAD double staining assay indicated that
the inhibition of autophagy by 3-MA enhanced berberine-induced
apoptosis, suggesting that berberine-induced autophagy protects
cells from apoptosis and, thus, might be a target for enhancing its
antitumor activity. In addition, treatment with berberine plus 3-MA
significantly enhanced the antiproliferation effects against
NCI-H2452 cells, relative to berberine alone.
The aforementioned results revealed that apoptosis
is the main form of death induced by berberine in MPM NCI-H2452
cells. Additionally, autophagy might have a protective response
through the degradation and recycling of damaged cellular
organelles and proteins caused by berberine treatment, which
protects MPM cells from berberine-induced apoptosis.
Our results were inconsistent with studies of
berberine in hepatocarcinoma cell lines conducted by Hou et
al (13) and Wang et al
(24). Their results showed that
berberine induced cell death through both apoptosis and autophagy,
and that the inhibition of autophagy decreased cell death. This may
be because the effects were studied in a different cell type.
Therefore, the results need to be validated in more cell lines and
in vivo studies in the future.
However, our study had limitations. According to
previous literature (25),
increases in the level of LC3-II, can reflect the induction of
autophagy and/or inhibition of autophagosome or amphisome
clearance. Autophagic flux reflects the entire process of
autophagy. Thus, at present, the actual mechanistic relationship
between LC3-II formation and the rest of the autophagic process is
not known; indeed, it may be possible to execute ‘self-eating’ in
the absence of LC3-II. It is more appropriate to detect autophagy
based on autophagic flux. Lysosomal degradation is an important
part of the autophagy pathway. This has been included in our other
study that is currently being conducted, and thus was not included
in the present study.
In conclusion, the present study demonstrated that
berberine is a potent antitumor agent for treating MPM. It can
induce apoptosis, possibly through a caspase-9-dependent intrinsic
mitochondrial pathway. Berberine can also induce autophagy in
NCI-H2452 cells, although apoptosis is the major form of
berberine-induced NCI-H2452 cell death. Berberine-induced autophagy
may be an adaptive response to antitumor agents and have a
protective role in MPM cells. Inhibition of autophagy enhanced
berberine-induced apoptosis. Therefore, the inhibition of autophagy
may be an effective treatment strategy for the management of
MPM.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science
Foundation of Shandong Province of China (grant no.
ZR2016HL27).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
DL and QL designed the study. ZY conducted the
experiments and analyzed the data. YW and BL assisted with the
western blot analysis. CZ and FY assisted with the
immunofluorescence experiments and the analysis. YK assisted with
collecting and analyzing the data. All authors read and approved
the final manuscript and agree to be accountable for all aspects of
the research in ensuring that the accuracy or integrity of any part
of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wagner JC, Sleggs CA and Marchand P:
Diffuse pleural mesothelioma and asbestos exposure in the North
Western Cape Province. Br J Ind Med. 17:260–271. 1960.PubMed/NCBI
|
|
2
|
Hodgson JT, McElvenny DM, Darnton AJ,
Price MJ and Peto J: The expected burden of mesothelioma mortality
in Great Britain from 2002 to 2050. Br J Cancer. 92:587–593. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu JC, Chan P, Chen YJ, Tomlinson B, Hong
SH and Cheng JT: The antihypertensive effect of the berberine
derivative 6-protoberberine in spontaneously hypertensive rats.
Pharmacology. 59:283–289. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moghaddam HK, Baluchnejadmojarad T,
Roghani M, Khaksari M, Norouzi P, Ahooie M and Mahboobi F:
Berberine ameliorate oxidative stress and astrogliosis in the
hippocampus of STZ-induced diabetic rats. Mol Neurobiol.
49:820–826. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou ZW, Zheng HC, Zhao LF, Li W, Hou JW,
Yu Y, Miao PZ and Zhu JM: Effect of berberine on
acetylcholine-induced atrial fibrillation in rabbit. Am J Transl
Res. 7:1450–1457. 2015.PubMed/NCBI
|
|
6
|
Zhang XJ, Deng YX, Shi QZ, He MY, Chen B
and Qiu XM: Hypolipidemic effect of the Chinese polyherbal
Huanglian Jiedu decoction in type 2 diabetic rats and its possible
mechanism. Phytomedicine. 21:615–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim S, Lee J, You D, Jeong Y, Jeon M, Yu
J, Kim SW, Nam SJ and Lee JE: Berberine suppresses cell motility
through downregulation of TGF-β1 in triple negative breast cancer
cells. Cell Physiol Biochem. 45:795–807. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li F, Dong X, Lin P and Jiang J:
Regulation of Akt/FoxO3a/Skp2 axis is critically involved in
berberine-induced cell cycle arrest in hepatocellular carcinoma
cells. Int J Mol Sci. 19:pii: E3272018. View Article : Google Scholar
|
|
9
|
Okubo S, Uto T, Goto A, Tanaka H, Nishioku
T, Yamada K and Shoyama Y: Berberine induces apoptotic cell death
via activation of caspase-3 and −8 in HL-60 human leukemia cells:
Nuclear localization and structure-activity relationships. Am J
Chin Med. 45:1497–1511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y, Jing Z, Lv J, Zhang Z, Lin J, Cao
X, Zhao Z, Liu P and Mao W: Berberine activates
caspase-9/cytochrome c-mediated apoptosis to suppress
triple-negative breast cancer cells in vitro and in vivo. Biomed
Pharmacother. 95:18–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang SX, Qi B, Yao WJ, Gu CW, Wei XF,
Zhao Y, Liu YZ and Zhao BS: Berberine displays antitumor activity
in esophageal cancer cells in vitro. World J Gastroenterol.
23:2511–2518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Wang N, Li H, Liu M, Cao F, Yu X,
Zhang J, Tan Y, Xiang L and Feng Y: Up-regulation of PAI-1 and
down-regulation of uPA are involved in suppression of invasiveness
and motility of hepatocellular carcinoma cells by a natural
compound berberine. Int J Mol Sci. 17:5772016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou Q, Tang X, Liu H, Tang J, Yang Y, Jing
X, Xiao Q, Wang W, Gou X and Wang Z: Berberine induces cell death
in human hepatoma cells in vitro by downregulating CD147. Cancer
Sci. 102:1287–1292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
La X, Zhang L, Li Z, Yang P and Wang Y:
Berberine-induced autophagic cell death by elevating GRP78 levels
in cancer cells. Oncotarget. 8:20909–20924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang Y, Ding M, Tian G, Guo H, Wan Y, Yao
Z, Li B and Lin D: Overexpression of Numb suppresses tumor cell
growth and enhances sensitivity to cisplatin in epithelioid
malignant pleural mesothelioma. Oncol Rep. 30:313–319. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patil JB, Kim J and Jayaprakasha GK:
Berberine induces apoptosis in breast cancer cells (MCF-7) through
mitochondrial-dependent pathway. Eur J Pharmacol. 645:70–78. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang X and Huang N: Berberine induces
selective apoptosis through the AMPK-mediated mitochondrial/caspase
pathway in hepatocellular carcinoma. Mol Med Rep. 8:505–510. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mantena SK, Sharma SD and Katiyar SK:
Berberine, a natural product, induces G1-phase cell
cycle arrest and caspase-3-dependent apoptosis in human prostate
carcinoma cells. Mol Cancer Ther. 5:296–308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Renz A, Berdel WE, Kreuter M, Belka C,
Schulze-Osthoff K and Los M: Rapid extracellular release of
cytochrome c is specific for apoptosis and marks cell death in
vivo. Blood. 98:1542–1548. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zou H, Li Y, Liu X and Wang X: An
APAF-1.cytochrome c multimeric complex is a functional apoptosome
that activates procaspase-9. J Biol Chem. 274:11549–11556. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wolf BB and Green DR: Suicidal tendencies:
Apoptotic cell death by caspase family proteinases. J Biol Chem.
274:20049–20052. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim H, Moon JY, Ahn KS and Cho SK:
Quercetin induces mitochondrial mediated apoptosis and protective
autophagy in human glioblastoma U373MG cells. Oxid Med Cell Longev.
2013:5964962013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren JH, He WS, Nong L, Zhu QY, Hu K, Zhang
RG, Huang LL, Zhu F and Wu G: Acquired cisplatin resistance in
human lung adenocarcinoma cells is associated with enhanced
autophagy. Cancer Biother Radiopharm. 25:75–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang N, Feng Y, Zhu M, Tsang CM, Man K,
Tong Y and Tsao SW: Berberine induces autophagic cell death and
mitochondrial apoptosis in liver cancer cells: The cellular
mechanism. J Cell Biochem. 111:1426–1436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Arozena Acevedo A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|