Introduction
Acute lung injury (ALI) and acute respiratory
distress syndrome (ARDS) are characterized by rapid pulmonary
inflammation and ARDS is considered the most severe form of ALI
(1). Pro-inflammatory cytokines
(TNF-α, IL-6 and IL-1β) have been identified as biomarkers of
ALI/ARDS (2,3). Neutrophil-derived reactive oxygen
species (ROS) promote lung injury in the pathogenesis of ALI/ARDS
(4) and increased ROS levels
indicate neutrophil activation. Furthermore, macrophage-derived
monocyte chemoattractant protein-1 (MCP-1) accelerates pulmonary
inflammation by inducing neutrophil/macrophage recruitment
(5). Lipopolysaccharide (LPS) is
used to induce ALI/ARDS in mouse models (6–8),
which show significant upregulation of inflammatory cytokines and
chemokines (TNF-α, IL-6, IL-1β and MCP-1). In addition, an increase
in inducible nitric oxide synthase (iNOS) protein levels in the
lung has been reported in clinical and pre-clinical studies of
ALI/ARDS (9–11).
In ALI/ARDS pathogenesis, inflammatory cytokines,
chemokines and mediators are expressed following NF-κB activation
(5,12). Mitogen-activated protein kinase
(MAPK) activation serves an important role in LPS-stimulated
inflammatory response (13,14). Cumulative evidence suggests that
an increase in the expression of heme oxygenase-1 (HO-1) relieves
inflammatory response in ALI and ARDS (15,16).
Methyl p-hydroxycinnamate (MH) is an esterified
derivative of p-Coumaric acid that has been shown to possess
anti-lipid peroxidation activity (17). Previously, the anti-inflammatory
properties of MH were confirmed in LPS-stimulated RAW264.7
macrophages (18); MH strongly
inhibited the production of TNF-α and IL-1β, which serve as
clinical predictors in ALI/ARDS (1,5).
Based on these previous results, MH may exert ameliorative effects
on ARDS. Therefore, the present study aimed to investigate the
potential protective role of MH against pulmonary inflammation in a
mouse model of LPS-induced ARDS.
Materials and methods
Cell culture
RAW264.7 cells were purchased from American Type
Culture Collection and maintained in DMEM supplemented with 10% FBS
and 1% antibiotic/antimycotic solution (all Invitrogen; Thermo
Fisher Scientific, Inc.) in 5% CO2 (37°C). To detect
HO-1 expression, cells were seeded into 6-well plates
(5×105 cells/well) and incubated at 37°C with 5, 10 and
20 µM MH (SynQuest Laboratories, Inc.) for 16 h.
Mouse model of LPS-induced ALI
Male C57BL/6 mice (n=24; age, 6 weeks; weight, 18–20
g) were purchased from Koatech Co., Ltd. and were housed in
specific-pathogen-free cages (22–23°C; 55–60% humidity; 12 h
light/dark cycle) with free access to food and water. The animal
experimental procedures were approved by the Institutional Animal
Care and Use Committee of the Korea Research Institute of
Bioscience and Biotechnology (Ochang, Korea). To induce ARDS in
mice, the dosage of LPS was selected based on previous studies
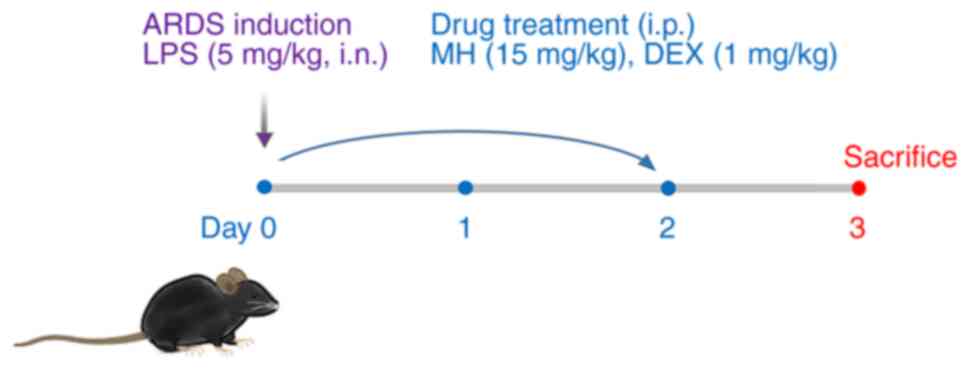
(5,8). In brief, mice were intranasally
exposed to LPS (5 mg/kg, 40 µl) on day 0. At 1 h after LPS
administration, MH and dexamethasone (DEX; positive control) were
intraperitoneally administered on days 0–2. The mice were randomly
divided into four groups (n=6/group) as follows: i) Normal control
(NC; treated with normal saline, i.p.); ii) ARDS (LPS); iii) DEX
(LPS + 1 mg/kg DEX, i.p.) and iv) MH 15 (LPS + 15 mg/kg MH, i.p.).
DEX and MH were dissolved with 1% DMSO and 1% Tween-20 in PBS.
Inflammatory cell count and ELISA
To evaluate the number of inflammatory cells and
levels of inflammatory molecules in bronchoalveolar lavage fluid
(BALF), BALF was collected as previously described (19). The mice were anesthetized with
Zoletil 50 (30–50 mg/kg i.p.; Virbac) and xylazine (5–10 mg/kg
i.p.; Bayer-Korea, Ltd.) on day 3, as previously described
(19). Tracheal PBS perfusion was
performed 24 h after the final intraperitoneal treatment of MH and
DEX, and BALF was collected from all groups. Then, BALF cells were
mounted on glass slides, stained with Diff-Quik®
solution at room temperature for 30 sec (Sysmex Co., Ltd.) to
distinguish the cells according to morphology and then manually
counted under a light microscope (magnification, ×400). The levels
of TNF-α, IL-6 and IL-1β in BALF were determined using ELISA kits
according to the manufacturer's instructions (cat. nos. 558534,
555240 and 559603, respectively; all BD Biosciences, Inc.). To
evaluate the level of intracellular ROS activity, BALF cells were
maintained with 20 µM 2′,7′-dichloroflurorescein diacetate
(Sigma-Aldrich; Merck KGaA) for 10 min at 37°C as previously
described (20). Then,
fluorescence was detected using a plate reader (wavelength, 488 nm
excitation and 525 nm emission). The levels of total protein
content in BALF were determined using a BCA assay kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
protocol.
Lung wet/dry (W/D) ratio
The degree of edema was determined as the ratio of
lung W/D weight. Briefly, lungs were collected from the mice,
washed with PBS to remove blood and weighed with electronic balance
(wet weight). Then, the lungs were dried at 60°C for 48 h in an
incubator before measuring dry weight. The calculation of W/D ratio
was determined to evaluate the lung edema as previously described
(13).
Western blotting
Following the collection of BALF, the mice were
sacrificed by cervical dislocation as previously described
(21). Lysate of mouse lung and
RAW264.7 whole cells were prepared using CelLytic™ MT Cell lysis
buffer (cat. no. c3228; SigmaAldrich; Merck KGaA) in the presence
of protease and phosphatase inhibitor cocktail as previously
described (19) and protein
quantification was performed using BCA assay. Then, the protein (50
µg/lane) was separated with 10–12% SDS-PAGE and transferred to PVDF
membranes. Each membrane was blocked at room temperature with 5%
skimmed milk in 0.3% (TBST for 1 h and incubated at 4°C for 16 h
with primary anti-phosphorylated (p)-p38 (cat. no. 9211; 1:1,000;
Cell Signaling Technology, Inc.), anti-p-IκBα (cat. no. 2859;
1:1,000; Cell Signaling Technology, Inc.), anti-p-p65 (cat. no.
3033; 1:1,000; Cell Signaling Technology, Inc.), anti-p65 (cat. no.
8242; 1:1,000; Cell Signaling Technology, Inc.), anti-β-actin (cat.
no. sc-69879; 1:2,000; Santa Cruz Biotechnology, Inc.), anti-p38
(cat. no. sc-7149; 1:1,000; Santa Cruz Biotechnology, Inc.),
anti-CD68 (cat. no. sc-20060; 1:1,000; Santa Cruz Biotechnology,
Inc.), anti-p-HO-1 (cat. no. 27338; 1:1,000; Invitrogen; Thermo
Fisher Scientific, Inc.) and anti-IκBα (cat. no. 15132; 1:1,000;
Invitrogen; Thermo Fisher Scientific, Inc.), Then, membranes were
washed at room temperature with TBST and incubated with horseradish
peroxidase-conjugated secondary antibodies (goat antimouse &
anti-rabbit; both 1:2,000; cat. nos. 115-035-003 and 111-035-003,
respectively; both Jackson ImmunoResearch Laboratories, Inc.) at
room temperature for 1 h. Finally, each membrane was developed with
Clarity™ Western ECL Substrate (cat. no. 170–5061; Bio-Rad
Laboratories Inc.). Protein bands were visualized using an
ImageQuant LAS 4000 mini luminescent Image Analyzer (GE Healthcare,
Inc) and the levels of protein expression were quantified using
ImageJ software (version 1.50e; National Institutes of Health).
β-actin was used as the loading control.
Histological examination
To confirm histological changes, lung tissue was
isolated from mice and fixed at room temperature for 48 h with 10%
formalin. Then, the lungs were embedded in paraffin, cut into 4-µm
sections using a microtome and stained with hematoxylin and eosin
(H&E) at room temperature for 5 and 1 min, respectively. The
levels of inflammatory cells in the airway were quantitatively
measured using ImageJ software (version 1.50e; National Institutes
of Health).
Statistical analysis
Data are presented as the mean ± standard deviation
of ≥3 independent experiments. One-way ANOVA followed by Tukey's
multiple comparison test was performed to analyze differences using
SPSS 20.0 software (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of MH on neutrophil/macrophage
recruitment in LPS-induced ARDS mice
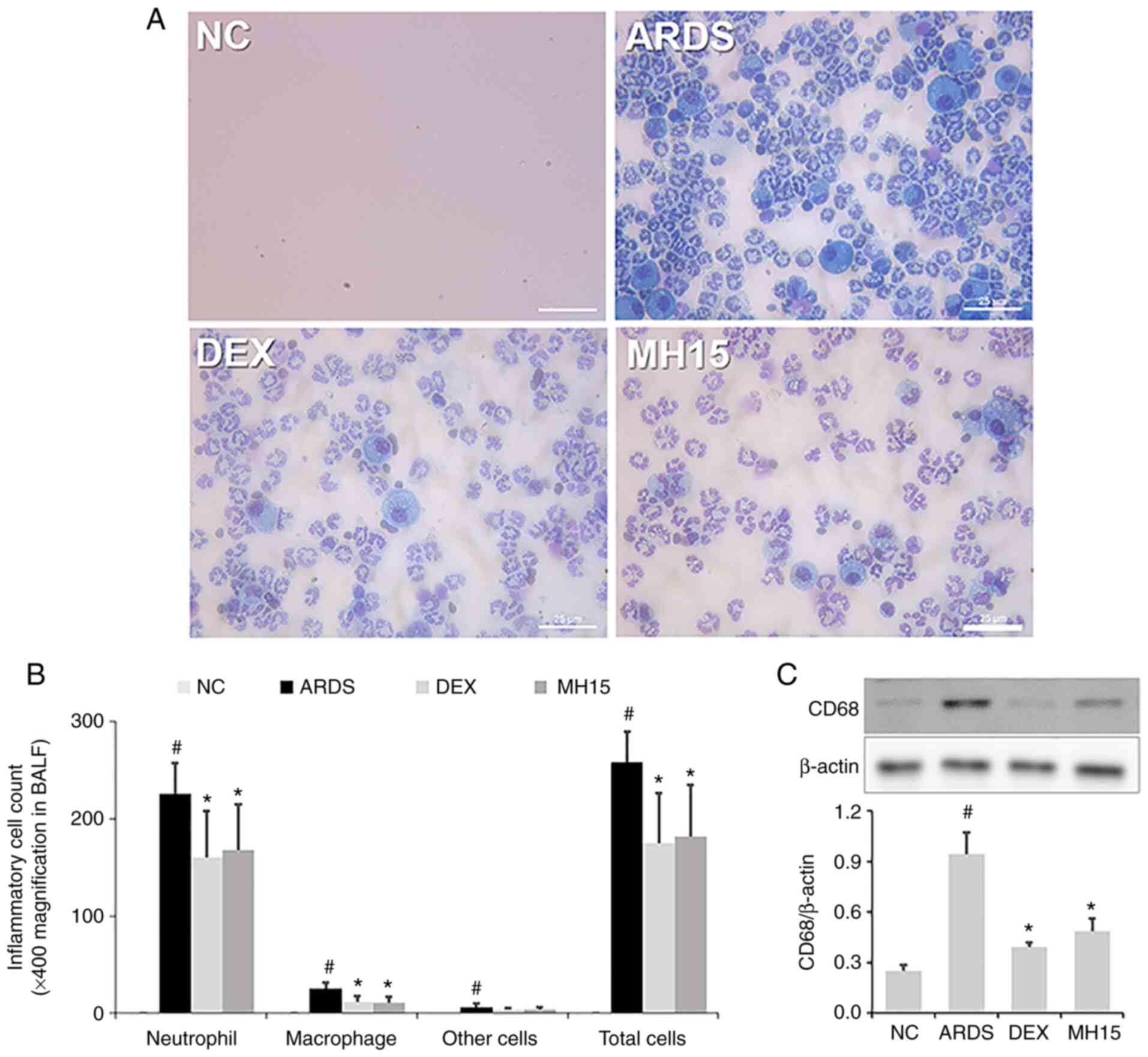
According to the procedure in Fig. 1, the protective effect of MH on
LPS-induced ARDS in mice was assessed. The inhibitory effect of MH
on neutrophil recruitment was investigated; a significant increase
in neutrophil numbers was confirmed in the BALF of LPS-induced ARDS
mice (Fig. 2A and B). This
increase was lower in BALF of the MH15 group compared with the ARDS
group. Similar to its effects on neutrophils, MH treatment also
suppressed the increase in numbers of macrophages in the BALF of
ARDS mice. In addition, western blotting indicated that the
expression of CD68, which is a marker of pro-inflammatory M1
macrophages, was significantly increased in lungs of LPS-induced
ARDS mice (Fig. 2C). However, MH
treatment decreased this increase in lungs of ARDS mice. The
inhibitory effects of 15 mg/kg MH were similar to those of 1 mg/kg
DEX, which was used as a positive control.
Effects of MH on inflammatory molecule
levels in LPS-induced ARDS mice
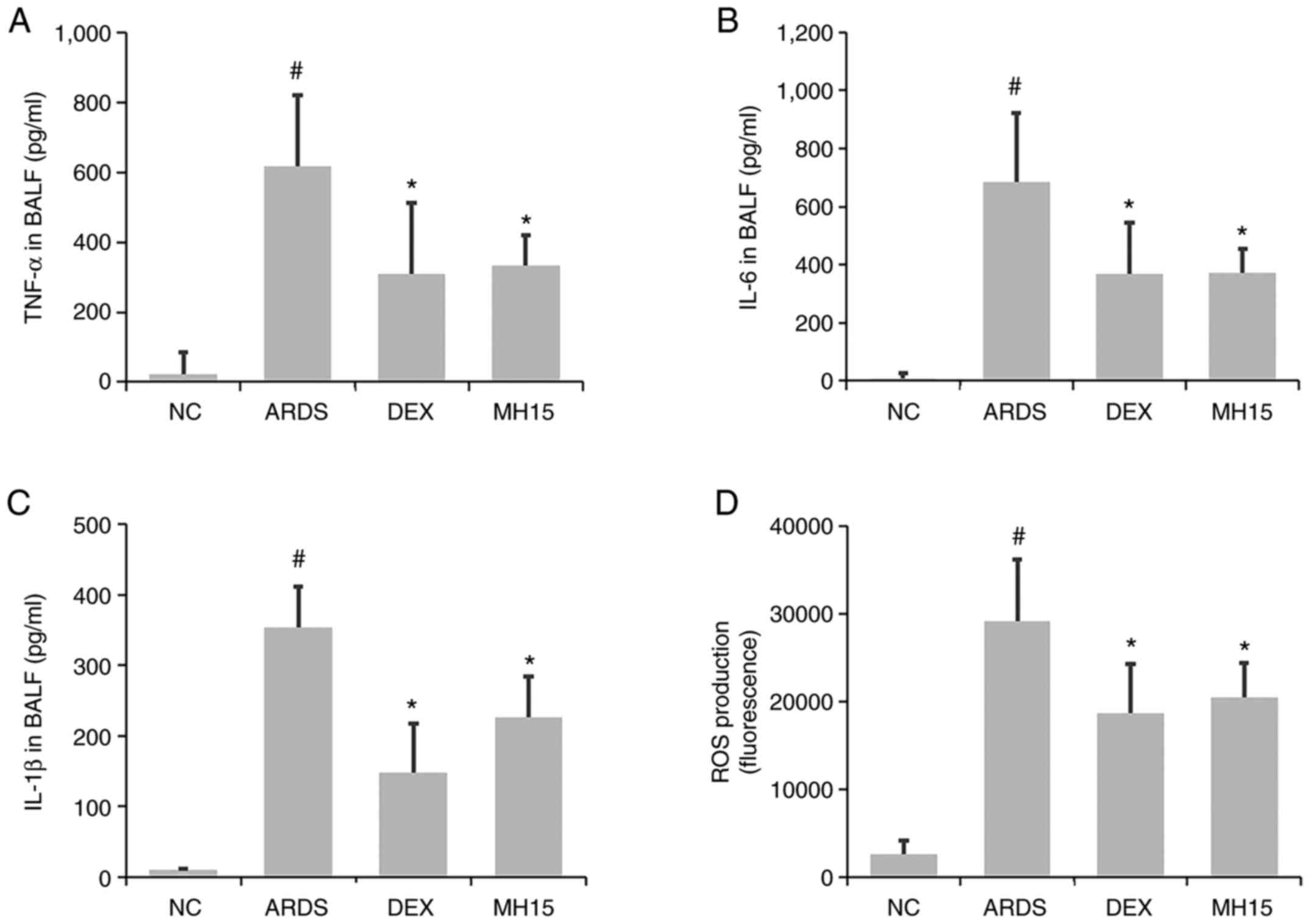
Next, it was investigated whether MH exerted
inhibitory effects on the levels of inflammatory molecules. A
significant increase in TNF-α, IL-6 and IL-1β levels was observed
in the BALF of LPS-induced ARDS mice (Fig. 3A-C). MH treatment significantly
inhibited this increase. The increased levels of ROS in the BALF of
ARDS mice was also decreased by MH treatment (Fig. 3D). The inhibitory ability of 15
mg/kg MH on the levels of these molecules was similar to that of 1
mg/kg DEX, which was used as a positive control.
Effects of MH on inflammatory cell
infiltration and lung edema in LPS-induced ARDS mice
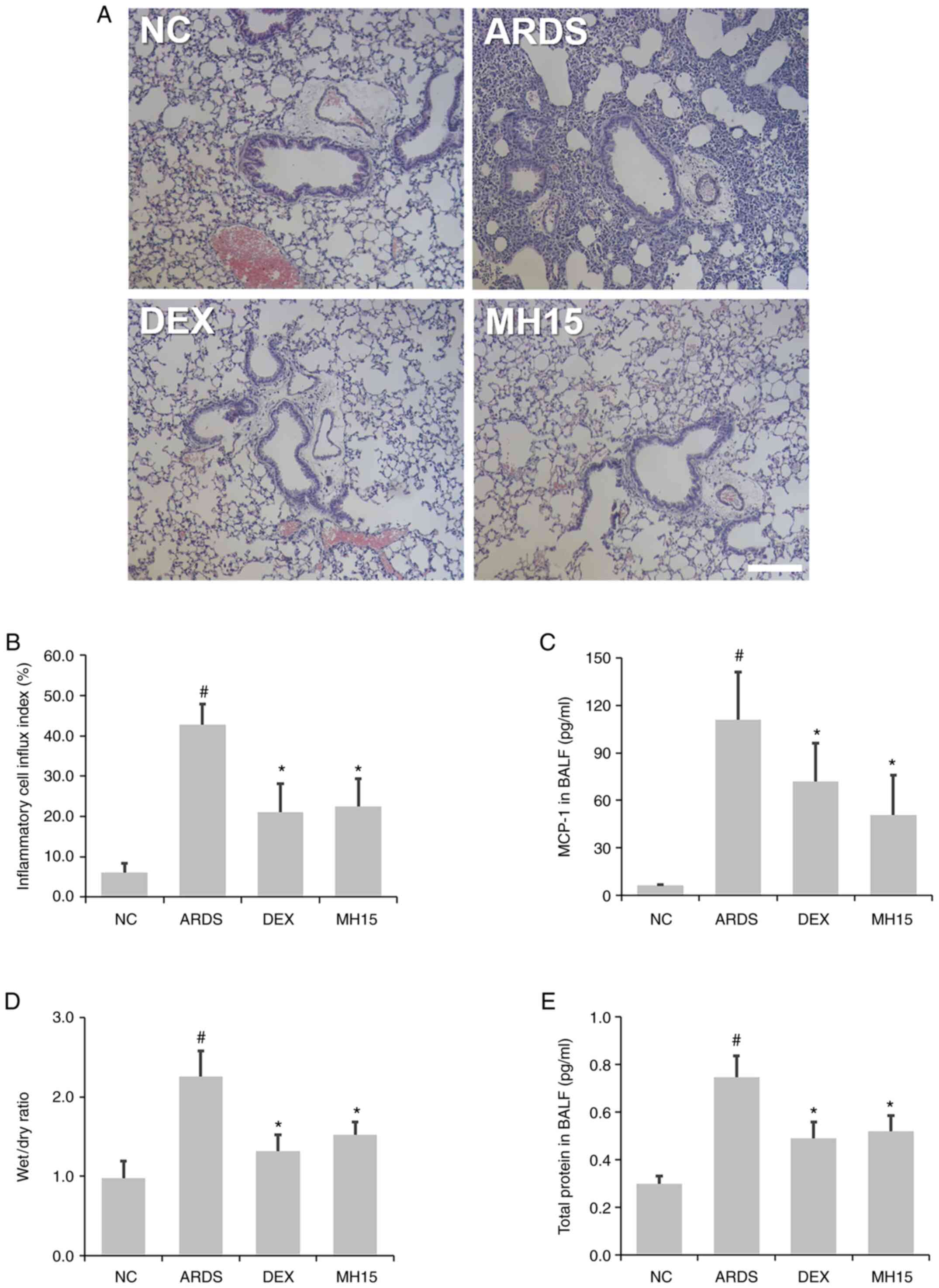
H&E staining showed that the influx of
inflammatory cells around the airway was higher in the lungs of
LPS-induced ARDS mice compared with the NC group, whereas this
trend was decreased in ARDS mice receiving MH treatment (Fig. 4A and B). In addition, the results
of ELISA demonstrated that MH treatment attenuated the LPS-induced
MCP-1 secretion in the BALF of mice (Fig. 4C). MH exerted an inhibitory effect
on LPS-induced increase of ratio of lung W/D weight (Fig. 4D). Furthermore, MH decreased
LPS-induced upregulation of total protein content in BALF of mice
(Fig. 4E), showing that MH
exerted a regulatory effect in LPS-induced inflammatory cell influx
and lung edema. The inhibitory effects of 15 mg/kg MH on the level
of inflammatory cell infiltration and lung edema were similar to
that of 1 mg/kg DEX, which was used as a positive control.
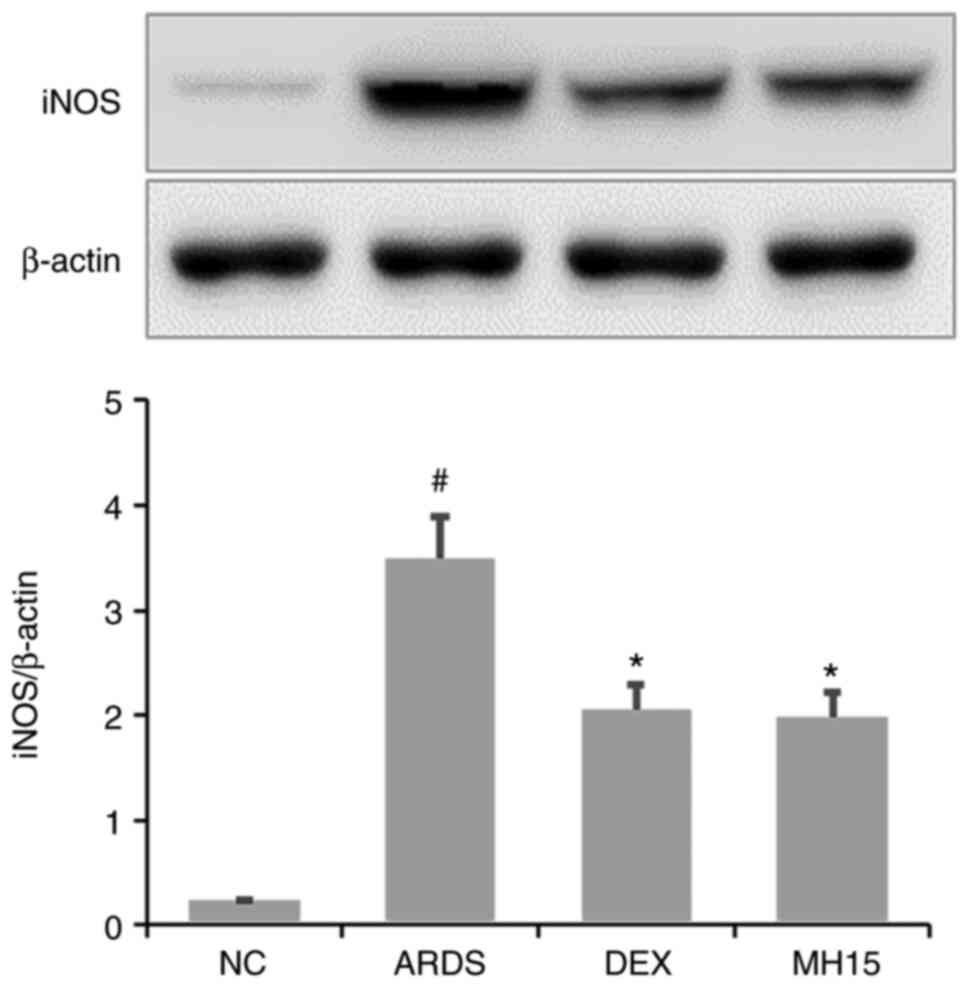
Effects of MH on iNOS expression in
LPS-induced ARDS mice
Western blotting results demonstrated that the
expression of iNOS protein was significantly upregulated in lungs
of LPS-induced ARDS mice (Fig.
5). However, MH treatment decreased this upregulation in the
lungs of ARDS mice. The inhibitory effects of 15 mg/kg MH on the
expression of iNOS were similar to that of 1 mg/kg DEX, which was
used as a positive control.
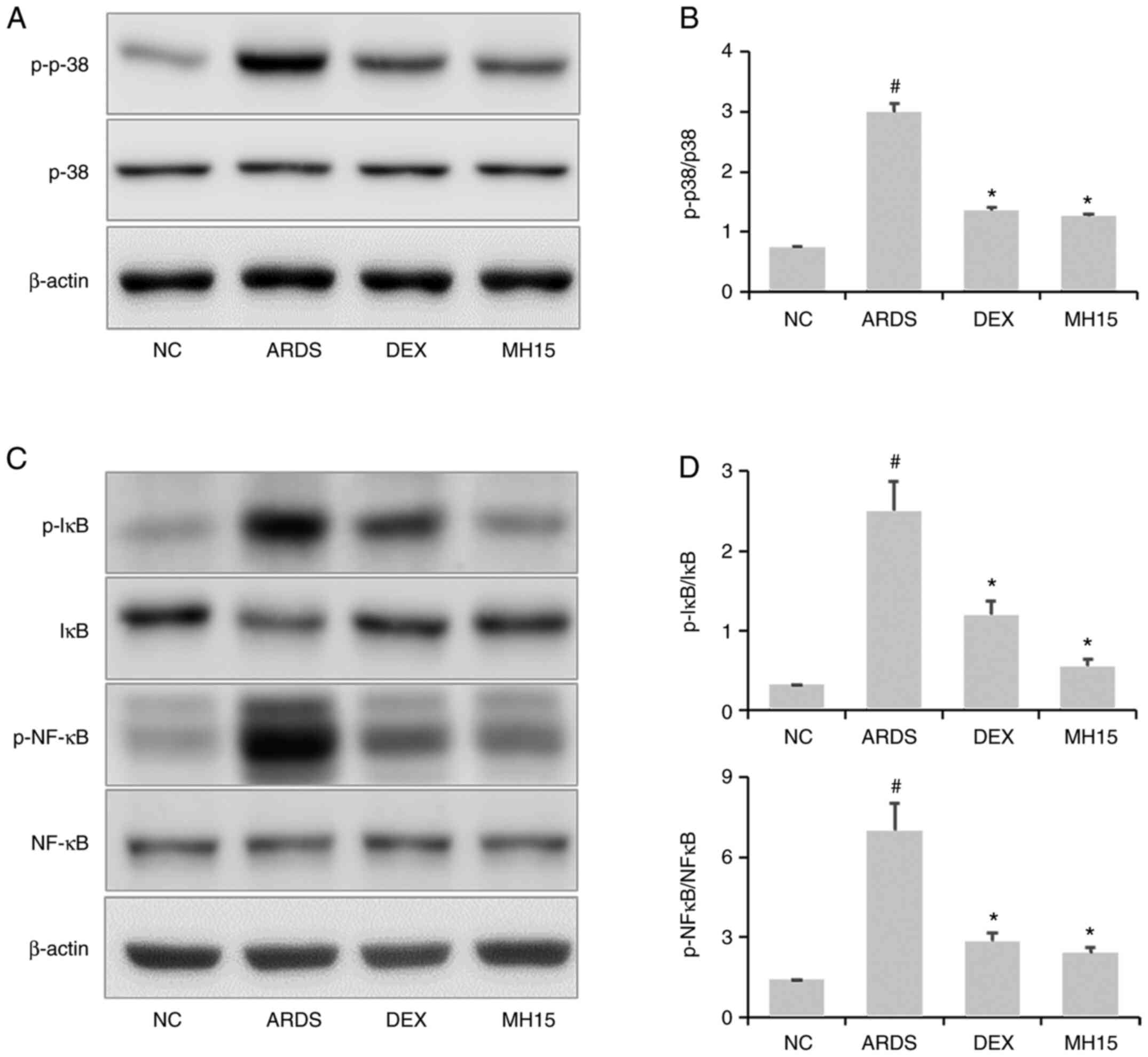
Effects of MH on p38MAPK/NF-κB
activation in LPS-induced ARDS mice
Increased p38 phosphorylation was confirmed in the
lungs of LPS-induced ARDS mice, whereas this was significantly
reversed by MH treatment (Fig. 6A and
B). Similar to these results, MH exerted inhibitory effects on
LPS-induced upregulation of IκB and NF-κB phosphorylation (Fig. 6C and D). In total, the inhibitory
ability of 15 mg/kg MH on the activation of p38MAPK and NF-κB was
similar to that of 1 mg/kg DEX, which was used as a positive
control.
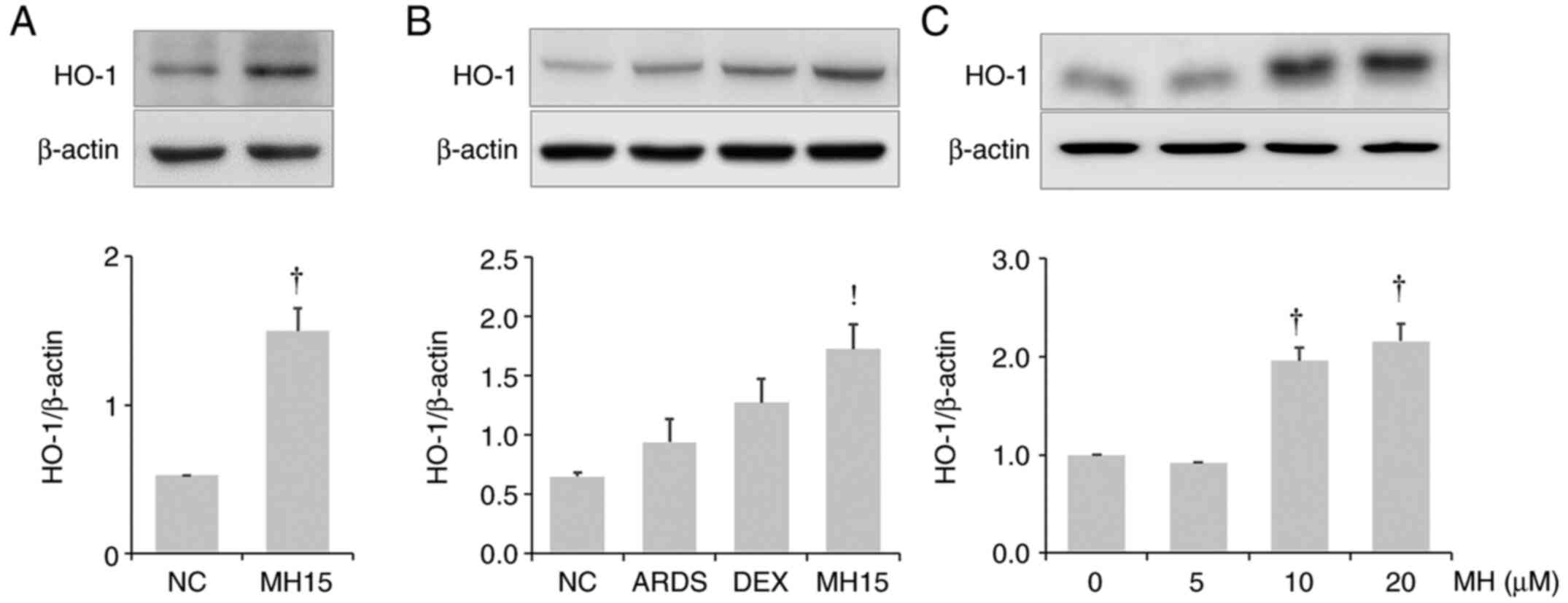
Effects of MH on HO-1 induction in
lungs of mice and RAW264.7 macrophages
It was investigated whether MH affects HO-1
expression in the lungs of control or ARDS mice. MH treatment
resulted in upregulation of HO-1 expression in the lungs of control
mice or ARDS mice (Fig. 7A and
B). The results also demonstrated that MH treatment at 10 and
20 µM led to an increase in HO-1 expression in RAW264.7 macrophages
(Fig. 7C).
Discussion
Accumulating evidence has shown that endotoxins
cause pulmonary inflammation by inducing excessive production of
TNF-α, IL-6, IL-1β, ROS and MCP-1, thereby leading to organ
dysfunction and lung edema during the pathogenesis of ALI/ARDS
(5,22–24). Macrophages and neutrophils
accelerate pulmonary inflammation by participating in the
production of these molecules (5). Thus, regulation of these
cell-derived inflammatory molecules may ameliorate the development
of ALI/ARDS. The aim of the present study was to confirm the
inhibitory effect of MH on neutrophil/macrophage recruitment and
the production of TNF-α, IL-6, IL-1β, ROS and MCP-1 in mice with
LPS-induced ARDS. The present study demonstrated that MH exerted an
ameliorative effect on the recruitment of inflammatory cells
(neutrophils and macrophages), production of cytokines and
chemokines and lung edema. In addition, the inhibitory effect of MH
on inflammatory cell infiltration of lung tissue was also confirmed
by histological examination. These results indicated that MH may
exert protective effects by suppressing pulmonary inflammation in
ALI/ARDS mice by controlling inflammatory cell recruitment and
inflammatory molecule secretion.
Increased iNOS-induced NO generation contributes to
the formation of peroxynitrite, which leads to vascular leakage and
organ injury in ALI/ARDS (25).
Researchers have reported that iNOS inhibitors effectively relieve
inflammatory response in in vivo studies of ALI/ARDS
(26,27). In a previous study, MH was shown
to exert inhibitory effects on both iNOS and NO production in
LPS-induced RAW264.7 macrophages (18). In the present study, the
inhibitory effect of MH on iNOS expression was confirmed in ARDS
mice. These results collectively suggest that MH may serve as an
iNOS inhibitor in ALI/ARDS.
Activation of p38 MAPK is associated with ROS
generation (28,29). Inhibition of p38 relieves
pulmonary inflammation in mice with ALI/ARDS (30,31). Accumulating evidence has shown
that suppression of NF-κB activation ameliorates airway
inflammation by downregulating inflammatory cytokines, chemokines
and mediators (6,32–34) in in vivo studies on
ALI/ARDS. Therefore, therapeutic approaches to inflammatory
diseases, including ALI/ARDS, have focused on the regulation of
these signaling pathways. Based on these studies, the role of MH in
the regulation of p38 MAPK and NF-κB activation was examined. In
the present study, MH was shown to exert suppressive effect on
LPS-induced p38 MAPK/NF-κB activation, indicating that the
protective effects of MH were mediated not only by regulating
inflammatory molecule production, but also by p38 MAPK/NF-κB
activation.
LPS-induced ROS generation leads to excessive
inflammation and oxidative damage (35,36) and the induction of the antioxidant
protein HO-1 exerts protective effects against LPS-induced
pulmonary inflammation and oxidative stress in mice (6,19,37,38). Inhibition of expression of
cytokines (TNF-α, IL-6 and IL-1β), chemokines (MCP-1) and
inflammatory mediators (iNOS) and of activation of p38MAPK/NF-κB is
accompanied by HO-1 upregulation. The present study confirmed that
MH inhibited the production of TNF-α, IL-6, IL-1β, MCP-1 and ROS,
expression of iNOS and p38 MAPK/NF-κB activation. Based on these
results, the present study investigated whether MH leads to HO-1
induction. The results revealed the ability of MH to induce HO-1
expression both in vivo and in vitro, suggesting that
MH has antioxidant properties that may be associated with its
anti-inflammatory activity.
In conclusion, the present study demonstrated that
MH effectively ameliorated pulmonary inflammation in LPS-induced
ARDS mice. This effect was accompanied by suppression of
p38MAPK/NF-κB activation. In addition, MH led to an increase in
HO-1 expression. Thus, MH may be of value as an adjuvant for the
treatment of ALI/ARDS.
Acknowledgements
Not applicable.
Funding
The present study was supported by Korea Research Institute of
Bioscience and Biotechnology Research Initiative Program (grant
nos. KGM5522113 and KGS1402113).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SMK, JHM, JHK, JC, JMP, JL, SHG, JHO, SHK, WC and
KSA performed the experiments and analyzed data. SK and JWL
designed and conceived the study and revised the manuscript. All
authors have read and approved the final manuscript. All authors
drafted and revised the manuscript. All authors have read and
approved the final manuscript. SMK, JHM, JHK, SK and JWL confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The experimental procedures were approved by the
Institutional Animal Care and Use Committee of the Korea Research
Institute of Bioscience and Biotechnology (Ochang, Korea; approval
no. KRIBB-AEC-21111).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Spadaro S, Park M, Turrini C, Tunstall T,
Thwaites R, Mauri T, Ragazzi R, Ruggeri P, Hansel TT, Caramori G
and Volta CA: Biomarkers for Acute Respiratory Distress syndrome
and prospects for personalised medicine. J Inflamm (Lond).
16:12019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhargava M and Wendt CH: Biomarkers in
acute lung injury. Transl Res. 159:205–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barnett N and Ware LB: Biomarkers in acute
lung injury-marking forward progress. Crit Care Clin. 27:661–683.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kellner M, Noonepalle S, Lu Q, Srivastava
A, Zemskov E and Black SM: ROS signaling in the pathogenesis of
acute lung Injury (ALI) and acute respiratory distress syndrome
(ARDS). Adv Exp Med Biol. 967:105–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JW, Chun W, Lee HJ, Min JH, Kim SM,
Seo JY, Ahn KS and Oh SR: The role of macrophages in the
development of acute and chronic inflammatory lung diseases. Cells.
10:8972021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JW, Chun W, Kwon OK, Park HA, Lim Y,
Lee JH, Kim DY, Kim JH, Lee HK, Ryu HW, et al:
3,4,5-Trihydroxycinnamic acid attenuates lipopolysaccharide
(LPS)-induced acute lung injury via downregulating inflammatory
molecules and upregulating HO-1/AMPK activation. Int
Immunopharmacol. 64:123–130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JW, Park HA, Kwon OK, Park JW, Lee G,
Lee HJ, Lee SJ, Oh SR and Ahn KS: NPS 2143, a selective
calcium-sensing receptor antagonist inhibits
lipopolysaccharide-induced pulmonary inflammation. Mol Immunol.
90:150–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu B, Cheng Y, Wu Y, Zheng X, Li X, Yang
G, He T, Li S and Shen F: Emodin improves alveolar hypercoagulation
and inhibits pulmonary inflammation in LPS-provoked ARDS in mice
via NF-κB inactivation. Int Immunopharmacol. 88:1070202020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frank JA, Pittet JF, Lee H, Godzich M and
Matthay MA: High tidal volume ventilation induces NOS2 and impairs
cAMP- dependent air space fluid clearance. Am J Physiol Lung Cell
Mol Physiol. 284:L791–L798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao Q, Cui Y, Zhao Y, Liu L, Wang H and
Yang L: Orientin relieves lipopolysaccharide-induced acute lung
injury in mice: The involvement of its anti-inflammatory and
anti-oxidant properties. Int Immunopharmacol. 90:1071892021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee SA, Lee SH, Kim JY and Lee WS: Effects
of glycyrrhizin on lipopolysaccharide-induced acute lung injury in
a mouse model. J Thorac Dis. 11:1287–1302. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moine P, McIntyre R, Schwartz MD, Kaneko
D, Shenkar R, Le Tulzo Y, Moore EE and Abraham E: NF-kappaB
regulatory mechanisms in alveolar macrophages from patients with
acute respiratory distress syndrome. Shock. 13:85–91. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong WT, Wu YC, Xie XX, Zhou X, Wei MM,
Soromou LW, Ci XX and Wang DC: Phillyrin attenuates LPS-induced
pulmonary inflammation via suppression of MAPK and NF-κB activation
in acute lung injury mice. Fitoterapia. 90:132–139. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo S, Jiang K, Wu H, Yang C, Yang Y, Yang
J, Zhao G and Deng G: Magnoflorine ameliorates
lipopolysaccharide-induced acute lung injury via suppressing NF-κB
and MAPK activation. Front Pharmacol. 9:9822018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lei J, Wei Y, Song P, Li Y, Zhang T, Feng
Q and Xu G: Cordycepin inhibits LPS-induced acute lung injury by
inhibiting inflammation and oxidative stress. Eur J Pharmacol.
818:110–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pooladanda V, Thatikonda S, Bale S,
Pattnaik B, Sigalapalli DK, Bathini NB, Singh SB and Godugu C:
Nimbolide protects against endotoxin-induced acute respiratory
distress syndrome by inhibiting TNF-α mediated NF-κB and HDAC-3
nuclear translocation. Cell Death Dis. 10:812019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwon YS and Kim CM: Antioxidant
constituents from the stem of Sorghum bicolor. Arch Pharm Res.
26:535–539. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vo VA, Lee JW, Shin SY, Kwon JH, Lee HJ,
Kim SS, Kwon YS and Chun W: Methyl p-Hydroxycinnamate suppresses
lipopolysaccharide-induced inflammatory responses through akt
phosphorylation in RAW264.7 cells. Biomol Ther (Seoul). 22:10–16.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JW, Ryu HW, Ahn HI, Min JH, Kim SM,
Kim MG, Kwon OK, Hwang D, Kim SY, Choi S, et al: The
anti-inflammatory effect of trichilia martiana C. DC. in the
lipopolysaccharide-stimulated inflammatory response in macrophages
and airway epithelial cells and in LPS-challenged mice. J Microbiol
Biotechnol. 30:1614–1625. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JW, Ryu HW, Lee SU, Kim MG, Kwon OK,
Kim MO, Oh TK, Lee JK, Kim TY, Lee SW, et al: Pistacia
weinmannifolia ameliorates cigarette smoke and
lipopolysaccharide-induced pulmonary inflammation by inhibiting
interleukin8 production and NF-κB activation. Int J Mol Med.
44:949–959. 2019.PubMed/NCBI
|
|
21
|
Tian M, Peng S, Wang S, Li X, Li H and
Shen L: Pristimerin reduces dextran sulfate sodium-induced colitis
in mice by inhibiting microRNA-155. Int Immunopharmacol.
94:1074912021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhatia M and Moochhala S: Role of
inflammatory mediators in the pathophysiology of acute respiratory
distress syndrome. J Pathol. 202:145–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parsons PE, Eisner MD, Thompson BT,
Matthay MA, Ancukiewicz M, Bernard GR and Wheeler AP; NHLBI Acute
Respiratory Distress Syndrome Clinical Trials Network, : Lower
tidal volume ventilation and plasma cytokine markers of
inflammation in patients with acute lung injury. Crit Care Med.
33:1–6; discussion 230-2. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong Z and Yuan Y: Accelerated
inflammation and oxidative stress induced by LPS in acute lung
injury: Inhibition by ST1926. Int J Mol Med. 41:3405–3421.
2018.PubMed/NCBI
|
|
25
|
Guimarães LMF, Rossini CVT and Lameu C:
Implications of SARS-Cov-2 infection on eNOS and iNOS activity:
Consequences for the respiratory and vascular systems. Nitric
Oxide. 111-112:64–71. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen JR, Tang Y, Wang YL, Cui Q, Inam M,
Kong LC and Ma HX: Serine protease inhibitor MDSPI16 ameliorates
LPS-induced acute lung injury through its anti-inflammatory
activity. Int Immunopharmacol. 88:1070152020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu YX, Zeng S, Wan BB, Wang YY, Sun HX,
Liu G, Gao ZQ, Chen D, Chen YQ, Lu MD and Pang QF: Sophoricoside
attenuates lipopolysaccharide-induced acute lung injury by
activating the AMPK/Nrf2 signaling axis. Int Immunopharmacol.
90:1071872021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun WH, Liu F, Chen Y and Zhu YC: Hydrogen
sulfide decreases the levels of ROS by inhibiting mitochondrial
complex IV and increasing SOD activities in cardiomyocytes under
ischemia/reperfusion. Biochem Biophys Res Commun. 421:164–169.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lan A, Liao X, Mo L, Yang C, Yang Z, Wang
X, Hu F, Chen P, Feng J, Zheng D and Xiao L: Hydrogen sulfide
protects against chemical hypoxia-induced injury by inhibiting
ROS-activated ERK1/2 and p38MAPK signaling pathways in PC12 cells.
PLoS One. 6:e259212011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fang W, Cai SX, Wang CL, Sun XX, Li K, Yan
XW, Sun YB, Sun XZ, Gu CK, Dai MY, et al: Modulation of
mitogen-activated protein kinase attenuates sepsis-induced acute
lung injury in acute respiratory distress syndrome rats. Mol Med
Rep. 16:9652–9658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu H, Zhao G, Jiang K, Chen X, Zhu Z, Qiu
C, Li C and Deng G: Plantamajoside ameliorates
lipopolysaccharide-induced acute lung injury via suppressing NF-κB
and MAPK activation. Int Immunopharmacol. 35:315–322. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jha P and Das H: KLF2 in Regulation of
NF-κB-Mediated immune cell function and inflammation. Int J Mol
Sci. 18:23832017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meng L, Li L, Lu S, Li K, Su Z, Wang Y,
Fan X, Li X and Zhao G: The protective effect of dexmedetomidine on
LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB
and PI3K/Akt/mTOR pathways. Mol Immunol. 94:7–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang J, Xu L, Zeng Y and Gong F: Effect of
gut microbiota on LPS-induced acute lung injury by regulating the
TLR4/NF-kB signaling pathway. Int Immunopharmacol. 91:1072722021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang WB, Yang F, Wang Y, Jiao FZ, Zhang
HY, Wang LW and Gong ZJ: Inhibition of HDAC6 attenuates LPS-induced
inflammation in macrophages by regulating oxidative stress and
suppressing the TLR4-MAPK/NF-κB pathways. Biomed Pharmacother.
117:1091662019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong Q, Li Y, Chen J and Wang N:
Azilsartan Suppressed LPS-Induced Inflammation in U937 Macrophages
through Suppressing Oxidative Stress and Inhibiting the TLR2/MyD88
Signal Pathway. ACS Omega. 6:113–118. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Q, Ci X, Wen Z and Peng L: Diosmetin
Alleviates Lipopolysaccharide-Induced acute lung injury through
activating the Nrf2 Pathway and inhibiting the NLRP3 Inflammasome.
Biomol Ther (Seoul). 26:157–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Song M, Zhu G, Xi X, Li K, Wu C and
Huang L: Corynoline attenuates LPS-induced acute lung injury in
mice by activating Nrf2. Int Immunopharmacol. 48:96–101. 2017.
View Article : Google Scholar : PubMed/NCBI
|