Introduction
Cancer is the second cause of death worldwide, with
increasing incidence and mortality rates, and it is the leading
cause of death in China. Data from the National Central Cancer
Registry of China indicate that an estimated 3,682,000 new cancer
cases and 2,229,300 cancer-related deaths occurred in 2013 in
China, and that 4,292,000 new cancer cases and 2,814,000 cancer
deaths occurred in 2015 (1,2). An increasing number of studies have
reported that social and psychological factors are implicated in
the process of cancer development, mainly through
pathophysiological mechanisms triggered in response to stress
(3). Therefore, the effect of
chronic stress on cancer patients should not be overlooked.
Currently available studies provide convergent
evidence that two pathways, namely the
hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic
nervous system (SNS), are activated under chronic psychological
stress conditions. The β2-adrenergic receptor (ADRB-2),
a SNS receptor, which can be activated by chronic stress,
contributes to the tumorigenesis and cancer development.
Hypoxia-inducible factor (HIF)-1α expression is induced by lack of
oxygen, which has been found to be closely associated with the
occurrence and development of several types of tumors. Our previous
study revealed that activation of the ADRB-2/HIF-1α pathway plays
an essential role in cancer growth and angiogenesis under chronic
stress conditions (4). Thus,
identifying a treatment targeting the ADRB-2/HIF-1α axis may serve
as an effective strategy for cancer therapy.
Resveratrol (Res) is a type of polyphenol abundantly
present in knotweed and grapes, and it has been confirmed to
inhibit tumor development (5–8). The
mechanism underlying the anticancer properties of Res has been
extensively investigated, and it includes metabolic shift (9), inhibition of migration and invasion of
cancer cells (10), induction of
autophagy (11), as well as direct
cytotoxicity (12). However, the
effect of Res on cancer cells under chronic stress remains unclear.
The aim of the present study was to determine whether Res inhibits
tumor cell proliferation and promotes apoptosis via suppressing
catecholamine neurotransmitters induced by the ADRB-2/HIF-1α axis
activation in cancer.
Materials and methods
Cell culture and major reagents
The BxPC-3 human pancreatic cancer cell line was
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The human hepatoblastoma HepG2 cell line
(13) was generously provided by Dr
Chang Liu (Medical College, Xi'an Jiaotong University). The SKOV-3
ovarian carcinoma cell line was kindly provided by the First
Affiliated Hospital Obstetrics and Gynecology Laboratory at Xi'an
Jiaotong University. BxPC-3 and HepG2 cells were cultured in DMEM,
and SKOV-3 cells were cultured in RPMI-1640 medium. The medium was
supplemented with 10% fetal bovine serum (FBS), and cells were
maintained under normal culture conditions of 5% CO2 at
37°C. Res, norepinephrine (NE), HIF-1α antibody (diluted 1:1,000,
ab51608), ADRB-2 antibody (diluted 1:1,000, ab61778) and MTT were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Proliferating cell nuclear antigen (PCNA) and Ki-67 antibodies
(both diluted 1:200, sc-56 and sc-15402) were obtained from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
MTT cell viability assay
BxPC-3, HepG2 and SKOV-3 cancer cells were plated in
96-well plates at a density of 5×103 cells/well and
treated with 10 µM norepinephrine (NE). After 24 h, the
NE-containing medium was removed and various concentrations (0, 25,
50, 75 and 100 µM) of Res were added to the cells. Cell
viability was assessed by the MTT assay at the indicated time
points (24, 36 and 48 h). A multi-well microplate reader (BIO-TEC,
Inc., Richmond, VA, USA) was used to measure the absorbance at 490
nm.
Colony formation assay
Cells (1,000 cells/well) were seeded in triplicate
and allowed to adhere overnight, and they were then incubated with
NE for 48 h. Subsequently, the cells were treated with Res without
NE-containing medium for 24 h followed by incubation with media
lacking Res for 7–10 days. The cells were then fixed with 4%
paraformaldehyde and stained with 0.1% crystal violet solution, and
the colonies (>50 cells) were counted.
Apoptosis assay
Apoptosis was determined by flow cytometry with an
Annexin V-FITC/PI apoptosis detection kit (Beyotime Institute of
Biotechnology, Shanghai, China). Briefly, cells were seeded at
density of 1×105 cells/well in 6-well plates and
cultured to the indicated density followed by treatment with 10
µM NE for 24 h. Cells were treated with fresh medium
containing various concentrations (0, 25 and 50 µM) of Res
for an additional 24 h. Subsequently, the cells were trypsinized,
washed with phosphate-buffered saline (PBS) and stained with
Annexin V and PI. Apoptosis was evaluated using flow cytometry
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA).
Western blotting
For analysis of the HIF-1α, ADRB-2, proliferating
cell nuclear antigen (PNCA) and Ki-67 protein expression, cell
lysates were prepared from BxPC-3, HepG2 and SKOV-3 cells, which
had been treated with medium containing 10 µM NE for 24 h
followed by treatment with fresh medium containing various
concentrations (0, 25 and 50 µM) of Res. Total proteins were
extracted using RIPA lysis buffer (Sigma Aldrich; Merck KGaA),
separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and transferred onto PVDF membranes. After blocking
with 5% non-fat milk in Tris-buffered saline Tween-20 (TBST) for 1
h, the membranes were incubated with primary antibody overnight at
4°C. The primary antibody was removed, and the membranes were then
incubated with 1:2,000 horseradish peroxidase-conjugated secondary
antibody for 2 h at room temperature. Protein expression was
visualized with enhanced chemiluminescence, and images were
captured using the ChemiDoc XRS imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Quantity One image software
was used for the densitometry analysis of each band. β-actin was
used as an internal loading control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total cell RNA was extracted using TRIzol reagent
following the manufacturer's instructions (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). cDNA synthesis was
performed using a PrimeScript RT reagent kit (Takara Biotechnology
Co., Ltd., Dalian, China). qPCR was performed with an iQ5
Multicolor Real-Time PCR Detection System (Bio-Rad Laboratories,
Inc.) using a SYBR-Green PCR kit (Takara Biotechnology Co., Ltd.)
according to the manufacturer's instructions. The amplification
consisted of predenaturation at 95°C for 5 min, denaturation at
95°C for 30 sec, annealing at 58°C for 30 sec and extension at 72°C
for 30 sec for 32 cycles. After the last cycle, a final extension
was performed at 72°C for 7 min. The primer sequences for ADRB-2
were as follows: Forward 5′-GGGTCTTTCAGGAGGCCAAA-3′ and reverse
5′-ATGCCTAACGTCTTGAGGGC-3′; the primer sequences for HIF-1α were as
follows: Forward 5′-CTTGGCAACCTTGGATTGGATG-3′ and reverse
5′-AATCTCCGTCCCTCAACCTCT-3′. The ΔΔCq method was used to calculate
the relative expression of the sample genes with β-actin as the
normalized reference gene.
Immunofluorescence staining
Cells were plated in chamber slides, incubated for
24 h, and then treated as indicated. The cells were fixed using 4%
paraformaldehyde for 15 min and permeabilized using PBS containing
0.5% Triton X-100 for 20 min at room temperature. The cells were
then incubated with the primary antibody overnight at 4°C, followed
by incubation with fluorescein-conjugated secondary antibody at
room temperature for 1 h. The cells were then visualized using a
fluorescence microscope.
Statistical analysis
Each experiment was performed at least three times.
The results are expressed as the mean ± standard deviation of the
number of experiments. One-way analysis of variance was used to
evaluate the statistical significance between groups. P-value
<0.05 was considered to indicate statistically significant
differences. All statistical analyses were performed using SPSS
software, version 15.0 (SPSS, Inc., Chicago, IL, USA).
Results
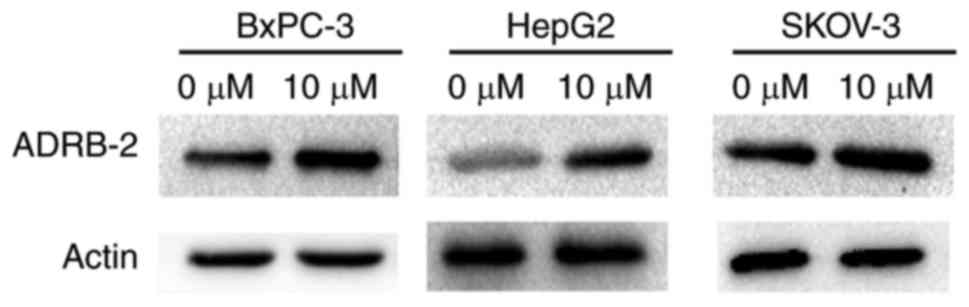
NE induces the expression of ADRB-2 in
BxPC-3, HepG2 and SKOV-3 cancer cell lines
To imitate a chronic stress condition in
vitro, three cell lines, namely BxPC-3, HepG2 and SKOV-3, were
treated with NE. After 24 h, western blotting was used to detect
ADRB-2 expression. The results demonstrated that ADRB-2 expression
was significantly increased after treatment, indicating that NE may
be used for in vitro experiments to simulate chronic stress
(Fig. 1).
Res inhibits the proliferation of
cancer cells under chronic stress conditions
First, the effects of Res on the viability of
chronically stressed cancer cells were examined. Res inhibited
chronically stressed cancer cells in a dose- and time-dependent
manner (Fig. 2A). Colony formation
ability was detected after the addition of Res to chronically
stressed cancer cells. Compared with untreated cells, treatment
with 25 µM Res markedly decreased the number of colonies,
and there was almost no colony formation when the concentration of
Res was increased to 50 µM (P<0.05) (Fig. 2B). PCNA is located in the nucleus
and plays an important role in cell proliferation. Ki-67 protein
expression is used as a marker to indicate cell proliferation. To
test whether Res inhibits the proliferation of chronically stressed
cancer cells, PCNA and Ki-67 protein expression was measured in the
three cell lines. Western blot analysis revealed that Res (0, 25
and 50 µM) suppressed PCNA and Ki-67 expression in
chronically stressed cancer cells in a dose-dependent manner
(Fig. 2C).
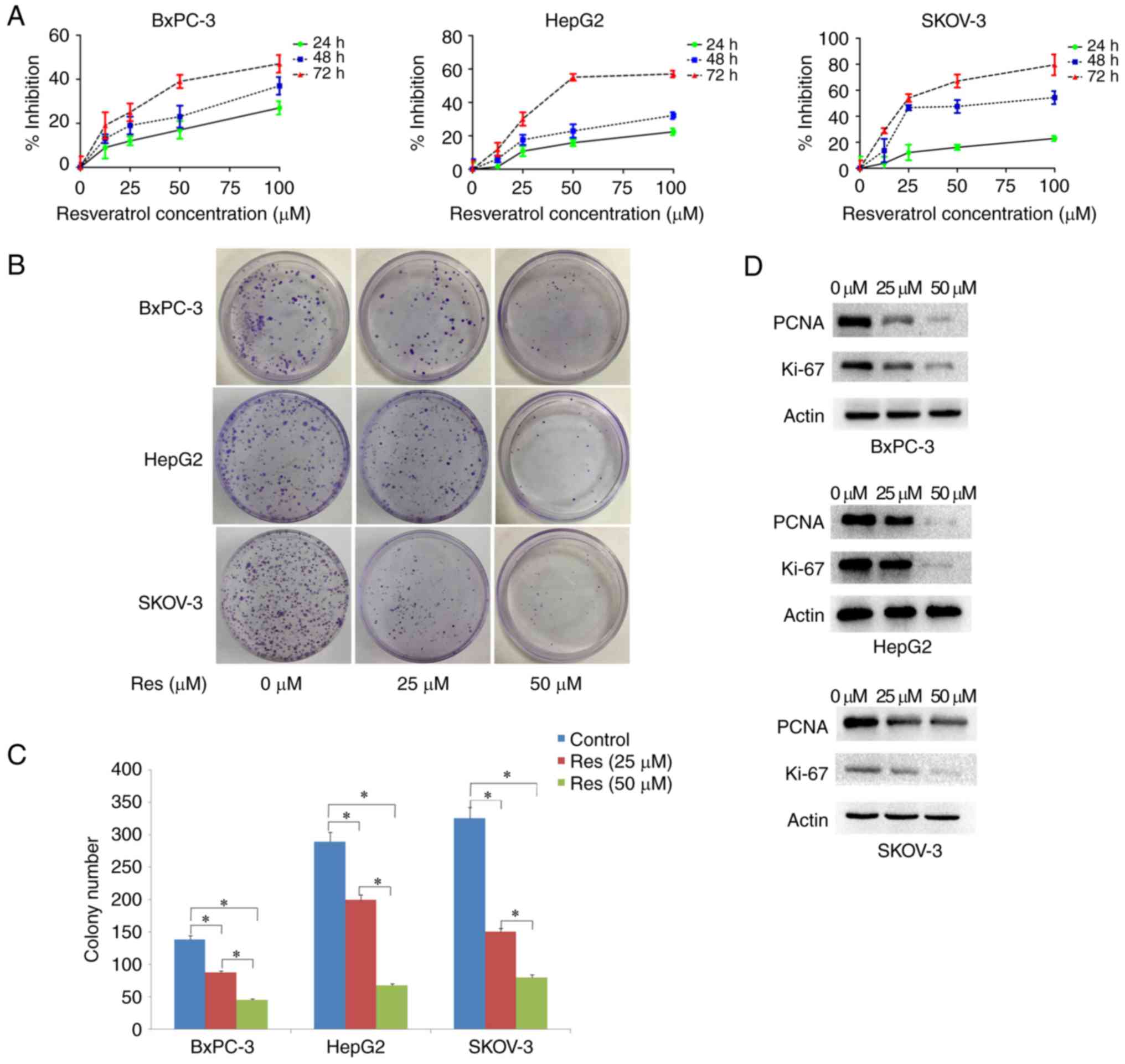 | Figure 2.Inhibitory effect of Res on the
proliferation of chronically stressed cancer cells. (A) Chronically
stressed cancer cells (BxPC-3, HepG2 and SKOV-3) were treated with
different concentrations of Res (0, 25, 50, 75 and 100 µM). After
24, 48 and 72 h, cell viability was assessed by an MTT assay. (B
and C) Res treatment inhibited the colony-forming ability of
chronically stressed BxPC-3, HepG2 and SKOV-3 cancer cells
(*P<0.05). (D) PNCA and Ki-67 protein expression was detected in
the chronically stressed BxPC-3, HepG2 and SKOV-3 cancer cells.
Treatment of the three chronically stressed cancer cell lines with
25 and 50 µM Res significantly reduced the expression of PNCA and
Ki-67. Res, resveratrol; PNCA, proliferating cell nuclear
antigen. |
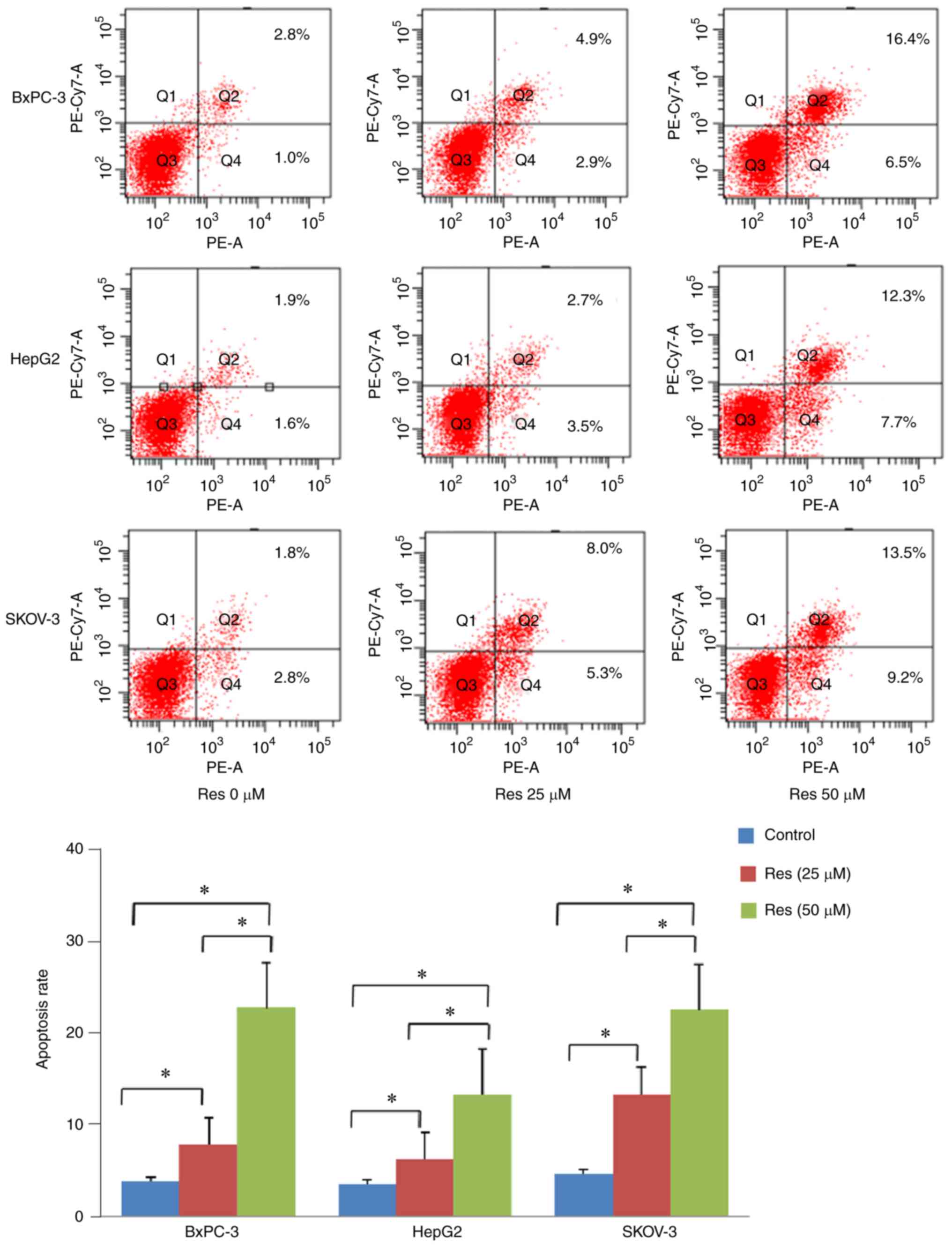
Res induces apoptosis of chronically
stressed cancer cells
To determine whether Res induces cancer cell
apoptosis under chronic stress conditions, flow cytometric analyses
were conducted after the cancer cells (BxPC-3, HepG2 and SKOV-3)
were treated with Res (25 and 50 µM) for 48 h in the
presence of NE. The apoptosis rates of the cancer cells were
calculated by summing up the quadrants of the early (lower right)
and late (upper right) apoptosis of the FITC graphs. Res increased
apoptosis of the three chronically stressed cancer cell types
compared with untreated cells (Fig.
3, P<0.05).
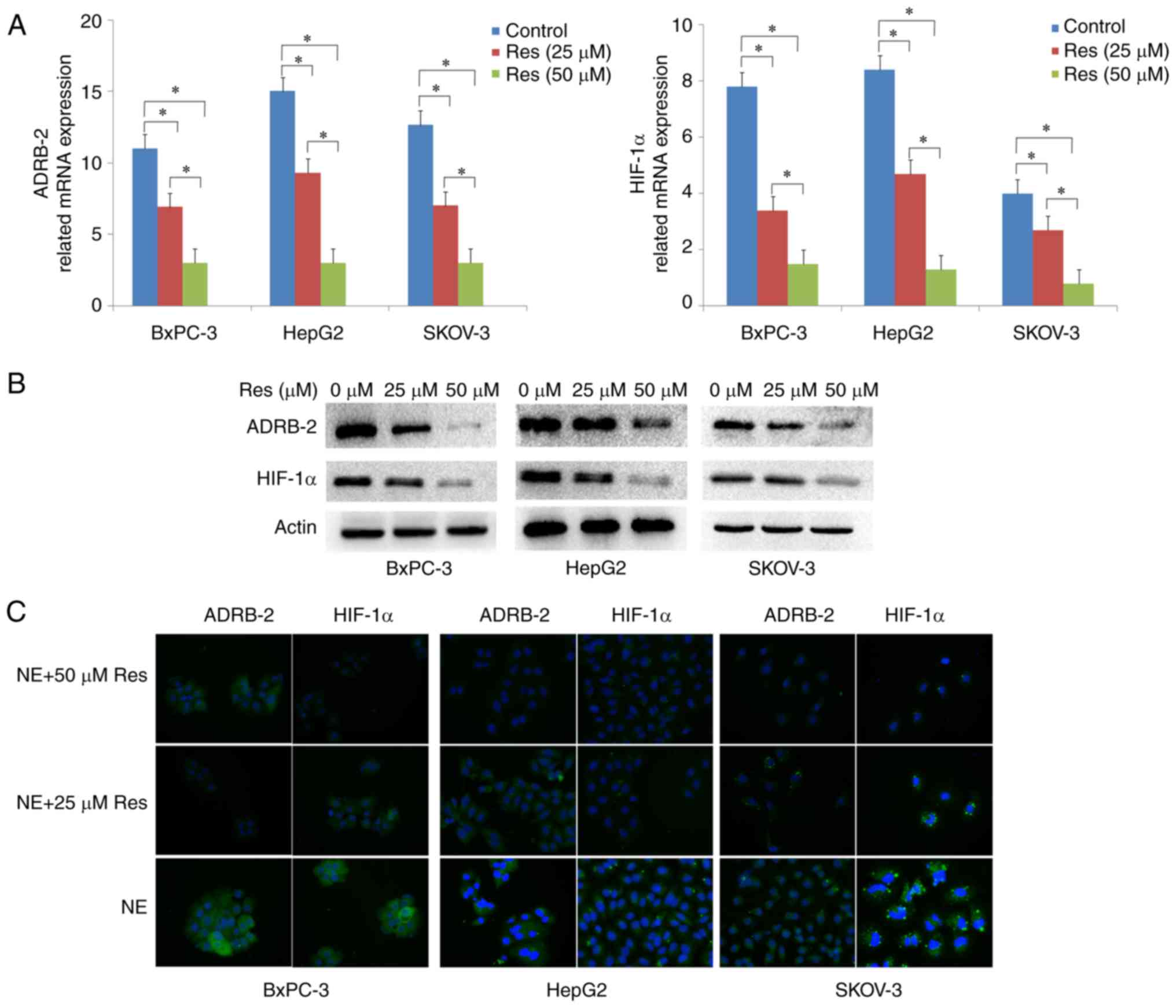
Res suppresses chronically stressed
cancer cell proliferation through the ADRB-2/HIF-1α axis
Previous studies provided evidence that ADRB-2 plays
an important role in chronic stress-induced tumor growth (4). Based on our previous findings, we
hypothesized that the inhibition of chronically stressed cancer
cells by Res may be mediated by the ADRB-2/HIF-1α axis. To verify
the effect of Res on the ADRB-2/HIF-1α axis, RT-qRCR, western
blotting and immunofluorescence assays were used to measure ADRB-2
and HIF-1α expression after treatment of chronically stressed
BxPC-3, HepG2 and SKOV-3 cancer cells with 0, 25 and 50 µM
Res. RT-qRCR demonstrated that Res treatment markedly decreased
ADRB-2 and HIF-1α expression at the mRNA level (P<0.05)
(Fig. 4A). The protein expression
levels of ADRB-2 and HIF-1α were confirmed by western blotting
(Fig. 4B). Consistently, the
immunofluorescence results revealed that the ADRB-2 and HIF-1α
protein levels were markedly decreased following Res treatment
(Fig. 4C). Taken together, these
findings confirmed that Res inhibits chronically stressed cancer
cell proliferation via inhibiting ADRB-2 and HIF-1α expression.
Discussion
Chronic stress is characterized by inability to
adjust to the circumstances or cope with emotional and
psychological pressure. A number of studies have reported that
chronic stress interferes with immune, endocrine and neural system
functions. A number of epidemiological and clinical studies have
reported that chronic stress plays an essential role in cancer
occurrence, development and mortality, and reducing stress may
prolong survival and decrease recurrence after therapy (14–17).
It has been demonstrated that chronic stress can activate the HPA
axis and the SNS, leading to a release of catecholamines (NE,
epinephrine and dopamine), glucocorticoids and other hormones. NE,
a stress hormone, has been found to stimulate cancer cell
proliferation, invasion and metastasis through β-adrenergic
receptors (18–20). In addition, our previous data
demonstrated that HIF-1α is a downstream target of ADRB, and that
the ADRB-2-HIF-1α axis induces cancer growth in animal stress
models (4). Thus, the
identification of drug targets within the ADRB-2-HIF-1α axis may be
of value in cancer therapy. Accumulating evidence has shown that
Res exerts a potent inhibitory effect on tumor growth in pancreatic
cancer (21), hepatoblastoma
(22) and ovarian cancer (6). However, it remains unknown whether Res
can inhibit tumor growth under chronic stress conditions. In the
present study, Res was shown to inhibit cancer development and
progression through the ADRB-2/HIF-1α axis under chronic stress
conditions.
HIF-1α is a pivotal molecule and its activation has
long been known to drive cancer cell responses to a hypoxic
microenvironment by regulating the expression of genes involved in
cell proliferation/survival, apoptosis, metastasis and resistance
to radio- and chemotherapy (23,24).
However, HIF-1α may also be expressed under normoxic conditions
(4). Sun et al reported
(25) that Res inhibited HIF-1α
protein accumulation without affecting the HIF-1α mRNA level, which
was not consistent with our results. Our study focused on
chronically stressed cancer cells, and the three cell lines were
treated with NE to simulate chronic stress conditions. The HIF-1α
mRNA levels in the three chronically stressed cancer cell lines
were found to be significantly inhibited by Res, although our
results were not consistent with those of Sun et al
(25). We inferred that NE may play
an important role.
NE is a catecholamine, the secretion of which is
increased during stress. Preclinical models have suggested that NE
plays a decisive role in cancer promotion. Thus, NE treatment of
cells or animals may mimic stress in in vitro and in
vivo models (26,27). Therefore, NE-treated cancer cells
were used to mimic a chronic stress state, which allowed testing
the effects of Res on chronically stressed cancer cells. First,
MTT, colony formation and western blotting data revealed that Res
inhibited chronically stressed cancer cell proliferation in a dose-
and time-dependent manner. Cell apoptosis was assessed after the
chronically stressed cancer cells (BxPC-3, HepG2, and SKOV-3) were
treated with or without Res. Our results indicated that Res
inhibited the proliferation and increased apoptosis in chronically
stressed cancer cells.
Mechanistic studies have focused on Res
chemoprevention and treatment of cancers, as well as on the effects
of Res on chronic diseases that may develop into cancer. The main
pathways involved are the AMPK/Akt/mTOR (28), Wnt/β-catenin (29), MAPK (30) and STAT3 (31) pathways. Ulcerative colitis is
associated with a high risk of colon cancer, which is abated by Res
treatment through downregulation of inflammatory stress (32). Epidemiological data have
demonstrated that there is a strong association between diabetes
mellitus and pancreatic cancer. Accumulating evidence has shown
that the antidiabetic properties of Res may inhibit glucose uptake
and suppress cancer cell metabolism (30,33).
It has been reported that the HBsAg prevalence for the Chinese
population aged 1–59 years was 7.2% (34), and chronic hepatitis B virus
infection may promote the development of liver cancers, such as
hepatoblastoma (35). However, the
mechanism underlying the suppressive effect of Res on chronic
stress-induced cancer has not been fully elucidated. In the present
study, Res was used to treat chronically stressed cancer cells in
order to demonstrate its anticancer effects on stress-induced
cancer cells and to determine whether the underlying mechanism may
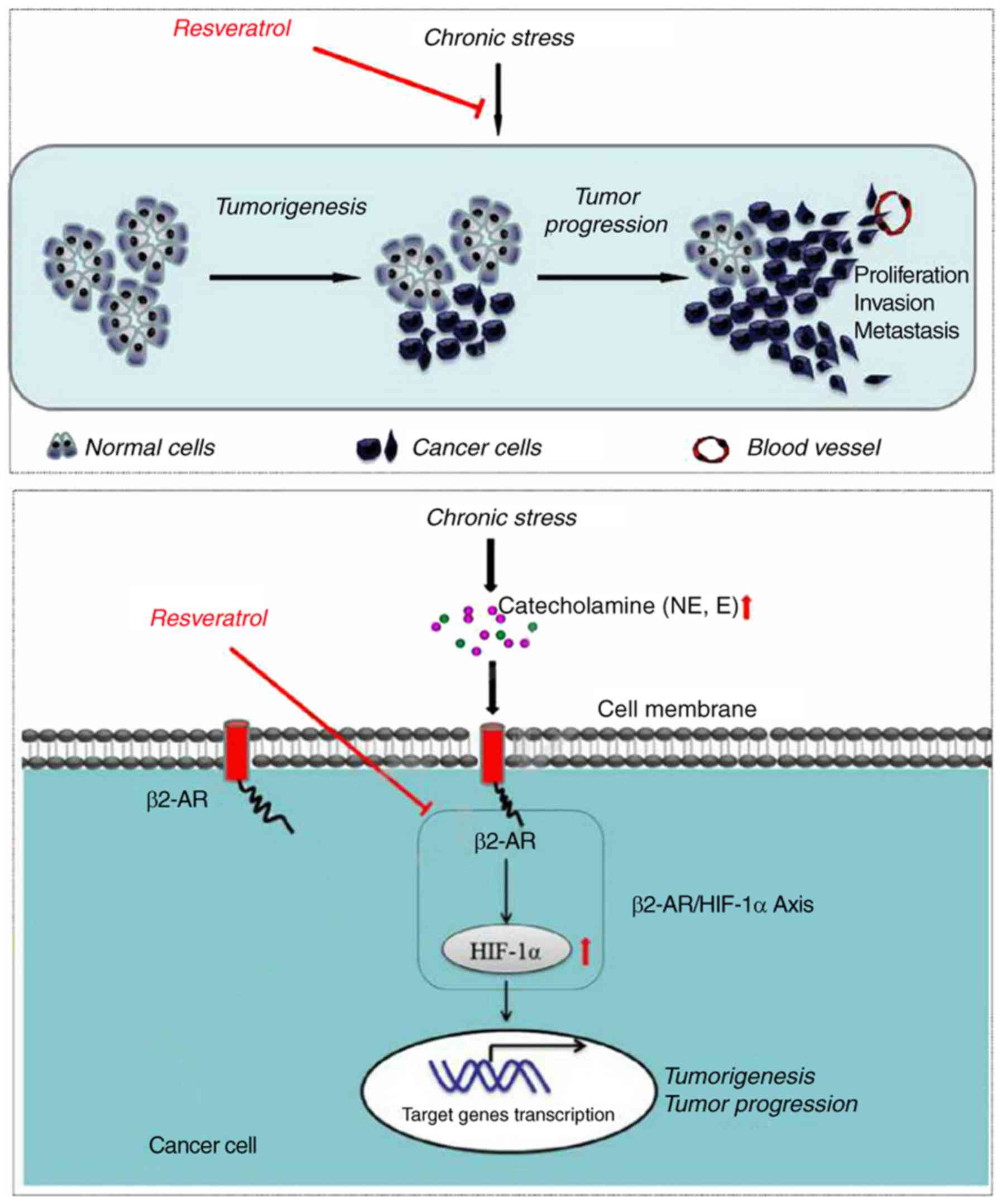
involve inhibition of the ADRB-2-HIF-1α axis (Fig. 5).
In conclusion, the findings of the present study
demonstrated that Res can significantly inhibit the growth of tumor
cells under chronic stress in a dose-dependent manner by
suppressing their proliferation and promoting their apoptosis of
tumor cells. The mechanism underlying these effects of Res may be
mediated by inhibiting the activation of the ADRB-2-HIF-1α axis in
tumor cells under chronic stress. Thus, Res may be an effective
treatment strategy for cancer patients who suffer from chronic
stress.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81402971 and
81702916).
Availability of data and materials
The datasets generated and analyzed in the present
study are included in the published manuscript.
Authors' contributions
JM and MX designed the experiments, performed most
of the experiments and drafted the manuscript; LC, WQ and SZ
performed cell lines culture and analyzed the data; WD and XS
designed the experiments and revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests to disclose.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zhang S, Zeng H, Xia C,
Zuo T, Yang Z, Zou X and He J: Cancer incidence and mortality in
China, 2013. Cancer Lett. 401:63–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Massetti GM, Thomas CC, King J, Ragan K
and Buchanan Lunsford N: Mental health problems and cancer risk
factors among young adults. Am J Prev Med. 53(3): Suppl 1:S30–S39.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shan T, Ma J, Ma Q, Guo K, Guo J, Li X, Li
W, Liu J, Huang C, Wang F, et al: β2-AR-HIF-1α: A novel regulatory
axis for stress-induced pancreatic tumor growth and angiogenesis.
Curr Mol Med. 13:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Q, Zong L, Chen X, Jiang Z, Nan L, Li
J, Duan W, Lei J, Zhang L, Ma J, et al: Resveratrol in the
treatment of pancreatic cancer. Ann NY Acad Sci. 1348:10–19. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vergara D, De Domenico S, Tinelli A,
Stanca E, Del Mercato LL, Giudetti AM, Simeone P, Guazzelli N,
Lessi M, Manzini C, et al: Anticancer effects of novel resveratrol
analogues on human ovarian cancer cells. Mol Biosyst. 13:1131–1141.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeng YH, Zhou LY, Chen QZ, Li Y, Shao Y,
Ren WY, Liao YP, Wang H, Zhu JH, Huang M, et al: Resveratrol
inactivates PI3K/Akt signaling through upregulating BMP7 in human
colon cancer cells. Oncol Rep. 38:456–464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan Y, Zhou C, Li J, Chen K, Wang G, Wei
G, Chen M and Li X: Resveratrol inhibits hepatocellular carcinoma
progression driven by hepatic stellate cells by targeting Gli-1.
Mol Cell Biochem. 434:17–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saunier E, Antonio S, Regazzetti A, Auzeil
N, Laprévote O, Shay JW, Coumoul X, Barouki R, Benelli C, Huc L, et
al: Resveratrol reverses the Warburg effect by targeting the
pyruvate dehydrogenase complex in colon cancer cells. Sci Rep.
7:69452017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bai Y, Yang H, Zhang G, Hu L, Lei Y, Qin
Y, Yang Y, Wang Q, Li R and Mao Q: Inhibitory effects of
resveratrol on the adhesion, migration and invasion of human
bladder cancer cells. Mol Med Rep. 15:885–889. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferraresi A, Phadngam S, Morani F, Galetto
A, Alabiso O, Chiorino G and Isidoro C: Resveratrol inhibits
IL-6-induced ovarian cancer cell migration through epigenetic
up-regulation of autophagy. Mol Carcinog. 56:1164–1181. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deus CM, Serafim TL, Magalhães-Novais S,
Vilaça A, Moreira AC, Sardão VA, Cardoso SM and Oliveira PJ:
Sirtuin 1-dependent resveratrol cytotoxicity and
pro-differentiation activity on breast cancer cells. Arch Toxicol.
91:1261–1278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu Y, Zhao X, Zhang Y, Kang Y, Wang J and
Liu Y: Antitumor activity of YM155, a selective survivin
suppressant, in combination with cisplatin in hepatoblastoma. Oncol
Rep. 34:407–414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kostev K, Jacob L and Kalder M: Risk of
depression, anxiety, and adjustment disorders in women with a
suspected but unconfirmed diagnosis of breast or genital organ
cancer in Germany. Cancer Causes Control. 28:1021–1026. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dzebo S, Mahmutovic J, Erkocevic H and
Foco F: Frequency of depression and its correlation with quality of
life of patients with oral cavity cancer. Mater Sociomed.
29:97–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gonzalez-Saenz de Tejada M, Bilbao A, Baré
M, Briones E, Sarasqueta C, Quintana JM and Escobar A: CARESS-CCR
Group: Association between social support, functional status, and
change in health-related quality of life and changes in anxiety and
depression in colorectal cancer patients. Psychooncology.
26:1263–1269. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia Y, Li F, Liu YF, Zhao JP, Leng MM and
Chen L: Depression and cancer risk: A systematic review and
meta-analysis. Public Health. 149:138–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vilardi BM, Bravo-Calderón DM, Bernabé DG,
Oliveira SH and Oliveira DT: VEGF-C expression in oral cancer by
neurotransmitter-induced activation of beta-adrenergic receptors.
Tumour Biol. 34:139–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang XY, Wang HC, Yuan Z, Huang J and
Zheng Q: Norepinephrine stimulates pancreatic cancer cell
proliferation, migration and invasion via β-adrenergic
receptor-dependent activation of P38/MAPK pathway.
Hepatogastroenterology. 59:889–893. 2012.PubMed/NCBI
|
|
20
|
Zhang D, Ma QY, Hu HT and Zhang M:
β2-adrenergic antagonists suppress pancreatic cancer cell invasion
by inhibiting CREB, NFκB and AP-1. Cancer Biol Ther. 10:19–29.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang Z, Chen X, Chen K, Sun L, Gao L,
Zhou C, Lei M, Duan W, Wang Z, Ma Q, et al: YAP inhibition by
resveratrol via activation of AMPK enhances the sensitivity of
pancreatic cancer cells to gemcitabine. Nutrients. 8:E5462016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tomas-Hernández S, Blanco J, Rojas C,
Roca-Martínez J, Ojeda-Montes MJ, Beltrán-Debón R, Garcia-Vallve S,
Pujadas G, Arola L and Mulero M: Resveratrol potently counteracts
quercetin starvation-induced autophagy and sensitizes HepG2 cancer
cells to apoptosis. Mol Nutr Food Res. 62:2018. View Article : Google Scholar
|
|
23
|
De Francesco EM, Maggiolini M and Musti
AM: Crosstalk between Notch, HIF-1α and GPER in Breast Cancer EMT.
Int J Mol Sci. 19:E20112018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schito L and Semenza GL: Hypoxia-inducible
factors: Master regulators of cancer progression. Trends Cancer.
2:758–770. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Y, Wang H, Liu M, Lin F and Hua J:
Resveratrol abrogates the effects of hypoxia on cell proliferation,
invasion and EMT in osteosarcoma cells through downregulation of
the HIF-1α protein. Mol Med Rep. 11:1975–1981. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barbieri A, Bimonte S, Palma G, Luciano A,
Rea D, Giudice A, Scognamiglio G, La Mantia E, Franco R, Perdonà S,
et al: The stress hormone norepinephrine increases migration of
prostate cancer cells in vitroin vivo. Int J Oncol.
47:527–534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang LP, Jin J, Lv FF, Cao J, Zhang J,
Wang BY, Shao ZM, Hu XC and Wang ZH: Norepinephrine attenuates
CXCR4 expression and the corresponding invasion of MDA-MB-231
breast cancer cells via β2-adrenergic receptors. Eur Rev Med
Pharmacol Sci. 19:1170–1181. 2015.PubMed/NCBI
|
|
28
|
Chang CH, Lee CY, Lu CC, Tsai FJ, Hsu YM,
Tsao JW, Juan YN, Chiu HY, Yang JS and Wang CC: Resveratrol-induced
autophagy and apoptosis in cisplatin-resistant human oral cancer
CAR cells: A key role of AMPK and Akt/mTOR signaling. Int J Oncol.
50:873–882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reddivari L, Charepalli V, Radhakrishnan
S, Vadde R, Elias RJ, Lambert JD and Vanamala JK: Grape compounds
suppress colon cancer stem cells in vitro and in a rodent model of
colon carcinogenesis. BMC Complement Altern Med. 16:2782016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao L, Chen X, Xiao X, Ma Q and Li W:
Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and
migration of pancreatic cancer cells via suppression of the ERK and
p38 MAPK signaling pathways. Int J Oncol. 49:735–743. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baek SH, Ko JH, Lee H, Jung J, Kong M, Lee
JW, Lee J, Chinnathambi A, Zayed ME, Alharbi SA, et al: Resveratrol
inhibits STAT3 signaling pathway through the induction of SOCS-1:
Role in apoptosis induction and radiosensitization in head and neck
tumor cells. Phytomedicine. 23:566–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cui X, Jin Y, Hofseth AB, Pena E, Habiger
J, Chumanevich A, Poudyal D, Nagarkatti M, Nagarkatti PS, Singh UP
and Hofseth LJ: Resveratrol suppresses colitis and colon cancer
associated with colitis. Cancer Prev Res. 3:549–559. 2010.
View Article : Google Scholar
|
|
33
|
León D, Uribe E, Zambrano A and Salas M:
Implications of resveratrol on glucose uptake and metabolism.
Molecules. 22:E3982017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang X, Bi S, Yang W, Wang L, Cui G, Cui
F, Zhang Y, Liu J, Gong X, Chen Y, et al: Reprint of:
Epidemiological serosurvey of Hepatitis B in China - declining HBV
prevalence due to Hepatitis B vaccination. Vaccine. 31 Suppl
9:J21–J28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Casciano JC and Bouchard MJ: Hepatitis B
virus X protein modulates cytosolic Ca2+ signaling in
primary human hepatocytes. Virus Res. 246:23–27. 2018. View Article : Google Scholar : PubMed/NCBI
|