Introduction
The lumen diameter directly affects the
physiological function of capillaries and understanding its
regulatory mechanism is important for the construction of
functional tissue-engineered capillaries (1,2). If
the diameter of the capillary lumen is too small, the passage of
blood cells will be affected, causing damage to blood and vascular
endothelial cells (3). When the
lumen diameter is too large, it may result in an ineffective
exchange of nutrients and metabolites between tissues and blood,
leading to tissue hypoxia and accumulation of metabolites (4,5).
However, the mechanisms underlying regulation of the capillary
lumen diameter remain to be fully elucidated.
During wound healing, the pH of the surface layer of
the granulation tissue is low (pH 5.8-6.0); the pH of the
granulation tissue in the proliferative stage is 6.4-7.0 and the pH
of the deep layer is close to that of the plasma (pH 7.3-7.6)
(6,7). In tumor specimens, the capillaries
near the center of the tumor tissue (pH 5.8-6.6) are relatively
small in diameter or even without any definite lumen formation,
while those near the edge of the tumor tissue (plasma pH 7.3-7.4)
have larger lumen diameters (7,8).
This suggests that the pH of the peripheral environment is one of
the key factors that regulate the lumen diameter of newly formed
capillaries.
However, pH, as a parameter for assessing the
overall outcome, is not well studied, as the effect of the pH on
the diameter of capillaries during neovascularization is rarely
reported. Therefore, the present study aimed to explore the role of
the pH in determining the vascular lumen diameter of new
vessels.
Materials and methods
Cell culture
Human dermal microvascular endothelial cells
(HDMECs; cat. no. 2000; ScienCell Research Laboratories, Inc.) were
cultured according to the manufacturer's protocol in an endothelial
cell medium (cat. no. 1001; ScienCell Research Laboratories, Inc.)
supplemented with 5% fetal bovine serum (ScienCell Research
Laboratories, Inc.), 100 U/ml penicillin-G, 100 U/ml streptomycin
and 1% endothelial cell growth supplement (ScienCell Research
Laboratories, Inc.). The cells were cultivated in 35-cm2
culture flasks at 37˚C under 5% CO2 in air. The culture
medium was replaced every 24 h. The cells in passage 4 to 5 were
used for experimentation. All experiments reported in this study
were performed in triplicate.
Preparation of different pH media
Different concentrations of NaOH and hydroxyethyl
piperazine ethanesulfonic acid were added into the endothelial cell
medium and the pH of the medium was adjusted to 6.4, 6.6, 6.8, 7.0,
7.2, 7.4, 7.6 and 7.8 using a pH meter (model PB-10; Sartorius AG).
A 0.22-µm membrane was used to filter the medium and remove
bacteria, after which it was stored at 4˚C until use.
Cytosolic pH determined using flow
cytometry
Cytosolic pH was assessed through flow cytometry
using the pH-sensitive fluorescent probe
2,7-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein,
acetoxymethyl ester (BCECF-AM; cat. no. ab143463; Abcam). The cells
incubated in media with different pH for 12 h and were harvested by
centrifugation and incubated in a culture medium containing 4 µM
BCECF-AM in the dark at 37˚C for 30 min. The cells were washed with
PBS and quantitative analysis of intracellular fluorescence was
performed using a flow cytometer (BD FACSCalibur™; BD Biosciences).
Data analysis was performed using the CELLQuest software tool
(version 5.1; BD Biosciences).
Cell proliferation assay
The cells (5x105 cell/ml) were grown on
polylysine-coated glass coverslips and stained with an
anti-bromodeoxyuridine (BrdU) antibody (1:500 dilution; cat. no.
B35128; Thermo Fisher Scientific, Inc.) at 4˚C overnight. The
coverslips were washed with PBS and the cells were fixed with 4%
paraformaldehyde for 15 min at 23˚C. The coverslips were placed in
2 mol/l HCl for 30 min and washed with PBS. The cells were
incubated with goat anti-mouse Cy3-conjugated secondary antibodies
(1:1,000; cat. no. ab97035; Abcam) at 37˚C for 2 h and then blocked
with PBS containing 5% goat serum (cat. no. ab7481; Abcam) at room
temperature for 1 h. Finally, the cells were stained with
4',6-diamidino-2-phenylindole for 10 min at room temperature and
examined using a fluorescence microscope (Olympus IX 71
fluorescence microscope; Olympus Corporation).
Wound-healing assay
A wound-healing assay was used to evaluate the
migratory behavior of HDMECs in media at varying pH. In brief, the
cells were labeled with CellTracker™ (cat. no. C2925; Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol, and then confluent cells were scratched with a 200-µl
micropipette tip to create a consistent wound gap in the middle.
The cells were washed with PBS to remove any cell debris and media
of varying pH without serum were added to allow wound healing.
Images of the wound gap were acquired using a fluorescence
microscope (Olympus IX 71; Olympus Corporation) at 0, 24 and 48 h
at three random locations in each well to examine the distance of
wound closure. Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc.) was used to calculate the migration rate.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed to detect the expression of
endothelial angiogenesis-related genes [VEGFA, angiopoietin 1
(ANG1) and CD31] at different pH. Total RNA was extracted from the
different groups of cells using a Takara MiniBEST Universal RNA
Extraction kit (cat. no. 9767; Takara Bio, Inc.), according to the
manufacturer's protocol. RT reactions were performed with
PrimeScript™ RT Master Mix (cat. no. RR036A; Takara Bio, Inc.)
according to the manufacturer's protocol. qPCR was performed using
TB Green™ Premix Ex Taq ІІ (cat. no. RR820A; Takara Bio, Inc.)
according to the manufacturer's protocol. The PCR amplifications
were performed in Real Time Instrument (CFX Connect Real Time PCR
system, Bio-Rad Laboratories, Inc.). Thermocycling conditions were:
Initial denaturation at 95˚C for 2 min; 40 cycles of denaturation
at 95˚C for 15 sec, annealing and elongation at 60˚C for 1 min;
then a final extension at 72˚C for 3 min. The mRNA expression
levels of the target genes were quantified using the
2-∆∆Cq (9) method and
normalized to those of GAPDH. The primers used for qPCR are listed
in Table SI.
Tube formation on
Matrigel®
Endothelial cells rapidly attach, align and form
capillary-like tubules on a reconstituted basement membrane matrix
(10,11). Cold liquid Matrigel (cat. no.
354234; BD Biosciences) was added to 96-well plates at a volume of
50 µl/well and incubated at 37˚C in an incubator containing
humidified air with 5% CO2 for 1 h. After the Matrigel
had solidified, fluorescently-labeled (CellTracker™; cat. no.
C2925; Invitrogen; Thermo Fisher Scientific, Inc.) HDMECs
(2x104 cells/100 µl) were seeded into each well and
incubated in the conditioned medium at 37˚C. Images were acquired
after 24 h with a fluorescence microscope (Olympus IX 71; Olympus
Corporation) to evaluate the degree of tube formation, which was
quantified by measuring the branch length in three randomly
selected fields using ImageJ software (version 1.51; National
Institutes of Health).
Tube formation in a 3D Matrigel
model
The cell suspensions with different pH values were
mixed with Matrigel and cultured in 48-well plates under 5%
CO2 at 37˚C. The cell tubes were stained using toluidine
blue at room temperature for 10 min after 48 h cultured, as
described previously (12). To
observe tube formation, the cells were photographed under a
microscope (Olympus IX 71; Olympus Corporation) after 3 days. The
degree of tube formation was quantified by measuring the lumen
diameter using ImageJ software (version 1.51; National Institutes
of Health).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean of at least three independent experiments and all
experiments were performed at least three independent repeats.
Differences among groups were analyzed using one-way analysis of
variance followed by Bonferroni correction for multiple
comparisons. Statistical analysis was performed using SPSS 23.0
software (IBM Corporation). Figures were plotted using Origin 8.5
software (OriginLab Corporation).
Results
pH of the medium is tightly associated
with the cytosolic pH
First, the effect of media with different pH on the
cytosolic pH was determined (Fig.
1). An association between the relative fluorescence and the pH
of the medium was observed; the cytosolic pH increased steadily
with the increase in the pH of the medium. The specific data for
each group are presented in Fig.
S1.
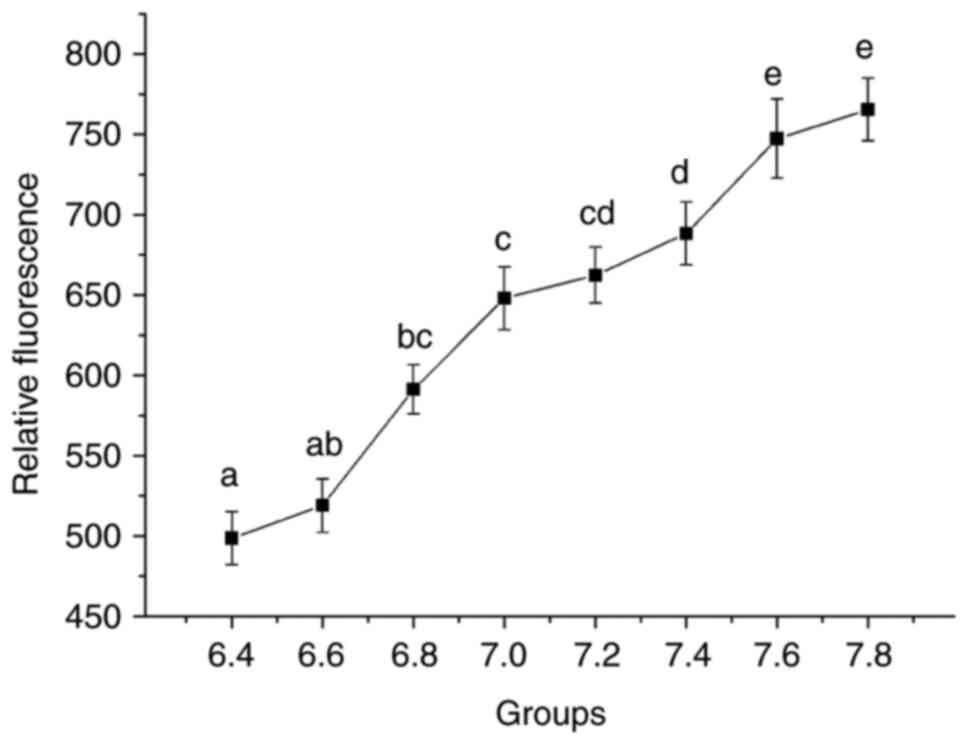 | Figure 1Relative fluorescence of each group in
endothelial cells and different cytosolic pH levels analyzed using
flow cytometry. The X-axis represents the different pH groups and
the Y-axis the relative fluorescence. One-way analysis of variance
and Bonferroni correction among groups was performed.
aP<0.05 vs. 6.8, 7.0, 7.2, 7.4, 7.6 and 7.8;
bP<0.05 vs. 7.0, 7.2, 7.4, 7.6 and 7.8;
cP<0.05 vs. 7.4, 7.6 and 7.8; dP<0.05
vs. 7.6 and 7.8; eP<0.05 vs. 6.4, 6.6, 6.8, 7.0, 7.2
and 7.4. |
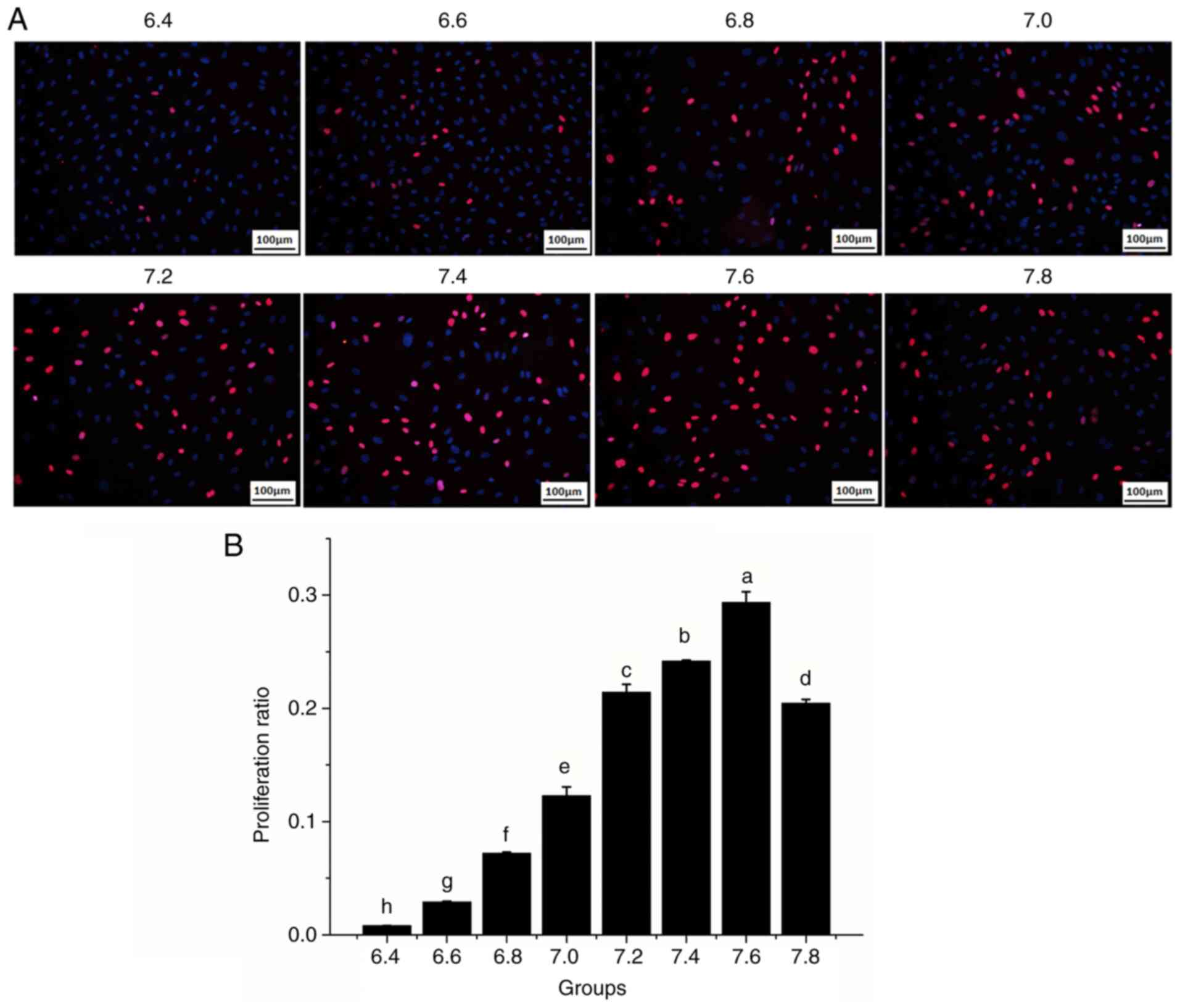
pH affects the proliferation and
migration of HDMECs
To investigate whether pH affects the biological
properties of vascular endothelial cells, the effects of media of
varying pH on cell proliferation and migration were examined. The
cell proliferation rate increased as the pH increased to 6.4-7.6,
particularly at pH 7.2-7.6; however, the cell proliferation rate
decreased at pH 7.8 (Fig. 2A and
B). The result of cell migration
is analogous to proliferation. The cell images after 24 and 48 h
revealed that HDMEC migration was significantly influenced by the
pH (Fig. 3A and B). At pH values between 6.4 and 6.8, the
migratory rate of HDMECs into the wound gap after 24 h of culture
was relatively low (~22%) and only moderate wound coverage (~50%)
was achieved after 48 h. By contrast, HDMEC migration into the
wound gap was markedly enhanced by increasing the pH from 7.0 to
7.6; the ‘wound gap’ was completely covered at pH 7.6 after 48 h.
However, compared with pH 7.6, the migratory rate of HDMECs
decreased significantly at pH 7.8 (P<0.05).
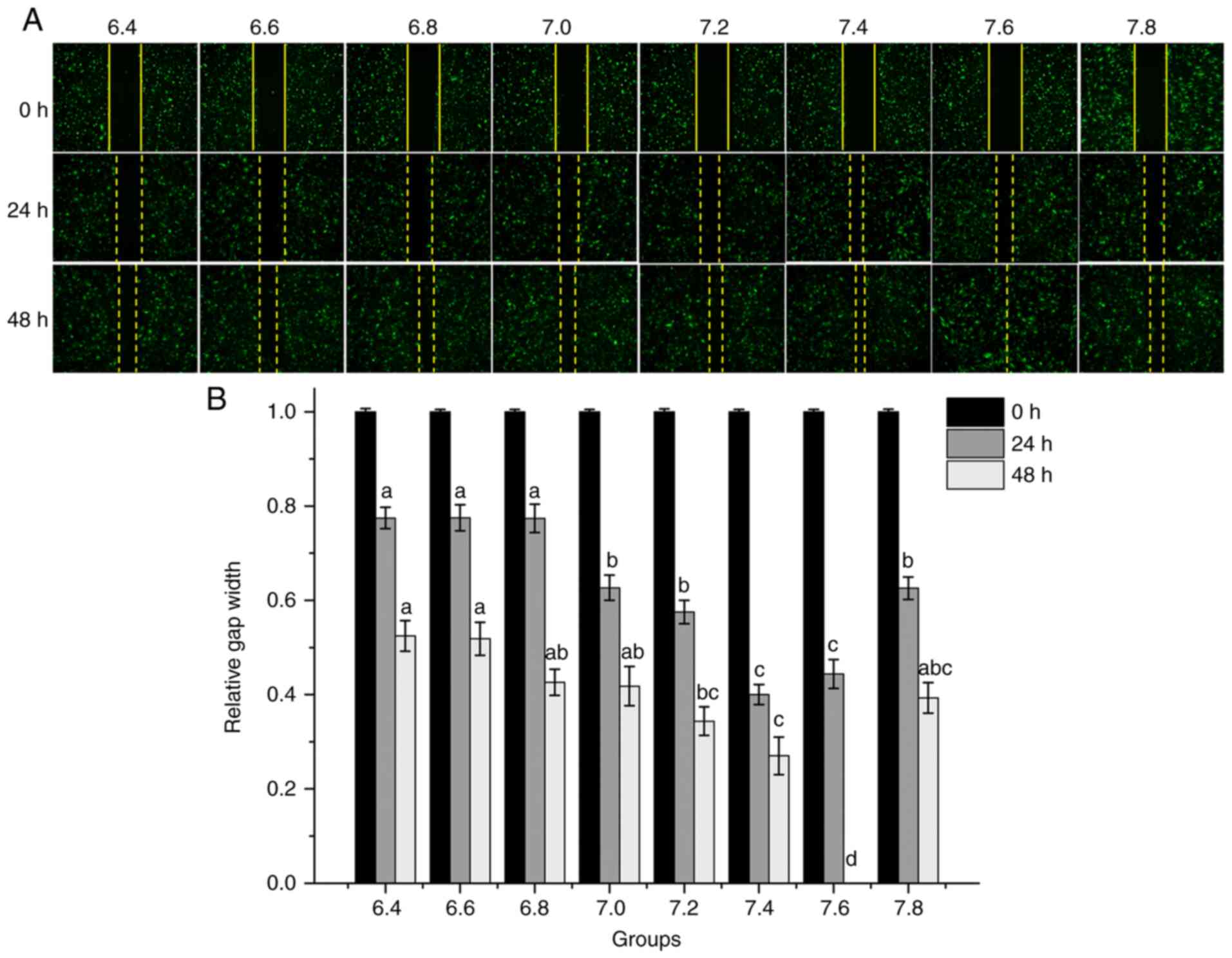 | Figure 3Migration of human dermal
microvascular endothelial cells at different pH levels determined
using a wound-healing assay. (A) Representative migration images
(magnification, x40). Cells were allowed to migrate after the wound
gaps were created and visualized after 24 and 48 h. (B)
Quantification of the wound gap distance between the front lines of
migrating cells. The X-axis represents the different pH groups.
One-way analysis of variance and Bonferroni correction among groups
was performed. 24 h: aP<0.05 vs. 7.0, 7.2, 7.4,7.6
and 7.8; bP<0.05 vs. 7.4 and 7.6; 48 h:
aP<0.05 vs. 7.2, 7.4 and 7.6; bP<0.05
vs. 7.4 and 7.6; cP<0.05 vs. 7.6;
dP<0.05 vs. 6.4, 6.6, 6.8, 7.0, 7.2, 7.4 and 7.8. |
pH influences the expression of
angiogenesis-related genes
RT-qPCR analysis of the expression of
angiogenesis-related genes further confirmed that the pH influenced
their expression (Fig. 4). The
expression of ANG1 gradually increased with the increase in pH and
reached a maximum at pH 7.6 (Fig.
4B). Similarly, the expression of VEGFA was significantly
higher at pH 7.0-7.8 compared with at pH 6.4-6.8; however, it
decreased from pH 7.6 to 7.8 (Fig.
4A). By contrast, the expression of CD31 was only slightly
altered with the increase in the medium pH and the differences
between the groups were not significant (Fig. 4C).
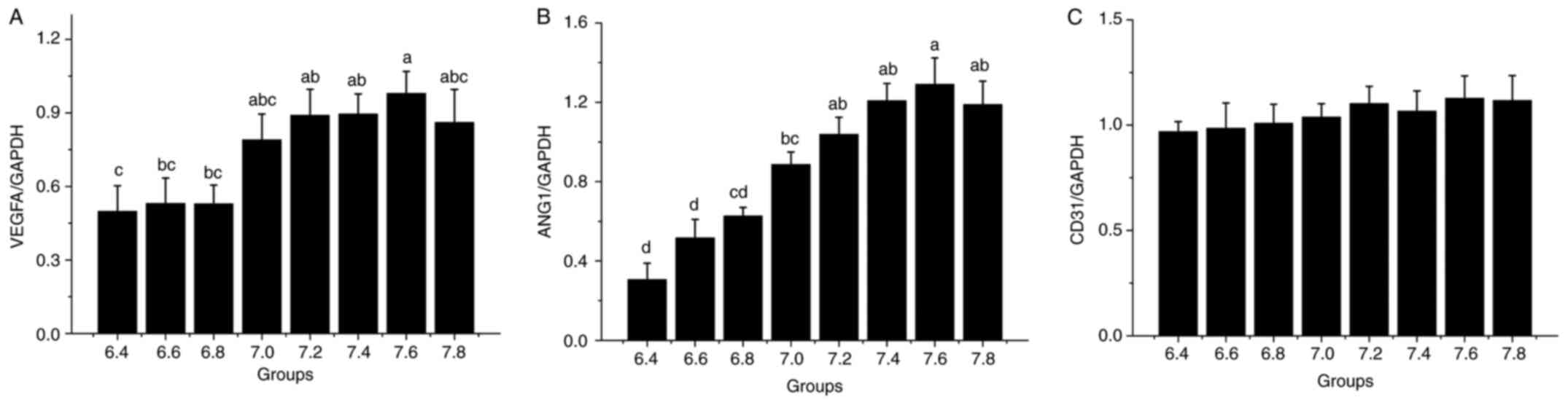 | Figure 4Reverse transcription-quantitative PCR
analysis of genes related to angiogenesis. The X-axis represents
the different pH groups. Relative levels of (A) VEGFA
(aP<0.05 vs. 6.4, 6.6 and 6.8; bP<0.05
vs. 6.4; cP<0.05 vs. 7.2, 7.4 and 7.6), (B) ANG1
(aP<0.05 vs. 6.4, 6.6, 6.8 and 7.0;
bP<0.05 vs. 6.4, 6.6 and 6.8; cP<0.05
vs. 6.4 and 6.6; dP<0.05 vs. 7.0, 7.2, 7.4, 7.6 and
7.8) and (C) CD31. ANG1, angiopoietin 1. |
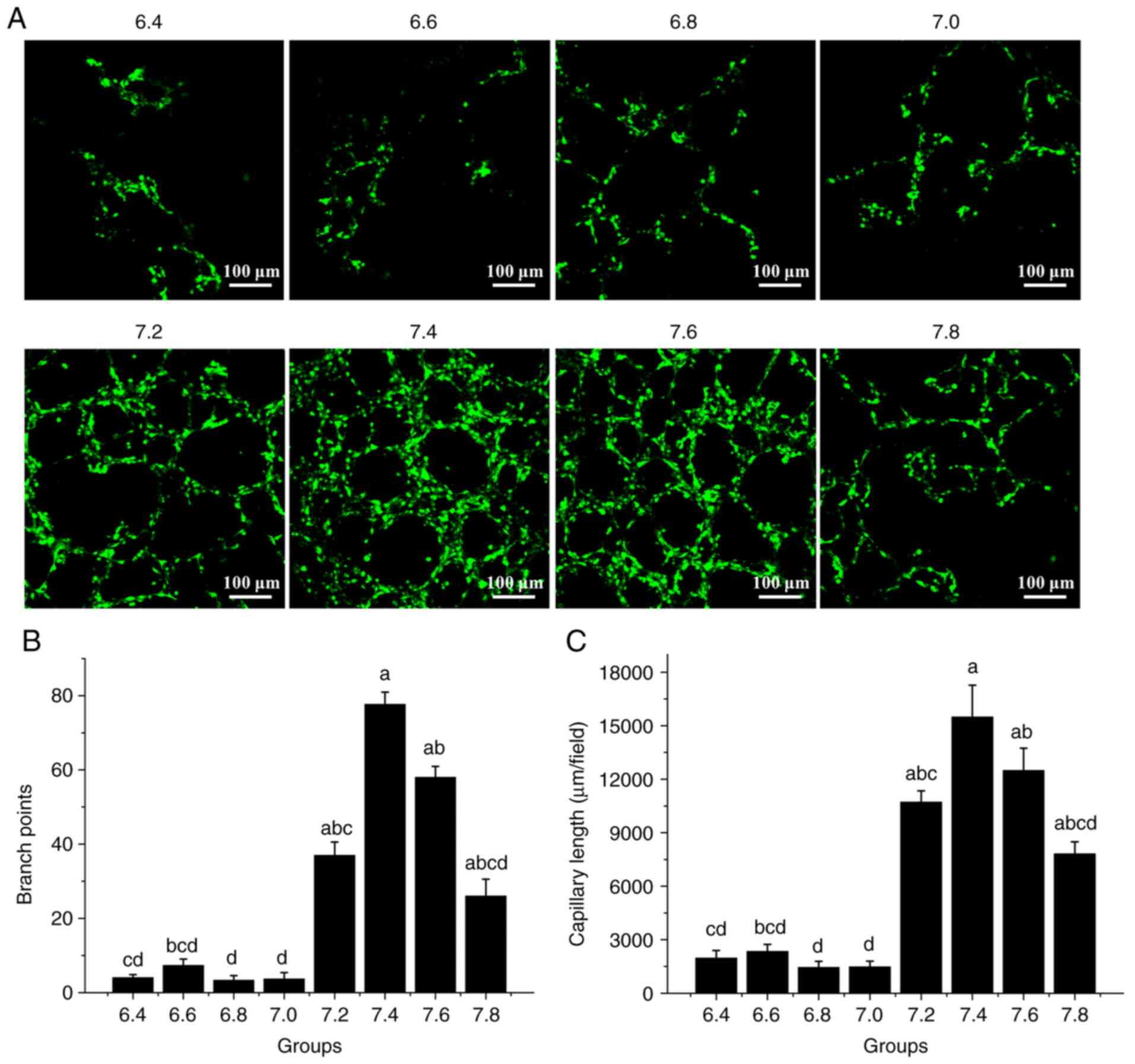
Medium pH affects tube formation
An HDMEC tube formation assay was performed to
evaluate the effect of the media at different pH on tube formation.
The cells were seeded on Matrigel and the formation of tube-like
cell arrangements was assessed. The tube formation capacity of the
cells was evaluated by determining the branch points and branch
length of the tubes (13,14). It was revealed that tube formation
was significantly reduced at pH 6.4-7.0 compared with higher pH
values (Fig. 5A). The histogram
indicated that in comparison with those at pH 6.4-7.0, the number
of branch points and the capillary length at pH 7.2, 7.4, 7.6 and
7.8 were significantly increased, the highest being at pH 7.4 and
7.6 (P<0.05; Fig. 5B and
C). Therefore, the results
indicated that 7.4 and 7.6 are the optimal pH values for HDMEC tube
formation.
pH regulates lumen diameter in
Matrigel
HDMECs formed tube-like structures in a 3D Matrigel
model in media with different pH (Fig.
6). HDMECs formed tubules (Fig.
6A) and the diameter of the vascular tubules increased with an
increase in the pH of the medium; however, at pH 7.8, the diameter
of the vascular tubules was decreased. The histogram in Fig. 6B indicated that the lumen diameter
increased steadily from pH 6.4-7.6, and significantly decreased at
pH 7.8 (P<0.05). These results indicated that the pH of the
medium has an important role in lumen formation.
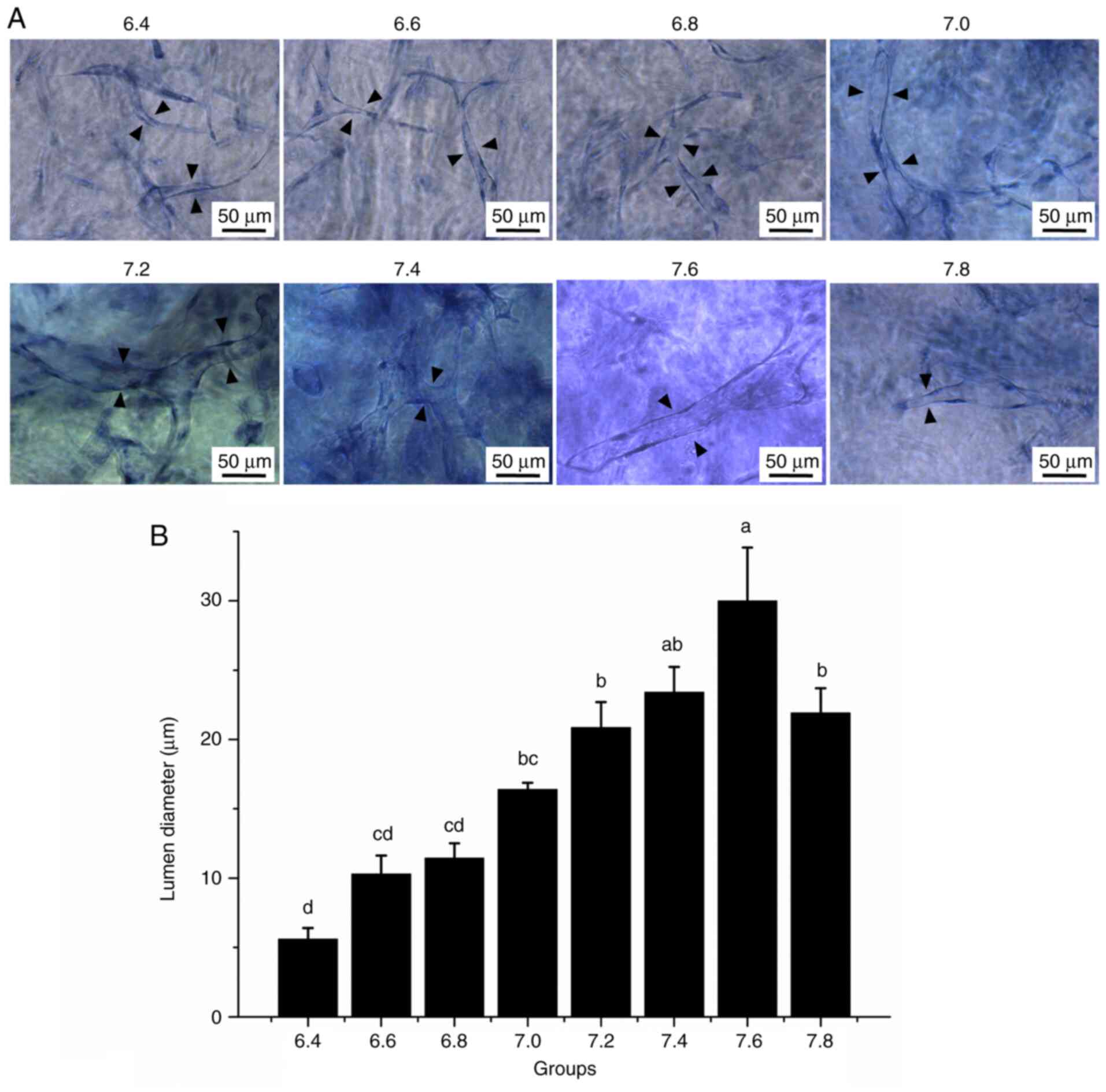 | Figure 6Three-dimensional HDMEC tubes in
Matrigel. (A) Representative images of HDMEC tubes in Matrigel. The
background of images may be different after toluidine blue staining
due to staining in Matrigel, and the images with clear lumen were
selected as the representative images. Black arrowheads indicate
the cell tubes (scale bars, 50 µm). (B) Quantitative analysis of
the lumen diameter of the endothelial cell tubes in each group. The
X-axis represents the different pH groups. One-way analysis of
variance and Bonferroni correction among groups was performed.
aP<0.05 vs. 6.4, 6.6, 6.8, 7.0, 7.2 and 7.8;
bP<0.05 vs. 6.4, 6.6 and 6.8; cP<0.05
vs. 6.4, 7.2, 7.4, 7.6, and 7.8; dP<0.05 vs. 7.0,
7.2, 7.4, 7.6 and 7.8. HDMEC, human dermal microvascular
endothelial cell. |
Discussion
The regulation of capillary lumen diameter is
important in the formation of tissue-engineered capillaries
(15-17).
The lumen diameter directly influences the physiological function
of capillaries and its regulation is of great significance for the
construction of tissue-engineered organs (18). Capillaries in normal tissues have
diameters of 5-10 µm (19,20). If the capillary lumen diameter is
too small, it will affect the passage of blood cells, resulting in
blood cell and vascular endothelial cell damage (21). On the other hand, if the capillary
lumen diameter is too large, the velocity of blood flow decreases,
affecting the rate of delivery of oxygen, nutrients, growth factors
and circulating cells necessary for the body (22,23).
In the present study, it was observed that the pH of
the peripheral environment is a key factor in regulating the lumen
diameter of newly formed capillaries. The extracellular pH affects
the pH of the cytoplasm (24,25).
Furthermore, the relationship between the pH of the medium and that
of the cell cytoplasm was studied. In HDMEC cultures, an increase
in the pH of the culture medium led to an increase in the pH of the
cytoplasm. However, these corresponding changes were not always
consistent. This may be attributed to the buffering capacity of
HDMECs. Therefore, a change in the pH of the culture medium was
able to affect the cytoplasmic pH of HDMECs, leading to a change in
vascular diameter.
In in vivo vascular systems, capillaries that
are between 5 and 10 µm in diameter are the only regions where
nutrients and metabolites are able to be exchanged with tissues
(19). The lumen diameter of
capillaries from HDMECs formed in Matrigel was ~10 µm when the pH
of the medium was 6.4-6.8. This size is suitable for forming
capillaries in tissue engineering. This possibly suggests that
smaller capillaries may be constructed using a medium with lower
pH. A higher cytosolic pH is associated with increased cell growth
and proliferation in mammalian cells (26), which is consistent with the results
of the present study. Cell proliferation tests indicated that the
proliferation ratio increased with an increase in the medium pH
from 6.4 to 7.6, particularly at pH 7.2-7.6, but it gradually
decreased at pH 7.8. The expression of ANG1 and VEGFA exhibited a
similar trend. These results reflect the difficulty in constructing
capillaries with small diameters; however, they further confirmed
that a medium pH of 7.2-7.6 is optimal for in vitro
neovascularization with HDMECs. The expression of CD31 remained
unaffected. Therefore, the pH possibly does not affect cell
adhesion. Both the capillary length and branch points of the tubes
were associated with an increased cytosolic pH. This is
particularly important for the construction of capillaries in
tissue-engineered organs. Furthermore, in a previous study by Ye
(27), wherein the acceptance rate
of skin grafts in patients with acute and chronic wounds of
different origin was observed, 99% of the skin grafts were
successfully taken in wounds at a pH of 7.4 and higher; however, no
skin graft was successfully taken in wounds at a pH <7.0. In
combination with the results of the present study, this may be due
to the presence of appropriate growth conditions for capillaries at
pH 7.4 and 7.6, which provide sufficient blood supply, leading to
improved wound healing.
Considering the results of cell proliferation,
migration and tube formation, it was determined that the most
optimal pH of the medium was 7.2-7.6 for in vitro
neovascularization with HDMECs. This may be attributed to the
closeness to the physiological pH of blood and extracellular
fluids, which is in the range of 7.35-7.45 (28-30).
At this appropriate pH range, the various enzymes had maximum
activity, enabling improved formation of new blood vessels.
In conclusion, the present study observed that the
pH regulated the diameter of the capillary lumen in HDMECs; the
optimal pH was 7.4-7.6. However, the mechanisms underlying the
regulation of the lumen diameter of capillaries by the pH remain to
be fully elucidated. This will be our next research direction.
Supplementary Material
BCECF-AM in each group was examined
using flow cytometry. The different pH ranged from 6.4 to 7.8
(displayed above each histogram). The abscissa represents the green
channel; the ordinate represents the emission wavelength, which is
positively correlated with the fluorescence intensity. BCECF-AM,
2,7-bis-(2-carboxyethyl)-5-(and-6)- carboxyfluorescein,
acetoxymethyl ester.
Sequences of RNA primers (human
species).
Acknowledgements
The authors would like to thank Professor Xiwang Hu
(Experimental Center of the Third Affiliated Hospital of the Air
Force Medical University) for his assistance with laser confocal
microscopy.
Funding
Funding: This work was supported by grants from the National
Natural Science Foundation of China (grant nos. 31570986 and
81701909).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW, JinqL and XL designed the study. XW and JingL
wrote the manuscript. XW, YB and CZ performed the experiments and
JingL analyzed the data. All authors read and approved the final
manuscript and confirm the authenticity of the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang Z, Mithieux SM and Weiss AS:
Fabrication techniques for vascular and vascularized tissue
Engineering. Adv Healthc Mater. 8(e1900742)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hati S, Agrawal S and Rai V: Vascular
regeneration and tissue engineering: Progress, clinical impact, and
future challenges. Regenerated Organs 153-166, 2021.
|
|
3
|
Munisso MC and Yamaoka T: Circulating
endothelial progenitor cells in small-diameter artificial blood
vessel. J Artif Organs. 23:6–13. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hamilton NB, Attwell D and Hall CN:
Pericyte-mediated regulation of capillary diameter: A component of
neurovascular coupling in health and disease. Front
Neuroenergetics. 2(5)2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Brewster L, Brey EM and Greisler HP: Blood
Vessels. Principles of Tissue Engineering. 5th edition. Academic
Press, Boston, 2020.
|
|
6
|
Schneider LA, Korber A, Grabbe S and
Dissemond J: Influence of pH on wound-healing: A new perspective
for wound-therapy? Arch Dermatol Res. 298:413–420. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Barar J and Omidi Y: Dysregulated pH in
tumor microenvironment checkmates cancer therapy. Bioimpacts.
3:149–162. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gerweck LE and Seetharaman K: Cellular pH
Gradient in tumor versus normal tissue: Potential exploitation for
the treatment of cancer. Cancer Res. 56:1194–1198. 1996.PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Arnaoutova I, George J, Kleinman HK and
Benton G: The endothelial cell tube formation assay on basement
membrane turns 20: State of the science and the art. Angiogenesis.
12:267–274. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Arnaoutova I and Kleinman HK: In vitro
angiogenesis: Endothelial cell tube formation on gelled basement
membrane extract. Nat Protoc. 5:628–635. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Koh W, Stratman AN, Sacharidou A and Davis
GE: In vitro three dimensional collagen matrix models of
endothelial lumen formation during vasculogenesis and angiogenesis.
Methods Enzymol. 443:83–101. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Baker CD, Ryan SL, Ingram DA, et al:
Endothelial colony-forming cells from preterm infants are increased
and more susceptible to hyperoxia. Am J Respir Crit Care Med.
180:454–461. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hou HH, Hammock BD, Su KH, et al:
N-terminal domain of soluble epoxide hydrolase negatively regulates
the VEGF-mediated activation of endothelial nitric oxide synthase.
Cardiovasc Res. 93:120–129. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nemeno-Guanzon JG, Lee S, Berg JR, Jo YH,
Yeo JE, Nam BM, Koh YG and Lee JI: Trends in tissue Engineering for
blood vessels. J Biomed Biotechnol. 2012(956345)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Catto V, Farè S, Freddi G and Tanzi MC:
Vascular tissue engineering: Recent advances in small diameter
blood vessel regeneration. ISRN Vasc Med. 2014(27)2014.
|
|
17
|
Ncube S, Akankwasa NT, Sibanda P and
Ndlovu LN: Nanofibres for blood vessel tissue engineering: A
review. MSAIJ. 12:218–225. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ziegler T and Nerem RM: Tissue engineering
a blood vessel: Regulation of vascular biology by mechanical
stresses. J Cell Biochem. 56:204–209. 1994.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Davey B, Halliday T and Hirst M: Human
Biology and Health: An Evolutionary Approach (Health and Disease).
Open University Press, Philadelphia, PH, 2001.
|
|
20
|
Tilton RG, Hoffmann PL, Kilo C and
Williamson JR: Pericyte degeneration and basement membrane
thickening in skeletal muscle capillaries of human diabetics.
Diabetes. 30:326–334. 1981.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tresoldi C, Pellegata AF and Mantero S:
Cells and stimuli in small-caliber blood vessel tissue engineering.
Regen Med. 10:505–527. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Iruela-Arispe ML and Davis GE: Cellular
and molecular mechanisms of vascular lumen formation. Dev Cell.
16:222–231. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schulz E and Münzel T: Lumen size matters:
Role of protein disulfide Isomerase A1 in vascular remodeling.
Hypertension. 67:488–489. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Smith GA, Howell GJ, Phillips C, Muench
SP, Ponnambalam S and Harrison MA: Extracellular and Luminal pH
Regulation by Vacuolar H+-ATPase isoform expression and targeting
to the plasma membrane and endosomes. J Biol Chem. 291:8500–8515.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao L, Cui L, Jiang X, Zhang J, Zhu M,
Jia J, Zhang Q, Zhang J, Zhang D and Huang Y: Extracellular pH
regulates autophagy via the AMPK-ULK1 pathway in rat
cardiomyocytes. FEBS Lett. 590:3202–3212. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dechant R, Saad S, Ibáñez AJ and Peter M:
Cytosolic pH regulates cell growth through distinct GTPases, Arf1
and Gtr1, to promote Ras/PKA and TORC1 activity. Mol Cell.
55:409–421. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ye RC: The relationship of pH of the
granulation tissue and the take of the skin graft. Plast Reconstr
Surg (1946). 19:213–217. 1957.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Findlay M and Isles C: Disorders of Acid
Base Balance. In: Clinical Companion in Nephrology. Springer
International Publishing, Switzerland, 2015.
|
|
29
|
Cheng HM and Jusof F: Acid-Base Balance.
Springer, Singapore, pp177-185, 2018.
|
|
30
|
Chaves MH, Wolf AR, Nascimento KA, Nawcki
D, Feustel GM, Bettega PV, Ignacio SA, Brancher JA, Tannous LA,
Werneck RI, et al: Sialochemical analysis in polytraumatized
patients in intensive care units. PLoS One.
14(e0222974)2019.PubMed/NCBI View Article : Google Scholar
|