Introduction
Ambient fine particulate matter (PM) is associated
with cardiovascular disease (CVD), including coronary artery
disease (CAD), heart failure and hypertension (1). However, the underlying mechanism is
not well studied. Different types of CVD may be caused by duration
of PM exposure; acute PM exposure increases myocardial infarction
(2), stroke (3) and other acute cardiovascular events
(4), while chronic exposure
contributes to development of hypertension, diabetes and other
cardiometabolic conditions (4).
Following six months exposure of PM with diameter ≤2.5 µm,
atherosclerosis development in apolipoprotein E knockout mice
significantly increases and is accompanied by vasomotor tone
alteration and vascular inflammation (5). However, the detailed mechanisms
underlying PM-potentiated atherosclerosis in hyperlipidemia is not
well studied.
Endothelial dysfunction or injury is a key factor
that contributes to the development of atherosclerosis and CAD
(6,7). Bone marrow (BM)-derived endothelial
progenitor cells (EPCs) serve a key role in vascular
reendothelialization, angiogenesis and promotion of neointima
formation following vascular injury (8–11).
In addition, EPC number and function are significantly decreased in
patients with CAD and hyperlipidemia (12,13). However, the exact mechanism
underlying decreased EPC levels in patients with hyperlipidemia
remains unknown. Oxidized low-density lipoprotein (ox-LDL) is a key
component in hyperlipidemia and serves an important role in
development of atherosclerosis, primarily via oxidative stress
(14). Our previous study
indicated that ox-LDL has a similar effect to chronic
hyperlipidemia on BM and blood EPC populations (15). However, the effect of PM exposure
on ox-LDL and EPC population in hyperlipidemia is still
unknown.
Reactive oxygen species (ROS) are small molecules,
such as hydrogen peroxide (H2O2), that
regulate tissue oxidative stress (16). ROS-induced oxidative stress also
regulates BM stem cell (BMSC) and BMSC-derived progenitor cell
self-renewal, proliferation, mobilization, homing, migration,
differentiation, apoptosis and senescence (17,18). Rate of ROS generation in
peripheral blood monocytes is increased in hyperlipidemic patients,
along with elevated plasma ox-LDL levels, which increases
intracellular ROS formation in cultured endothelial cells (19,20). Our previous data showed that PM
exposure also increases EPC intracellular ROS production in
wild-type (WT) mice (21). To the
best of our knowledge, however, ROS production following short- and
long-term PM exposure in mice with hyperlipidemia has not been
evaluated.
N-acetyl cysteine (NAC) is an antioxidant widely
used to investigate CVD (22).
Our previous data indicated that NAC prevents atherosclerosis by
maintaining EPC population, decreasing both intracellular and
extracellular ROS production in hyperlipidemic mice and preventing
LDL oxidation in WT mice (15,23). NAC also protects EPCs from
apoptosis and decreases levels of inflammatory cytokines following
PM exposure in WT mice (21). To
the best of our knowledge, however, the effects of NAC on
development of atherosclerosis in hyperlipidemic mice with acute
and chronic PM exposure has not been investigated. The present
study amid to determine the protective effect and underlying
mechanism of NAC on atherosclerosis in mice with hyperlipidemia
following acute and chronic PM exposure.
Materials and methods
Animal model
All animal experiments were performed in accordance
with the Guidelines of the Animal Care Committee of Shandong
Provincial Hospital affiliated to Shandong First Medical
University, Jinan, China. The experimental protocols for the
present study were reviewed and approved by the Animal Care
Committee of Shandong First Medical University (approval no.
2021-228). A total of 48 4–6-week-old male homozygous C57BL/6J LDL
receptor knockout (LDLR KO) mice were obtained from Shanghai Model
Organisms Center, Inc. and randomized into 6 groups (n=8/group) as
follows: Normal diet (ND); high-fat diet (HFD); HFD + NAC; PM + ND;
PM + HFD and PM + HFD + NAC. In addition to ad libitum
access to food and water, all mice were kept at room temperature
with 40–60% humidity and a 12/12-h light/dark cycle. Following 1
week acclimation, mice were challenged with HFD (17% anhydrous milk
fat; 0.2% cholesterol) to induce hyperlipidemia for 1 week or 6
months, as previously described (23). PM (cat. no. NIST2786; mean
particle diameter, <4 µm) was purchased from MilliporeSigma. PM
was dispersed in solution by ultrasonication in endotoxin-free
water for 30 min at a concentration of 0.5 µg/µl as previously
described (24,25). Hyperlipidemic mice were treated
with 10 µg PM three times/week for 1 week or 6 months via
intranasal instillation, as previously described (26). Endotoxin-free water was used as
control. There are multiple routes for PM to invade the body
following inhalation. The primary route is via the respiratory
system; other routes include olfactory epithelium and digestive
(swallowing sputum with PM) (27). Intranasal instillation was used to
mimic natural PM exposure. To evaluate the protective effect of NAC
on hyperlipidemic mice with acute and chronic PM exposure, animals
were pre-treated with NAC (1 mg/ml in drinking water) for 1 week
and continued NAC treatment for 1 week or 6 months, as previously
described (23). Mice fed ND were
used as control. Although animal health and behavior were monitored
every 7 days, 6 mice in the 6-month PM group died before the end of
experiment; this may have been due to advanced atherosclerosis
development.
Following 1-week or 6-month PM, HFD and NAC
treatment, 3.0 and 1.5% isoflurane was used to induce and maintain
anesthesia, respectively. A total of 300–500 µl murine blood was
collected via cardiac puncture. Then, carbon dioxide (50–70% of
chamber volume/min) was used to euthanize the animals. Death was
confirmed by cardiac and respiratory arrest or fixed and dilated
pupils. Murine aorta was isolated after confirming the death of
mice.
Blood lipid, plasma ROS and
inflammatory cytokine measurement and atherosclerotic plaque ratio
calculation
At the end of the experiment, murine blood was
centrifuged at 300 g and room temperature for 20 min to collect
plasma for lipid profile, ox-LDL, plasma ROS and inflammatory
cytokine analysis. A total of 40 µl plasma was applied in the lipid
profile test cassette (Cholestech LDX; cat. no. 10–989; Thermo
Fisher Scientific, Inc.) for measuring total cholesterol (TC),
triglyceride (TG), LDL, high-density lipoprotein (HDL), non-HDL
cholesterol (28) and the ratio
of TC/HDL by using Alere Cholestech LDX System. Murine plasma
ox-LDL was measured with Ox-LDL ELISA kit (cat. no. CSB-E07933m;
Cusabio Technology LLC) according to the manufacturer's
instruction. Murine plasma was collected to detect blood ROS levels
by using In Vitro ROS/Reactive Nitrogen Species (RNS) Assay
(cat. no. STA-347; Cell Biolabs, Inc.) and Pierce™ Quantitative
Peroxide Assay kit (lipid-compatible formulation; cat. no. 23285;
Thermo Fisher Scientific, Inc.) following the manufacturer's
instructions. Following incubation of plasma with reagent at room
temperature for 30 min, samples were transferred to a 48-well plate
for analysis using a microplate reader (Multiskan™ FC Microplate
Photometer) at the wavelength of 595 nm to measure the optical
density (OD). Plasma levels of inflammatory cytokines TNF-α (cat.
no. 430915), IL-1β (cat. no. 432615) and IL-6 (cat. no. 431315)
were measured using ELISA kits (BioLegend, Inc.) according to the
manufacturer's instructions. Murine aorta was dissected and
directly stained with oil red (MilliporeSigma) for plaque formation
measurement at room temperature for 5 min. The ratio of plaque area
to total inner surface of aorta was calculated as previously
described (29).
Mononuclear cell and EPC intracellular
ROS detection and EPC measurement
An endothelial cell marker combined with a stem cell
marker CD34+/CD133+ was used to identify EPC
as previously described (30).
Murine blood cells were harvested after sacrifice to detect EPC and
mononuclear cell intracellular ROS formation via flow cytometry by
using ROS Detection Reagents-FITC (cat. no. D399; Invitrogen;
Thermo Fisher Scientific, Inc.) as previously described (31). After removing red blood cells
(RBCs) using 1X RBC lysis buffer (cat. no. 00433357; Thermo Fisher
Scientific), a total of 50,000 cells in each sample were incubated
with 5 µg/ml ROS Detection Reagents-FITC for 10 min at 37°C. BD™
LSRII (BD Biosciences) at a wavelength of 525 nm was used to
calculate the positively fluorescent cells for intracellular ROS
detection. For EPC measurement, cells were incubated with
anti-mouse CD34-AF700 (cat. no. 560518; BD Biosciences) and
CD133-PE (cat. no. 12-1331-82; eBioscience; Thermo Fisher
Scientific, Inc.) as previously described (21), then incubated with 5 µg/ml ROS
Detection Reagents-FITC for 10 min at 37°C. Labeled cells were
washed twice with PBS and suspended in warm PBS for analysis by
flow cytometry on a BD™ LSRII system (BD Biosciences). All
antibodies were diluted 1:100.
Statistical analysis
All data are presented as the mean ± SD (5–8
repeats) and were analyzed using one- or two-way ANOVA (PRISM
Version 5.0.; GraphPad Software, Inc.) followed by post hoc
conservative Bonferroni's test to minimize type I error. Normal
distribution of data was tested using Shapiro-Wilk W-test; equal
variance was tested using F-test. When the null hypothesis of
normality and/or equal variance was rejected, non-parametric
Mann-Whitney U-test was used. Two-tailed P<0.05 was considered
to indicate a statistically significant difference.
Results
NAC prevents PM-potentiated
atherosclerosis in hyperlipidemia
To test the effect of NAC on atherosclerosis
development in hyperlipidemic mice following PM exposure, LDLR KO
mice were pre-treated with NAC for 1 week, and continued with NAC,
HFD and PM exposure for 6 months. Atherosclerotic plaque formation
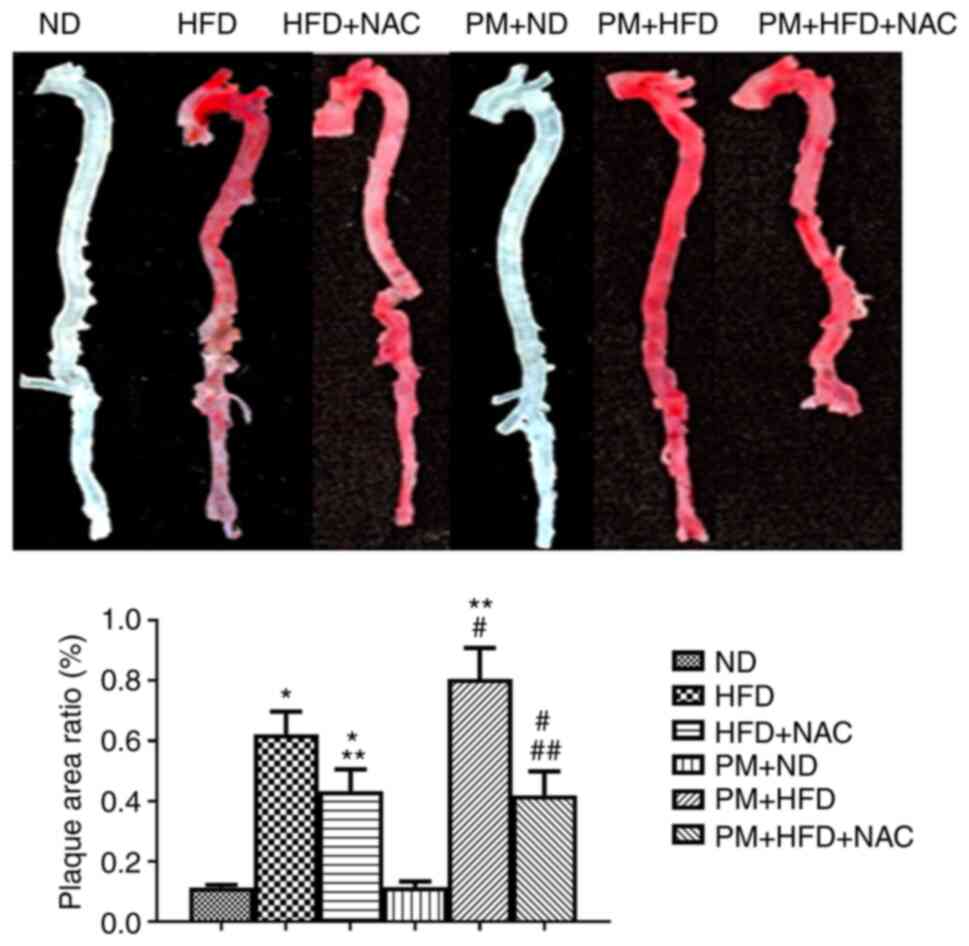
was increased up to 1.3 fold in PM + HFD compared with HFD mice
(Fig. 1). NAC significantly
decreased plaque formation in PM + HFD + NAC (0.43±0.20%) and HFD +
NAC (0.42±0.22%) compared with PM + HFD (0.80±0.29%) and HFD
(0.62±0.21%) (Fig. 1). There was
no plaque formation in any 1 week treatment (data not shown). These
results indicated that NAC prevented PM-potentiated atherosclerosis
formation.
NAC decreases blood ox-LDL levels
following chronic PM exposure in hyperlipidemic mice
The blood lipid levels, including TC, TG and LDL,
were significantly increased in LDLR KO mice treated with HFD for 1
week or 6 months with or without PM exposure compared with ND mice
(Table I). NAC had no effect on
blood lipid profile in hyperlipidemic mice with or without PM
exposure (Table I). Blood ox-LDL
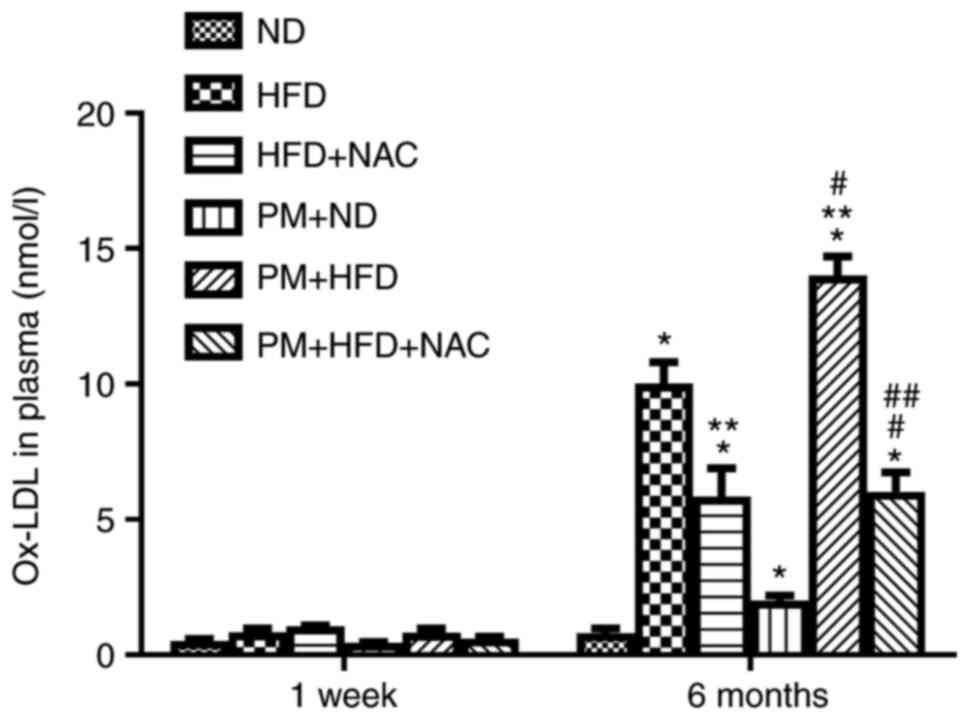
levels raised to 2.0±0.5 nmol/ml in LDLR KO 6-month PM + ND mice
compared with 1-week PM + ND mice (0.44±0.10 nmol/ml; Fig. 2). Furthermore, blood ox-LDL level
significantly increased to 14.00±2.00 nmol/ml in 6-month PM + HFD
mice compared with 6-month HFD mice (10.02±2.21 nmol/ml; Fig. 2). NAC treatment significantly
decreased murine plasma ox-LDL levels in LDLR KO mice following 6
month HFD treatment with (PM + HFD + NAC, 6.03±0.21 nmol/ml) or
without PM (HFD + NAC, 5.80±3.00 nmol/ml) compared with 6-month PM
+ HFD (14.01±2.00 nmol/ml) or HFD groups (10.02±2.21 nmol/ml;
Fig. 2). Blood ox-LDL levels were
not elevated in any 1 week group compared with ND (Fig. 2). These data suggested that NAC
prevented atherosclerosis in association with reducing PM-induced
elevation of ox-LDL level in hyperlipidemia.
 | Table I.Lipid profile of LDL receptor
knockout mice. |
Table I.
Lipid profile of LDL receptor
knockout mice.
| A, 1 week |
|---|
|
|---|
| Lipid | ND | HFD | HFD + NAC | PM + ND | PM + HFD | PM + HFD + NAC |
|---|
| TC, mg/dl | 228.60±25.66 |
1162.80±148.13a |
1132.80±123.22a | 230.60±27.88 |
1252.80±233.23b |
1255.60±114.34b |
| HDL, mg/dl | 88.00±9.64 | 82.80±6.87 | 85.40±7.54 | 90.00±10.24 | 85.50±7.07 | 82.50±6.34 |
| TG, mg/dl | 148.40±35.47 |
673.20±123.82a |
632.80±87.32a | 150.80±36.62 |
658.30±130.94b |
642.50±90.54b |
| LDL, mg/dl | 140.00±0.74 |
920.60±119.92a |
983.80±108.50a | 139.00±1.55 |
898.40±152.72b |
884.20±110.30b |
| Non-HDL, mg/dl | 146.40±24.25 |
1074.80±136.13a |
1054.80±103.22a | 148.30±26.72 |
1088.80±140.24b |
1059.20±112.32b |
| TC/HDL | 2.61±0.32 |
14.00±0.74a |
13.20±0.85a | 2.56±0.52 |
14.65±0.24b |
15.22±0.62b |
|
| B, 6
months |
|
| Lipid | ND | HFD | HFD +
NAC | PM + ND | PM +
HFD | PM + HFD +
NAC |
|
| TC, mg/dl | 228.70±29.02 |
1704.0±228.70a |
1728.00±234.80a | 232.50±28.77 |
1789.00±242.30b |
1732.00±243.80b |
| HDL, mg/dl | 76.00±9.80 | 74.00±24.40 | 92.00±21.30 | 78.00±10.10 | 78.00±25.20 | 95.00±32.40 |
| TG, mg/dl | 103.70±35.00 |
597.50±217.30a |
545.50±197.20a | 105.50±42.00 |
566.40±220.40b |
567.40±188.40b |
| LDL, mg/dl | 132.00±23.10 |
1583.00±100.50a |
1576.00±112.20a | 140.00±34.20 |
1602.00±103.20b |
1610.00±122.20b |
| Non-HDL, mg/dl | 119.30±83.30 |
1700.00±90.50a |
1720.00±100.60a | 122.50±90.50 |
1689.00±89.50b |
1656.00±112.40b |
| TC/HDL | 2.80±0.40 |
23.00±5.40a |
18.78±7.20a | 2.98±0.70 |
22.90±4.40b |
18.23±6.90b |
NAC maintains EPC levels following
acute and chronic PM exposure in mice with hyperlipidemia
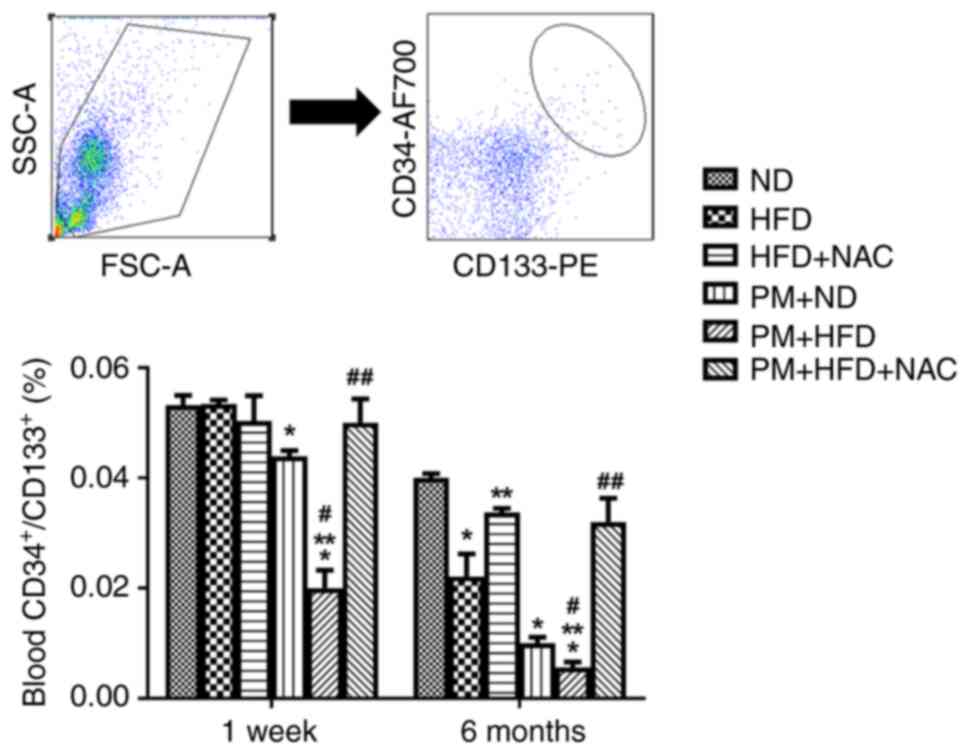
PM significantly decreased levels of circulating
CD34+/CD133+ cell in LDLR KO mice fed ND for
1 week (0.044±0.003%) and 6 months (0.010±0.003%) and further
decreased the cell population in PM + HFD mice (1 week, 0.02±0.01%;
6 months, 0.006±0.003%; Fig. 3).
NAC treatment effectively reversed the effects of PM and
hyperlipidemia on EPC (PM + HFD + NAC) in both 1 week (0.05±0.01%)
and 6 month (0.03±0.01%) groups compared with 1-week PM + HFD
(0.02±0.01%) and 6-month PM + HFD (0.0056±0.0029%) groups
respectively. Of note, EPC levels were not altered in 1-week HFD
mice (HFD, 0.050±0.002% vs. ND, 0.050±0.005%) and were
significantly decreased in 6-month HFD mice (HFD, 0.02±0.01% vs.
ND, 0.040±0.002%). These data demonstrated that NAC prevented
PM-induced decrease of EPC in hyperlipidemia.
NAC blocks plasma, mononuclear cell
and EPC intracellular ROS production in hyperlipidemic mice with
acute and chronic PM exposure
Our previous study indicated that increased blood
ROS production is associated with increased ox-LDL levels (23) and circulating EPCs are derived
from mononuclear cells (32). To
determine the mechanism underlying the protective effect of NAC on
elevated plasma ox-LDL and decreased circulating EPC levels in mice
with hyperlipidemia following acute and chronic PM exposure, plasma
ROS, as well as circulating mononuclear cell and EPC intracellular
ROS production, was measured in hyperlipidemic mice following
1-week and 6-month PM exposure with or without NAC. Total plasma
ROS/RNS and H2O2 levels were significantly
increased in 1-week PM + HFD mice (530±48 µM/ml; 0.33±0.02 OD) or 6
months (1,255±110 µM/ml; 0.60±0.01 OD) compared with 1 week HFD
mice (239±57 µM/ml; 0.12±0.05 OD) or 6 month (845±107 µM/ml;
0.45±0.04 OD). NAC significantly blocked plasma ROS production in
1-week (PM + HFD + NAC, 318±35 µM/ml; 0.13±0.01 OD) or 6-month (PM
+ HFD + NAC, 480±88 µM/ml; 0.24±0.02 OD) PM and HFD treated mice
compared with 1 week or 6-month PM + HFD mice (Fig. 4A and B). Similarly, mononuclear
cell and EPC intracellular ROS production were significantly
elevated up to 1.3-1.6-fold in LDLR KO 1-week or 6-month PM + HFD
mice compared with HFD mice (Fig. 4C
and D). The increased EPC and mononuclear cell intracellular
ROS production were effectively blocked by NAC in 1 week and 6
month PM + HFD + NAC mice (Fig. 4C
and D). Of note, ROS production increased in 1 week and 6 month
PM + ND mice and 6 month HFD mice compared with 1 week and 6 month
ND mice. There was no ROS elevation in 1-week HFD compared with ND
mice (Fig. 4A-D). These results
indicated that NAC prevented atherosclerosis formation mainly
through inhibition of ROS production in hyperlipidemic mice
following PM exposure.
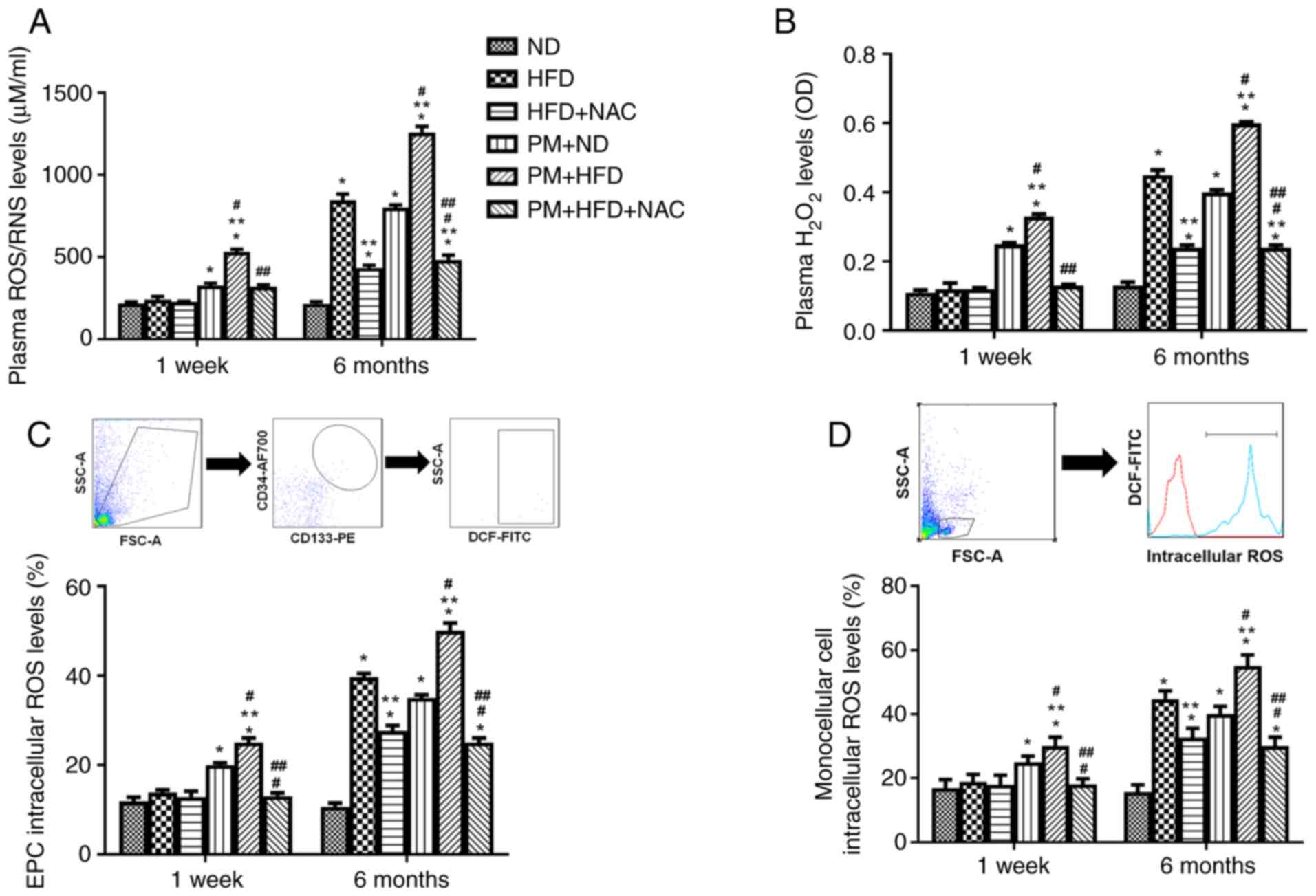 | Figure 4.NAC blocks plasma ROS and both
mononuclear and EPC intracellular ROS production in LDLR KO PM +
HFD mice fed HFD. Plasma (A) ROS/RNS and (B)
H2O2, (C) EPC and (D) mononuclear cell
intracellular ROS production were significantly increased in 1 week
and 6 month PM + HFD LDLR KO mice. Addition of NAC effectively
decreased both plasma ROS and intracellular ROS production. n=5-8.
*P<0.01 vs. ND; **P<0.01 vs. HFD; #P<0.01 vs.
PM + ND; ##P<0.01 vs. PM + HFD. ND, normal diet; HFD,
high-fat diet; NAC, N-acetyl cysteine; PM, ambient fine particulate
matter; H2O2, hydrogen peroxide; OD, optical
density; LDLR, low-density lipoprotein receptor; KO, knockout; EPC,
endothelial progenitor cell; ROS, reactive oxygen species; RNS,
reactive nitrogen species. |
NAC effectively inhibits plasma
inflammatory cytokine production in hyperlipidemic mice with acute
and chronic PM exposure
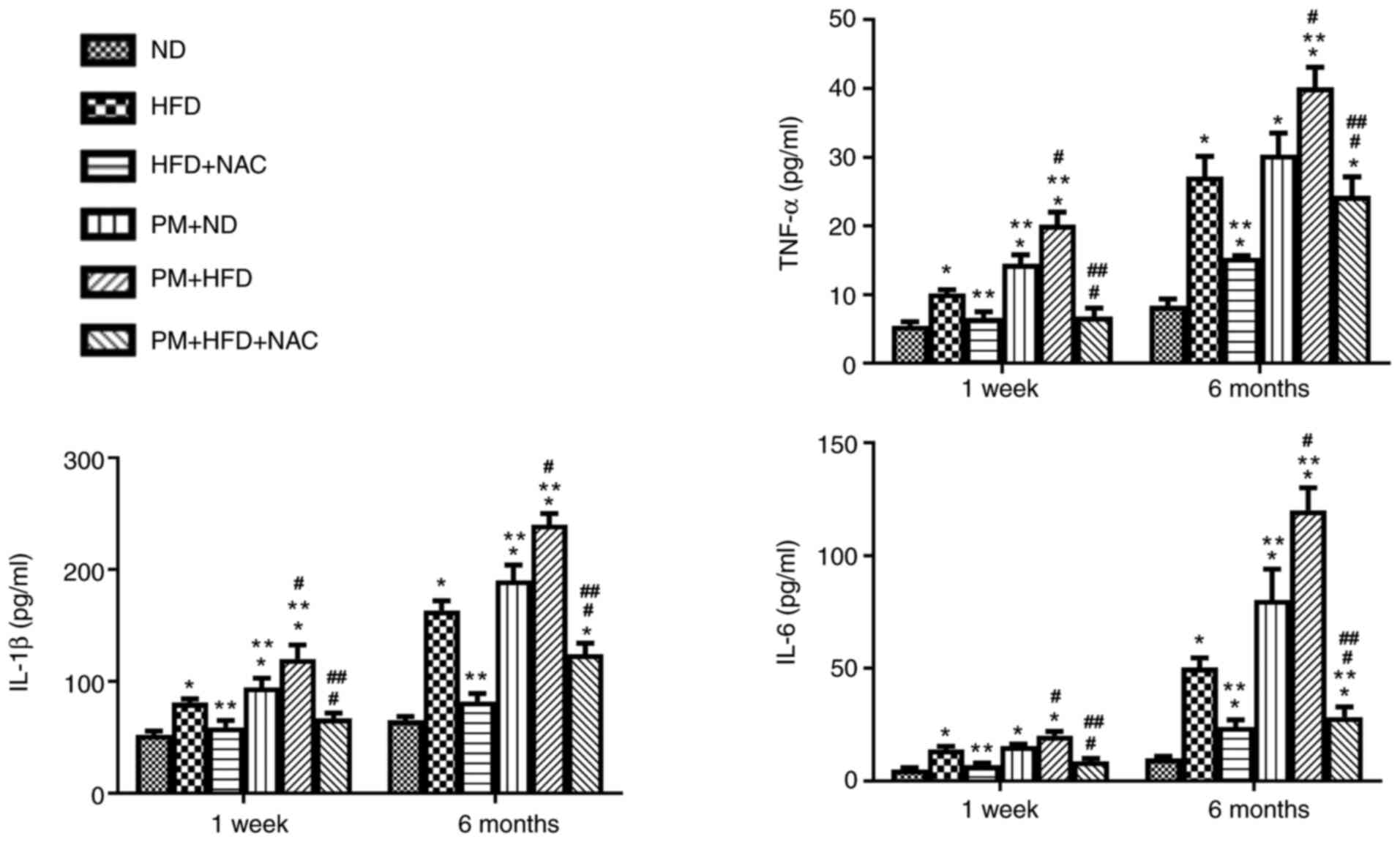
Our previous study reported that NAC exerts powerful
anti-inflammatory effects in WT mice with PM exposure (21). Therefore, the present study
investigated whether PM inhibits production of inflammatory
cytokines, including TNF-α, IL-1β and IL-6, in LDLR KO HFD mice.
Inflammatory cytokine levels were significantly increased up to
1.3-2.0-fold in LDLR KO 1-week and 6-month PM + HFD mice compared
with mice with either HFD or PM + ND (Fig. 5). NAC effectively prevented
production of all three cytokines in PM + HFD mice (Fig. 5).
Discussion
The present study demonstrated that PM exposure
potentiated atherosclerosis formation by promoting ox-LDL levels
and inflammatory cytokine production and decreasing circulating EPC
levels in hyperlipidemic mice. The mechanisms primarily involved
elevated ROS production. NAC effectively reversed both acute and
chronic PM exposure-potentiated effects in LDLR KO HFD mice.
Cardiovascular risk factors, such as hyperlipidemia,
diabetes and age are associated with incomplete revascularization
and decreased re-reendothelialization following arterial injury in
both humans and animals (33–35). Chronic hyperlipidemia exerts
harmful effects on the cardiovascular system, including aggravation
of atherosclerosis due to elevation of ox-LDL and TG levels and
decreased HDL (36). In addition,
short-term hyperlipidemia affects organs primarily via initiating
inflammation (37–39). On the other hand, PM exposure is
associated with atherosclerosis development in both human and
animal studies (1,40). The mechanisms primarily involved
in PM-potentiated atherosclerosis include early changes in vascular
tone via elevated oxidative stress and inflammation, cholesterol
modification and promotion of pro-thrombotic reactions (40). The present data indicated that
acute and chronic PM exposure in hyperlipidemic mice further
increased plasma ox-LDL and inflammatory cytokine levels and
decreased circulating EPCs, which serve an important role in
endothelial repair (1). The
mechanisms primarily involve increased plasma ROS as well as EPC
and mononuclear intracellular ROS production. In addition, males
have a higher risk for CVD than females; females are better
protected than males against CVD due to undefined mechanisms
(41). Our previous study
demonstrated that CD34+/CD133+ EPC population
was selectively decreased in male mice following PM exposure via
elevated oxidative stress, while PM exposure had no effects on
estrogen-independent EPC changes in female mice (42). PM exposure-induced decrease in EPC
population in male mice may be due to decreased expression of
pulmonary superoxide dismutase 1 (43). Therefore, the regulation of
anti-atherosclerotic mechanisms in females requires further
investigation. To the best of our knowledge, there is no way to
determine the exact amount of PM in respiratory tissue following
exposure; however, our previous study indicated that there is
increased inflammation in murine pulmonary tissue following PM
exposure compared with control (21).
NAC is an antioxidant with therapeutic value for
decreasing endothelial dysfunction, inflammation, fibrosis,
invasion, cartilage erosion, acetaminophen detoxification and
prolong transplant life (44). In
cardiovascular studies, NAC protects diabetic heart at risk of
myocardial infarction and prevents ischemia- and
non-ischemia-associated cardiac damage and
ischemia-reperfusion-induced injury primarily via inhibition of
oxidative stress in humans (22,45). NAC is also reported to prevent
hypertrophy and fibrosis in β-MyHC-Q403 transgenic rabbits and
cTnT-Q92 transgenic mice with hypertrophic cardiomyopathy via
multiple thiol-responsive mechanisms (46) and inhibit platelet aggregation and
reperfusion injury in both human and animals (47). In accordance with our and other
previous studies (23,48–50), the present study demonstrated that
NAC had no effect on lipid profile in hyperlipidemia, whereas Korou
et al (51) found NAC
decreases serum LDL levels in hyperlipidemic mice. This difference
may be due to different administration methods. Additionally, NAC
decreases ox-LDL levels via inhibition of oxidative stress; to the
best of our knowledge, however, the detailed mechanism has not
previously been investigated (52–54). NAC prevents atherosclerosis
formation via suppression of inflammation by inhibiting
NF-κB-induced TNF-α production and blood homocysteine levels
(55,56). NAC maintains EPC population,
decrease conversion of LDL to ox-LDL in vivo, prevent
atherosclerosis in humans and mice with hyperlipidemia and promote
hind limb ischemia recovery in mice (15,23,30,57).
NAC exhibits protective effects against
PM-associated damage in different organs (1). NAC could abolishes PM-induced
suppression of lymphocyte function in rats and mice (58), inhibits pulmonary inflammation in
mice following 5 h and 1 month PM exposure (21,59) and prevents PM-induced ROS
production in human endothelial cells (60). To the best of our knowledge,
however, the effect of NAC on air pollution-potentiated
atherosclerosis has not been reported. Certain studies have
reported that NAC prevents senescence of porcine coronary artery
endothelial cells in vitro (61), attenuates carbon monoxide-induced
ischemic heart failure (62),
prevents cardiomyocyte apoptosis (63) and inhibits vascular smooth muscle
proliferation (64) and heart
arrhythmia (65) in rats
following PM exposure. The mechanisms include inhibition of
oxidative stress, deactivation of c-Jun N-terminal kinase, p38 MAP
kinase and inhibition of inflammation via prevention of NF-κB
transcription factor activity (44). NAC prevents apoptosis and promotes
cell survival via extracellular signal-regulated kinase activation
(44). Our previous studies
indicated that NAC inhibits EPC apoptosis, promotes BMSC
proliferation and inhibits inflammatory cytokine production via
blocking ROS generation in mice following PM exposure for 1 month
(21,66). The present data showed that NAC
effectively attenuated PM-potentiated atherosclerosis formation via
inhibition of plasma ROS-induced ox-LDL elevation and mononuclear
cell and EPC intracellular ROS-induced decreases in circulating EPC
levels. Of note, blood inflammatory cytokine levels in mice with
hyperlipidemia following 1 week or 6 month PM exposure were also
significantly decreased by NAC treatment.
Further studies are needed to determine the
mechanism of NAC against PM-potentiated atherosclerosis including
that underlying NAC-induced decrease in ox-LDL levels, inability of
NAC to completely prevent atherosclerosis development and the cell
signaling pathways involved in NAC suppression of inflammatory
cytokine production.
In conclusion, the present study demonstrated that
NAC effectively prevented PM-potentiated atherosclerosis via
inhibition of plasma ROS-induced ox-LDL elevation and mononuclear
cell and EPC intracellular ROS-induced decrease in circulating EPC
levels, as well as suppression of inflammatory cytokine
production.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National Nature Science
Foundation of China (grant nos. 81600222 and 81800255), Young
Experts of Taishan Scholar Program of Shandong Province (grant no.
tsqn201812142), Academic Promotion Programme of Shandong First
Medical University (grant nos. 2019RC017), The Natural Science
Foundation of Shandong Province (grant nos. ZR2016HM22,
ZR2018BH002, ZR2020MH044 and ZR2021MH112) and Clinical Medical
Science and Technology Innovation Development Plan Project of Jinan
in China (grant no. 201704106).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YQC, XCM and LQC designed the experiments. YXX, HRB,
YFJ, QWL, XWG and XQZ performed the experiments. KH, ZHS, QYZ, YC
and HHS collected and analyzed the data. YQC and YXX wrote the
manuscript. All authors have read and approved the final
manuscript. YQC, XCM and YXX confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
All animal procedures were performed in accordance
with the Guidelines of the Animal Care Committee of the Shandong
Provincial Hospital affiliated to Shandong First Medical University
(Jinan, China). The Animal Care Committee of Shandong Provincial
Hospital affiliated to Shandong First Medical University (Jinan,
China) approved the experimental protocols (approval no.
2021-228).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cui Y, Sun Q and Liu Z: Ambient
particulate matter exposure and cardiovascular diseases: A focus on
progenitor and stem cells. J Cell Mol Med. 20:782–793. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Y, Pan J, Fan C, Xu R, Wang Y, Xu C,
Xie S, Zhang H, Cui X, Peng Z, et al: Short-term exposure to
ambient air pollution and mortality from myocardial infarction. J
Am Coll Cardiol. 77:271–281. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah AS, Lee KK, McAllister DA, Hunter A,
Nair H, Whiteley W, Langrish JP, Newby DE and Mills NL: Short term
exposure to air pollution and stroke: Systematic review and
meta-analysis. BMJ. 350:h12952015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajagopalan S, Al-Kindi SG and Brook RD:
Air pollution and cardiovascular disease: JACC state-of-the-art
review. J Am Coll Cardiol. 72:2054–2070. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun Q, Wang A, Jin X, Natanzon A, Duquaine
D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, et al:
Long-term air pollution exposure and acceleration of
atherosclerosis and vascular inflammation in an animal model. JAMA.
294:3003–3010. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davignon J and Ganz P: Role of endothelial
dysfunction in atherosclerosis. Circulation. 109 (23 Suppl
1):III27–III32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heitzer T, Schlinzig T, Krohn K, Meinertz
T and Munzel T: Endothelial dysfunction, oxidative stress, and risk
of cardiovascular events in patients with coronary artery disease.
Circulation. 104:2673–2678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rauscher FM, Goldschmidt-Clermont PJ,
Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C
and Taylor DA: Aging, progenitor cell exhaustion, and
atherosclerosis. Circulation. 108:457–463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strehlow K, Werner N, Berweiler J, Link A,
Dirnagl U, Priller J, Laufs K, Ghaeni L, Milosevic M, Böhm M and
Nickenig G: Estrogen increases bone marrow-derived endothelial
progenitor cell production and diminishes neointima formation.
Circulation. 107:3059–3065. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: Characterization and role in vascular biology.
Circ Res. 95:343–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Werner N, Junk S, Laufs U, Link A, Walenta
K, Bohm M and Nickenig G: Intravenous transfusion of endothelial
progenitor cells reduces neointima formation after vascular injury.
Circ Res. 93:e17–e24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen JZ, Zhang FR, Tao QM, Wang XX and Zhu
JH and Zhu JH: Number and activity of endothelial progenitor cells
from peripheral blood in patients with hypercholesterolaemia. Clin
Sci (Lond). 107:273–280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vasa M, Fichtlscherer S, Aicher A, Adler
K, Urbich C, Martin H, Zeiher AM and Dimmeler S: Number and
migratory activity of circulating endothelial progenitor cells
inversely correlate with risk factors for coronary artery disease.
Circ Res. 89:E1–E7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kattoor AJ, Pothineni NVK, Palagiri D and
Mehta JL: Oxidative stress in atherosclerosis. Curr Atheroscler
Rep. 19:422017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui Y, Narasimhulu CA, Liu L, Li X, Xiao
Y, Zhang J, Xie X, Hao H, Liu JZ, He G, et al: Oxidized low-density
lipoprotein alters endothelial progenitor cell populations. Front
Biosci (Landmark Ed). 20:975–988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Auten RL and Davis JM: Oxygen toxicity and
reactive oxygen species: The devil is in the details. Pediatr Res.
66:121–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogasawara MA and Zhang H: Redox regulation
and its emerging roles in stem cells and stem-like cancer cells.
Antioxid Redox Signal. 11:1107–1122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pervaiz S, Taneja R and Ghaffari S:
Oxidative stress regulation of stem and progenitor cells. Antioxid
Redox Signal. 11:2777–2789. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cominacini L, Garbin U, Pasini AF, Davoli
A, Campagnola M, Pastorino AM, Gaviraghi G and Lo Cascio V:
Oxidized low-density lipoprotein increases the production of
intracellular reactive oxygen species in endothelial cells:
Inhibitory effect of lacidipine. J Hypertens. 16:1913–1919. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vasconcelos EM, Degasperi GR, de Oliveira
HC, Vercesi AE, de Faria EC and Castilho LN: Reactive oxygen
species generation in peripheral blood monocytes and oxidized LDL
are increased in hyperlipidemic patients. Clin Biochem.
42:1222–1227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui Y, Xie X, Jia F, He J, Li Z, Fu M, Hao
H, Liu Y, Liu JZ, Cowan PJ, et al: Ambient fine particulate matter
induces apoptosis of endothelial progenitor cells through reactive
oxygen species formation. Cell Physiol Biochem. 35:353–363. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dludla PV, Dias SC, Obonye N, Johnson R,
Louw J and Nkambule BB: A systematic review on the protective
effect of N-Acetyl cysteine against diabetes-associated
cardiovascular complications. Am J Cardiovasc Drugs. 18:283–298.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui Y, Narasimhulu CA, Liu L, Zhang Q, Liu
PZ, Li X, Xiao Y, Zhang J, Hao H, Xie X, et al: N-acetylcysteine
inhibits in vivo oxidation of native low-density lipoprotein. Sci
Rep. 5:163392015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mutlu GM, Green D, Bellmeyer A, Baker CM,
Burgess Z, Rajamannan N, Christman JW, Foiles N, Kamp DW, Ghio AJ,
et al: Ambient particulate matter accelerates coagulation via an
IL-6-dependent pathway. J Clin Invest. 117:2952–2961. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gavett SH, Haykal-Coates N, Highfill JW,
Ledbetter AD, Chen LC, Cohen MD, Harkema JR, Wagner JG and Costa
DL: World Trade Center fine particulate matter causes respiratory
tract hyperresponsiveness in mice. Environ Health Perspect.
111:981–991. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shadie AM, Herbert C and Kumar RK: Ambient
particulate matter induces an exacerbation of airway inflammation
in experimental asthma: Role of interleukin-33. Clin Exp Immunol.
177:491–499. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Holt GR: Effects of air pollution on the
upper aerodigestive tract. Otolaryngol Head Neck Surg. 114:201–204.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aggarwal DJ, Kathariya MG and Verma DPK:
LDL-C, NON-HDL-C and APO-B for cardiovascular risk assessment:
Looking for the ideal marker. Indian Heart J. 73:544–548. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhuo X, Bu H, Hu K, Si Z, Chen L, Chen Y,
Yang L, Jiang Y, Xu Y, Zhao P, et al: Differences in the reaction
of hyperlipidemia on different endothelial progenitor cells based
on sex. Biomed Rep. 15:642021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cui Y, Liu L, Xiao Y, Li X, Zhang J, Xie
X, Tian J, Sen CK, He X, Hao H and Liu Z: N-acetylcysteine
differentially regulates the populations of bone marrow and
circulating endothelial progenitor cells in mice with limb
ischemia. Eur J Pharmacol. 881:1732332020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rosenkranz AR, Schmaldienst S, Stuhlmeier
KM, Chen W, Knapp W and Zlabinger GJ: A microplate assay for the
detection of oxidative products using
2′,7′-dichlorofluorescin-diacetate. J Immunol Methods. 156:39–45.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eggermann J, Kliche S, Jarmy G, Hoffmann
K, Mayr-Beyrle U, Debatin KM, Waltenberger J and Beltinger C:
Endothelial progenitor cell culture and differentiation in vitro: A
methodological comparison using human umbilical cord blood.
Cardiovasc Res. 58:478–486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ii M, Takenaka H, Asai J, Ibusuki K,
Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, et
al: Endothelial progenitor thrombospondin-1 mediates
diabetes-induced delay in reendothelialization following arterial
injury. Circ Res. 98:697–704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kinnaird T, Stabile E, Zbinden S, Burnett
MS and Epstein SE: Cardiovascular risk factors impair native
collateral development and may impair efficacy of therapeutic
interventions. Cardiovasc Res. 78:257–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sodha NR, Clements RT, Boodhwani M, Xu SH,
Laham RJ, Bianchi C and Sellke FW: Endostatin and angiostatin are
increased in diabetic patients with coronary artery disease and
associated with impaired coronary collateral formation. Am J
Physiol Heart Circ Physiol. 296:H428–H434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Panahi Y, Ahmadi Y, Teymouri M, Johnston
TP and Sahebkar A: Curcumin as a potential candidate for treating
hyperlipidemia: A review of cellular and metabolic mechanisms. J
Cell Physiol. 233:141–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Krauzova E, Kracmerova J, Rossmeislova L,
Mališová L, Tencerová M, Koc M, Štich V and Šiklová M: Acute
hyperlipidemia initiates proinflammatory and proatherogenic changes
in circulation and adipose tissue in obese women. Atherosclerosis.
250:151–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He L, Hao L, Fu X, Huang M and Li R:
Severe hypertriglyceridemia and hypercholesterolemia accelerating
renal injury: A novel model of type 1 diabetic hamsters induced by
short-term high-fat/high-cholesterol diet and low-dose
streptozotocin. BMC Nephrol. 16:512015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krysiak R, Zmuda W, Marek B and Okopien B:
Comparison of the effects of short-term hypolipidaemic treatment on
plasma adipokine levels in men and women with isolated
hypercholesterolaemia. Endokrynol Pol. 66:114–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bevan GH, Al-Kindi SG, Brook R and
Rajagopalan S: Ambient air pollution and atherosclerosis: Recent
updates. Curr Atheroscler Rep. 23:632021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kander MC, Cui Y and Liu Z: Gender
difference in oxidative stress: A new look at the mechanisms for
cardiovascular diseases. J Cell Mol Med. 21:1024–1032. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu X, Xiao Y, Zhu Q, Cui Y, Hao H, Wang
M, Cowan PJ, Korthuis RJ, Li G, Sun Q and Liu Z: Circulating
endothelial progenitor cells are preserved in female mice exposed
to ambient fine particulate matter independent of estrogen. Int J
Mol Sci. 22:72002021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu X, Wang A, Chen Z, Cui Y, Hao H,
Domeier TL, Sun Q and Liu Z: Tempol preserves endothelial
progenitor cells in male mice with ambient fine particulate matter
exposure. Biomedicines. 10:3272022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zafarullah M, Li WQ, Sylvester J and Ahmad
M: Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life
Sci. 60:6–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bartekova M, Barancik M, Ferenczyova K and
Dhalla NS: Beneficial effects of N-acetylcysteine and
N-mercaptopropionylglycine on ischemia reperfusion injury in the
heart. Curr Med Chem. 25:355–366. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Marian AJ: Experimental therapies in
hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2:483–492.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nikbakht M, Ahmadi F, Vaseghi G, Talasaz
AH, Eshraghi A, Naderi J and Daneshmehr MA: The role of
N-acetylcysteine in platelet aggregation and reperfusion injury in
recent years. Curr Clin Pharmacol. 12:83–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin CP, Huang PH, Lai CF, Chen JW, Lin SJ
and Chen JS: Simvastatin attenuates oxidative stress, NF-κB
activation, and artery calcification in LDLR-/-mice fed with high
fat diet via down-regulation of tumor necrosis factor-α and TNF
receptor 1. PLoS One. 10:e01436862015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Subrahmanian S, Varshney R, Subramani K,
Murphy B, Woolington S and Ahamed J: N-Acetylcysteine inhibits
aortic stenosis progression in a murine model by blocking
Shear-induced activation of platelet latent transforming growth
factor beta 1. Antioxid Redox Signal. Dec 7–2021.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Spartalis M, Siasos G, Mastrogeorgiou M,
Spartalis E, Kaminiotis VV, Mylonas KS, Kapelouzou A, Kontogiannis
C, Doulamis IP, Toutouzas K, et al: The effect of per os colchicine
administration in combination with fenofibrate and N-acetylcysteine
on triglyceride levels and the development of atherosclerotic
lesions in cholesterol-fed rabbits. Eur Rev Med Pharmacol Sci.
25:7765–7776. 2021.PubMed/NCBI
|
|
51
|
Korou LM, Agrogiannis G, Pantopoulou A,
Vlachos IS, Iliopoulos D, Karatzas T and Perrea DN: Comparative
antilipidemic effect of N-acetylcysteine and sesame oil
administration in diet-induced hypercholesterolemic mice. Lipids
Health Dis. 9:232010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Meng XP, Yin CS, Cui JH, Li ZX, Wang L,
Wang YW and Li YL: Inhibitory effect of N-acetylcysteine upon
atherosclerotic processes in rabbit carotid. Zhonghua Yi Xue Za
Zhi. 89:1850–1853. 2009.(In Chinese). PubMed/NCBI
|
|
53
|
Sung HJ, Kim J, Kim Y, Jang SW and Ko J:
N-acetyl cysteine suppresses the foam cell formation that is
induced by oxidized low density lipoprotein via regulation of gene
expression. Mol Biol Rep. 39:3001–3007. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Thiele H, Hildebrand L, Schirdewahn C,
Eitel I, Adams V, Fuernau G, Erbs S, Linke A, Diederich KW, Nowak
M, et al: Impact of high-dose N-acetylcysteine versus placebo on
contrast-induced nephropathy and myocardial reperfusion injury in
unselected patients with ST-segment elevation myocardial infarction
undergoing primary percutaneous coronary intervention. The
LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled,
Randomized Leipzig Immediate PercutaneouS Coronary Intervention
Acute Myocardial Infarction N-ACC) Trial. J Am Coll Cardiol.
55:2201–2209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Poitevin S, Garnotel R, Antonicelli F,
Gillery P and Nguyen P: Type I collagen induces tissue factor
expression and matrix metalloproteinase 9 production in human
primary monocytes through a redox-sensitive pathway. J Thromb
Haemost. 6:1586–1594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wiklund O, Fager G, Andersson A, Lundstam
U, Masson P and Hultberg B: N-acetylcysteine treatment lowers
plasma homocysteine but not serum lipoprotein(a) levels.
Atherosclerosis. 119:99–106. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhu Q, Hao H, Xu H, Fichman Y, Cui Y, Yang
C, Wang M, Mittler R, Hill MA, Cowan PJ, et al: Combination of
antioxidant enzyme overexpression and N-acetylcysteine treatment
enhances the survival of bone marrow mesenchymal stromal cells in
ischemic limb in mice with type 2 diabetes. Am Heart Assoc.
10:e0234912021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Omara FO, Fournier M, Vincent R and
Blakley BR: Suppression of rat and mouse lymphocyte function by
urban air particulates (Ottawa dust) is reversed by
N-acetylcysteine. J Toxicol Environ Health A. 59:67–85. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rhoden CR, Lawrence J, Godleski JJ and
Gonzalez-Flecha B: N-acetylcysteine prevents lung inflammation
after short-term inhalation exposure to concentrated ambient
particles. Toxicol Sci. 79:296–303. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang T, Wang L, Moreno-Vinasco L, Lang GD,
Siegler JH, Mathew B, Usatyuk PV, Samet JM, Geyh AS, Breysse PN, et
al: Particulate matter air pollution disrupts endothelial cell
barrier via calpain-mediated tight junction protein degradation.
Part Fibre Toxicol. 9:352012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sharma K, Lee HH, Gong DS, Park SH, Yi E,
Schini-Kerth V and Oak MH: Fine air pollution particles induce
endothelial senescence via redox-sensitive activation of local
angiotensin system. Environ Pollut. 252((Pt A)): 317–329. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Reboul C, Boissiere J, Andre L, Meyer G,
Bideaux P, Fouret G, Feillet-Coudray C, Obert P, Lacampagne A,
Thireau J, et al: Carbon monoxide pollution aggravates ischemic
heart failure through oxidative stress pathway. Sci Rep.
7:397152017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kim JB, Kim C, Choi E, Park S, Park H, Pak
HN, Lee MH, Shin DC, Hwang KC and Joung B: Particulate air
pollution induces arrhythmia via oxidative stress and calcium
calmodulin kinase II activation. Toxicol Appl Pharmacol. 259:66–73.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tzeng HP, Yang RS, Ueng TH and Liu SH:
Upregulation of cyclooxygenase-2 by motorcycle exhaust
particulate-induced reactive oxygen species enhances rat vascular
smooth muscle cell proliferation. Chem Res Toxicol. 20:1170–1176.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rhoden CR, Wellenius GA, Ghelfi E,
Lawrence J and Gonzalez-Flecha B: PM-induced cardiac oxidative
stress and dysfunction are mediated by autonomic stimulation.
Biochim Biophys Acta. 1725:305–313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cui Y, Jia F, He J, Xie X, Li Z, Fu M, Hao
H, Liu Y, Liu DZ, Cowan PJ, et al: Ambient fine particulate matter
suppresses in vivo proliferation of bone marrow stem cells through
reactive oxygen species formation. PLoS One. 10:e01273092015.
View Article : Google Scholar : PubMed/NCBI
|