Introduction
Serotonin (5-hydroxytryptamine, 5-HT) has important
biological functions that are mediated via 5-HT receptors. Seven
families of 5-HT receptors, designated from 5-HT1 to 5-HT7, are
currently recognized and more than sixteen subtypes have been
identified in humans. With the exception of the 5-HT3 receptor, the
receptors are members of the seven transmembrane domain G
protein-coupled receptor family, while the 5-HT3 receptor is a
ligand-gated ion channel belonging to the Cys-loop superfamily of
pentameric proteins (1).
The majority of 5-HT in the body is produced by
enterochromaffin cells in the gut. 5-HT receptors are widely
distributed in the gastrointestinal mucosa and muscle layers, and
play an important role in the functional mediation of the
gastrointestinal tract (2). It has
been found that 5-HT receptors are widely expressed in the
gastrointestinal tracts of mammals, such as the rat and opossum
(3). However, there is little
information available concerning 5-HT receptor expression in the
human lower esophageal sphincter (LES). The LES is an important
physiological structure at the esophagogastric junction.
Abnormalities in the LES are closely associated with dysfunction in
gastrointestinal motility disorders such as achalasia and
gastroesophageal reflux disease (GERD) (4).
The objective of the present study was to detect
5-HT receptors in the human LES, in particular, within the clasp
and sling fibers of the LES. Following the identification of their
expression patterns, the role of the 5-HT receptors in the
modulation of human LES function was further investigated.
Materials and methods
Patients and tissue retrieval
The experimental protocol has been approved by the
Research Ethics Committee of the Fourth Hospital of Hebei Medical
University (Shijiazhuang, China). Written informed consent was
obtained from the patients. Muscle strips were collected from 28
patients who underwent esophagectomy for mid-third esophageal
carcinoma in the Department of Thoracic Surgery at this hospital
from March 2012 to August 2012. There were 16 males and 12 females,
with an average age of 58 years (range, 50 to 67 years). Patients
with a history of GERD, achalasia, scleroderma, or other diseases
associated with a disorder of the LES were excluded from the study.
Each specimen was resected en bloc in the operating room, and the
fresh specimen was placed immediately in ice-cold Krebs solution.
The Krebs solution had the following composition (in mM): sodium,
143.0; potassium, 5.0; calcium, 2.5; magnesium, 1.2; chloride,
128.0; phosphate, 2.2; bicarbonate, 24.9; sulfate, 1.2; and
glucose, 10.0. Specimens were not included in this study if any
segment that was required for study contained a macroscopically
visible tumor.
In the laboratory, the fresh specimens of the
gastroesophageal junction were opened along the long axis of the
esophagus and the greater curvature of the stomach. Specimens were
pinned to a wax plate in the presence of Krebs solution at 37°C to
maintain its approximate in situ dimensions, with a
continuous supply of mixed gas of 95% O2 and 5%
CO2. The mucosa and submucosa were then removed by
dissection.
The sling and clasp fibers were identified as
thickened bands of circular oriented smooth muscle in the gastric
cardia, adjacent to the greater and lesser curvature of the
stomach, respectively. The sling and clasp muscle strips were
prepared using a previously described method (5,6). In
addition, circular muscle fibers from the esophagus above the LES,
and circular muscle fibers from the gastric fundus below the LES,
were dissected for use as control specimens. These circular muscle
strips were obtained 3 cm proximal and distal to the
gastro-esophageal junction. All specimens were removed carefully to
ensure that the myenteric plexus and the longitudinal muscle in the
wall of the esophagus and stomach were excluded. The dissected
muscle strips from the four parts were frozen in liquid nitrogen
and stored at −80°C for subsequent RNA extraction.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR) for 5-HT receptor
analysis
Tissue was homogenized in TRIzol reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA) at a ratio of 100 mg tissue
to 1 ml TRIzol, and then centrifuged at 12,000 × g for 5 min. Total
RNA was extracted by acid guanidinium thiocyanate-phenol-chloroform
extraction. The quality of the RNA was verified by agarose gel
electrophoresis using ethidium bromide staining. First-strand cDNA
synthesis (reaction volume, 20 μl) using 2 mg RNA was performed by
RT reaction in the presence of RevertAid Moloney Murine Leukemia
Virus (M-MuLV) reverse transcriptase (Fermentas, Thermo Fisher
Scientific, Waltham, MA, USA). RT was conducted with 0.5 mg
oligo(dT)18, using diethypyrocarbonate (DEPC)-treated
water to achieve a volume of 11 μl, and the reaction mixture was
incubated at 70°C for 5 min, prior to being chilled on ice. Next, 4
μl 5X reaction buffer (Fermentas), 2 μl 10 mM 4 dNTP mix and 20
units RNasin (both from Fermentas) were added, using DEPC-treated
water to provide a reaction volume of 19 μl, and the mixture was
incubated at 37°C for 5 min. Finally, 200 units (1 μl) RevertAid
M-MuLV reverse transcriptase was added, and the reaction mixture
was incubated at 37°C for 50 min, before the reaction was stopped
and held at 70°C for 15 min.
PCR amplification of the cDNA was performed using
primers designed specifically to match the 5-HT receptor mRNA
(primers listed in Table I). In
each PCR reaction, 2 μl cDNA reaction mixture was used. PCR was
performed in a 20-μl reaction volume. The amplification conditions
were: 5 min initial denaturation at 95°C, then 36 cycles of 95°C
for 35 sec, 60°C for 35 sec and 72°C for 45 sec followed by 72°C
for 5 min. A negative control in which all the components of the
reaction were added, except the cDNA template was tested in
parallel with each sample to identify any risk of false positive
results. The amplified products were electrophoresed on a 1.5%
agarose gel and PCR products were determined using the Gel-Pro gel
image analysis system (Media Cybernetics, Silver Spring, Maryland,
USA). Densitometry for analysis of the PCR product bands was
conducted by the imaging software. The relative expression level of
each gene was normalized by the value of β-actin. The amplified
products were analyzed by electrophoresis on 1.5% agarose gels and
visualized by ethidium bromide staining, with images captured by
photography under a UV transilluminator. The integrated optical
density (IOD) of the gel was calculated with Gel-Pro software
(Media Cybernetics). The relative expression level of the mRNA of
each 5-HT receptor type was normalized by the value of β-actin.
 | Table IPrimer sequences and expected product
sizes for RT-PCR. |
Table I
Primer sequences and expected product
sizes for RT-PCR.
| Gene | Primer pair
sequence (sense/antisense) | Product size
(bp) |
|---|
|
5-HT1AR |
5′-GGCGGCAACACTACTGGTAT-3′
5′-AGCCAAGTGAGCGAGATGAG -3′ | 422 |
|
5-HT2AR |
5′-ACTCGCCGATGATAACTTTGTCCT-3′
5′-TGACGGCCATGATGTTTGTGAT-3′ | 359 |
|
5-HT3AR |
5′-CCGGCGGCCCCTCTTCTAT-3′
5′-GCAAAGTAGCCAGGCGATTCTCT-3′ | 448/352 |
|
5-HT4R |
5′-GGCCTTCTACATCCCATTTCTCCT-3′
5′-CTTCGGTAGCGCTCATCATCACA-3′ | 411 |
|
5-HT5AR |
5′-CCCTTCTGCAAGTACCCCAG-3′
5′-ATGACGTTGGAGACGCACTT-3′ | 522 |
|
5-HT6R |
5′-CCGCCGGCCATGCTGAACG-3′
5′-GCCCGACGCCACAAGGACAAAAG-3′ | 342 |
|
5-HT7R |
5′-GCGCTGGCCGACCTCTC-3′
5′-TCTTCCTGGCAGCCTTGTAAATCT-3′ | 436 |
| β-actin |
5′-GTGGGGCGCCCCAGGCACCA-3′
5′-CTCCTTAATGTCACGCACGATTTC-3′ | 540 |
Quantification by quantitative PCR
(qPCR)
The qPCR experiments were conducted using an Applied
Biosystem 7500 Real-Time PCR System (Applied Biosystems, Foster
City, CA, USA) and data were analyzed with ABI 7500 (version 2.0.6)
software. All oligonucleotide primers for qPCR were designed using
Primer 3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3www.cgi)
and synthesized by Invitrogen Life Technologies. The diluted cDNA
(1 μl from each sample) was used as PCR template. The reaction
composition contained: 1 μl diluted cDNA; 12.5 μl 2X TransStart Top
Green qPCR SuperMix (TransGen Biotech, Beijing, China.), 10 μl
ddH2O, 0.5 μl forward primer and 0.5 μl reverse primer,
and 0.5 μl Passive Reference Dye (TransGen Biotech) in a final
volume of 25 μl. The cDNA was amplified by one cycle at 94°C for 30
sec, followed by 42 cycles of 95°C for 5 sec, 60°C for 34 sec and
melt curve analysis. Reactions were performed in triplicate
according to the manufacturers’ instructions (TransGen Biotech).
Melting point and melting curve analyses were undertaken on each
set of reactions to confirm that only a single product was
produced. No primer-dimers were detected by melting point analysis
and this was confirmed in preliminary runs with gel
electrophoresis. The expression level of the target gene was
calculated by the 2−ΔΔCt method (7). The relative expression of target gene
mRNA was indexed to the reference gene β-actin using the formula:
10,000 × 2ΔCt, in which ΔCt = Ctβ-actin -
Cttarget gene.
Western blot analysis of 5-HT
receptors
Total proteins were extracted from the muscle strips
using a protein extraction kit (Solarbio, Beijing, China). Protein
concentration was determined using a colorimetric bicinchoninic
acid (BCA) protein assay reagent (Multisciences, Hangzhou, China).
Following denaturation at 100°C for 10 min, aliquots of protein
samples (30 μg) were separated by electrophoresis on
SDS-polyacrylamide gel 10% separation gel and 4% pycnotic gel,
separated at 150 V for 1 h, and transferred onto a polyvinylidene
difluoride (PVDF) membrane, which was then blocked for 1 h with 5%
non-fat milk in Tris-buffered saline with Tween 20 (TBST) at room
temperature, and incubated with an anti-human, polyclonal primary
antibody (dilutions: rabbit anti-5HT1AR, 1:300; rabbit
anti-5HT2AR, 1:400; rabbit anti-5HT3AR,
1:500; rabbit anti-5HT4R, 1:500; rabbit
anti-5HT5AR, 1:300; rabbit anti-5HT6R, 1:300;
rabbit anti-5HT7R, 1:300; 1:10,000 for the rabbit
anti-β-actin; Abcam Trading, Shanghai, China) at 4°C overnight.
After washing three times with TBST at room temperature for 30 min
in total, the membrane was incubated with a goat anti-rabbit IgG
polyclonal secondary antibody (1:2,000, anti-rabbit IgG; Abcam
Trading) for 1 h. After three washes with TBST, the membrane was
analyzed using an infrared fluorescence imaging instrument (Odyssey
Infrared Imaging System, American LI-COR, Lincoln, Nebraska, USA).
The IOD value was calculated by the Gel-Pro software and the
relative expression level of each protein was normalized by the
value of β-actin.
Statistical analysis
Results are expressed as the mean ± standard
deviation (SD). SAS software, version 9.2 (SAS Institute Inc.,
Cary, NC, USA) was used to conduct the statistical analysis.
Differences in the mRNA and protein expression levels were analyzed
with one-way analysis of variance, and the Student-Newman-Keuls
multiple range (SNK-q) test was used to evaluate comparisons within
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Characterization of mRNA encoding 5-HT
receptors
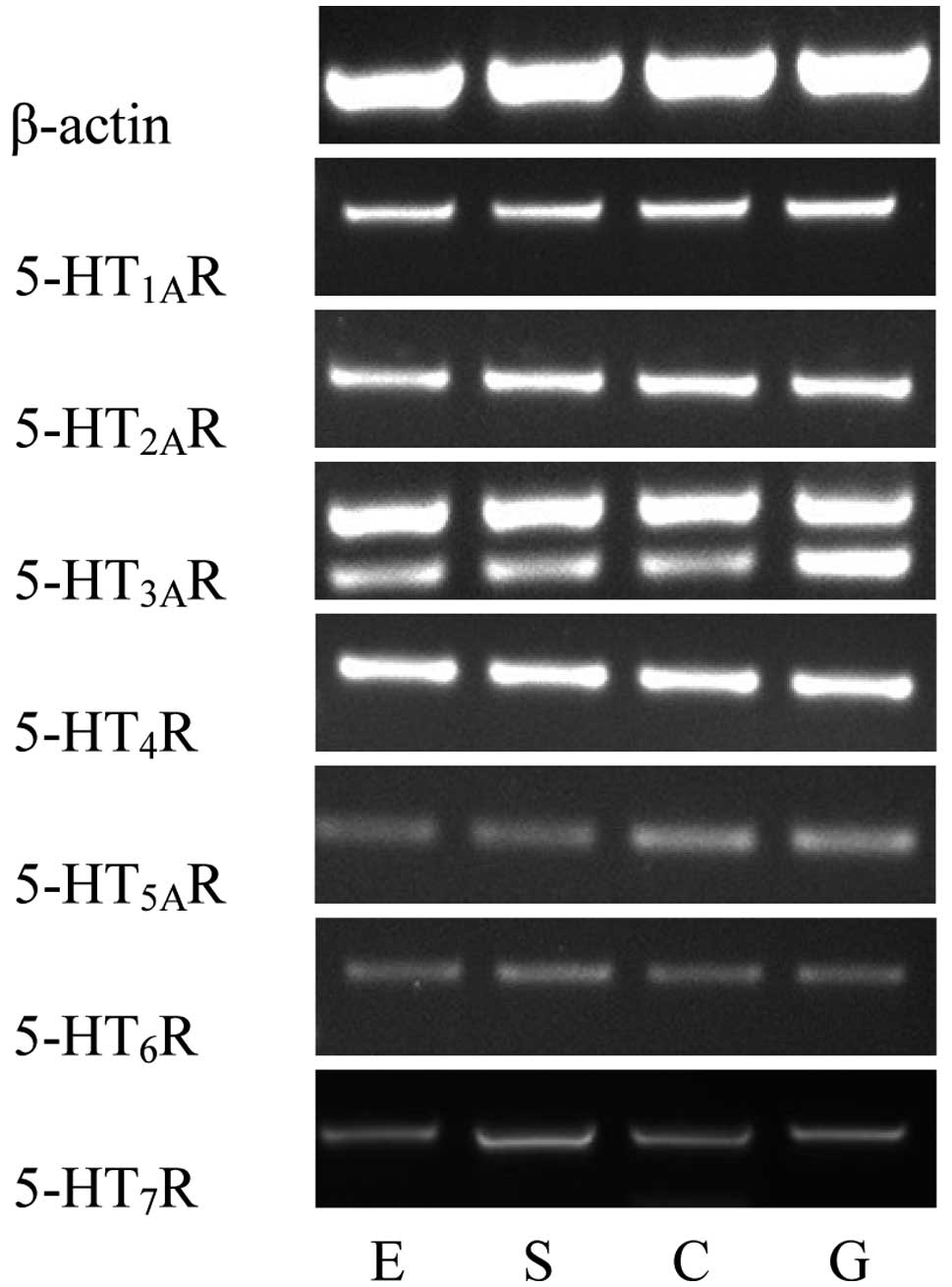
Using RT-PCR, the mRNA expression levels of seven
5-HT receptors in the human LES were determined. Distinct bands of
the expected sizes were detected for each of the seven 5-HT
receptor mRNAs and their levels of expression appeared to differ.
Similar results were obtained in all PCR assays performed on mRNA
extracted from the 28 patients. The primer pairs designed to
recognize the 5-HT3AR and 5-HT4R mRNA
generated strong bands, indicating high expression levels of the
5-HT3AR and 5-HT4R mRNA. The
5-HT1AR and 5-HT2AR mRNA also generated
comparatively strong bands. However, the primer pairs for the
5-HT7R, 5-HT5AR and 5-HT6R mRNA
produced relatively weak bands (Fig.
1).
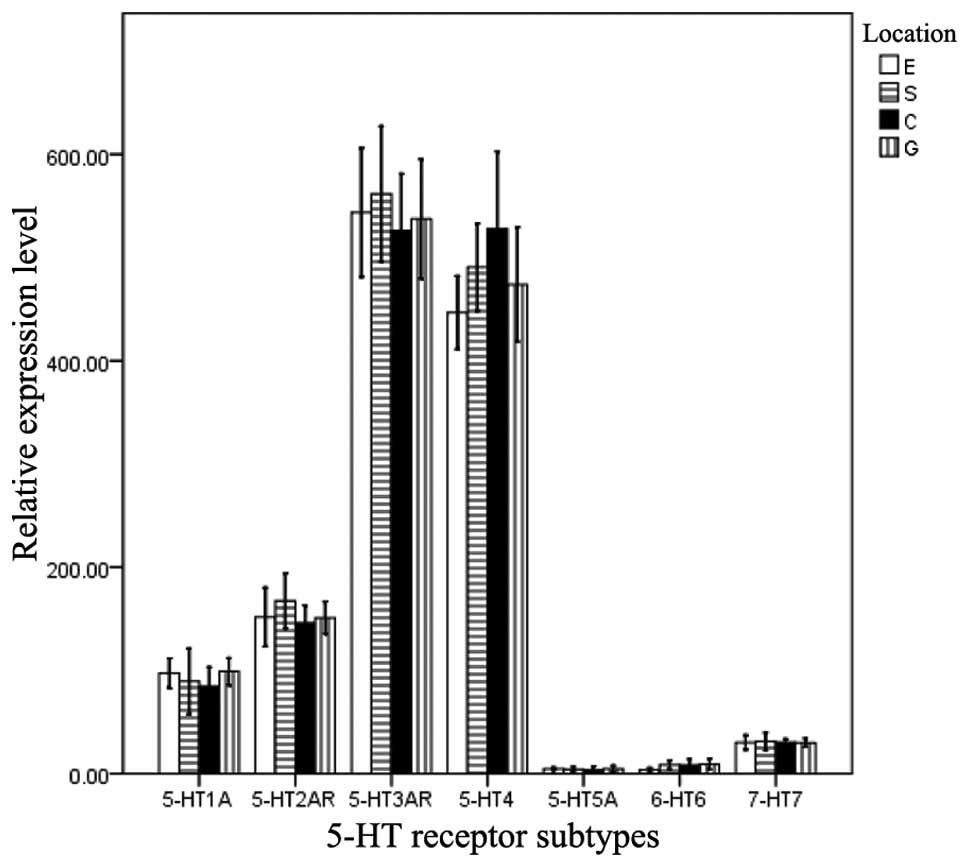
Quantification of 5-HT receptor mRNA
expression
To compare the expression levels of the seven
different 5-HT receptor mRNAs, qPCR was performed. Significant
differences were identified when the mRNA expression levels of the
5-HT receptors were compared in the same muscle strip (F, 78.281;
P=0.000). The rank order of expression was as follows:
5-HT3AR = 5-HT4R > 5-HT1AR =
5-HT2AR > 5-HT5AR = 5-HT6R =
5-HT7R. However, no significant difference was observed
in the mRNA expression levels of the 5-HT receptors among the four
types of muscle strip (F, 0.232; P=0.731; Fig. 2).
Expression of 5-HT receptor proteins
With the exception of 5-HT5AR and
5-HT6R proteins, the other five receptors were
identified. Significant differences in the IOD values for the
different 5-HT receptors in the same muscle strip were observed (F,
657.357; P=0.000). The rank order of the IOD values was as follows:
5-HT3AR = 5-HT4R > 5-HT1AR =
5-HT2AR > 5-HT7R. No significant
differences in IOD values were identified among the four type of
muscle strip (F, 0.194; P=0.801; Fig.
3).
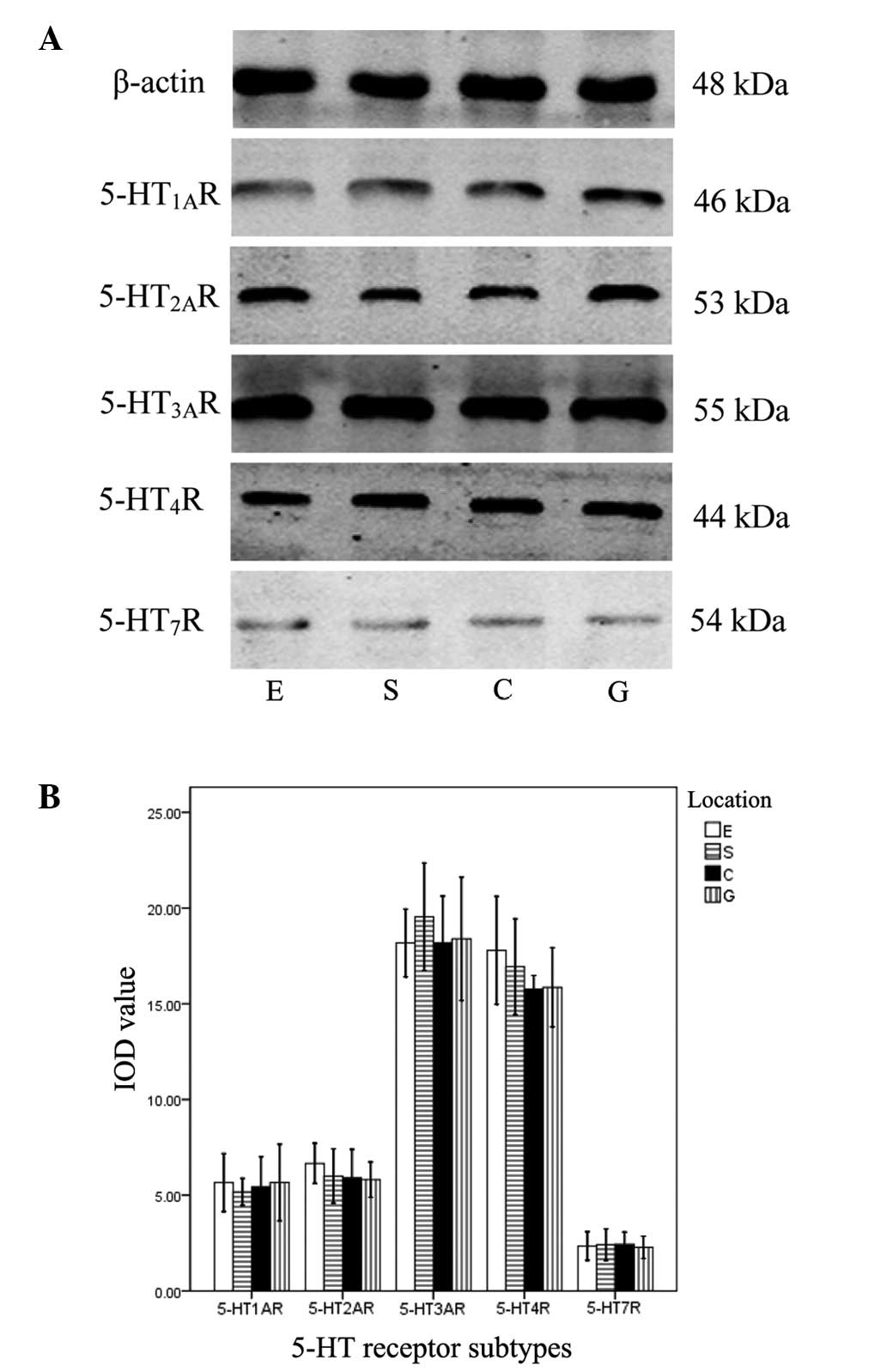 | Figure 3Expression of 5-HT receptor subtypes
determined by western blot analysis in the sling and clasp fibers
of the LES and circular muscle strips of the esophagus and stomach.
(A) Bands of five 5-HT receptor subtypes in E, S, C and G were
identified by western blotting. (B) IOD values of the bands. There
were significant differences between the 5-HT subtypes in the same
muscle strip (P<0.05), but no significant differences were
identified in a single subtype between the four types of muscle
strip (P>0.05). 5-HT, serotonin; LES, lower esophageal
sphincter; IOD, integrated optical density; E, circular muscle
strips of esophagus; S, sling fibers; C, clasp fibers; G,
stomach. |
Discussion
Serotonin (5-HT), as a predominant neurotransmitter,
controls a variety of functions, including locomotor activity,
cognition, emotion, food intake and endocrine regulation, via
effects on 5-HT receptors. Currently, seven 5-HT receptors and more
than sixteen 5-HT receptor subtypes have been identified as members
of the G protein-coupled receptor or ligand-gated ion channel
families. More specifically, these are the receptor subtypes of
5-HT1A, 5-HT1B, 5-HT1C, 5-HT1D, 5-HT1E, 5-HT1F, 5-HT2A, 5-HT2B,
5-HT2C, 5-HT3A, 5-HT3B, 5-HT4, 5-HT5A, 5-HT5B, 5-HT6 and 5-HT7, of
which the 5-HT7 receptor is divided into 5-HT7(a), 5-HT7(b) and
5-HT7(c) in humans. The 5-HT receptors are widely distributed in
the central and peripheral nervous system, cardiovascular system
and gastrointestinal tract in mammals, and have been shown to play
significant physiological roles (8–10).
Various esophageal motility disorders, such as achalasia, diffuse
esophageal spasm and nutcracker esophagus, are associated with
motor dysfunction of the LES (11). The regulatory mechanism of the LES
involves various receptors (12),
neurotransmitters and signal transduction pathways., including CCK,
muscarinic and dopamine receptors (5,6,13).
5-HT receptors are widely distributed in smooth
muscle in various parts of the body, including the gastrointestinal
tract, where they control aspects of gastrointestinal motility and
secretion (14). 5-HT and
serotonergic agonists and antagonists have been found to exert
pharmacological effects on various regions of the gut. For example,
5-HT3 receptor antagonists have antiemetic activity and
5-HT4 receptor agonists are used to promote
gastrointestinal peristalsis in the clinic (15,16).
Previous studies have evaluated the distribution of 5-HT receptors
in the gastrointestinal tract. Mader et al (17) found that 5-HT4 receptor
mRNA expression was present throughout the gastrointestinal tract
in humans and primates, which supports findings that
5-HT4 receptors exhibit multiple effects in the
gastrointestinal system. Champaneria et al (18) identified the expression of the
5-HT3 receptor in rat gut. Irving et al (19) evaluated 5-HT3,
5-HT4 and 5-HT7 receptor expression and
compared 5-HT4 and 5-HT7 receptor function in
the circular muscle of the human colon. In addition, the
5-HT1A receptor was identified in guinea pig and human
intestine by Wang et al (20). These findings prompt the hypothesis
that 5-HT receptors are located throughout the gastrointestinal
tract. However, prior to the present study, little information was
available concerning the distribution of 5-HT receptors in the LES.
The present study was designed to determine whether 5-HT receptors
exist in the region of the human LES. The LES is a complex
structure comprising clasp and sling fiber muscle strips in the
gastric cardia, and circular muscle fibers in the distal end of the
esophagus immediately above the gastroesophageal junction. In the
present study, the clasp and sling fiber muscle strip component of
the LES was investigated. 5-HT5 and 5-HT6
receptors mainly exist in the central nervous system and are
associated with memory, mood, pain and cognition (21–25).
The gastrointestinal tract is known to express 5-HT1,
5-HT2, 5-HT3, 5-HT4 and
5-HT7 receptors, which regulate the function of
gastrointestinal tract. The 5-HT1 and 5-HT7
receptors are considered to be relaxant and the 5-HT2,
5-HT3 and 5-HT4 receptors to be contractile
(26–30).
The selective 5-HT1A receptor agonists
sumatriptan and buspirone have been identified to enhance
esophageal peristalsis and LES function (31). Cohen et al (32) found that 5-HT induced contractions
mediated by the 5-HT2 receptor in guinea pig and rabbit
esophagus. In the present study, the presence of the
5-HT2 receptor in distal esophageal muscle and in the
human LES was clearly identified. Among all 5-HT receptors, the
5-HT3 and 5-HT4 receptors have been the most
thoroughly studied in the gastrointestinal system. 5-HT3
receptors mainly exist in the nervous system and gastrointestinal
tract; the most well established physiological roles of the
5-HT3 receptor are in the coordination of emesis and
regulation of gastrointestinal motility (33). 5-HT3 receptor
antagonists such as ondansetron and granisetron can mediate
gastrointestinal contraction and intestinal secretion, and
antagonize 5-HT-induced relaxation of the esophagus by increasing
LES tone (34). The
5-HT4 receptor-mediated response is predominant in the
5-HT-induced acceleration of motility associated with acetylcholine
release in the gastrointestinal tract (35,36),
and previous studies have identified the 5-HT4 receptor
is localized on the myenteric plexus (37). The 5-HT7 receptor was
the last member of 5-HT receptor family to be discovered (35,38).
5-HT7 receptors have been implicated in the
pathophysiology of several disorders; they play a role in smooth
muscle relaxation within the vasculature and in the
gastrointestinal tract (39,40).
Liu et al (41) identified
the expression of four subtypes of 5-HT7 receptor
throughout the rat gastrointestinal tract. Yang et al
(42) concluded that a 5-HT
signaling pathway disorder may be a major factor in the
pathogenesis of gastroesophageal reflux and reflux esophagitis in
experiments on rats.
Through the use of RT-PCR and qPCR, the present
study identified the mRNA of all seven 5-HT receptors in human LES
sling fibers, clasp fibers, circular muscles of the esophageal body
and gastric fundus. 5-HT3AR and 5-HT4R
expression levels were the highest, followed by those of
5-HT1AR and 5-HT2AR; the lowest expression
levels were found for 5-HT5AR, 5-HT6R and
5-HT7R. Western blotting confirmed the expression of
five of the 5-HT receptors, with the exception of
5-HT5AR and 5-HT6R. It is speculated that the
low levels of 5-HT5AR and 5-HT6R caused them
to be undetectable. No significant difference in the extent of 5-HT
receptor expression for each of the different receptor types was
identified among the four types of muscle strip.
To the best of our knowledge, the present study is
the first to identify 5-HT receptor mRNA and protein expression in
the human LES. Although little information concerning the
physiological and pharmacological effects of 5-HT receptors on the
LES is available, the detection of 5-HT receptors in the present
study supports the notion that the serotonergic system is an
important modulator of esophageal motility. In the future, the
development of specific ligands, as well as the use of gene
deletion animal models such as knock-out mice for each 5-HT
receptor, should allow precise evaluation of the physiological and
pharmacological effects of specific 5-HT receptors in the LES.
Acknowledgements
This study was funded by China Natural Science
Foundation and Hebei Provincial Natural Science Foundation.
References
|
1
|
Hoyer D, Hannon JP and Martin GR:
Molecular, pharmacological and functional diversity of 5-HT
receptors. Pharmacol Biochem Behav. 71:533–554. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beattie DT and Smith JA: Serotonin
pharmacology in the gastrointestinal tract: a review.
Naunyn-Schmiedebergs Arch Pharmacol. 377:181–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li JP, Chang TM and Chey WY: Roles of 5-HT
receptors in the release and action of secretin on pancreatic
secretion in rats. Am J Physiol Gastrointest Liver Physiol.
280:G595–602. 2001.PubMed/NCBI
|
|
4
|
Martinucci I, de Bortoli N, Giacchino M,
et al: Esophageal motility abnormalities in gastroesophageal reflux
disease. World J Gastrointest Pharmacol Ther. 5:86–96.
2014.PubMed/NCBI
|
|
5
|
Liu JF, Gao LP, Wen SW, et al: Responses
of human sling and clasp fibers to cholecystokinin (CKK) and
gastrin through CCK receptors. J Gastroenterol Hepatol.
23:1608–1612. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu JF, Lu HL, Wen SW and Wu RF: Effects
of acetylcholine on sling and clasp fibers of the human lower
esophageal sphincter. J Gastroenterol Hepatol. 26:1309–1317. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
8
|
Meneses A: 5-HT systems: emergent targets
for memory formation and memory alterations. Rev Neurosci.
24:629–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kato S: Role of serotonin 5-HT3 receptors
in intestinal inflammation. Biol Pharm Bull. 36:1406–1409. 2013.
View Article : Google Scholar
|
|
10
|
Hayes DJ and Greenshaw AJ: 5-HT receptors
and reward-related behaviour: a review. Neurosci Biobehav Rev.
35:1419–1449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schneider JH, Küper MA, Königsrainer A and
Brücher BL: Transient lower esophageal sphincter relaxation and
esophageal motor response. J Surg Res. 159:714–719. 2010.
View Article : Google Scholar
|
|
12
|
Gershon MD: Review article: serotonin
receptors and transporters - roles in normal and abnormal
gastrointestinal motility. Aliment Pharmacol Ther. 20:3–14. 2004.
View Article : Google Scholar
|
|
13
|
Liu XB and Liu JF: Expression of dopamine
receptors in human lower esophageal sphincter. J Gastroenterol
Hepatol. 27:945–950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hansen MB and Jaffe BM: 5-HT receptor
subtypes involved in luminal serotonin-induced secretion in rat
intestine in vivo. J Surg Res. 56:277–287. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thompson AJ and Lummis SC: The 5-HT3
receptor as a therapeutic target. Expert Opin Ther Targets.
11:527–540. 2012. View Article : Google Scholar
|
|
16
|
Walstab J, Rappold G and Niesler B: 5-HT
(3) receptors: role in disease and target of drugs. Pharmacol Ther.
128:146–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mader R, Kocher T, Haier J, et al:
Investigation of serotonin type 4 receptor expression in human and
non-human primate gastrointestinal samples. Eur J Gastroenterol
Hepatol. 18:945–950. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Champaneria S, Costall B, Naylor RJ and
Robertson DW: Identification and distribution of 5-HT3 recognition
sites in the rat gastrointestinal tract. Br J Pharmacol.
106:693–696. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Irving HR, Tan YY, Tochon-Danguy N, et al:
Comparison of 5-HT4 and 5-HT7 receptor expression and function in
the circular muscle of the human colon. Life Sci. 80:1198–1205.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang GD, Wang XY, Zou F, et al: Mast cell
expression of the serotonin1A receptor in guinea pig and human
intestine. Am J Physiol Gastrointest Liver Physiol. 304:G855–G863.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Waeber C, Grailhe R, Yu XJ, Hen R and
Moskowitz MA: Putative 5-ht5 receptors: localization in the mouse
CNS and lack of effect in the inhibition of dural protein
extravasation. Ann NY Acad Sci. 861:85–90. 1998S. View Article : Google Scholar
|
|
22
|
Nelson DL: 5-HT5 receptors. Curr Drug
Targets CNS Neurol Disord. 3:53–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marazziti D, Baroni S, Catena Dell’Osso M,
Bordi F and Borsini F: Serotonin receptors of type 6 (5-HT6): what
can we expect from them? Curr Med Chem. 18:2783–2790. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonasera SJ, Chu HM, Brennan TJ and Tecott
LH: A null mutation of the serotonin 6 receptor alters acute
responses to ethanol. Neuropsychopharmacology. 31:1801–1813. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiu HJ, Wang YC, Liou JH, et al:
Serotonin 6 receptor polymorphism in schizophrenia: frequency, age
at onset and cognitive function. Neuropsychobiology. 43:113–116.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Janssen P, Prins NH, Moreaux B, et al:
Characterization of 5-HT7-receptor-mediated gastric relaxation in
conscious dogs. Am J Physiol Gastrointest Liver Physiol.
289:108–115. 2005. View Article : Google Scholar
|
|
27
|
Janssen P, Prins NH, Moreaux B, et al: In
vivo characterization of 5-HT1A receptor-mediated gastric
relaxation in conscious dogs. Br J Pharmacol. 140:913–920. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Janssen P, Prins NH, Meulemans AL, et al:
Smooth muscle 5-HT2A receptors mediating contraction of porcine
isolated proximal stomach strips. Br J Pharmacol. 137:1217–1224.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prins NH, van der Grijn A, Lefebvre RA, et
al: 5-HT(4) receptors mediating enhancement of contractility in
canine stomach; an in vitro and in vivo study. Br J Pharmacol.
132:1941–1947. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fox A and Morton IK: An examination of the
5-HT3 receptor mediating contraction and evoked [3H]-acetylcholine
release in the guinea-pig ileum. Br J Pharmacol. 101:553–558. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Stefano M, Papathanasopoulos A,
Blondeau K, et al: Effect of buspirone, a 5-HT1A receptor agonist,
on esophageal motility in healthy volunteers. Dis Esophagus.
25:470–476. 2012. View Article : Google Scholar
|
|
32
|
Cohen ML, Susemichel AD, Bloomquist W and
Robertson DW: 5-HT4 receptors in rat but not guinea pig, rabbit or
dog esophageal smooth muscle. Gen Pharmacol. 25:1143–1148. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Machu TK: Therapeutics of 5-HT3 receptor
antagonists: current uses and future directions. Pharmacol Ther.
130:338–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miyata K, Yamano M, Kamato T and Akuzawa
S: Effect of serotonin (5-HT)3-receptor antagonists YM060, YM114
(KAE-393), ondansetron and granisetron on 5-HT4 receptors and
gastric emptying in rodents. Jpn J Pharmacol. 69:205–214. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Poole DP, Xu B, Koh SL, et al:
Identification of neurons that express 5-hydroxytryptamine4
receptors in intestine. Cell Tissue Res. 325:413–422. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saegusa Y, Takeda H, Muto S, et al:
Decreased motility of the lower esophageal sphincter in a rat model
of gastroesophageal reflux disease may be mediated by reductions of
serotonin and acetylcholine signaling. Biol Pharm Bull. 34:704–711.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ren J, Zhou X and Galligan JJ: 5-HT4
receptor activation facilitates recovery from synaptic rundown and
increases transmitter release from single varicosities of myenteric
neurons. Am J Physiol Gastrointest Liver Physiol. 294:G1376–1383.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taniyama K, Kaibara M, Uezono Y and
Hayashi H: Functional difference of prokinetics depending on
subtypes and localization of receptor in alimentary tract. Nihon
Yakurigaku Zasshi. 122(Suppl): 63PP–66PP. 2003.(In Japanese).
|
|
39
|
Vanhoenacker P, Haegeman G and Leysen JE:
5-HT7 receptors: current knowledge and future prospects. Trends
Pharmacol Sci. 21:70–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tonini M, Vicini R, Cervio E, et al: 5-HT7
receptors modulate peristalsis and accommodation in the guinea pig
ileum. Gastroenterology. 129:1557–1566. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu H, Irving HR and Coupar IM: Expression
patterns of 5-HT7 receptor isoforms in the rat digestive tract.
Life Sci. 69:2467–2475. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang L, Cai H, Tou J, et al: The role of
the 5-hydroxytryptamine pathway in reflux-induced esophageal
mucosal injury in rats. World J Surg Oncol. 10:2192012. View Article : Google Scholar : PubMed/NCBI
|