Introduction
Gastric cancer is one of the major malignancies in
the world (1,2). Although its incidence and mortality
rates have declined dramatically in Western countries for decades
(1,2),
GC still constitutes a huge health threat in some parts of the
world, such as in China and Japan (3,4). Surgery
with radical intention is suitable for only a limited percentage of
patients, while many patients are often diagnosed at later stages,
or experience postsurgical disease relapse or metastasis, which
make their prognoses even worse (5).
Comprehensive treatment of advanced GC remains unsatisfactory, so
it is of vital importance to elucidate the molecular mechanisms
that lead to disease progression of GC.
Metallocarboxypeptidases (MCPs) are zinc-dependent
peptidases that cleave off C-terminal amino acid residues from
their substrates by catalysis of peptide bond hydrolysis. More than
26 MCPs have been discovered and are categorized into four major
subgroups of the M14 family of peptidases based on their structural
and functional relevance, namely M14A subfamily or digestive
carboxypeptidases, M14B subfamily or regulatory carboxypeptidases,
M14C subfamily or bacterial peptidoglycan hydrolyzing enzymes, and
M14D subfamily or cytosolic carboxypeptidases. MCPs induce
substrate degradation and participate in a variety of physiological
and pathological processes such as digestion, development,
inflammation, and type 2 diabetes, as reviewed by several authors
(6–8).
Furthermore, dysregulation of MCPs has been associated with
oncogenesis. For example, carboxypeptidase A4 (CPA4) is
elevated in GCs and is associated with an aggressive phenotype and
unfavorable prognosis (9), and
carboxypeptidase E (CPE) and its splice isoform are
correlated with metastases in a variety of malignancies (10), while carboxypeptidase M (CPM)
is dysregulated in several tumor types (11), highlighting the importance of some
carboxypeptidases in modulating tumor behavior. Although the
underlying mechanisms of their oncogenic roles are largely unknown,
their interaction with growth factors could be of some interest
(11). In addition, successful
isolation of endogenous inhibitors and synthesis of exogenous
inhibitors make MCPs ideal targets for anticancer therapy (8).
Carboxypeptidase X, M14 family member 2
(CPXM2), one of the less characterized MCPs, has been
associated with developmental diseases (12,13),
late-onset Alzheimer's disease, and cognitive decline in
schizophrenia (14,15). However, whether CPXM2 is
involved in oncogenesis or tumor progression remains unclear. In
the present study, we determined the expression of CPXM2 and
its clinical and prognostic relevance in GCs. We also investigated
its in vitro activities in cultured GC cells and
characterized the potential underlying mechanisms of action. We
aimed to shed light on the oncogenic roles of CPXM2 in GC,
one of the most fatal malignancies in the world.
Materials and methods
Patients and specimens
Fifteen paired human GC samples and their matched
gastric non-cancerous tissues (NTs) were collected at the time of
surgical resection at Shanghai Fifth People's Hospital (Shanghai,
China) from February 2017 to February 2018. There were 10 males and
5 females, with a median age of 63 (range, 52–77 years). Samples
were snap-frozen in liquid nitrogen and stored at −80°C.
Paraffin-embedded tissues were retrieved from the Tissue Bank of
the Shanghai Fifth People's Hospital, and 4-µm tissue sections were
prepared by the Department of Pathology at the same hospital.
Tissue microarrays (TMAs) of GCs and adjacent NTs were prepared by
Shanghai Outdo Biotech (Shanghai, China). The TMA sections
contained paired GCs and NTs from 90 patients with a median
follow-up of 30 months. The clinicopathological characteristics of
these patients are summarized in Table
I. This study was approved by the institutional Ethics
Committee of Shanghai Fifth People's Hospital (Ethical approval
form no. 2017–097) and adhered to the principles of the Declaration
of Helsinki. Informed consent was obtained from each patient before
collection of tissues.
 | Table I.Clinical and pathological features of
the gastric cancer patientsa (n=90). |
Table I.
Clinical and pathological features of
the gastric cancer patientsa (n=90).
| Variable | No. of patients
(%) |
|---|
| Sex |
|
|
Male | 69 (76.7) |
|
Female | 21 (23.3) |
| Age (years) |
|
|
<70 | 45 (50) |
|
≥70 | 45 (50) |
|
Differentiation |
|
| G2 | 28 (31.1) |
| G3 | 62 (68.9) |
| Tumor size
(cm) |
|
|
<5 | 35 (38.9) |
| ≥5 | 54 (60.0) |
| NA | 1 (1.1) |
| TNM stage |
|
| I | 12 (13.3) |
| II | 27 (30.0) |
|
III | 49 (54.5) |
| IV | 2 (2.2) |
| Tumor stage |
|
| T1 | 5 (5.6) |
| T2 | 15 (16.7) |
| T3 | 46 (51.1) |
| T4 | 24 (26.6) |
| Nodal stage |
|
| N0 | 24 (26.7) |
| N1 | 15 (16.7) |
| N2 | 23 (25.5) |
| N3 | 28 (31.1) |
| Vessel
invasion |
|
| No | 74 (82.2) |
|
Yes | 16 (17.8) |
| Nerve invasion |
|
| No | 74 (82.2) |
|
Yes | 15 (16.7) |
| NA | 1 (1.1) |
| Expression of
CPXM2 |
|
|
Low | 41 (45.6) |
|
High | 49 (54.4) |
Immunohistochemistry (IHC)
Sections were stained with an anti-CPXM2 polyclonal
antibody using IHC in the Department of Pathology at our hospital.
In brief, after deparaffination, rehydration in graded ethanol,
antigen retrieval with citrate buffer pH 6.0 (1:300 dilution;
ZLI-9065; ZSGB-Bio, Beijing, China) and blocking, slides were
stained with a rabbit anti-CPXM2 polyclonal antibody (1:50
dilution; cat. no. abs127533a; Absin Bioscience, Shanghai, China)
at 4°C overnight. Normal rat IgG (1:50 dilution; cat. no. D110504;
Sangon Biotech, Shanghai, China) instead of the primary antibody
was used as a control. Subsequently, after washing with PBS, a
horseradish peroxidase (HRP)-conjugated secondary antibody
(1:2,000; goat anti-rat, cat. no. A0192; Beyotime Institute of
Biotechnology, Haimen, China) was added and incubated at room
temperature for 1 h. Then, these sections were stained using
3,3′-diaminobenzidine (DAB) (cat. no. GK500705; Shanghai GeneTech
Co., Ltd., Shanghai, China) and counterstained with hematoxylin. A
modified H score system was used to semi-quantitate CPXM2
expression, as previously described (16). Briefly, the maximal intensity of
staining (0, negative; 1, weak; 2, moderate; and 3, strong) was
multiplied by the percentage of positive tumor cells (0–100%) to
generate the modified H score (range, 0–300). CPXM2 expression was
categorized as high or low based on the median H score.
Access to public datasets
The mRNA expression of CPXM2 in GC tissues
and normal mucosae was acquired from Oncomine (http://www.oncomine.org) (17,18). The
original data for prognostic analysis of CPXM2 were
downloaded from the Kaplan-Meier Plotter (http://www.kmplot.com) (19) and UCSC Xena (https://xenabrowser.net/heatmap/).
Cell lines and culture conditions
A gastric epithelial cell line (GES-1) and five GC
cell lines (AGS, HGC27, MGC803, NCI-N87 and SNU-1) were obtained
from the Type Culture Collection of the Chinese Academy of Science
(Shanghai, China) and were validated by short tandem repeat DNA
profiling. Cells were cultured in RPMI-1640 (BBI Life Sciences,
Shanghai, China) or F12K medium (Zhong Qiao Xin Zhou Biotechnology,
Shanghai, China) supplemented with 10% fetal bovine serum (FBS),
100 µg/ml penicillin, and 100 mg/ml streptomycin at 37°C with 5%
CO2 in a humidified incubator (Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Construction of CPXM2-targeting shRNAs
and packaging of lentiviruses
Four targeting shRNAs and a nontargeting scrambled
RNA (scramble) were subcloned into the GV248 lentivirus vector by
Shanghai GeneChem Co., Ltd., (Shanghai, China). The shCPXM2 target
sequences were AGGTTCATCGTGGCATTAA (shCPXM2-1), ACGATGGAATTGACATCAA
(shCPXM2-2), TCCCAATATCACCAGAATT (shCPXM2-3) and
CTCAGTCCTGGTTTGATAA (shCPXM2-4). Lentiviral stocks were prepared
and purified as previously described (20).
Infection of GC cells with
lentiviruses
Cells were seeded in 6-well plates at a density of
2×105/ml and cultivated for 24 h. Then, 20 µl of
lentivirus solution and 1 ml fresh medium containing 10 µg/ml
polybrene (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
were added to each well. The medium was changed after 24 h and an
efficient lentiviral transduction was confirmed by a fluorescence
microscope at 72 h after infection.
RNA extraction and the quantitative
polymerase chain reaction (qPCR)
Total RNA was isolated from cell cultures or from
snap-frozen tissues from GC patients using RNAiso Plus (Takara Bio,
Kusatsu, Japan) according to the manufacturer's instructions,
reverse transcribed with HiScript Q Select RT SuperMix (R132-01;
Vazyme, Jiangsu, China) according to the manufacturer's protocol,
and subjected to real-time reverse transcription (RT)-PCR using the
2−ΔΔCq method (21). The
thermocycling conditions were as follows: 95°C for 30 sec, followed
by 40 cycles of 95°C for 10 sec, 60°C for 32 sec, 95°C for 15 sec,
60°C for 60 sec and 95°C for 15 sec. Each sample was determined in
duplicate. All PCR products were confirmed by 2.0% agarose gel
electrophoresis. The sequences for RT-PCR primers were:
CPXM2 forward primer, 5′-GTGCGCGGGAAGAAATGAC-3′ and reverse
primer, 5′-CCTCCCTTGAGTGATGACACC-3′. The specificity of primers was
validated by sequencing. Glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) (forward primer, 5′-GTCAAGGCTGAGAACGGGAA-3′ and
reverse primer 5′-AAATGAGCCCCAGCCTTCTC-3′) served as an internal
control. Experiments were repeated three times in duplicate.
Western blot analysis
Total protein was extracted from cell cultures or
homogenized tissues from GC patients using radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Shanghai,
China) containing phenylmethylsulfonyl fluoride (Beyotime Institute
of Biotechnology) and proteinase inhibitor cocktail solution
(Roche, Basel, Switzerland), and quantitated using the
bicinchoninic acid protein assay (Beyotime Institute of
Biotechnology) as recommended by the manufacturers. Western
blotting was performed according to standard methods as previously
described (21). Briefly, the
proteins (30 µg) were separated by 10% SDS-PAGE and then
transferred to a polyvinylidene fluoride (PVDF) membrane.
Subsequently, the membranes were blocked with 5% fat-free dry milk
at room temperature for 1 h. Then, the blots were incubated with a
rabbit anti-CPXM2 polyclonal antibody (1:1,000 dilution; cat. no.
abs127533a; Absin Bioscience, Shanghai, China), and rabbit
monoclonal antibodies against E-cadherin, N-cadherin, vimentin and
ZEB1 (1:1,000 dilution; cat. nos. 3195, 13116, 5741 and 3396; Cell
Signaling Technology, Danvers, MA, USA) overnight at 4°C. GAPDH
(1:2,000; cat. no. AF1186, rabbit anti-human; Beyotime Institute of
Biotechnology) or β-actin (1:2,000; cat. no. AF0003, mouse
anti-human; Beyotime Institute of Biotechnology) was detected as a
loading control. The membranes were again washed with TBST and
incubated with respective horseradish peroxidase (HRP)-conjugated
secondary antibodies (1:2,000 dilution; goat anti-rat cat. no.
A0192, goat anti-mouse cat. no. A0216 and goat anti-rabbit cat. no.
A0239; Beyotime Institute of Biotechnology) at room temperature for
1 h. The proteins were finally examined by an enhanced
chemiluminescence system (ECL) (P0018AS; Beyotime Institute of
Biotechnology). The grayscale values of protein bands were analyzed
using ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Cell proliferation assay
Stably transfected AGS and HGC-27 cells
(2×103 cells/well) were seeded in 96-well plates and
cultivated for 24, 48, 72 or 96 h. Then, 10 µl cholecystokinin
octapeptide (CCK-8) reagent [10% (v/v) in serum-free RPMI-1640
medium; Beyotime Biotechnology] was added to each well and
incubated at 37°C for 1 h. The absorbance at 450 nm was measured
using a microplate reader (BioTek Synergy 2; BioTek Instruments
Inc., Winooski, VT, USA).
Cell colony formation assay
A plate colony formation assay was performed as
previously described (22). Briefly,
stably transfected AGS or HGC-27 cells (5×102
cells/well) were seeded in 6-well plates and cultivated in F12K or
RPMI-1640 complete medium at 37°C for 14 days. The cell colonies
were washed with phosphate-buffered saline (PBS) twice, fixed with
methanol for 20 min, and stained with 0.1% crystal violet in PBS
(Beyotime Institute of Biotechnology) for 15 min. The plates were
scanned and colonies containing >50 cells were counted manually,
and the experiments were performed in triplicate.
Cell migration assay
A Transwell® migration assay was
performed as previously reported (22). In brief, cells (4×105
cells/ml) were seeded in serum-free RPMI-1640 or F12K medium in the
top chamber of a Transwell® insert. The medium
containing 20% FBS in the lower chamber served as a
chemoattractant. After incubation for 24 h at 37°C, the cells on
the top side of the membrane were removed with a cotton swab, and
those on the bottom side were fixed with methanol for 20 min and
then stained with crystal violet (0.1% in PBS) for 15 min. Six
randomly selected fields per well were photographed under a
microscope using the ImageScope software (Leica Biosystems Nussloch
GmbH, Nussloch, Germany) with a magnification of ×200, and the
numbers of migrated cells were counted manually.
Scratch wound healing assay
A monolayer scratch wound assay was employed as
previously described (23). Briefly,
cells (4×105 cells/well) were seeded in 12-well plates
and grown to nearly 100% confluence. A scratch wound was generated
with a 200-µl pipette tip. Wound closure was photographed at 0 and
48 h under a microscope using the ImageScope software (Leica
Biosystems Nussloch GmbH) with a magnification of ×40.
Statistical analysis
Statistical analyses were performed using Microsoft
Excel 2010 (Microsoft, Redmond, WA, USA), GraphPad Prism7 (GraphPad
Software Inc., San Diego, CA, USA), and SPSS statistical software
for Windows, version 22 (IBM Corp., Armonk, NY, USA). Paired
Student's t-tests were performed for continuous variables between
two groups and Wilcoxon signed-rank tests were used if the
differences between pairs of data did not follow a Gaussian
distribution. One-way analysis of variance (ANOVA) was performed
for statistical comparisons among multiple groups along with
Bonferroni's post hoc test. Pearson's χ2 test and
Fisher's exact test were used for categorical comparisons.
Pearson's R correlation test was used to describe correlations
between continuous variables. Survival analyses were conducted
using the Kaplan-Meier method and differences in survival were
examined using the log-rank test. Univariate and multivariate
survival analyses were conducted using the Cox proportional hazards
regression model. Statistical significance was defined as a value
of P<0.05. All statistical tests were two-sided.
Results
CPXM2 is upregulated in GCs
To explore whether CPXM2 is dysregulated in
GCs, we first measured CPXM2 expression in 15 GCs and their
matched (normal tissues) NTs by qPCR and western blotting. The
results indicated that CPXM2 mRNA and protein were elevated
in the GC cases compared with the NTs (Fig. 1A-D). We then examined CPXM2 expression
in primary GC tissues by IHC. As shown in Fig. 1E and F, CPXM2 was expressed mainly in
the cytoplasm and on the cytosolic side of the membrane, while its
expression was stronger in GCs than in NTs, as quantified by H
scores. To validate these observations further, we analyzed
CPXM2 expression in two GC datasets [Chen Gastric, n=111
(17); Derrico Gastric, n=69
(18)] with Lauren's classification
in the Oncomine database and found that CPXM2 was
unanimously overexpressed in each tumor subtype compared with NTs
(Fig. 1G and H). Taken together,
these results clearly showed that CPXM2 is upregulated in
GCs.
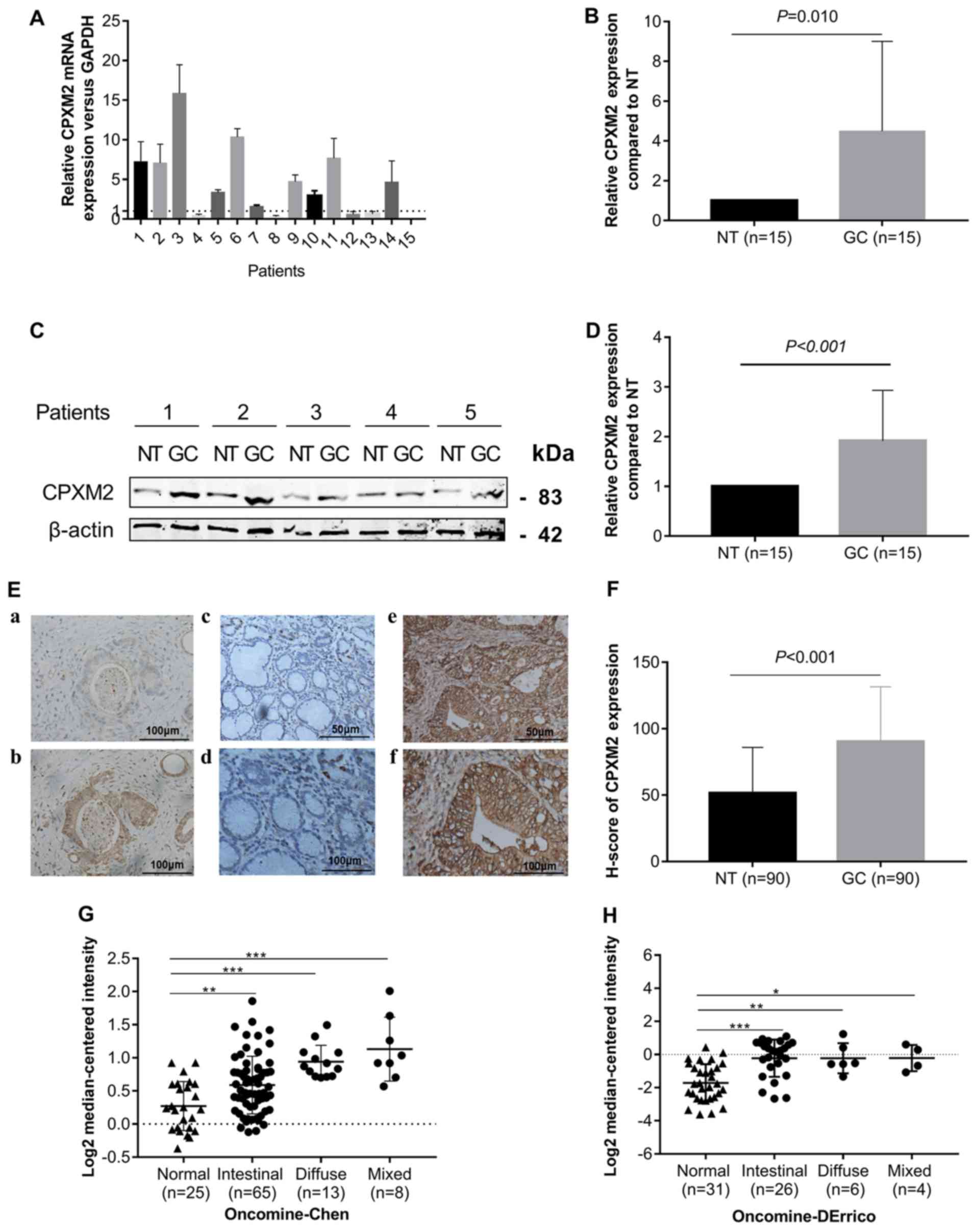 | Figure 1.CPXM2 is upregulated in
gastric cancers. (A and B) CPXM2 mRNA expression levels in
15 paired GCs and their adjacent NTs. (C and D) Representative
blots and quantification of CPXM2 protein expression levels from
samples in (A and B). The average CPXM2 expression was normalized
to the expression of β-actin. Three replicates were conducted for
each experiment. (E) Immunohistochemical staining of CPXM2 in GCs
and NTs. (a and b) IgG and CPXM2 staining in GCs (×400
magnification). (c-f) CPXM2 staining in NTs (c, ×200; d, ×400
magnification) and GCs (e, ×200; f, ×400 magnification). (F) H
scores of CPXM2 staining in NTs and GCs. (G and H) A logarithmic
2−ΔΔCq scale was used to represent the fold-changes in
CPXM2 mRNA expression in microarray datasets from the
Oncomine database: Chen gastric and Derrico gastric, grouped by
Lauren classification. *P<0.05, **P<0.01, ***P<0.001 vs.
the control group. CPXM2, carboxypeptidase X, M14 family
member 2; GC, gastric cancer; NT, normal tissue. |
Overexpression of CPXM2 in GCs
correlates with an unfavorable prognosis
To test whether CPXM2 overexpression in GCs
contributes to increased invasiveness, we analyzed the
relationships between CPXM2 expression and clinicopathological
variables. As shown in Table II,
high CPXM2 expression was significantly associated with larger
tumor size and later pTNM stages.
 | Table II.Association between CPXM2 expression
and clinicopathological variables in the gastric cancer cases
(n=90). |
Table II.
Association between CPXM2 expression
and clinicopathological variables in the gastric cancer cases
(n=90).
|
|
| CPXM2
expression |
|---|
|
|
|
|
|---|
| Clinicopathological
features | N | Low (n=45) | High (n=45) | P-value |
|---|
| Sex |
|
Male | 68 | 32 (47.1) | 36 (52.9) |
|
|
Female | 21 | 13 (61.9) | 8 (38.1) | 0.319 |
| Age (years) |
|
<70 | 45 | 26 (57.8) | 19 (42.2) |
|
|
≥70 | 45 | 19 (42.2) | 26 (57.8) | 0.206 |
| Histological
grade |
| G2 | 28 | 14 (50.0) | 14 (50.0) |
|
| G3 | 62 | 31 (50.0) | 31 (50.0) | >0.999 |
| Tumor size
(cm) |
|
<5 | 34 | 22 (64.7) | 12 (35.3) |
|
| ≥5 | 54 | 22 (40.7) | 32 (59.3) | 0.048 |
| pT stage |
|
T1/T2 | 29 | 12 (41.4) | 17 (58.6) |
|
|
T3/T4 | 60 | 32 (53.3) | 28 (46.7) | 0.367 |
| pN stage |
| N0 | 25 | 15 (60.0) | 10 (40.0) |
|
|
N1-N3 | 65 | 30 (46.2) | 35 (53.8) | 0.347 |
| pStage |
|
I/II | 42 | 27 (64.3) | 15 (35.7) |
|
|
III/IV | 48 | 18 (37.5) | 30 (62.5) | 0.020 |
| Vessel
invasion |
| No | 74 | 40 (54.1) | 34(45.9) |
|
|
Yes | 16 | 5 (31.3) | 11 (68.7) | 0.167 |
| Nerve invasion |
| No | 75 | 38 (50.7) | 37 (49.3) |
|
|
Yes | 15 | 7 (46.7) | 8 (53.3) | >0.999 |
We next determined the relationship between CPXM2
expression and patient outcomes. Kaplan-Meier survival analysis
revealed that patients with high CPXM2 expression had shorter
overall survival (OS) times than did those with low expression
[estimated mean OS, 30.7 months; 95% confidence interval (CI),
23.0–38.5 months vs. OS, 48.0 months; 95% CI, 39.0–56.9 months;
log-rank test, P=0.007; Fig. 2A]. In
multivariate analysis with a Cox proportional hazards model, CPXM2
overexpression was significantly associated with a shorter OS
[hazard ratio (HR), 1.92; 95% CI, 1.08–3.40; P=0.026], after
adjustment for age, tumor size, T stage, and N stage (Table III). To confirm the adverse
prognostic roles of CPXM2 in patients with GC, we downloaded
and analyzed CPXM2 transcription data from TCGA and the
Kaplan-Meier Plotter. The results showed that high CPXM2
expression was correlated with worse OS in both datasets (Fig. 2B and C). To eliminate potential
influences from confounding factors, we stratified patients in the
Kaplan-Meier Plotter dataset by Lauren classification and TNM
staging. As indicated by Fig. 2D-G,
high CPXM2 expression was consistently associated with
unfavorable OS in GC patients, regardless of the Lauren
classification and TNM staging. In addition, we explored the
potential effects of CPXM2 expression on recurrence-free
survival (RFS) or disease-free survival (DFS) in GC patients. The
results demonstrated that high CPXM2 expression was also
associated with tumor relapse (Fig. 2H
and I). Taken together, these findings indicate that
CPXM2 is a prognostic marker in GCs and correlates with an
unfavorable prognosis.
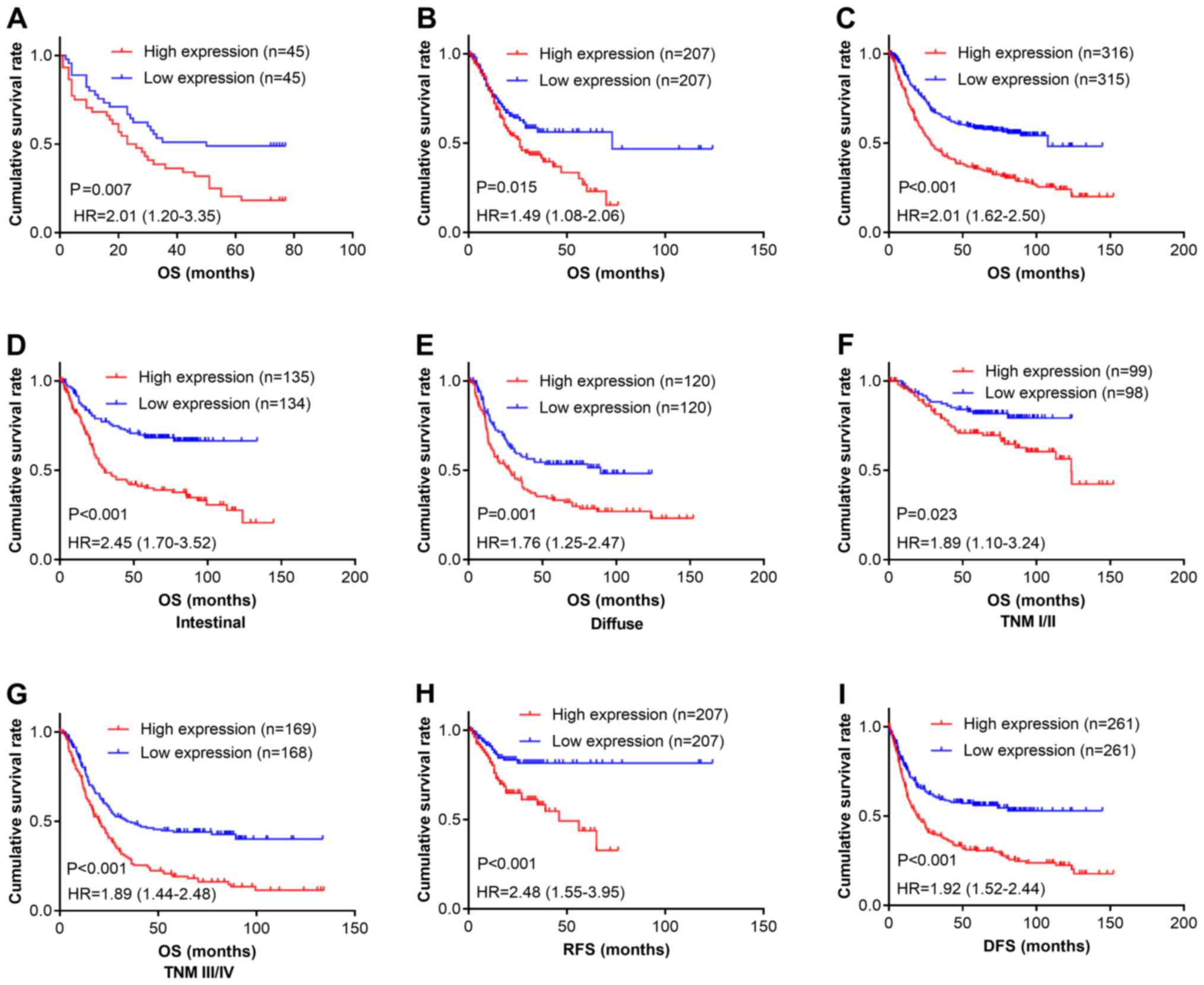 | Figure 2.CPXM2 overexpression in GCs is
associated with poor patient survival. (A-C) Kaplan-Meier plots for
OS of patients in the TMA cohort (A), TCGA cohort (B) and
Kaplan-Meier Plotter cohort (C). (D-G) Kaplan-Meier plots for OS of
patients with intestinal type (D), diffuse type (E), early stage
(F), and late stage (G) GC in the Kaplan-Meier Plotter cohort. (H
and I) Kaplan-Meier plots for RFS of patients in the TCGA cohort
(H) and Kaplan-Meier Plotter cohort (I). Patients were stratified
into low and high CPXM2 expression groups according to
CPXM2 mRNA expression (< median vs. ≥ median) in the TCGA
and Kaplan-Meier Plotter cohorts, or H scores of CPXM2 staining
(< median vs. ≥ median) in the TMA cohort. P-values were
obtained using the log-rank test. Censored data are indicated by
the + symbol. CPXM2, carboxypeptidase X, M14 family member
2; DFS, disease-free survival; GC, gastric cancer; HR, hazard
ratio; NT, adjacent normal tissue; OS, overall survival; RFS,
recurrence-free survival; TCGA, The Cancer Genome Atlas; TMA,
tissue microarray; TNM, tumor node metastasis. |
 | Table III.Univariate and multivariate Cox
proportional hazard models for overall survival of the GC patients
(n=90). |
Table III.
Univariate and multivariate Cox
proportional hazard models for overall survival of the GC patients
(n=90).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathological
features | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
|
Male | 1 (Reference) |
|
|
|
|
Female | 0.80
(0.42–1.51) | 0.487 |
|
|
| Age (years) |
|
<70 | 1 (Reference) |
| 1 (Reference) |
|
|
≥70 | 1.82
(1.08–3.05) | 0.024 | 1.23
(0.69–2.18) | 0.487 |
| Histological
grade |
| G2 | 1 (Reference) |
|
|
|
| G3 | 1.45
(0.83–2.55) | 0.194 |
|
|
| Tumor size
(cm) |
|
<5 | 1 (Reference) |
| 1 (Reference) |
|
| ≥5 | 3.68
(1.97–6.87) |
<0.001 | 2.29
(1.15–4.55) | 0.018 |
| pT stage |
|
T1/T2 | 1 (Reference) |
| 1 (Reference) |
|
|
T3/T4 | 1.74
(0.98–3.10) | 0.060 | 1.52
(0.79–2.94) | 0.208 |
| pN stage |
| N0 | 1 (Reference) |
| 1 (Reference) |
|
|
N1-N3 | 2.78
(1.40–5.50) | 0.003 | 2.20
(1.01–4.80) | 0.048 |
| pStage |
|
I/II | 1 (Reference) |
|
|
|
|
III/IV | 3.38
(1.94–5.88) |
<0.001 |
|
|
| Vessel
invasion |
| No | 1 (Reference) |
|
|
|
|
Yes | 1.55
(0.82–2.92) | 0.176 |
|
|
| Nerve invasion |
| No | 1 (Reference) |
|
|
|
|
Yes | 1.56
(0.81–3.00) | 0.185 |
|
|
| CPXM2
expression |
|
Low | 1 (Reference) |
| 1 (Reference) |
|
|
High | 2.07
(1.23–3.48) | 0.006 | 1.92
(1.08–3.40) | 0.026 |
CPXM2 promotes the proliferation and
migration of cultured GC cells
To illustrate further the potential role of
CPXM2 in GC progression, we first used qRT-PCR and western
blotting to examine the intrinsic expression of CPXM2 in a
gastric epithelial cell line, GES-1, and five GC cell lines. The
results indicated that CPXM2 expression was significantly
increased in GC cells relative to GES-1 cells (Fig. 3A and B). We then knocked down
CPXM2 expression in AGS and HGC-27 cells with lentiviruses
carrying CPXM2-specific shRNAs (Fig. 3C
and D), and selected shCPXM2-2 and shCPXM2-3 for subsequent
in vitro experiments. In cultured GC cells, knockdown of
CPXM2 expression significantly inhibited cell proliferation,
as indicated by the CCK-8 and colony formation assays (Fig. 4A-D). In addition, knockdown of
CPXM2 expression significantly decreased cell migration in
the scratch wound-healing and Transwell® migration
assays (Fig. 4E-H). Collectively,
these results clearly indicate that CPXM2 promoted the
proliferation and migration of GC cells.
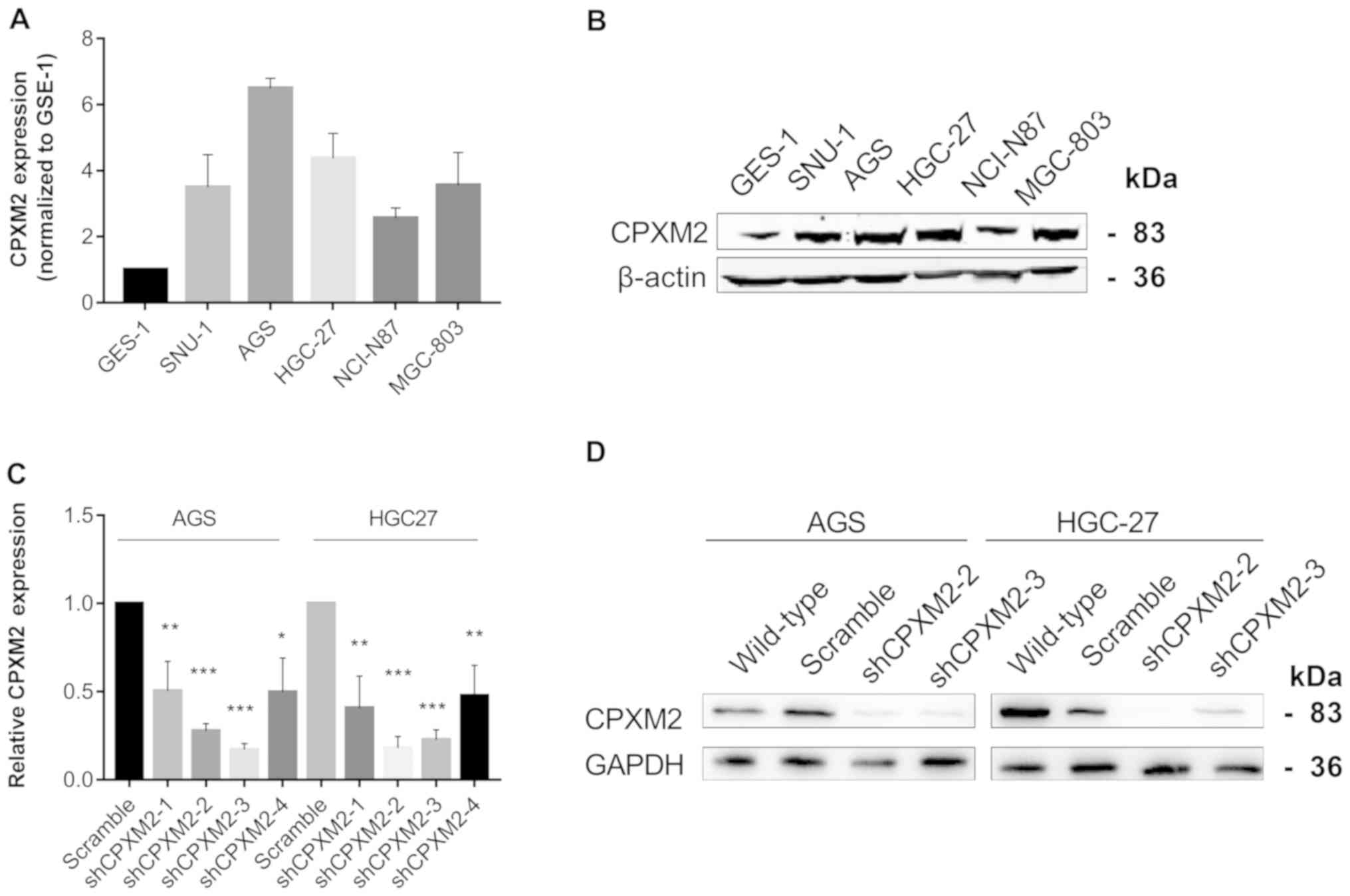 | Figure 3.Silencing of CPXM2 in GC cell
lines. (A and B) Expression of CPXM2 in a gastric mucosa
cell line (GES-1) and five GC cell lines. (C) AGS and HGC-27 cells
were infected with lentiviruses carrying shCPXM2-1, shCPXM2-2,
shCPXM2-3, shCPXM2-4, or scrambled control shRNA, and then
underwent quantitative reverse transcription polymerase chain
reaction. (D) AGS and HGC-27 cells were infected with lentiviruses
carrying shCPXM2-2, shCPXM2-3, or scrambled control shRNA, and then
underwent western blotting validation. *P<0.05, **P<0.01,
***P<0.001 vs. the control group. CPXM2, carboxypeptidase
X, M14 family member 2; GC, gastric cancer. |
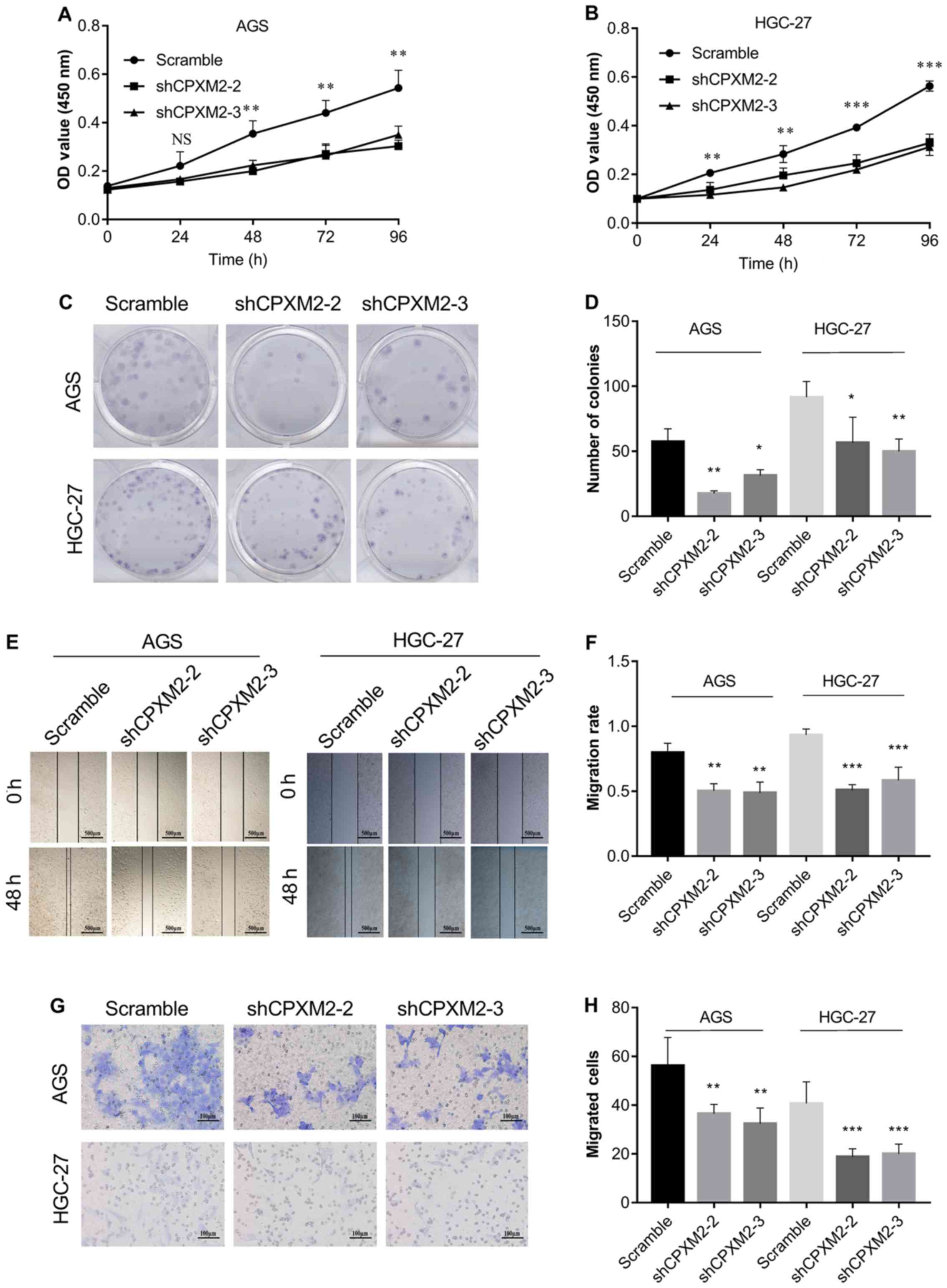 | Figure 4.In vitro activities of
CPXM2 in GC cells. AGS and HGC-27 cells were infected with
lentiviruses carrying shCPXM2-2, shCPXM2-3, or scrambled control
shRNA, and then underwent CCK-8 assay (A and B), colony formation
assay (C and D), scratch wound-healing assay (E and F), and
Transwell® migration assay (G and H). CPXM2
silencing in these cells significantly inhibited cell proliferation
and migration. Three replicates were conducted for each experiment.
*P<0.05, **P<0.01, ***P<0.001 vs. the control group.
CCK-8, Cell Counting Kit-8; OD, optical density; CPXM2,
carboxypeptidase X, M14 family member 2; GC, gastric cancer. |
CPXM2 may promote GC progression by
modulating EMT
To characterize the potential mechanism of
CPXM2 in promoting tumor progression, we used the RNA-seq
data for GCs from TCGA to conduct a gene set enrichment analysis
(GSEA) (18). We found that
CPXM2 expression was positively correlated with the
HALLMARK_APICAL_JUNCTION and
HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION gene sets (Fig. 5A). In addition, CPXM2 was
significantly correlated with key genes involved in EMT (Fig. 5B). Furthermore, the epithelial marker
E-cadherin was increased while the mesenchymal markers N-cadherin
and vimentin and EMT-related transcription factor ZEB1 were
decreased, after CPXM2 was silenced in GC cells (Fig. 5C), Taken together, these results imply
that CPXM2 plays an active role in promoting GC tumor
aggressiveness via EMT modulation.
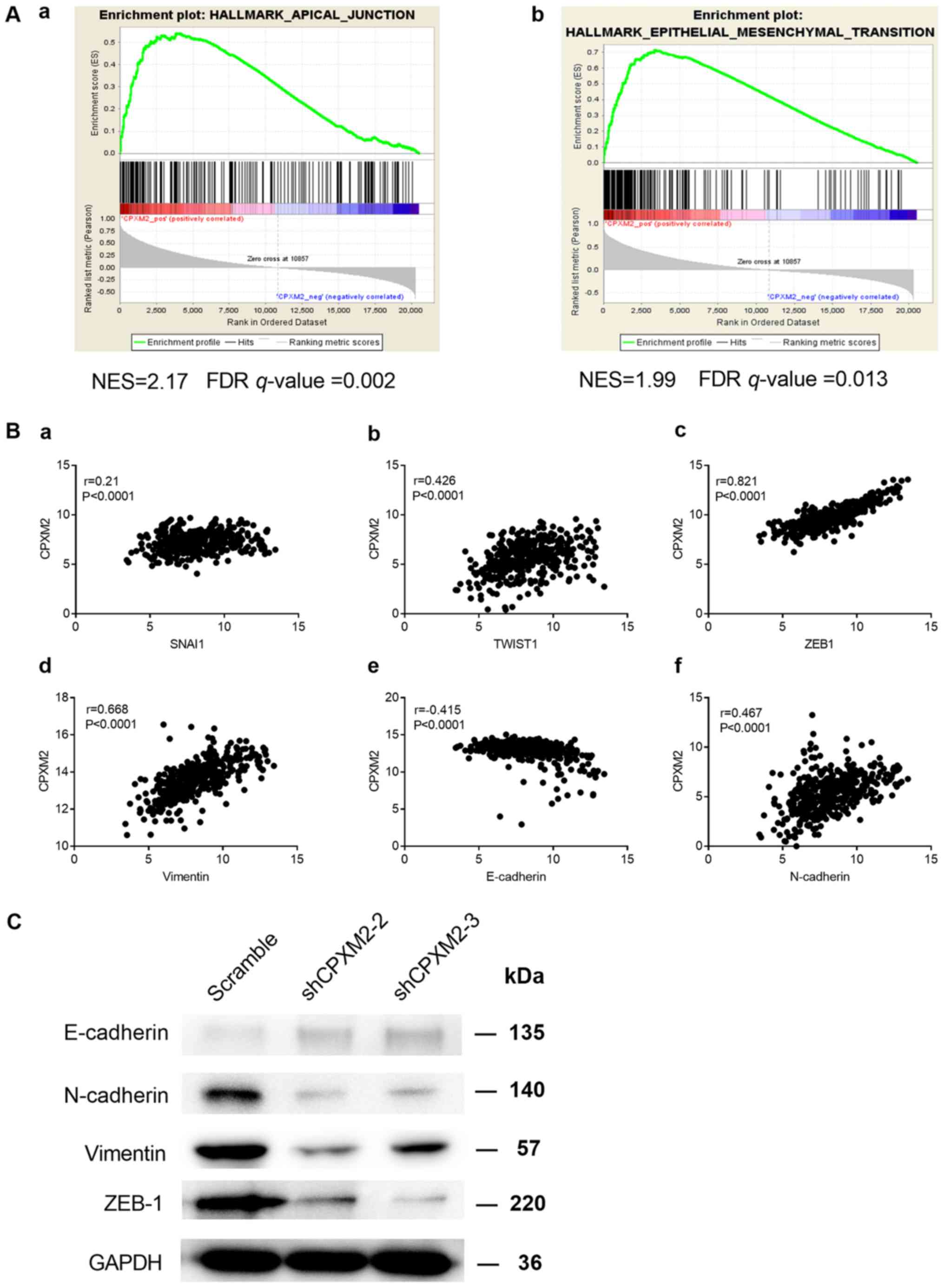 | Figure 5.Potential involvement of CPXM2
in the regulation of EMT. (A) GSEA enrichment plots indicate that
CPXM2 expression was positively correlated with apical
junction and EMT gene signatures. High CPXM2 expression in
GC patients was positively correlated with the (a)
HALLMARK_APICAL_JUNCTION and (b)
HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION gene sets. (B)
CPXM2 was significantly correlated with several key genes
associated with EMT: (a) SNAI1, (b) TWIST1, (c)
ZEB1, (d) vimentin, (e) E-cadherin and (f) N-cadherin. (C)
Western blotting analysis was used to compare expression of
epithelial and mesenchymal markers between GC cells infected with
shCPXM2 or scramble. GAPDH was used as loading control.
CPXM2, carboxypeptidase X, M14 family member 2; FDR, false
discovery rate; GC, gastric cancer; EMT, epithelial to mesenchymal
transition; GAPDH, glyceraldehyde-3-phosphate dehydrogenase;
GSEA, gene set enrichment analysis; NES, normalized enrichment
score. |
Discussion
Our knowledge concerning carboxypeptidase X, M14
family member 2 (CPXM2) is still quite limited. Most studies
have related CPXM2 to developmental diseases, mental
disorders, and neurodegenerative diseases (12–15). By
analyzing differentially expressed genes in dermal fibroblasts from
patients with Apert syndrome and controls, Çetinkaya et al
showed CPXM2 to be a gene with Gene Ontology terms
associated with extracellular matrix organization, which may
regulate early differentiation of connective tissues (12). Using both growth-restricted and
normal-term placentas, Sabri et al recognized CPXM2
as one of the most upregulated genes in fetal growth restriction.
Using bioinformatic analysis, these authors also suggested a
potential connection between fetal growth restriction and
gastrointestinal diseases (13).
There are at least 26 metallocarboxypeptidases (MCPs), several of
which have already been identified as potential tumor biomarkers
(6,9–11,24). However, the underlying mechanism to
explain the roles of these MCPs in modulating oncogenesis and tumor
progression is still lacking.
In the present study, we showed that CPXM2 is
a candidate tumor biomarker for gastric cancer (GC) (Fig. 6), regardless of the Lauren
classification or TNM stage. Therefore, targeting CPXM2 may
be a possible solution to impede GC progression. In fact, several
endogenous and synthesized carboxypeptidase inhibitors have been
reported, although their applications as anticancer reagents are
only at a preliminary stage (6,8). Latexin,
known for its activity as an endogenous carboxypeptidase inhibitor,
is dysregulated and exhibits tumor suppressor potential in several
malignancies (25–27). However, analysis of microarray data
comparing latexin-overexpressing MGC-803 GC cells with control
cells from GEO (GSE15787) (25) found
no significant association between latexin and CPXM2
expression (fold change=1.0). Thus, there may be other endogenous
inhibitors of CPXM2 that control its enzymatic activity.
We also showed that CPXM2 may function by
modulating epithelial to mesenchymal transition (EMT). As EMT is a
critical process that mediates tumor progression and metastasis
(28), the present study provides
further evidence that CPXM2 is closely associated with tumor
progression in GCs. Considering the catalytical activity of
CPXM2, we postulate that CPXM2 may catalyze key
molecules that regulate EMT in GCs. Several other MCPs also induce
tumor progression and metastasis. For example, CPM is one of
the most well-characterized carboxypeptidases. By modulating key
molecules such as kinins and chemokines, CPM affects
proliferation, angiogenesis, and metastasis in a number of
malignancies (11). CPE is
another MCP that has been extensively investigated. Both CPE
and its splice variant have been proposed as prognostic biomarkers
in a variety of tumors, while the latter promotes tumor invasion
and metastasis (10). Sun et
al demonstrated that CPA4 was associated with tumor
invasion and metastasis in a cohort of GC patients. Using
correlation analyses, these authors revealed a possible interaction
of CPA4 with p53 and Ki-67, which are closely
associated with tumor progression (9). Although the tumor-promoting roles of
some MCPs have been identified, the underlying mechanisms are
largely unknown. We provide a new mechanistic understanding of the
effects of these MCPs that centers on their potential modulation of
EMT.
To the best of our knowledge, this study is the
first to investigate the prognostic value and molecular function of
CPXM2 in cancers. However, there are several unanswered
questions in this study. First, as mentioned above, we do not know
whether there are any endogenous inhibitors of CPXM2. Second, we do
not know the substrates of CPXM2. Third, further studies are needed
to confirm whether CPXM2 can be used as a serum prognostic
biomarker for GC patients.
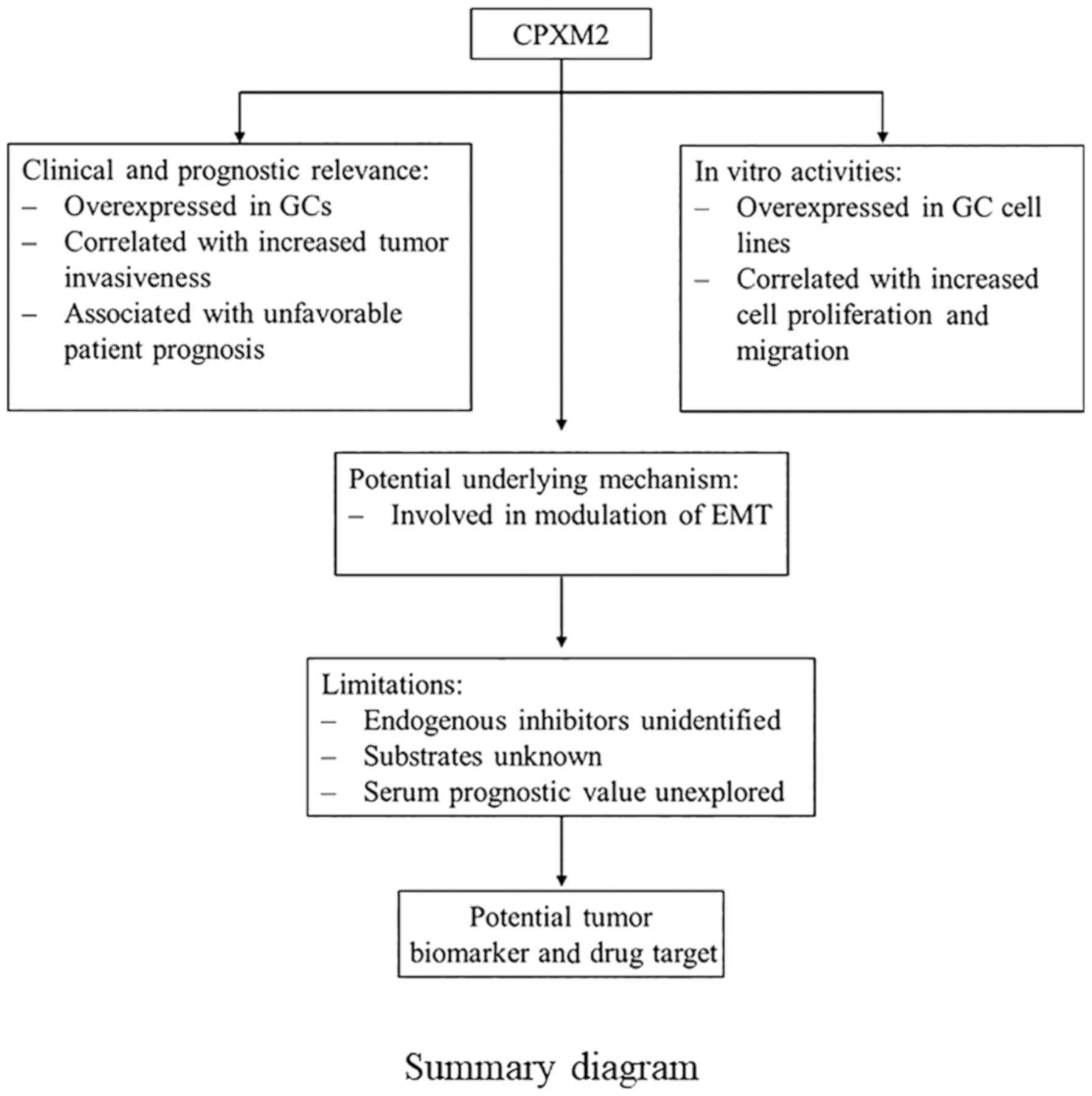
In summary, we identified CPXM2 as a novel
prognostic marker for GC. It was found to accelerate tumor
progression by promoting GC cell proliferation and migration via
modulation of EMT-associated central pathways, indicating that a
detailed study of this putative marker is warranted.
Acknowledgements
We greatly appreciate the technological help from
the Department of Pathology of our hospital for the IHC staining
and data analysis. We also appreciate the valuable work carried out
by Dr Jun Hou at Zhongshan Hospital (Shanghai, China) for her
interpretation of the IHC staining. We would like to thank Dr Lijie
Ma for his work in packaging the lentiviral particles.
Funding
The present study was supported by the Shanghai
Fifth People's Hospital (grant nos. 2016WYRC01 and 2017WYRCSG01);
the Medical System of Shanghai Minhang District (grant no.
2017MWDXK01); the Shanghai Minhang District Health and Family
Planning Commission (grant no. 2016MW03); and the Shanghai Minhang
District Science and Technology Commission (grant no. 2017MHZ02).
The funding sources were not involved in the study design; in the
collection, analysis, or interpretation of data; in the writing of
the report; or in the decision to submit the article for
publication.
Availability of data and materials
The datasets supporting the conclusions of this
article are included within this article and its additional images.
Raw data are available from the corresponding author on reasonable
request.
Authors' contributions
CK designed the study. GN, XW, YY, JR, TS, ZH, LC,
JX and RH performed the experiments and analyzed the data. XW, YY
and GN wrote the manuscript. CK and JR helped to revise the
manuscript. All authors read and approved the final manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
This study was approved by the Institutional Ethics
Committee of the Fifth People's Hospital of Shanghai, Fudan
University (Ethical approval form no. 2017-097) and adhered to the
principles in the Declaration of Helsinki. Informed consent was
obtained from each patient before tissue collection for
experimentation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malvezzi M, Bonifazi M, Bertuccio P, Levi
F, La Vecchia C, Decarli A and Negri E: An age-period-cohort
analysis of gastric cancer mortality from 1950 to 2007 in Europe.
Ann Epidemiol. 20:898–905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernandez D, Pallares I, Vendrell J and
Aviles FX: Progress in metallocarboxypeptidases and their small
molecular weight inhibitors. Biochimie. 92:1484–1500. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gomis-Rüth FX: Structure and mechanism of
metallocarboxypeptidases. Crit Rev Biochem Mol Biol. 43:319–345.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fernandez D, Pallares I, Covaleda G,
Aviles FX and Vendrell J: Metallocarboxypeptidases and their
inhibitors: Recent developments in biomedically relevant protein
and organic ligands. Curr Med Chem. 20:1595–1608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun L, Guo C, Yuan H, Burnett J, Pan J,
Yang Z, Ran Y, Myers I and Sun D: Overexpression of
carboxypeptidase A4 (CPA4) is associated with poor prognosis in
patients with gastric cancer. Am J Transl Res. 8:5071–5075.
2016.PubMed/NCBI
|
|
10
|
Cawley NX, Wetsel WC, Murthy SR, Park JJ,
Pacak K and Loh YP: New roles of carboxypeptidase E in endocrine
and neural function and cancer. Endocr Rev. 33:216–253. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Denis CJ and Lambeir AM: The potential of
carboxypeptidase M as a therapeutic target in cancer. Expert Opin
Ther Targets. 17:265–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Çetinkaya A, Taskiran E, Soyer T,
Şimşek-Kiper PÖ, Utine GE, Tuncbilek G, Boduroğlu K and
Alikaşifoğlu M: Dermal fibroblast transcriptome indicates
contribution of WNT signaling pathways in the pathogenesis of Apert
syndrome. Turk J Pediatr. 59:619–624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sabri A, Lai D, D'Silva A, Seeho S, Kaur
J, Ng C and Hyett J: Differential placental gene expression in term
pregnancies affected by fetal growth restriction and macrosomia.
Fetal Diagn Ther. 36:173–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YC, Hsiao CJ, Jung CC, Hu HH, Chen
JH, Lee WC, Chiou JM, Chen TF, Sun Y, Wen LL, et al: Performance
metrics for selecting single nucleotide polymorphisms in Late-onset
Alzheimer's disease. Sci Rep. 6:361552016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hashimoto R, Ikeda M, Ohi K, Yasuda Y,
Yamamori H, Fukumoto M, Umeda-Yano S, Dickinson D, Aleksic B, Iwase
M, et al: Genome-wide association study of cognitive decline in
schizophrenia. Am J Psychiatry. 170:683–684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Howitt BE, Sun HH, Roemer MG, Kelley A,
Chapuy B, Aviki E, Pak C, Connelly C, Gjini E, Shi Y, et al:
Genetic basis for PD-L1 expression in squamous cell carcinomas of
the cervix and vulva. JAMA Oncol. 2:518–522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Leung SY, Yuen ST, Chu KM, Ji J,
Li R, Chan AS, Law S, Troyanskaya OG, Wong J, et al: Variation in
gene expression patterns in human gastric cancers. Mol Biol Cell.
14:3208–3215. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
D'Errico M, de Rinaldis E, Blasi MF, Viti
V, Falchetti M, Calcagnile A, Sera F, Saieva C, Ottini L, Palli D,
et al: Genome-wide expression profile of sporadic gastric cancers
with microsatellite instability. Eur J Cancer. 45:461–469. 2009.
View Article : Google Scholar
|
|
19
|
Szasz AM, Lanczky A, Nagy A, Forster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A and Győrffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget.
7:49322–49333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi JY, Ma LJ, Zhang JW, Duan M, Ding ZB,
Yang LX, Cao Y, Zhou J, Fan J, Zhang X, et al: FOXP3 Is a HCC
suppressor gene and Acts through regulating the TGF-β/Smad2/3
signaling pathway. BMC Cancer. 17:6482017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao S, Zhou L, Niu G, Li Y, Zhao D and
Zeng H: Differential regulation of orphan nuclear receptor TR3
transcript variants by novel vascular growth factor signaling
pathways. FASEB J. 28:4524–4533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu HM, Chen Y, Liu L, Zhang CG, Wang W,
Gong K, Huang Z, Guo MX, Li WX and Li W: C1orf61 acts as a tumor
activator in human hepatocellular carcinoma and is associated with
tumorigenesis and metastasis. FASEB J. 27:163–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niu G, Ye T, Qin L, Bourbon PM, Chang C,
Zhao S, Li Y, Zhou L, Cui P, Rabinovitz I, et al: Orphan nuclear
receptor TR3/Nur77 improves wound healing by upregulating the
expression of integrin β4. FASEB J. 29:131–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kos J, Vižin T, Fonović UP and Pišlar A:
Intracellular signaling by cathepsin X: Molecular mechanisms and
diagnostic and therapeutic opportunities in cancer. Semin Cancer
Biol. 31:76–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Basang Z, Ding H, Lu Z, Ning T, Wei
H, Cai H and Ke Y: Latexin expression is downregulated in human
gastric carcinomas and exhibits tumor suppressor potential. BMC
Cancer. 11:1212011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue Z, Zhou Y, Wang C, Zheng J, Zhang P,
Zhou L, Wu L, Shan Y, Ye M, He Y and Cai Z: Latexin exhibits
tumor-suppressor potential in pancreatic ductal adenocarcinoma.
Oncol Rep. 35:50–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ni QF, Tian Y, Kong LL, Lu YT, Ding WZ and
Kong LB: Latexin exhibits tumor suppressor potential in
hepatocellular carcinoma. Oncol Rep. 31:1364–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|