Introduction
Lung cancer is a leading cause of cancer-related
mortality, with non-small cell lung cancer (NSCLC) accounting for
~80% of all lung cancer cases. Lung cancer incidence and mortality
have significantly increased worldwide (1). The 5-year overall survival (OS) rate is
only 15% among patients treated with traditional chemotherapeutic
drugs due to the development of side effects and drug resistance.
Approximately 1.7 million patients worldwide succumb to lung cancer
yearly (2). It is therefore important
to identify new therapies that may prolong the survival of lung
cancer patients.
Cytokines, including inflammatory factors in the
microenvironment, affect tumor cell proliferation and survival
(3,4).
Various inflammatory factors, including TNF-α, IL-6 and IL-8,
promote tumor growth through Toll-like receptor (TLR)-mediated
signaling pathways, thus promoting ERK, NF-κB and STAT3 activation
(5–7).
Damage-associated molecular patterns (DAMPs) are released by
stressed, injured or dying cells, and initiate non-infectious
inflammatory responses (8,9). High mobility group protein B1 (HMGB1),
one of the DAMPs, is released from damaged, inflamed, and cancerous
cells, in turn promoting tumor cell survival through its receptor
RAGE. HMGB1, which organizes DNA and regulates transcription, has
various biological functions in and outside the cell, and promotes
inflammation and tumorigenesis. The HMGB1/RAGE axis may cause
pro-inflammatory gene activation (10). Due to enhanced levels of HMGB1 in
certain chronic diseases, the RAGE receptor is believed to exert a
promoting effect in inflammatory diseases and various tumors. NF-κB
is a heterodimeric protein belonging to the Rel family, and plays a
major role in stress-induced immune and inflammatory responses
(11). It was previously demonstrated
that the NF-κB transcription factor group has an important
association with tumor progression by affecting programmed cell
death, proliferation control and tumorigenesis (12). Recent studies suggest that STAT
proteins (particularly STAT3) play a crucial role in the
carcinogenic inflammatory microenvironment at the early stages of
malignant transformation, as well as during cancer progression
(13,14). Several human malignancies, including
lymphoma, leukemia and multiple myeloma, are associated with STAT3
(15). Therefore, inhibiton of STAT3
signaling may be a promising approach to cancer treatment.
Ethyl pyruvate (EP), a lipophilic ester, is derived
from pyruvic acid and is a non-toxic food additive (16,17). EP
has pharmacological benefits, namely alleviation of redox-caused
cellular and tissue damage (18),
anti-inflammatory properties (19,20) and
promotion of apoptosis (21). EP
inhibits HMGB1 release (22).
Although EP may suppress various tumors to different degrees, its
effect on lung cancer remains unclear. The aim of the present study
was to investigate whether EP exerts antitumor effects on lung
cancer cells and elucidate the underlying mechanism.
Materials and methods
Materials
The NSCLC cell lines A549, H520 and PC-9 were
obtained from Tianjin Medical University Cancer Institute. The
primers for HMGB1, RAGE, MMP-9, PCNA, P53 and ACTIN were all
obtained from Invitrogen; Thermo Fisher Scientific, Inc. HMGB1
(cat. no. ab227168), RAGE (cat. no. ab3611), MMP-9 (cat. no.
ab38898) antibodies were all purchased from Abcam. ACTIN (cat. no.
sc-58673), GAPDH (cat. no. sc-365062), P53 (cat. no. sc-126), pCNA
(cat. no. sc-9857), Bax (cat. no. sc-4239), Bcl-2 (cat. no.
sc-7382), Mcl-1 (cat. no. sc-12756) and STAT3 (cat. no. sc-8019)
antibodies were purchased from Santa Cruz Biotechnology, Inc. The
antibodies against NF-κB (cat. no. 8242), p-NF-κB (cat. no. 3033
and p-STAT3 (cat. no. 9131) were all purchased from Cell Signaling
Technology, Inc.
Drugs and reagents
MTT was purchased from Roche Molecular Biochemicals.
EP was purchased from Sigma-Aldrich; Merck KGaA and RPMI-1640 from
Thermo Fisher Scientific, Inc. Fetal bovine serum (FBS) was
obtained from Gibco; Thermo Fisher Scientific, Inc. and TRIzol
reagent was purchased from Invitrogen; Thermo Fisher Scientific,
Inc. The PrimeScript RT Reagent kit (Perfect Real-Time) and SYBR
Premix Ex Taq (Tli RNaseH Plus) were obtained from Takara
Biotechnology Co., Ltd. The cell apoptosis kit with propidium
iodide and Annexin V-FITC was from BD Pharmingen.
Cell culture
The cell lines A549, H520 and PC-9 were all cultured
in RPMI-1640 medium. All three cultures were supplemented with 10%
FBS, penicillin (100 U/ml) and streptomycin (100 mg/ml). The cell
lines were all maintained at 37°C in a humidified atmosphere
containing 5% CO2.
MTT assay
The MTT assay was used to analyze cell growth.
Following treatment with EP, the cells were incubated in a
96-well-plate at a density of 3×104 cells/well. MTT (10
µl, 5 mg/ml) was added to the wells at 0, 1, 2, 3 and 4 days of
culture. After incubation for 2 h, the crystals were dissolved by
adding DMSO (150 µl/well) and mixed well with a multichannel
pipette. The optical density (OD) of the soluble formazan in each
well was measured at 490 nm with a microplate reader (Thermo Fisher
Scientific, Inc.). All experiments were performed in
triplicate.
RT-qPCR
mRNA expression levels were determined by RT-qPCR.
Total RNA was extracted with TRIzol according to the manufacturer's
specifications. Reverse transcription was carried out using a Prime
Script RT Reagent kit (Perfect Real-Time) and cDNA amplification
was conducted using SYBR Premix Ex Taq (Tli RNaseH Plus) according
to the manufacturer's instructions. Target genes were amplified
using oligonucleotide primers. The ACTIN gene was used as
endogenous control. The PCR primer sequences are listed in Table I. Data were analyzed using the
comparative Cq method (2−ΔΔCq) (23). Each experiment was conducted in
triplicate.
 | Table I.Primer sequences for PCR. |
Table I.
Primer sequences for PCR.
| Genes | Primer sequences
(5′→3′) |
|---|
| HMGB1 | Forward
ATATGGCAAAAGCGGACAAG |
|
| Reverse
AGGCCAGGATGTTCTCCTTT |
| RAGE | Forward
GTCATGGAACTGCCCAAACT |
|
| Reverse
TCCTTCTGCGGATCTGTCTT |
| PCNA | Forward
GCCGAGATCTCAGCCATATT |
|
| Reverse
ATGTACTTAGAGGTACAAAT |
| MMP-9 | Forward
CGCAGACATCGTCATCCAGT |
|
| Reverse
GGATTGGCCTTGGAAGATG |
| P53 | Forward
AACGGTACTCCGCCACC |
|
| Reverse
CGTGTCACCGTCGTGGA |
| ACTIN | Forward
CTGGAACGGTGAAGGTGACA |
|
| Reverse
AAGGGACTTCCTGTAACAATGCA |
Western blot analysis
The cells of each group were collected and treated
with EP (0, 5, 10, 20 and 30 mmol/l). Subsequently, proteins were
extracted with CellLytic M cell Lysis Reagent with protease
inhibitor and phosphatase inhibitor cocktails (all from
Sigma-Aldrich; Merck KGaA). The proteins were quantified using a
BCA protein assay kit. Proteins (30 µg) were separated by 10%
SDS-PAGE. Polyvinylidene difluoride (PVDF) membrane were blocked
with a blocking reagent [Tris-buffered saline-0.1% Tween-20 (TBST)
containing 5% polyvinyl pyrrolidone (PVP), 5% FBS] at room
temperature for 1 h and then incubated with primary antibody
(dilution 1:1,000) overnight at 4°C. After washing the membranes
three times, PVDF membranes were incubated with HRP-conjugated
secondary antibodies (Cell Signaling Technology; dilution 1:2,000)
for 1 h at normal temperature. Immunoreactive bands were visualized
by an Imaging System (Tanon Science and Technology) after adding
chemiluminescent HRP substrate (Millipore Corp.). Each experiment
was conducted in triplicate.
Colony formation assay
The A549, H520 and PC-9 cells were treated with EP
and seeded in 6-well plates with 800 cells/clone. The culture
medium was replaced every 3 days. Colonies were counted when
visible with the naked eye. The cells were then fixed with methanol
and stained with 5% crystal violet solution. Each experiment was
conducted in triplicate.
Cell apoptosis analysis
The percentage of apoptotic cells was calculated by
means of an Annexin-V fluorescein isothiocyanate (FITC) and
propidium iodide (PI) apoptosis detection kit (BD Biosciences)
according to the manufacturer's instructions. The percentage of
apoptotic cells (Annexin+/PI+) was analyzed
via flow cytometry (BD Biosciences). Each experiment was conducted
in triplicate.
Transwell assay
Transwell chambers (8 µm) were used for cell
migration and invasion assays (Corning, Inc.). For the migration
assay, 1×105 cells were harvested and seeded on the
upper chamber. For the invasion assay, 3×105 cells were
harvested and placed in the upper chamber coated with Matrigel (300
µg/ml, 100 µl). RPMI-1640 (600 µl) supplemented with 20% FBS was
added to the lower chamber. The migrating or invading cells were
fixed with 100% methanol for 30 min and stained with 5% crystal
violet solution for 8 min post 24 h. Cells in 5 random fields (×4)
were calculated using Nikon MA100 optical microscope (Nikon Corp.,
Tokyo. Japan). Each assay was performed in triplicate.
Statistical analysis
All statistical analyses were carried out using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). Results are presented
as mean ± standard deviation. The capability of cell migration and
invasion between 20 mmol/l EP and 0 mmol/l EP in Fig. 3 (bar graph) were calculated using
independent sample t-tests. Furthermore, the statistical analysis
method used for the data of mRNA expression level and cell
apoptosis index in Figs. 1, 2 and 4 (bar
graphs) was the Kruskal-Wallis followed by Dunnett's post hoc test
when the 0 mmol/l EP group was compared with the 5, 10, 20 and 30
mmol/l EP groups. P<0.05 was considered to indicate a
statistically significant difference.
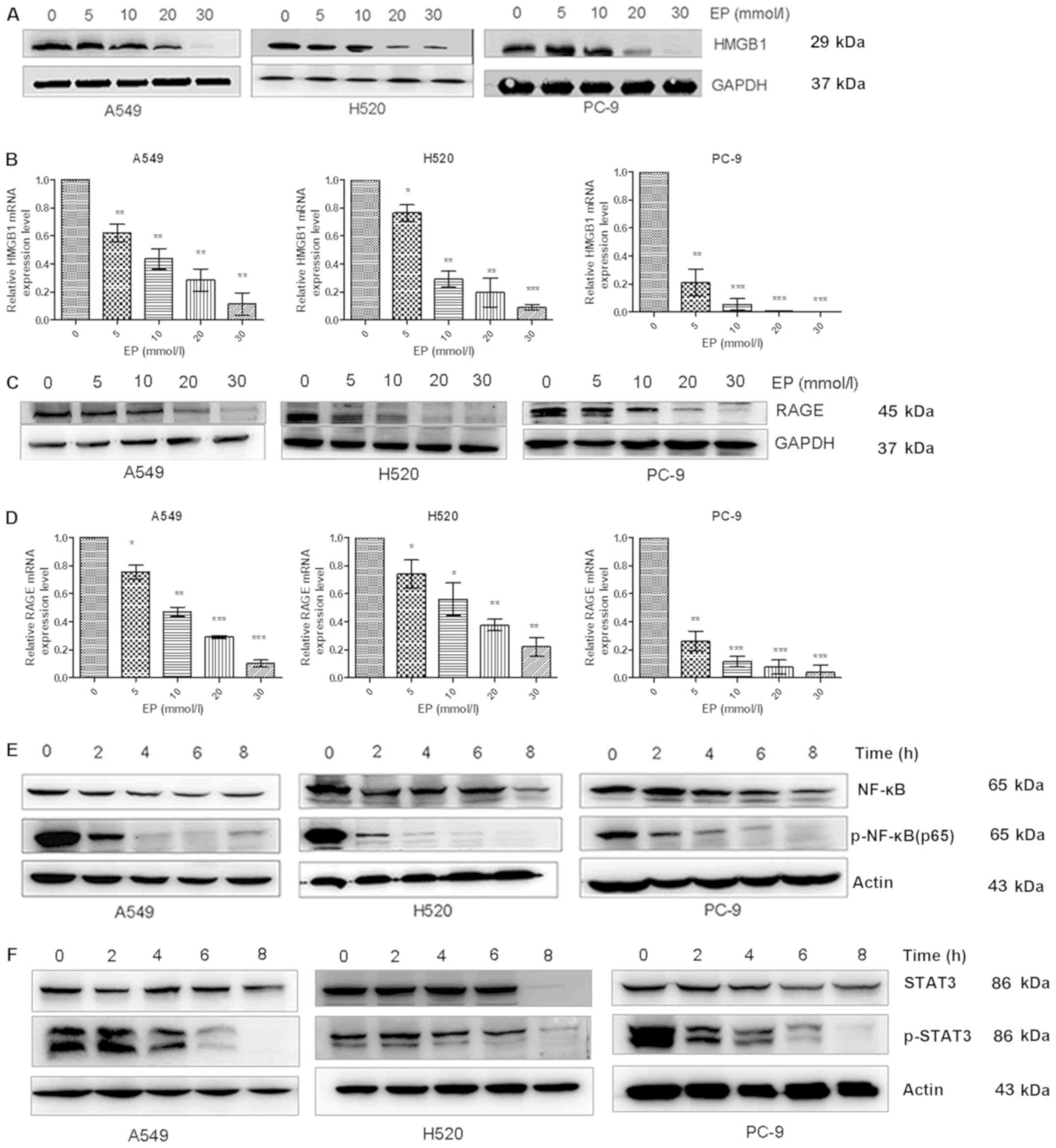 | Figure 1.Effects of EP administration on the
expression of the HMGBI/RAGE axis and the NF-κB/STAT3 pathway in
lung cancer cell lines. (A and B) The protein and mRNA expression
levels of HMGB1 as determined by western blot analysis and qPCR
were decreased in EP-treated groups in a dose-dependent manner
compared with the control group. (C and D) qPCR and western blot
analysis were used to measure the RAGE mRNA and protein levels,
respectively, in A549, H520 and PC-9 cells. The RAGE levels were
significantly reduced in EP-treated groups in a dose-dependent
manner compared with the control group. (E) Western blot analysis
revealed that the expression level of p-NF-κB was decreased in the
EP-treated groups in a time-dependent manner (at 2, 4, 6 and 8 h)
compared with the control group (0 h). (F) The protein levels of
p-STAT3 were determined by western blot analysis in A549, H520 and
PC-9 cells, and were found to be significantly reduced by treatment
with 30 mmol/l EP in a time-dependent manner compared with the
control group. *P<0.05, **P<0.01 and ***P<0.001 as
compared to the 0 mmol/l EP group. EP, ethyl pyruvate; HMGB1, high
mobility group protein B1. |
Results
Administration of EP suppresses the
expression of the HMGB1/RAGE axis and the NF-κB/STAT3 pathway in
lung cancer cells
RAGE, also known as AGER, is a transmembrane
receptor that binds to a variety of ligands, including HMGB1
(24,25). A549, H520 and PC-9 cells were treated
with EP (0, 5, 10, 20 and 30 mmol/l) in order to investigate its
effects on HMGB1 and RAGE protein as well as mRNA expression. The
protein and mRNA expression levels of HMGB1 were significantly
decreased with increasing EP dose (Fig.
1A and B). Similarly, the mRNA and protein expression levels of
RAGE were also decreased with increasing EP dose (Fig. 1C and D).
NF-κB regulates DNA transcription, cytokine
production and cell survival (26).
In the present study, A549, H520 and PC-9 cells were treated with
30 mmol/l EP for 2, 4, 6 and 8 h in order to examine NF-κB and
p-NF-κB levels. The protein expression of p-NF-κB was gradually
decreased over time (Fig. 1E). STAT3
is a member of the STAT protein family (27). A549, H520, PC-9 cells were treated
with 30 mmol/l EP for 2, 4, 6 and 8 h in order to examine STAT3 and
p-STAT3 levels. The protein expression level of p-STAT3 was also
decreased over the specified time period (Fig. 1F).
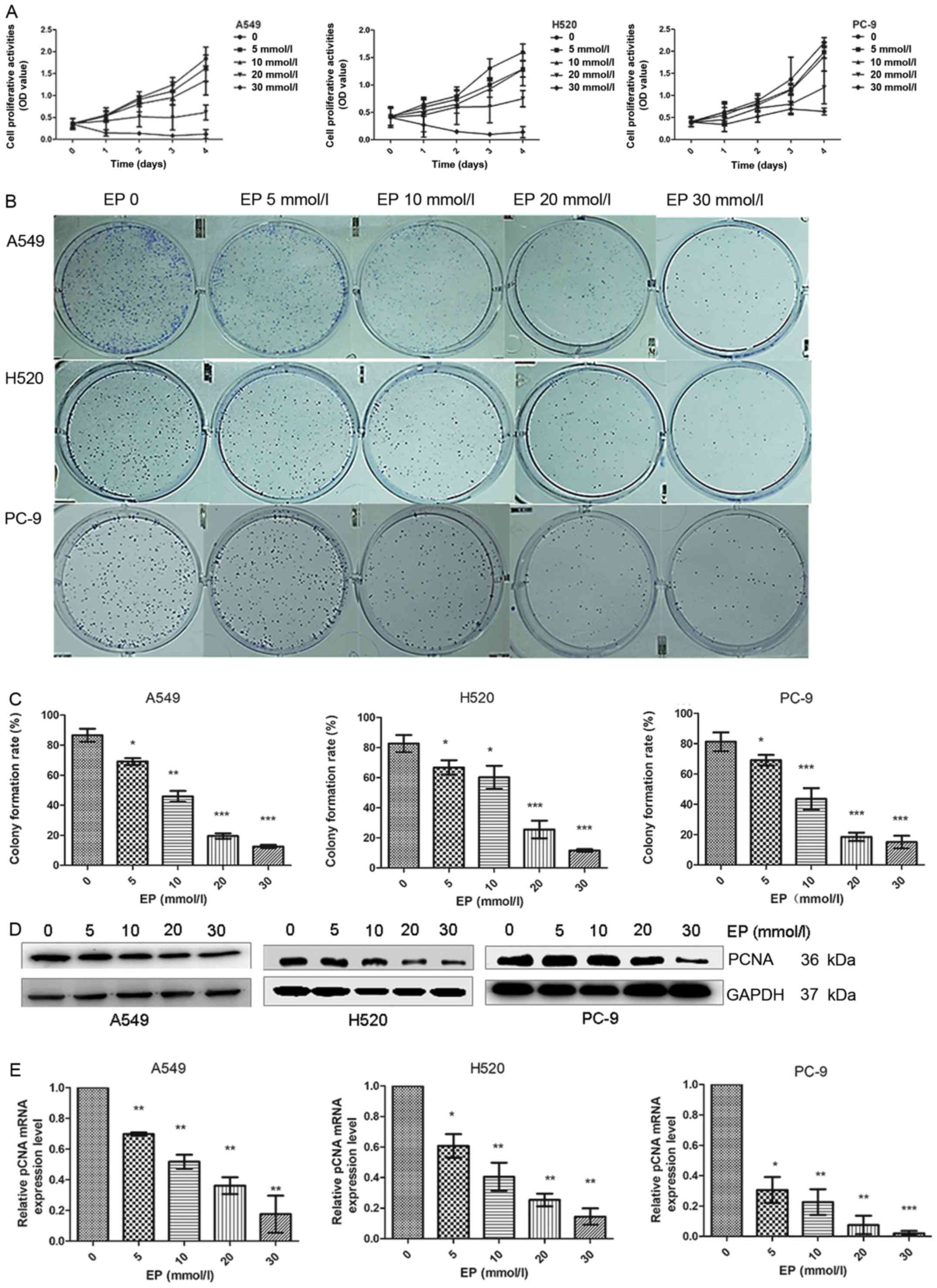
Administration of EP suppresses the
growth of lung cancer cells
A hallmark of cancer is enhanced cell proliferation
(28). A549 H520 and PC-9 cell growth
was assessed via MTT and colony formation assays. It was observed
that A549, H520 and PC-9 cell growth was markedly suppressed by EP
in a dose/time-dependent manner (Fig.
2A). The colony formation rate of A549, H520 and PC-9 cells was
significantly decreased in the EP-treated groups (Fig. 2B and C).
Proliferating cell nuclear antigen (PCNA) is a DNA
clamp that is essential for replication (29) and tumor progression. Therefore,
anticancer treatment effectiveness may be determined by PCNA
(30). PCNA was investigated by
western blot analysis and RT-qPCR. The data demonstrated that the
expression of PCNA was decreased in the EP-treated groups (Fig. 2D and E).
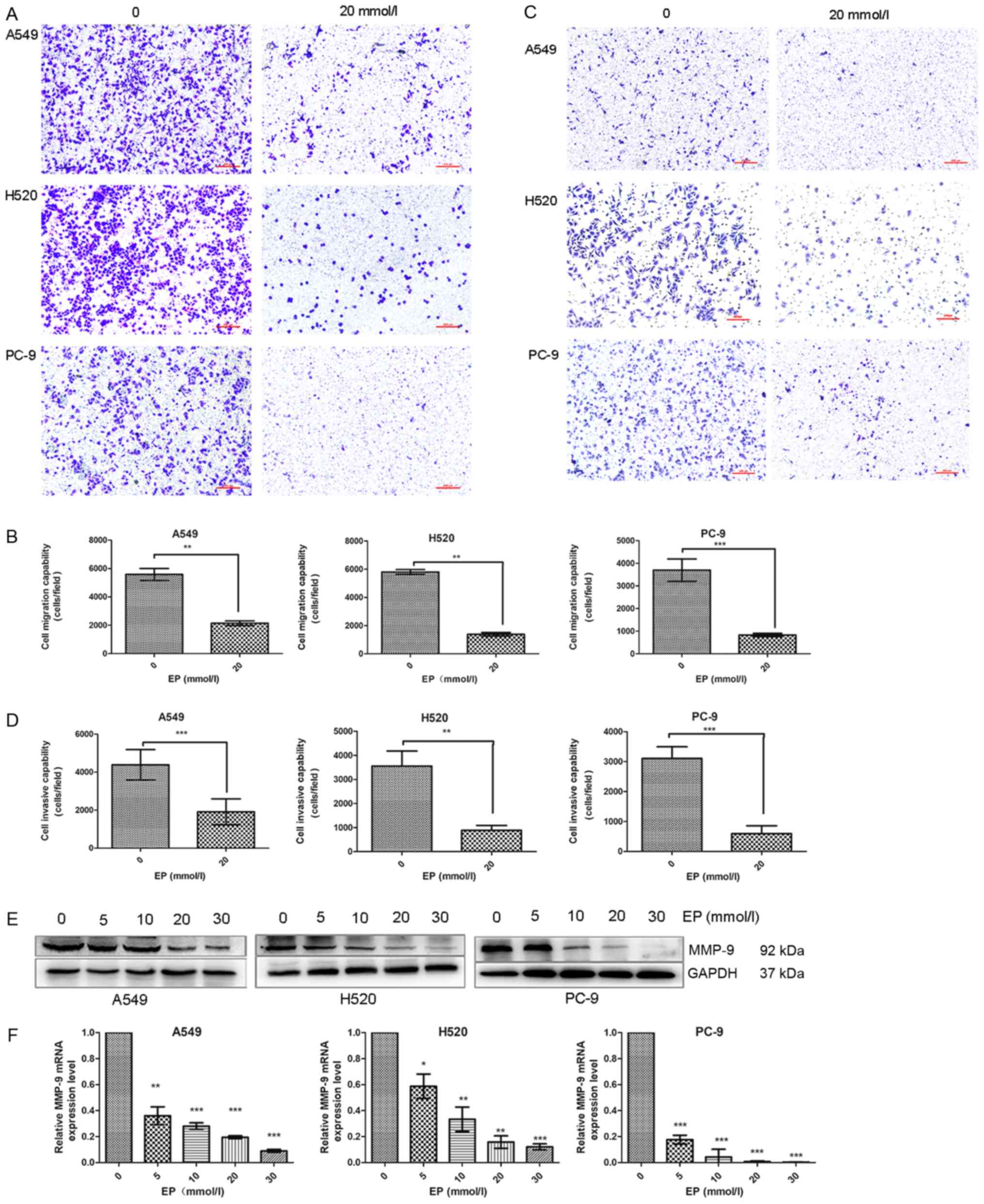
Administration of EP inhibits the
migration and invasion of lung cancer cells
A Transwell assay demonstrated that the migration
and invasion potential of A549, H520 and PC-9 cells in the
EP-treated groups was reduced compared with that of the control
group (Fig. 3A-D).
Matrix metallopeptidase 9 (MMP-9) participates in
the degradation of the extracellular matrix (31). MMP-9 may be associated with the
development of various human malignancies by affecting invasion,
metastasis, growth and angiogenesis (32,33).
Western blot analysis revealed downregulation of MMP-9 (Fig. 3E). The results were consistent with
those of RT-qPCR (Fig. 3F).
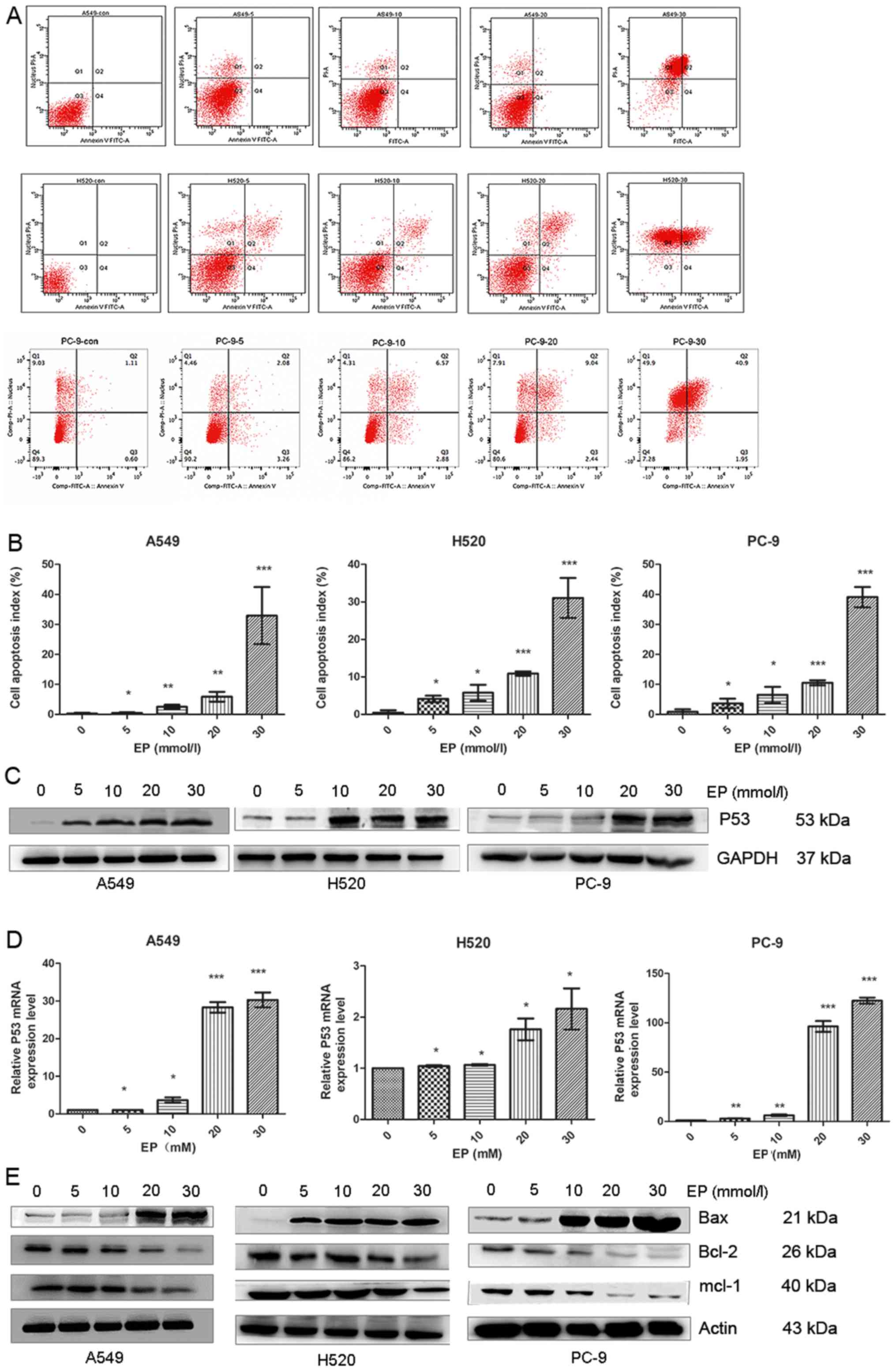
Administration of EP promotes
apoptosis of lung cancer cells
Flow cytometry was used to determine whether EP
affects the apoptotic process of lung cancer cells. The results
demonstrated that the apoptotic index of A549, H520 and PC-9 cells
in the EP-treated groups was significantly higher compared with
that of the control group (Fig. 4A and
B).
P53, a tumor suppressor, directly participates in
the endogenous apoptotic pathway by interacting with members of the
Bcl-2 family (34,35). Western blot analysis and qPCR were
used to determine P53 changes. The data demonstrated that the
protein and mRNA levels of P53 increased with increasing EP
concentration (Fig. 4C and D).
The Bcl-2 family plays an important role in the
regulation of apoptosis, a type of programmed cell death. Bcl-2
family members may promote or inhibit apoptosis (36). Western blot analysis revealed that EP
treatment increased the pro-apoptotic protein Bax and decreased the
anti-apoptotic proteins Bcl-2 and Mcl-1 (Fig. 4E).
Discussion
In the present study, it was established that ethyl
pyruvate (EP), a lipophilic ester, suppressed lung cancer cell
growth, invasion and migration in vitro, and promoted
apoptosis. Our results showed that the EP-mediated suppression of
lung cancer cell growth was mediated via inhibition of the
HMGB1/RAGE axis and the NF-κB/STAT3 pathway.
The results revealed that inflammatory factors play
an important role in tumor initiation and progression. As an
anti-inflammatory drug, EP has been shown to decrease organ
dysfunction in several inflammation-induced disease models
(19,20). Several trials have attempted to
improve the overall surivial (OS) rate of cancer patients via EP
administration. It was previously demonstrated that tumor
progression may be suppressed by EP. The OS of animals in several
tumor models, including hepatic, gastric, gallbladder cancer and
mesothelioma, may be increased via EP (37–42). As an
HMGB1 inhibitor, the antitumor effect of EP is mediated by
inhibiting the HMGB1/RAGE axis (37,40,42).
Previous studies have investigated the expression levels of HMGB1
and its receptor RAGE in lung cancer (43), and reported that growth, invasion and
migration of lung cancer cells are dependent on these signaling
molecules (44). In the present
study, a significant reduction in HMGB1 and RAGE protein levels was
observed in EP-treated lung cancer cells. Furthermore, RT-qPCR
demonstrated that HMGB1-induced RAGE mRNA expression was
specifically suppressed by EP treatment. Therefore, the antitumor
effects exerted by EP on lung cancer cell lines may be explained by
disruption of the HMGB1/RAGE loop. Using varying concentrations of
EP, decreased non-small cell lung cancer (NSCLC) cell growth,
invasion and migration, as well as increased apoptosis, were
observed. To the best of our knowledge, this is the first study to
investigate EP as a potential therapeutic agent for NSCLC. NF-κB
has been found to be active in tumor cells; therefore, NF-κB
suppression may prevent tumor cell proliferation, thus enhancing
the effectiveness of antitumor agents (45,46). STAT3
regulates gene expression and plays a critical role in a number of
cellular processes, such as cell growth and apoptosis (47). In the present study, EP suppressed the
RELA protein expression in a time-dependent manner in three lung
cancer cell lines via phosphorylation of Ser536. In regards to the
STAT3 pathway, EP suppressed STAT3 in a time-dependent manner via
phosphorylation of its Tyr705 residue.
EP may be proven to be useful as an antitumor agent.
Unlike other chemical compounds, EP is associated with no toxicity
and has a good safety profile (16).
EP is widely used as a food additive and is found in caramel,
brandy, rum, chocolate and other food spices. Its lack of toxicity
has been verified in several previous studies conducted on animal
models. EP treatment in humans was also proven to be risk-free
(48). Preclinical research has
demonstrated that tumor cell sensitivity to other antitumor agents
is significantly increased by inhibiting the release of HMGB1 via
EP, and chemotherapy-related cytotoxicity is decreased by EP
(49). This evidence strongly
supports the use of EP as an adjuvant lung cancer therapy.
Moreover, human malignant mesothelioma (MM) arises
from the malignant transformation of mesothelial cells in the
pleura, peritoneum and pericardial cavity. In a recent study by
Pellegrini et al, it was observed that HMGB1 targeting by EP
inhibited the development of MM (39). EP was shown to suppress the viability,
motility and migration of MM cell lines (REN, HP3 and PPM-MILL) by
inhibiting the HMGB1/RAGE and NF-κB pathways. Furthermore,
Pellegrini et al found that EP inhibited orthotopic tumor
growth in MM xenografts. Similarly, in our present study, we found
that EP suppressed the growth, invasion and migration and induced
apoptosis of NSCLC cells via the HMGB1/RAGE axis and the
NF-κB/STAT3 pathway. However, our findings were not tested in
vivo. Taken together, the findings of Pellegrini et al
(39) and those of the present study
indicate that EP may be useful as an adjuvant treatment for
thoracic malignancies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Foundation (NNSF) of China (grant no. 81570194 to LJ).
Availability of data and materials
All the datasets generated and analyzed in the
present study are included in this published manuscript.
Authors' contributions
QL, PW, LJ and YH conceived and designed the study.
QL, HZ and JZ performed the experiments. QL, PW and YH wrote the
manuscript. QL, YH, HZ, JZ and LJ reviewed and edited the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zou S, Pan X, Hua C, Wu M, He B and Chen
Z: Myeloperoxidase-463 G/A polymorphism is associated with lung
cancer risk: A meta-analysis with 7420 cases and 9132 controls. J
Cancer Res Ther. 14 (Suppl):S282–S287. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ziebarth NR: Lung cancer risk perception
biases. Prev Med. 110:16–23. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu FT, Jia L, Wang P, Farren T, Li H, Hao
X and Agrawal SG: CD126 and targeted therapy with tocilizumab in
chronic lymphocytic leukemia. Clin Cancer Res. 22:2462–2469. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park BS, Jo HW, Park C, Huh Y, Jung J and
Jeong NY: A novel effect of ethyl pyruvate in Schwann cell
de-differentiation and proliferation during Wallerian degeneration.
Anim Cells Syst. 19:262–268. 2015. View Article : Google Scholar
|
|
5
|
Li X, Jiang S and Tapping RI: Toll-like
receptor signaling in cell proliferation and survival. Cytokine.
49:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gruffaz M, Vasan K, Tan B, Ramos da Silva
S and Gao SJ: TLR4-mediated inflammation promotes KSHV-induced
cellular transformation and tumorigenesis by activating the STAT3
pathway. Cancer Res. 77:7094–7108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su X, Wang H, Zhao J, Pan H and Mao L:
Beneficial effects of ethyl pyruvate through inhibiting
high-mobility group box 1 expression and TLR4/NF-κB pathway after
traumatic brain injury in the rat. Mediators Inflamm.
2011:8071422012.
|
|
8
|
Shao Y, Nanayakkara G, Cheng J, Cueto R,
Yang WY, Park JY, Wang H and Yang X: Lysophospholipids and their
receptors serve as conditional DAMPs and DAMP receptors in tissue
oxidative and inflammatory injury. Antioxid Redox Signal. Apr
26–2017.(Epub ahead of print). doi: 10.1089/ars.2017.7069.
|
|
9
|
Lin TJ, Lin HT, Chang WT, Mitapalli SP,
Hsiao PW, Yin SY and Yang NS: Shikonin-enhanced cell immunogenicity
of tumor vaccine is mediated by the differential effects of DAMP
components. Mol Cancer. 14:1742015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bierhaus A, Schiekofer S, Schwaninger M,
Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting
I, et al: Diabetes-associated sustained activation of the
transcription factor nuclear factor-kappaB. Diabetes. 50:2792–2808.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: New discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Müller M, Morotti A and Ponzetto C:
Activation of NF-kappaB is essential for hepatocyte growth
factor-mediated proliferation and tubulogenesis. Mol Cell Biol.
22:1060–1072. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grivennikov S, Karin E, Terzic J, Mucida
D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H,
Eckmann L and Karin M: IL-6 and Stat3 are required for survival of
intestinal epithelial cells and development of colitis-associated
cancer. Cancer Cell. 15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen Y, Devgan G, Darnell JE Jr and
Bromberg JF: Constitutively activated Stat3 protects fibroblasts
from serum withdrawal and UV-induced apoptosis and antagonizes the
proapoptotic effects of activated Stat1. Proc Natl Acad Sci USA.
98:1543–1548. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pathak M, Mishra R, Agarwala PK, Ojha H,
Singh B, Singh A and Kukreti S: Binding of ethyl pyruvate to bovine
serum albumin: Calorimetric, spectroscopic and molecular docking
studies. Thermochim Acta. 633:140–148. 2016. View Article : Google Scholar
|
|
17
|
Cook VL, Holcombe SJ, Gandy JC, Corl CM
and Sordillo LM: Ethyl pyruvate decreases proinflammatory gene
expression in lipopolysaccharide-stimulated equine monocytes. Vet
Immunol Immunopathol. 141:92–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wagner N, Dieteren S, Franz N, Köhler K,
Mörs K, Nicin L, Schmidt J, Perl M, Marzi I and Relja B: Ethyl
pyruvate ameliorates hepatic injury following blunt chest trauma
and hemorrhagic shock by reducing local inflammation, NF-kappaB
activation and HMGB1 release. PLoS One. 13:e0192171doi:
10.1371/journal.pone.0192171. eCollection. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang FC, Xie Y and Zhou NJ: Ethyl pyruvate
reduced h pylori- induced inflammation through inhibition of
HMGB1/TLR4 pathways. J Gastroenterol Hepatol. 29:27. 2014.
|
|
20
|
Shen HX, Hu XM, Liu C, Wang SP, Zhang WT,
Gao H, Steder RA, Gao YQ and Chen J: Ethyl pyruvate protects
against hypoxic-ischemic brain injury via anti-cell death and
anti-inflammatory mechanisms. Neurobiol Dis. 37:711–722. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen M, Lu J, Dai W, Wang F, Xu L, Chen K,
He L, Cheng P, Zhang Y, Wang C, et al: Ethyl pyruvate ameliorates
hepatic ischemia-reperfusion injury by inhibiting intrinsic pathway
of apoptosis and autophagy. Mediators Inflamm. 2013:4615362013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chakhtoura M, Chain R, Varghese L and
Gallucci S: Ethyl pyruvate, an inhibitor of high-mobility group box
1 (HMGB1) release, modulates dendritic cell activation and survival
(TRAN3P.897). J Immunol 192 (1 Suppl). S202–S236. 2014.
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2011.
View Article : Google Scholar
|
|
24
|
Ibrahim ZA, Armour CL, Phipps S and Sukkar
MB: RAGE and TLRs: Relatives, friends or neighbours? Mol Immunol.
56:739–744. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han SH, Kim YH and Mook-Jung I: RAGE: The
beneficial and deleterious effects by diverse mechanisms of
actions. Mol Cells. 31:91–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Actis M, Inoue A, Evison B, Perry S,
Punchihewa C and Fujii N: Small molecule inhibitors of PCNA/PIP-box
interaction suppress translesion DNA synthesis. Bioorg Med Chem.
21:1972–1977. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abike F, Tapisiz OL, Zergeroglu S, Dunder
I, Temizkan O and Payasli A: PCNA and Ki-67 in endometrial
hyperplasias and evaluation of the potential of malignancy. Eur J
Gynaecol Oncol. 32:77–80. 2011.PubMed/NCBI
|
|
31
|
Himelstein BP, Canete-Soler R, Bernhard
EJ, Dilks DW and Muschel RJ: Metalloproteinases in tumor
progression: The contribution of MMP-9. Invasion Metastasis.
14:246–258. 1994.PubMed/NCBI
|
|
32
|
Morini M, Mottolese M, Ferrari N, Ghiorzo
F, Buglioni S, Mortarini R, Noonan DM, Natali PG and Albini A: The
alpha 3 beta 1 integrin is associated with mammary carcinoma cell
metastasis, invasion, and gelatinase B (MMP-9) activity. Int J
Cancer. 87:336–342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Farina AR and Mackay AR: Gelatinase
B/MMP-9 in tumour pathogenesis and progression. Cancers (Basel).
6:240–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Ma L, Yan T, Han X, Xu J, Xu J
and Xu X: Activated mitochondrial apoptosis in hESCs after
dissociation involving the PKA/p-p53/Bax signaling pathway. Exp
Cell Res. 369:226–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yao TH, Pataer P, Regmi KP, Gu XW, Li QY,
Du JT, Ge SM and Tu JB: Propranolol induces hemangioma endothelial
cell apoptosis via a p53-BAX mediated pathway. Mol Med Rep.
18:684–694. 2018.PubMed/NCBI
|
|
36
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Zhu JS, Zhou Z, Chen WX and Chen
NW: Inhibitory effects of ethyl pyruvate administration on human
gastric cancer growth via regulation of the HMGB1-RAGE and Akt
pathways in vitro and in vivo. Oncol Rep.
27:1511–1519. 2012.PubMed/NCBI
|
|
38
|
Birkenmeier G, Hemdan NYA, Kurz S, Bigl M,
Pieroh P, Debebe T, Buchold M, Thieme R, Wichmann G and Dehghani F:
Ethyl pyruvate combats human leukemia cells but spares normal blood
cells. PLoS One. 11:e01615712016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pellegrini L, Xue J, Larson D, Pastorino
S, Jube S, Forest KH, Saad-Jube ZS, Napolitano A, Pagano I, Negi
VS, et al: HMGB1 targeting by ethyl pyruvate suppresses malignant
phenotype of human mesothelioma. Oncotarget. 8:22649–22661. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng P, Dai W, Wang F, Lu J, Shen M, Chen
K, Li JJ, Zhang Y, Wang C, Yang J, et al: Ethyl pyruvate inhibits
proliferation and induces apoptosis of hepatocellular carcinoma via
regulation of the HMGB1-RAGE and AKT pathways. Biochem Biophys Res
Commun. 443:1162–1168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baunacke M, Horn LC, Trettner S, Engel KM,
Hemdan NY, Wiechmann V, Stolzenburg JU, Bigl M and Birkenmeier G:
Exploring glyoxalase 1 expression in prostate cancer tissues:
Targeting the enzyme by ethyl pyruvate defangs some
malignancy-associated properties. Prostate. 74:48–60. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li ML, Wang XF, Tan ZJ, Dong P, Gu J, Lu
JH, Wu XS, Zhang L, Ding QC, Wu WG, et al: Ethyl pyruvate
administration suppresses growth and invasion of gallbladder cancer
cells via downregulation of HMGB1-RAGE axis. Int J Immunopathol
Pharmacol. 25:955–965. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Queisser MA, Kouri FM, Königshoff M,
Wygrecka M, Schubert U, Eickelberg O and Preissner KT: Loss of RAGE
in pulmonary fibrosis: Molecular relations to functional changes in
pulmonary cell types. Am J Respir Cell Mol Biol. 39:337–345. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu X, Zhu H, Wang T, Sun Y, Ni P, Liu Y,
Tian S, Amoah Barnie P, Shen H, Xu W, et al: Exogenous
high-mobility group box 1 inhibits apoptosis and promotes the
proliferation of lewis cells via RAGE/TLR4-dependent signal
pathways. Scand J Immunol. 79:386–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Escárcega RO, Fuentes-Alexandro S,
Garcia-Carrasco M, Gatica A and Zamora A: The transcription factor
nuclear factor-kappa B and cancer. Clin Oncol (R Coll Radiol).
19:154–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yuan ZL, Guan YJ, Wang L, Wei W, Kane AB
and Chin YE: Central role of the threonine residue within the p+1
loop of receptor tyrosine kinase in STAT3 constitutive
phosphorylation in metastatic cancer cells. Mol Cell Biol.
24:9390–9400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bennett-Guerrero E, Swaminathan M, Grigore
AM, Roach GW, Aberle LG, Johnston JM and Fink MP: A phase II
multicenter double-blind placebo-controlled study of ethyl pyruvate
in high-risk patients undergoing cardiac surgery with
cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 23:324–329.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tang D, Kang R, Cheh CW, Livesey KM, Liang
X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, et
al: HMGB1 release and redox regulates autophagy and apoptosis in
cancer cells. Oncogene. 29:5299–5310. 2010. View Article : Google Scholar : PubMed/NCBI
|