Introduction
Atherosclerosis is a progressive chronic
inflammatory and metabolic disease with lipid deposition, focal
intimal thickening, smooth muscle cell proliferation and plaque
formation (1). With the changes in
diet, atherosclerosis and its complications have increased and
caused elevated morbidity and mortality worldwide (2). Atherosclerosis of the internal
carotid artery, leading to narrowing of the vessel lumen by >50%
of the original size, affects nearly 10% of the population above
the age of 70 years and causes ~15% of ischemic strokes (3). According to the statistics of
patients with carotid atherosclerosis, males are more prone to
carotid atherosclerosis and the incidence rate of carotid
atherosclerosis in males is higher than that in females (4). Elucidating the mechanisms of male
carotid atherosclerosis may help to explain the high incidence rate
in males and this may prevent male carotid atherosclerosis in the
future.
The mechanisms of atherosclerosis have remained to
be fully elucidated. However, lipid metabolism disorders,
endothelial dysfunction (5),
inflammation (6) and oxidative
stress are involved in the formation of atherosclerosis. In the
Gene Expression Omnibus (GEO) dataset GSE100927, atherosclerosis
was assessed in numerous parts of the human artery, including the
atherosclerotic femoral artery, infra-popliteal artery and carotid
artery (7). The GSE100927 dataset
contains 96 samples, including 31 healthy arterial samples and 65
late-stage atherosclerotic arterial samples. For the present study,
the gene expression profile of GSE100927 was selected and the data
of 10 male normal samples and 21 male late-stage atherosclerosis
samples were acquired. To date, the diagnosis rate of
atherosclerotic femoral artery and infra-popliteal artery is less
than that of carotid artery. So, carotid atherosclerosis rather
than atherosclerotic femoral artery and infra-popliteal artery was
selected in this study.
In the present study, key genes in carotid
atherosclerosis were screened out via bioinformatics, including
MMP7, MMP9, IL1β, C-C motif chemokine ligand 4 (CCL4), secreted
phosphoprotein 1 (SPP1), CCL3 and interferon regulatory factor 5
(IRF5). Subsequently, the mRNA levels of these genes were measured
in human umbilical vein endothelial cells (HUVECs), human aortic
vascular smooth muscle cells (HAVSMCs) and Tohoku Hospital
Pediatrics-1 (THP-1)-induced macrophages.
Materials and methods
Data sources
The GSE100927 gene expression dataset was obtained
from the GEO database (https://www.ncbi.nlm.nih.gov/gds/), which may be used
for genome-wide expression analyses. The gene expression dataset is
based on the GPL17077 platform (Agilent-039494 SurePrint G3 Human
GE v2 8x60K Microarray 039381). The GSE100927 dataset contains 31
healthy artery samples and 65 atherosclerotic artery samples. Male
carotid atherosclerosis samples were selected for analysis,
including 10 healthy samples (control samples) and 21 late-stage
atherosclerosis samples. The raw data were downloaded as MINiML
files. The extracted data were normalized. A fold change of at
least 2 and P≤0.05 was considered to indicate a statistically
significant difference. A total of 32 upregulated and 13
downregulated genes were identified.
Functional annotation of DEGs using
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes
(KEGG) analysis
The cluster Profiler package in R is a tool to
implement methods when analyzing and visualizing the functional
profiles of genomic coordinates and was used to perform GO and KEGG
analysis (8). GO analysis is a
useful method, which includes three biological aspects: Biological
Process (BP), Molecular Function (MF) and Cellular Component (CC)
(9). KEGG is a commonly used
bioinformatics database, which analyzes gene functions and enriched
genes with their pathways (10,11).
The GO and KEGG enrichment analyses were performed for DEGs using
the cluster Profiler package. The top 10 terms were selected.
Construction of the protein-protein
interaction (PPI) network and Venn diagram
The Search Tool for the Retrieval of Interacting
Genes and proteins (STRING) database (https://string-db.org/) may be used for the prediction
of PPIs. A total of 45 genes were inputted into STRING. In
addition, disconnected nodes in the network were hidden and the
minimum required interaction score was 0.700. The line thickness
indicated the strength of data support. Genes ranking first in BP,
MF and CC were inputted into a Venn diagram and the common genes
were acquired.
Cell lines
Cells were cultured in 5% CO2 at
36.5±0.5˚C and under 95% relative humidity. HAVSMCs were purchased
from Shenzhen Kuyuan Biotechnology Co., Ltd. HUVECs (CL-0122) and
THP-1 (CL-0233) cells were purchased from Procell Life Science
& Technology Co., Ltd. THP-1 cells were cultured in RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc.) +10% FBS + 0.05 mM
β-mercaptoethanol (PB180633) + 1% antibiotics. HAVSMCs and HUVECs
were cultured in 10% FBS + 89% DMEM (Gibco; Thermo Fisher
Scientific, Inc.) + 1% antibiotics. HAVSMCs, HUVECs and THP-1 cells
were immortalized cell lines.
Reagents
RNAiso Plus was acquired from Takara Biotechnology
Co., Ltd. SYBR®-Green Premix qPCR, an Evo M-MLV RT-PCR
kit and RNase-free water (cat. nos. AG11701, AG11602 and AG11012,
respectively) were obtained from Accurate Biotechnology Co., Ltd.
2',7'-dichloro-dihydrofluorescein diacetate (DCFH-DA; cat. no.
D6883) and a Cell Counting Kit-8 (CCK-8; cat. no. 96992) were
acquired from MilliporeSigma. Hoechst 33342 (cat. no. M5112) was
obtained from Guangzhou Juyan Biological Co., Ltd. Palmitic acid
and solvent (SYSJ-KJ0040) were acquired from Xi'an Quantum
Technology Development Co., Ltd. The Annexin V APC Apoptosis
Detection Kit I (cat. no. 62700-80) was purchased from Guangzhou
Squirrel Biological Co., Ltd.
Cell viability and cytotoxicity
assays
The viability of cells was determined using a CCK-8
assay. First, all cells were seeded into 96-well plates at a
density of 6x103 cells/well and incubated for 24 h. To
assess the effect of palmitic acid, the cells were then incubated
with palmitic acid at various concentrations (0, 25, 50, 75, 100,
125, 150, 175 or 200 µM) for 6, 12, 24 or 36 h, and then subjected
to the CCK-8 assay at 37˚C for 1 h. The absorbance at 450 nm was
measured using a microplate reader (BioTek Instruments, Inc.).
Experimental grouping
The groups were as follows: Control group, solvent
control group (equal volume of solvent) and palmitic acid group
(200 µM palmitic acid). Following co-cultivation with palmitic acid
for 24 h, cells were used in a series of experiments.
Intracellular reactive oxygen species
(ROS) measurement
Cells (1x106) in a 6-well plate or
collected in an Eppendorf tube were incubated at 37˚C for 20 min in
PBS containing 20 µM DCFH-DA. After the DCFH-DA was removed, cells
were washed three times with PBS. Subsequently, intracellular ROS
production was measured using an inverted fluorescence microscope
(Axio Vert.A1; Carl Zeiss, Inc.) or a flow cytometer (CytExpert
2.3; Beckman Coulter, Inc.).
Cell apoptosis detection via flow
cytometry
Annexin V allophycocyanin (APC) and PI were used to
evaluate the apoptotic rates of cells in different groups. Cells
were collected with trypsin (Gibco; Thermo Fisher Scientific, Inc.)
and washed with PBS. Subsequently, 1x106 cells were
placed in binding buffer and double-stained with Annexin
V-allophycocyanin and propidium iodide in the dark for 15 min at
4˚C. The proportion of apoptotic cells was then analyzed on a flow
cytometer (CytExpert 2.3; Beckman Coulter, Inc.) to determine the
apoptotic rate.
Hoechst 33258 staining
Cells were incubated for 20 min with 5 µl Hoechst
33258 in 0.995 ml PBS at 37˚C. After washing twice with PBS, the
fluorescence images were captured using an inverted fluorescence
microscope (Axio Vert.A1; Carl Zeiss, Inc.), and Image-Pro Plus 6.0
(Media Cybernetics, Inc.) was used for analysis to measure the
fluorescence intensity.
Reverse transcription-quantitative PCR
(RT-qPCR)
According to the manufacturer's protocol, total RNA
from cells was isolated using RNAiso Plus. Subsequently, cDNA was
synthesized based on the instructions of the RT-PCR kit (catalog
no. AG11602). Subsequently, a Bio-Rad CFX96 Real-Time PCR System
(Bio-Rad Laboratories, Inc.) was used to perform qPCR. The
amplification parameters were as follows: 95˚C for 30 sec, followed
by 40 cycles of 95˚C for 5 sec and 60˚C for 34 sec, 95˚C for 15
sec, 60˚C for 60 sec and 95˚C for 15 sec. Relative mRNA expression
was calculated using the 2-ΔΔCq method after
normalization to β-actin (12,13).
For this procedure, SYBR®-Green Premix qPCR and primers
(Table I) were used.
 | Table IPrimer sequences used for PCR. |
Table I
Primer sequences used for PCR.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| IL1β |
ATGATGGCTTATTACAGTGGCAA |
GTCGGAGATTCGTAGCTGGA |
| CCL3 |
AGTTCTCTGCATCACTTGCTG |
CGGCTTCGCTTGGTTAGGAA |
| CCL4 |
TCGCAACTTTGTGGTAGA |
TTCAGTTCCAGGTCATACAC |
| IRF5 |
GGGCTTCAATGGGTCAACG |
GCCTTCGGTGTATTTCCCTG |
| MMP7 |
GAGTGAGCTACAGTGGGAACA |
CTATGACGCGGGAGTTTAACAT |
| MMP9 |
GGGACGCAGACATCGTCATC |
TCGTCATCGTCGAAATGGGC |
| SPP1 |
GAAGTTTCGCAGACCTGACAT |
GTATGCACCATTCAACTCCTCG |
| β-actin |
GGGAAATCGTGCGTGACATTAAGG |
CAGGAAGGAAGGCTGGAAGAGTG |
Statistical analysis
Values are expressed as the mean ± standard
deviation. The experiments were repeated three times. GraphPad
Prism 8 (GraphPad Software, Inc.) was used to perform statistical
analysis. The data were analyzed by one-way ANOVA. Bonferroni's
test was used as the post-hoc test after ANOVA. P<0.05 was
considered to indicate a statistically significant difference.
Results
Genes detected in DEG analysis and
interaction diagram of proteins
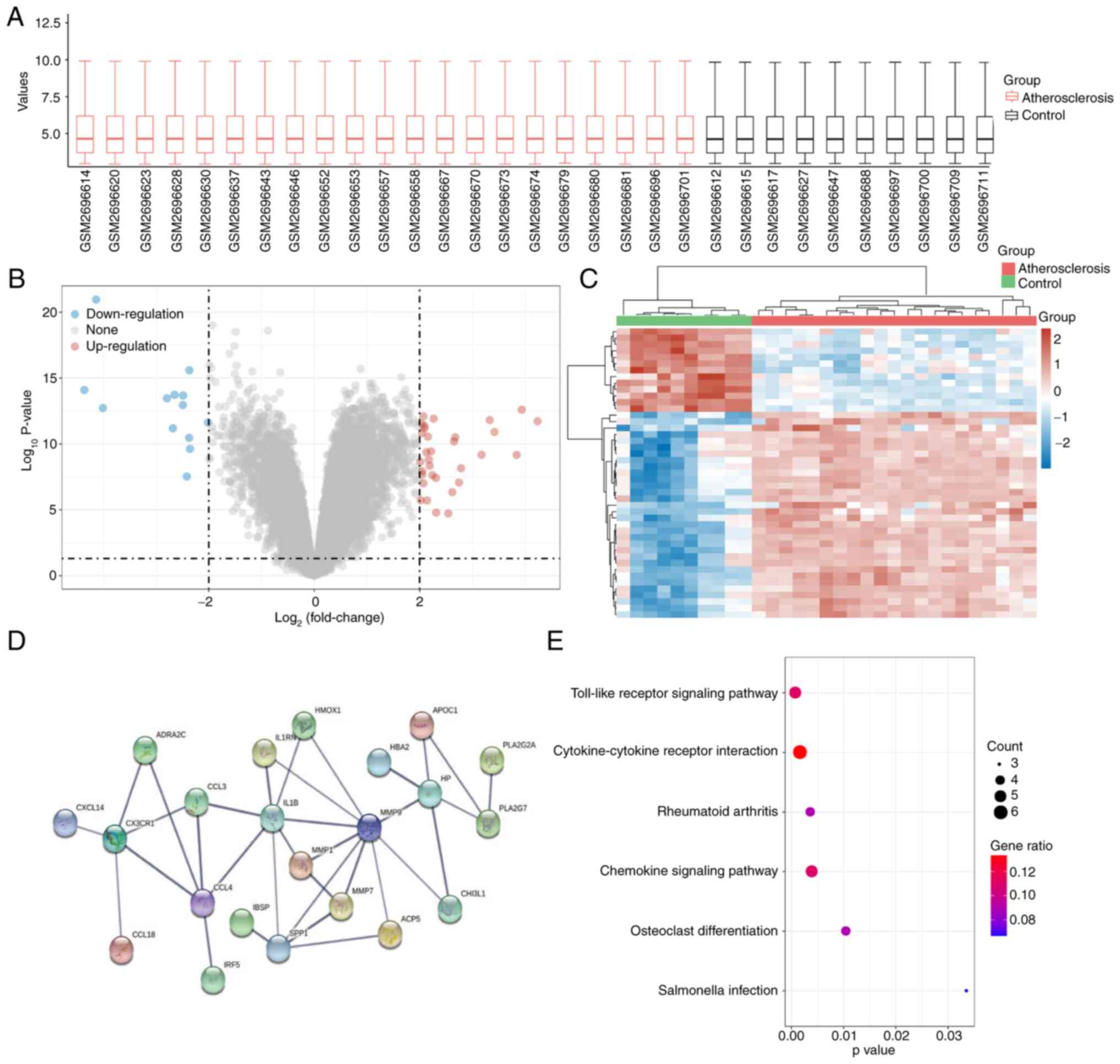
DEG analysis was performed on the GSE100927 dataset
(Fig. 1A). The extracted data were
processed by log2 transformation, and P≤0.05 was deemed
significant. A total of 32 upregulated and 13 downregulated genes
were identified (Fig. 1B). The
expression matrices of the identified genes were selected from the
GSE100927 dataset and the clustered heat map was constructed using
the heatmap function (Fig. 1C). A
total of 45 genes were inputted into STRING and the PPI
relationship was determined (Fig.
1D).
Functional enrichment analysis of
DEGs
The top 10 significant terms in the GO annotation
(Fig. 2A-C) and 6 most significant
terms in the pathway enrichment analysis (Fig. 1E; Table II) were identified. The results of
the pathway enrichment analysis revealed that the ‘Toll-like
receptor signaling pathway’ ranked first and this was selected for
experimental verification (Fig.
1E). As for the GO annotation, the terms ranking first in BP
(Table III), CC (Table IV) and MF (Table V) were selected. The Venn diagram
indicated two genes (MMP7 and MMP9) that were involved in these
three categories of terms. Subsequently, the expression levels of
IL1β, CCL4, SPP1, CCL3, IRF5, MMP7 and MMP9 were examined. The
results indicated that IL1β, CCL4, SPP1, CCL3, IRF5, MMP7 and MMP9
were highly expressed in the atherosclerosis group (Fig. 2E-K).
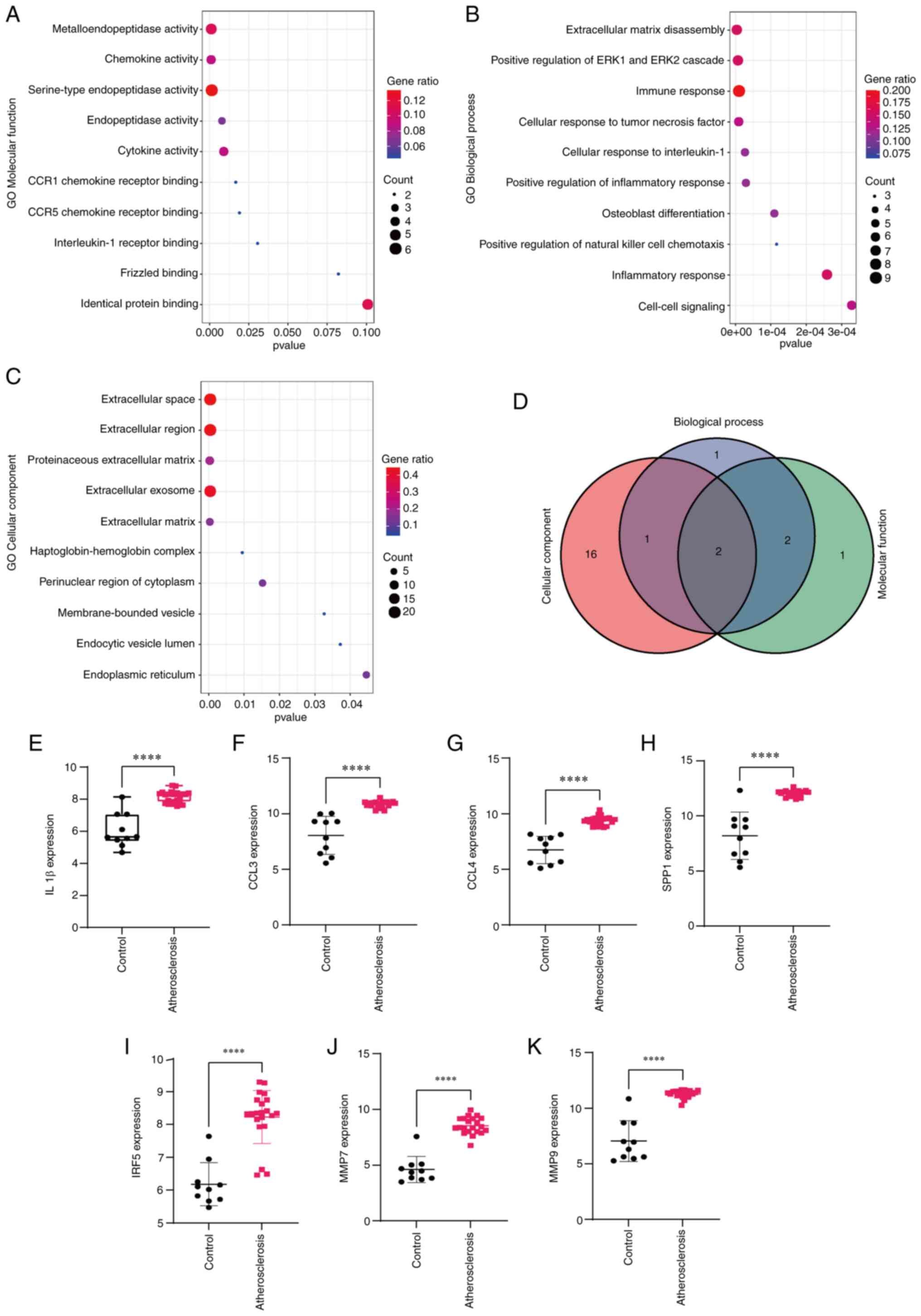 | Figure 2Functional enrichment analysis of
differentially expressed genes. GO annotation, including (A)
Molecular Function, (B) Biological Process and (C) Cellular
Component. (D) Venn diagram for two genes (MMP7 and MMP9) involved
in three vital GO annotations. (E-K) Expression levels of (E) IL1β,
(F) CCL3, (G) CCL4, (H) SPP1, (I) IRF5, (J) MMP7 and (K) MMP9 in
the control and atherosclerosis groups. ****P<0.05.
CCL3, C-C motif chemokine ligand 3; CCR1, C-C motif chemokine
receptor 1; GO, Gene Ontology; IRF5, interferon regulatory factor
5; SPP1, secreted phosphoprotein 1. |
 | Table IIKyoto Encyclopedia of Genes and
Genomes pathway enrichment. |
Table II
Kyoto Encyclopedia of Genes and
Genomes pathway enrichment.
| Term | Pathway | P-value | Genes |
|---|
| hsa04620 | Toll-like receptor
signaling pathway | 0.0004 | IL1B, CCL4, SPP1,
CCL3, IRF5 |
| hsa04060 | Cytokine-cytokine
receptor interaction | 0.0013 | CX3CR1, IL1B, CCL4,
CCL3, CCL18, CXCL14 |
| hsa05323 | Rheumatoid
arthritis | 0.0034 | MMP1, IL1B, CCL3,
ACP5 |
| hsa04062 | Chemokine signaling
pathway | 0.0036 | CX3CR1, CCL4, CCL3,
CCL18, CXCL14 |
| hsa04380 | Osteoclast
differentiation | 0.0102 | FCGR3A, IL1B, ACP5,
TREM2 |
| hsa05132 | Salmonella
infection | 0.0334 | IL1B, CCL4,
CCL3 |
 | Table IIIGO annotation (biological process,
top 10). |
Table III
GO annotation (biological process,
top 10).
| Term | Pathway | P-value | Genes |
|---|
| GO:0022617 | Extracellular
matrix disassembly |
3.24073x10-8 | MMP12, MMP7, MMP1,
SPP1, ADAM8, CAPG, MMP9 |
| GO:0070374 | Positive regulation
of ERK1 and ERK2 cascade |
4.51858x10-6 | HAND2, PLA2G2A,
CCL4, CCL3, CHI3L1, TREM2, CCL18 |
| GO:0071356 | Cellular response
to tumor necrosis factor |
7.71998x10-6 | SFRP1, CCL4, CCL3,
CHI3L1, CCL18, HAMP |
| GO:0006955 | Immune
response |
8.16275x10-6 | IL1RN, FCGR3A,
IL1B, AQP9, CCL4, CCL3, CCL18, HAMP, CXCL14 |
| GO:0071347 | Cellular response
to interleukin-1 |
2.90881x10-5 | SFRP1, CCL4, CCL3,
CHI3L1, CCL18 |
| GO:2000503 | Positive regulation
of natural killer cell chemotaxis |
1.2724x10-4 | CCL4, CCL3,
CXCL14 |
| GO:0001649 | Osteoblast
differentiation |
1.29681x10-4 | SFRP1, IBSP, MYOC,
SPP1, CCL3 |
| GO:0006954 | Inflammatory
response |
3.3532x10-4 | IL1B, CCL4, SPP1,
CCL3, CHI3L1, ADAM8, CCL18 |
| GO:0007267 | Cell-cell
signaling |
4.09983x10-4 | IL1B, CCL4, CCL3,
ADRA2C, CCL18, CXCL14 |
| GO:0045780 | Positive regulation
of bone resorption |
4.68124x10-4 | CA2, SPP1,
ADAM8 |
 | Table IVGO annotation (cellular component,
top 10). |
Table IV
GO annotation (cellular component,
top 10).
| Term | Pathway | P-value | Genes |
|---|
| GO:0005615 | Extracellular
space |
1.0927x10-11 | IL1RN, SPON1, MMP7,
MYOC, PLA2G2A, HP, CXCL14, MMP9, SFRP1, IBSP, CA2, IL1B, CCL4,
SPP1, CCL3, HMOX1, CHI3L1, APOD, CCL18, SCRG1, HAMP |
| GO:0005576 | Extracellular
region |
1.64812x10-8 | MMP7, MMP1,
PLA2G2A, HP, HBA2, TREM2, CXCL14, MMP9, MMP12, IL4I1, SFRP1, IBSP,
IL1B, CCL4, APOC1, SPP1, CCL3, APOD, HAMP |
| GO:0005578 | Proteinaceous
extracellular matrix |
2.65218x10-7 | MMP12, SPON1,
SFRP1, MMP7, MYOC, MMP1, TFPI2, CHI3L1, MMP9 |
| GO:0070062 | Extracellular
exosome |
1.48988x10-5 | IL1RN, MMP7, MYOC,
PLA2G2A, HP, HBA2, CAPG, MMP9, FCGR3A, SFRP1, DES, CA2, IL1B,
APOC1, SPP1, ACP5, CHI3L1, APOD, PI16, FBP1 |
| GO:0031012 | Extracellular
matrix |
8.34484x10-5 | SPON1, SFRP1, MMP7,
IBSP, MYOC, MMP1, TFPI2 |
| GO:0031838 |
Haptoglobin-hemoglobin complex |
9.841369x10-3 | HP, HBA2 |
| GO:0048471 | Perinuclear region
of cytoplasm |
1.7958995x10-2 | CX3CR1, PLA2G2A,
SPP1, HMOX1, CHI3L1, APOD |
| GO:0031988 | Membrane-bounded
vesicle |
3.4032333x10-2 | IBSP, SPP1 |
| GO:0071682 | Endocytic vesicle
lumen |
3.8800705x10-2 | HP, HBA2 |
| GO:0005783 | Endoplasmic
reticulum |
5.2251773x10-2 | MYOC, APOC1,
PLA2G2A, HMOX1, CHI3L1, APOD |
 | Table VGO annotation (molecular function,
top 10). |
Table V
GO annotation (molecular function,
top 10).
| Term | Pathway | P-value | Genes |
|---|
| GO:0004222 |
Metalloendopeptidase activity | 0.000159247 | MMP12, MMP7, MMP1,
ADAM8, MMP9 |
| GO:0008009 | Chemokine
activity | 0.000226706 | CCL4, CCL3, CCL18,
CXCL14 |
| GO:0004252 | Serine-type
endopeptidase activity | 0.000363433 | MMP12, MMP7, MMP1,
HP, ADAM8, MMP9 |
| GO:0004175 | Endopeptidase
activity | 0.007603833 | MMP12, MMP1,
MMP9 |
| GO:0005125 | Cytokine
activity | 0.008882175 | IL1RN, IL1B, CCL4,
SPP1 |
| GO:0031726 | CCR1 chemokine
receptor binding | 0.016880964 | CCL4, CCL3 |
| GO:0031730 | CCR5 chemokine
receptor binding | 0.019269721 | CCL4, CCL3 |
| GO:0005149 | Interleukin-1
receptor binding | 0.031128822 | IL1RN, IL1B |
| GO:0042802 | Identical protein
binding | 0.033983341 | SFRP1, DES, CCL4,
CCL3, FBP1, MMP9 |
| GO:0005109 | Frizzled
binding | 0.083902905 | SFRP1, MYOC |
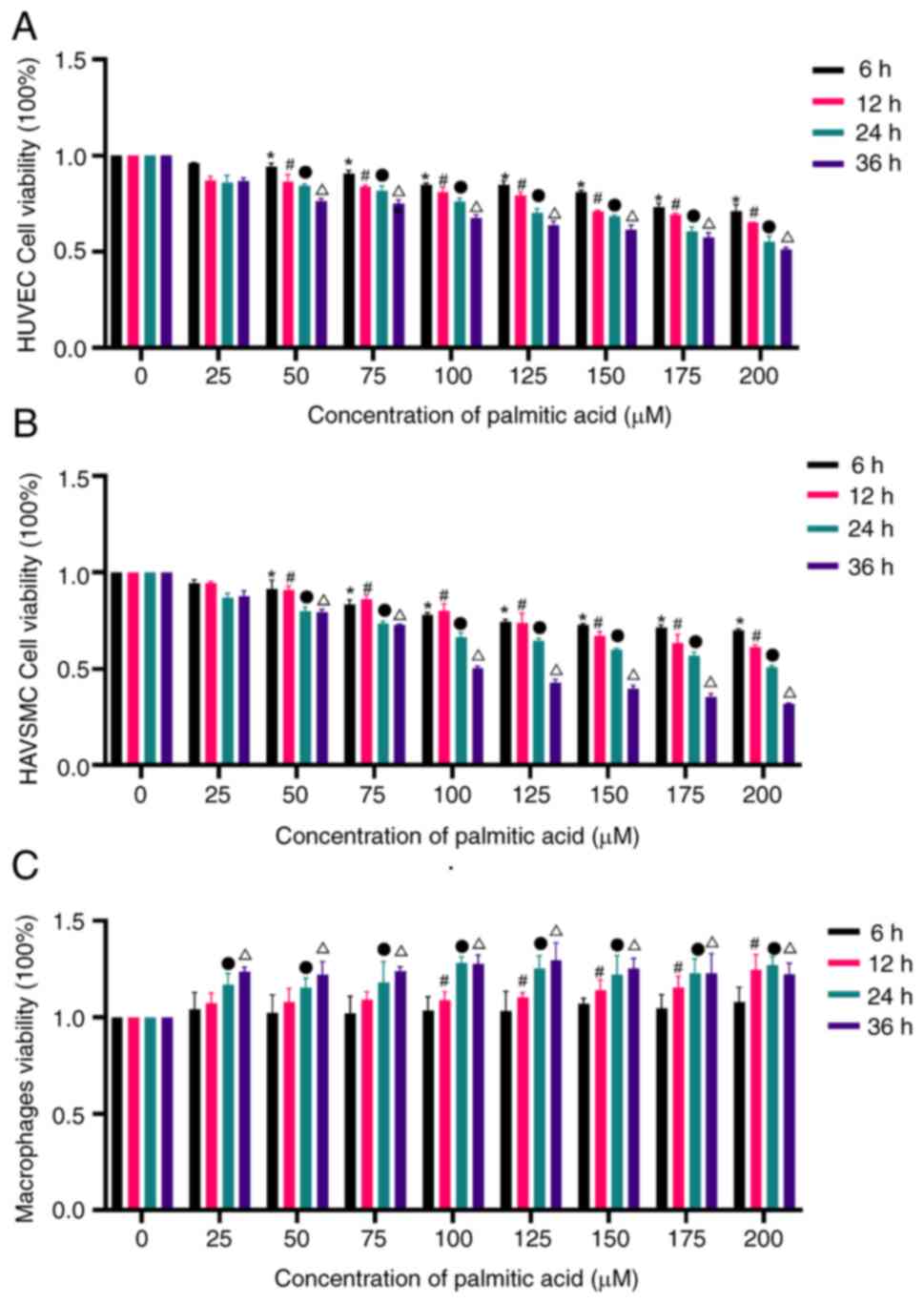
Palmitic acid affects cell
viability
To examine the cytotoxicity of palmitic acid,
HUVECs, HAVSMCs and THP-1-induced macrophages were incubated with
different doses of palmitic acid in the culture medium containing
10% FBS for 6, 12, 24 or 36 h. Cytotoxicity/growth inhibition was
determined by a CCK-8 assay. Palmitic acid (50, 75, 100, 125, 150,
175 or 200 µM) significantly lowered the viability of HUVECs and
HAVSMCs at different time-points (P<0.05; Fig. 3A and B), with a maximum response at 200 µM,
while 100, 125, 150, 175 and 200 µM palmitic acid increased the
viability of THP-1-induced macrophages at different time-points
(P<0.05) with a maximum response at 200 µM (Fig. 3C). Since incubation with 200 µM
palmitic acid for 36 h markedly decreased cell viability and was
associated with increased cell death, incubation with 200 µM
palmitic acid for 24 h was considered more suitable.
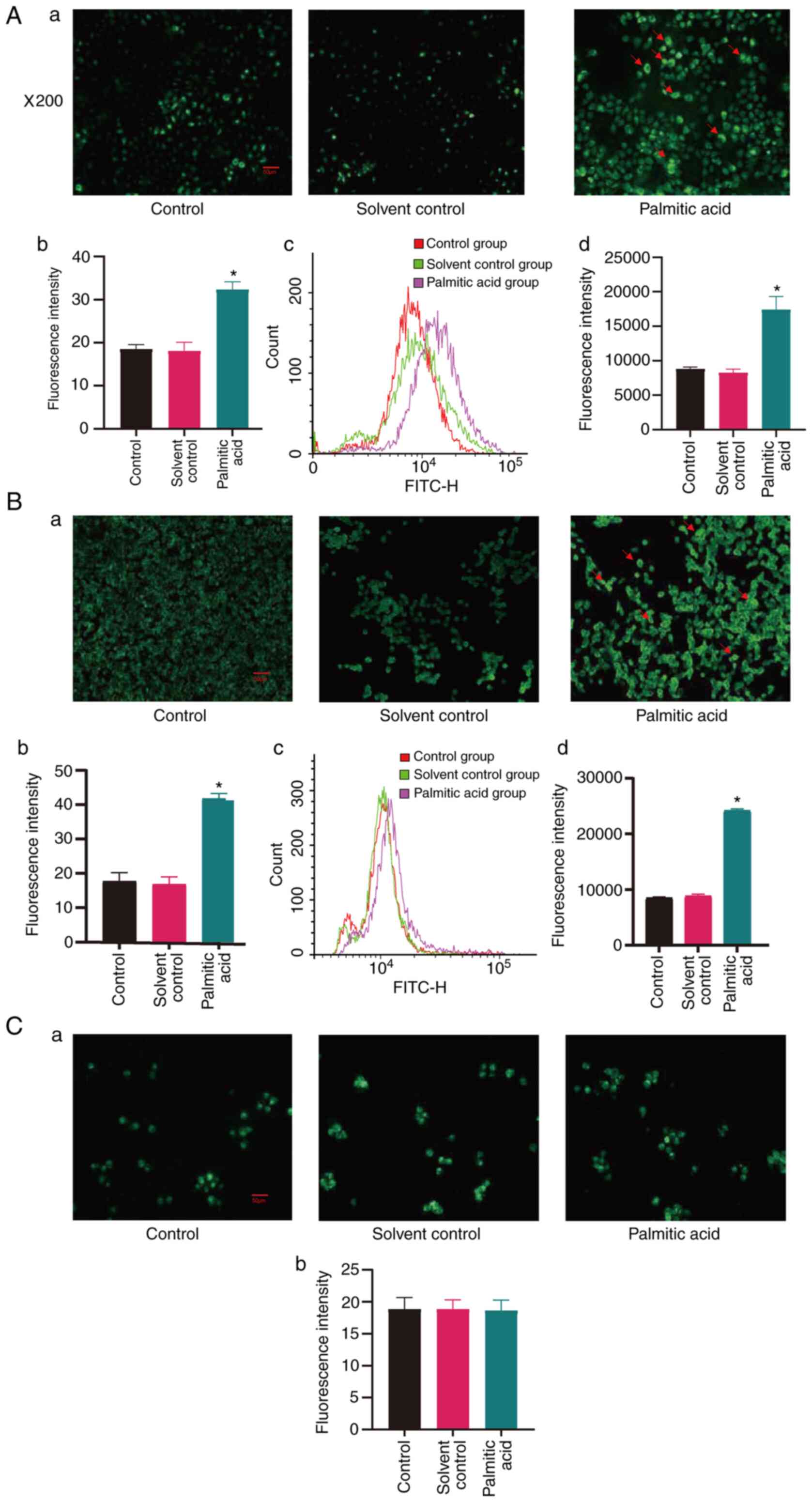
Palmitic acid induces ROS production
of cells
To determine whether palmitic acid has an effect on
intracellular oxidative stress, it was investigated whether
palmitic acid had effects on the production of ROS in cells.
Intracellular ROS levels in the different groups of cells were
examined after incubation with palmitic acid for 24 h via
fluorescence microscopy and flow cytometry. As indicated in
Fig. 4Aa-d, incubation with
palmitic acid increased intracellular ROS levels of HUVECs
(P<0.05), while the solvent alone did not. As presented in
Fig. 4Ba-d, incubation with
palmitic acid increased intracellular ROS levels of HAVSMCs
(P<0.05), while the solvent alone had no such effect. Of note,
palmitic acid and solvent incubation did not change the ROS levels
of THP-1-induced macrophages (Fig.
4Ca-b).
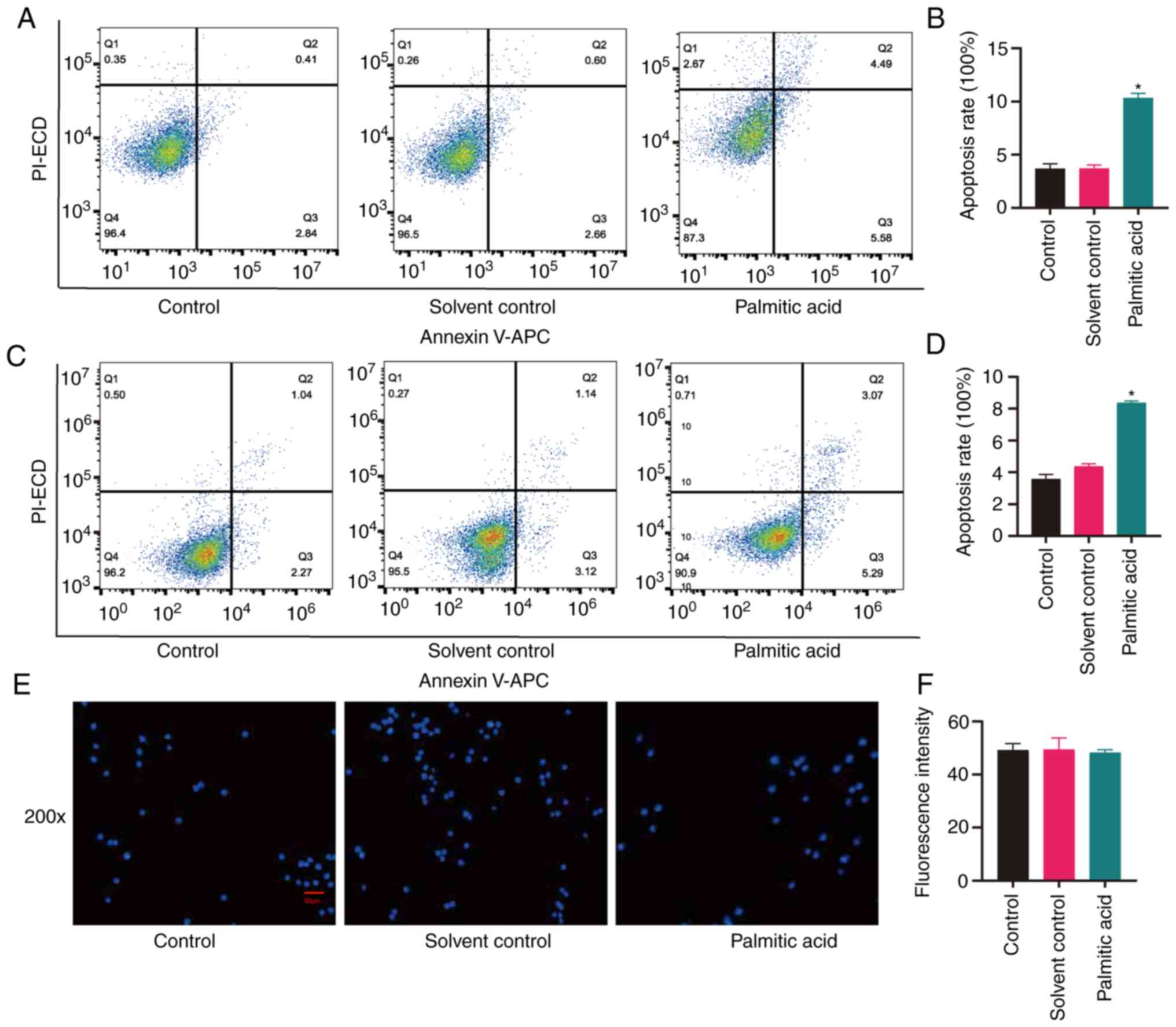
Effects of palmitic acid on cell
apoptosis
Certain studies have reported the use of palmitic
acid to represent atherosclerosis (14,15).
To determine the damaging effects of palmitic acid, it was
investigated whether palmitic acid had effects on cell apoptosis
and cell nuclear changes. In the present study, cell apoptosis was
examined after incubation with palmitic acid for 24 h via
fluorescence microscopy and nuclear changes were observed via
Hoechst 33258 staining. As presented in Fig. 5A and B, palmitic acid increased the proportion
of apoptotic HAVSMCs (P<0.05), while the solvent alone did not.
Palmitic acid also increased the apoptosis rate of HUVECs
(P<0.05), while the solvent did not (Fig. 5C and D). However, palmitic acid and solvent did
not affect the nuclei of THP-1-induced macrophages (Fig. 5E and F), as the fluorescence intensity did not
vary in the three groups.
mRNA levels of IL1β, CCL4, SPP1, CCL3,
IRF5, MMP7 and MMP9
The mRNA expression levels of IL1β, CCL4, SPP1,
CCL3, IRF5, MMP7 and MMP9 were detected in HAVSMCs, HUVECs and
THP-1-induced macrophages (Fig.
6). Palmitic acid increased the levels of IL1β, CCL4, SPP1,
CCL3, IRF5, MMP7 and MMP9 both in THP-1-induced macrophages and
HUVECs (P<0.05). In addition, palmitic acid increased the levels
of CCL4 and MMP9 (P<0.05), while the expression levels of IRF5
were only slightly altered.
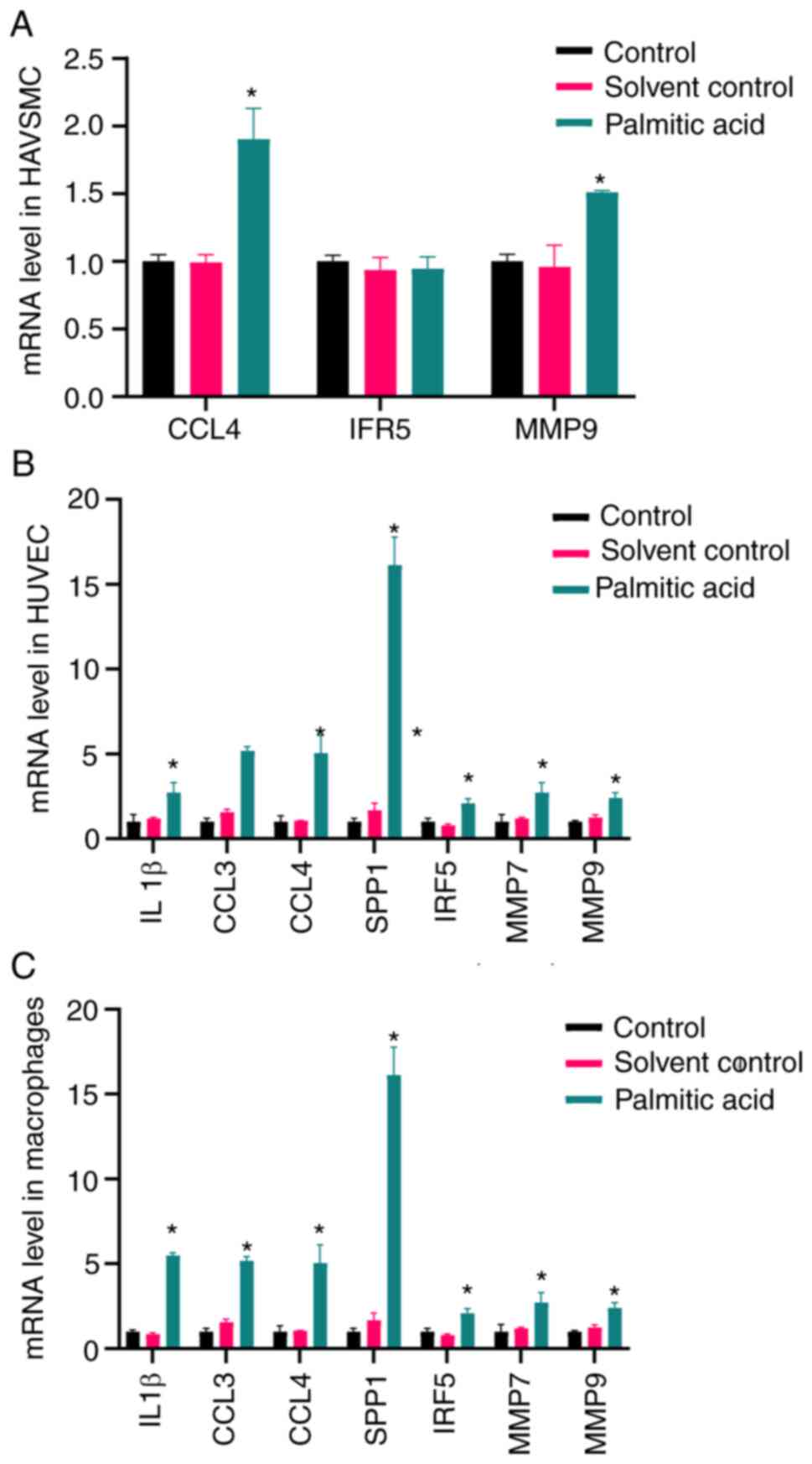 | Figure 6mRNA expression levels of IL1β, CCL4,
SPP1, CCL3, IRF5, MMP7 and MMP9. (A) Palmitic acid increased the
levels of CCL4 and MMP9, while the levels of IRF5 were not
significantly altered. Palmitic acid increased the levels of IL1β,
CCL4, SPP1, CCL3, IRF5, MMP7 and MMP9 both in (B) HUVECs and (C)
Tohoku Hospital Pediatrics-1-induced macrophages.
*P<0.05 compared with the control group. CCL3, C-C
motif chemokine ligand 3; HAVSMC, human aortic vascular smooth
muscle cell; HUVEC, human umbilical vein endothelial cell; IRF5,
interferon regulatory factor 5; SPP1, secreted phosphoprotein
1. |
Discussion
Atherosclerosis may occur throughout the arterial
vascular system and lead to various diseases. The present study
provided evidence that inflammation markers serve a vital role in
male late-stage carotid atherosclerosis. The major findings were as
follows: i) Carotid atherosclerosis is closely related to arterial
inflammation, including pathways such as ‘Toll-like receptor
signaling pathway’, ‘Cytokine-cytokine receptor interaction’ and
‘Chemokine signaling pathway’; ii) macrophages and vascular
endothelial cells are involved in vascular inflammation; iii)
palmitic acid causes apoptosis of HUVECs and HAVSMCs, indicating
that hyperlipidemia may cause blood vessel damage, while it does
not affect THP-induced macrophages; and iv) palmitic acid increased
the levels of oxidative stress in HUVECs and HAVSMCs, while it did
not increase the levels of oxidative stress in THP-induced
macrophages.
Steenman et al (7) performed a canonical pathway analysis,
revealing the important involvement of immune system processes in
atherosclerotic and healthy carotid arteries. In the original study
providing the GSE100927 dataset, it was demonstrated that the
immune system served a key role in atherosclerosis. MMP7 was the
top differentially expressed gene between the three arterial
territories in both atherosclerotic and healthy arteries and
carotid atherosclerosis displayed higher expression levels of MMP7,
MMP9 and MMP12 than those in the atherosclerotic femoral artery and
infra-popliteal artery (7). In the
present study, MMP7 and MMP9 were indicated to be involved in
biological aspects of carotid atherosclerosis and were higher
expressed after palmitic acid treatment, which was consistent with
the results of GSE100927.
The pathogenesis of atherosclerosis is closely
related to hyperlipidemia. Free fatty acids may cause endothelial
damage and lipid deposition. The effect of palmitic acid on
endothelial cells, smooth muscle cells and macrophages is able to
induce the high-fat model in vitro (14,15).
Palmitic acid may lead to inflammation and apoptosis of vascular
endothelial cells by mediating the PI3K/Akt/endothelial nitric
oxide synthase signaling pathway (16). Palmitic acid induced endothelial
lipotoxicity and lectin-like oxidized LDL receptor-1 upregulation
by reducing endoplasmic reticulum stress in HUVECs. High glucose-
and palmitic acid-induced apoptosis, oxidative stress and
inflammatory response in HUVECs (17). Nearly all studies included
inflammation, and oxidative stress was involved as well. Thus,
inhibiting inflammation requires further investigation in future
experiments.
Oxidative stress serves a vital role in the process
of atherosclerotic plaque formation (18). Oxidative stress is associated with
systemic inflammation, endothelial cell proliferation and
apoptosis, as well as vasoconstriction, which contribute to
endothelial dysfunction, leading to atherosclerosis (19). Toualbi et al (20) suggested that cardiovascular
diseases, mainly atherosclerosis, may be diagnosed indirectly by
measuring oxidative stress markers. In addition, oxidative stress
and inflammation are two major proatherogenic factors, responsible
for the modification of vascular wall integrity (21). The present study revealed that
palmitic acid may induce increased levels of oxidative stress in
HUVECs and HAVSMCs, which is consistent with previous research.
Furthermore, attenuating oxidative stress may potentially
decelerate the progression of atherosclerotic plaque formation
(22). Ji et al (23) revealed that propolis ameliorated
restenosis in hypercholesterolemia rabbits with carotid balloon
injury by inhibiting lipid accumulation, oxidative stress and the
Toll-like receptor 4/NF-κB signaling pathway. Zhang et al
(24) reported that quercetin is a
potential therapeutic agent ameliorating atherosclerotic
pathophysiology in rat carotid arteries by inhibiting oxidative
stress and inflammatory response, the mechanism of which involved
modulating the AMP-activated protein kinase/sirtuin 1/NF-κB
signaling pathway. Overproduction of ROS was reported to cause
vascular endothelial damage (11,13,25).
Endothelial dysfunction-induced lipid retention is an early feature
of atherosclerotic lesion formation (26). Apoptosis of vascular smooth muscle
cells is one of the major modulating factors of atherogenesis,
which accelerates atherosclerosis progression by causing plaque
destabilization and rupture (27).
The present study revealed that palmitic acid induces apoptosis of
HUVECs and HAVSMCs, which may induce arterial lipid accumulation
and exacerbation of atherosclerosis.
Inflammation is one of the major proatherogenic
factors, destroying the structure of blood vessels (21). IL1 is a critical factor in the
process of atherosclerosis. Mice with knockout of apolipoprotein E
(ApoE) and IL1β have markedly smaller sizes of atherosclerotic
lesions in the aortic sinus and ratios of atherosclerotic areas of
the aorta compared with single ApoE-knockout mice (28). Furthermore, artificial IL1β
expression on one side of the coronary artery led to increases in
coronary stenosis and aggravation of vascular diseases (29). The present study demonstrated that
the expression levels of IL1β in the control group were higher than
those in other groups in HUVECs and THP-1 induced macrophages,
which was consistent with the previous conclusions.
The circulating levels of C-C chemokine ligand (CCL)
are increased in atherosclerotic patients (30). CCL4 may be detected in T-cells,
smooth muscle cells and macrophages in atherosclerotic plaques
(31), and is further upregulated
in vulnerable plaques (32). In
the present study, the levels of CCL4 in HAVSMCs, HUVECs and
THP-1-induced macrophages were determined. Chang et al
(31) considered that direct
inhibition of CCL4 stabilized atheroma and reduced endothelial and
macrophage activation. Komissarov et al (33) reported that T-cell migration into
human atherosclerotic plaques may predominantly occur via C-C motif
chemokine receptor 5-CCL3 and C-X3-C motif chemokine receptor
1-C-X3-C motif chemokine ligand 1 interactions. Munjal and Khandia
(34) suggested that the
chemokines (family of small cytokines) involved in atherosclerotic
plaque formation are CCL3, chemokine (C-X-C motif) ligand 4 and
macrophage migration-inhibitory factor. Bai et al (35) indicated that the serum levels of
SPP1, CD36, ATPase H+ transporting V0 subunit d2, chitinase 3 like
1, myosin heavy chain 11 and brain-derived neurotrophic factor in
patients with coronary heart disease differed from those in healthy
subjects. The present study revealed that the levels of CCL3 and
SPP1 in HUVECs and THP-1-induced macrophages were higher than those
in the control group, which was consistent with these reports.
IRF5 serves a central role in inflammation,
mediating the production of proinflammatory cytokines, such as IL6,
IL12, IL23 and TNF-α (36).
Seneviratne et al (37) and
Posadas-Sanchez et al (38)
reported that IRF5 was detrimental in atherosclerosis, promoting
the maintenance of proinflammatory CD11c+ macrophages.
Gaubatz et al (39)
suggested a positive association between MMP-7 and the
calcification of the carotid arteries. Polonskaya et al
(40) considered that the relative
risk of coronary artery calcification was associated with MMP-9 and
the MMP-7 levels were markedly higher in patients with coronary
heart disease and verified coronary artery atherosclerosis than in
the control group. The present results were consistent with these
reports.
There are certain deficiencies in the present study.
It was not possible to obtain male primary endothelial cells, VSMCs
and macrophages to perform validation in vitro; thus, HVSMC,
HUVEC and THP-1 cell lines were used. These cell lines are more
widely used in cardiovascular disease, although these three cell
types do not optimally represent the carotid artery. It may be
attempted to generate stable male cell cultures in the future.
Furthermore, pre-term and mid-term atherosclerosis was not
investigated in the present study. These dynamic processes should
be included in future research.
In conclusion, inflammation is closely related to
atherosclerosis, as demonstrated using bioinformatics and
experimental verification. IL1β, CCL3, CCL4, SPP1, IRF5, MMP7 and
MMP9 are markers of carotid atherosclerosis.
Acknowledgements
The authors would like to thank Miss Yixuan Li
(College of Traditional Chinese Medicine, Jinan University,
Guangzhou, China) for her valuable comments on English language
revision.
Funding
Funding: The National Natural Science Foundation of China (grant
no. 81874404), Guangdong Medical Research Fund project (grant no.
B2021324), TCM Research Project of Guangdong Provincial Bureau of
TCM (grant no. 20222171) and the Guangzhou TCM and Integrated
Traditional Chinese and Western Medicine Science and Technology
Project (grant no. 202185151002) supported this study.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The gene expression datasets generated and/or analyzed
during the current study are available in the GEO repository,
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE100927.
Authors' contributions
DZ and GZ designed the experiments and wrote this
manuscript. GQ was involved in the experimental design and
manuscript revision. DZ, BJ and XL completed the experiments. HS,
SC, ZC, YZ and YP provided help with the English language revision
and were involved in data analysis. DZ, XL, BJ, GQ and GZ confirmed
the authenticity of the data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kraaijenhof JM, Hovingh GK, Stroes ESG and
Kroon J: The iterative lipid impact on inflammation in
atherosclerosis. Curr Opin Lipidol. 32:286–292. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Barrett TJ: Macrophages in atherosclerosis
regression. Arterioscler Thromb Vasc Biol. 40:20–33.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Arba F, Vit F, Nesi M, Rinaldi C,
Silvestrini M and Inzitari D: Carotid revascularization and
cognitive impairment: The neglected role of cerebral small vessel
disease. Neurol Sci. 43:139–152. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sinning C, Wild PS, Echevarria FM, Wilde
S, Schnabel R, Lubos E, Herkenhoff S, Bickel C, Klimpe S, Gori T,
et al: Sex differences in early carotid atherosclerosis (From The
Community-Based Gutenberg-Heart Study). Am J Cardiol.
107:1841–1847. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Arnlov J, Sang Y, Ballew SH, Vaidya D,
Michos ED, Jacobs DR Jr, Lima J, Shlipak MJ, Bertoni AG, Coresh J,
et al: Endothelial dysfunction and the risk of heart failure in a
community-based study: The multi-ethnic study of atherosclerosis.
ESC Heart Fail. 7:4231–4240. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ji E and Lee S: Antibody-based
therapeutics for atherosclerosis and cardiovascular diseases. Int J
Mol Sci. 22(5770)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Steenman M, Espitia O, Maurel B, Guyomarch
B, Heymann MF, Pistorius MA, Ory B, Heymann D, Houlgatte R,
Gouëffic Y and Quillard T: Identification of genomic differences
among peripheral arterial beds in atherosclerotic and healthy
arteries. Sci Rep. 8(3940)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yu G, Wang LG, Han Y and He QY:
Clusterprofiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gene Ontology Consortium. The gene
ontology (GO) project in 2006. Nucleic Acids Res. 34:D322–D326.
2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Altermann E and Klaenhammer TR:
Pathwayvoyager: Pathway mapping using the kyoto encyclopedia of
genes and genomes (KEGG) database. BMC Genomics.
6(60)2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang D, Yang B, Chang SQ, Ma SS, Sun JX,
Yi L, Li X, Shi HM, Jing B, Zheng YC, et al: Protective effect of
paeoniflorin on H2O2 induced Schwann cells injury based on network
pharmacology and experimental validation. Chin J Nat Med. 19:90–99.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang D, Sun J, Chang S, Li X, Shi H, Jing
B, Zheng Y, Lin Y, Qian G, Pan Y and Zhao G: Protective effect of
18 beta-glycyrrhetinic acid against H2O2-induced injury in schwann
cells based on network pharmacology and experimental validation.
Exp Ther Med. 22(1241)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Karbasforush S, Nourazarian A, Darabi M,
Rahbarghazi R, Khaki-Khatibi F, Avci CB, Salimi L, Bagca BG,
Bahador TN, Rezabakhsh A and Khaksar M: Docosahexaenoic acid
reversed atherosclerotic changes in human endothelial cells induced
by palmitic acid in vitro. Cell Biochem Funct. 36:203–211.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Novinbahador T, Nourazarian A, Asgharzadeh
M, Rahbarghazi R, Avci CB, Bagca BG, Ozates NP, Karbasforoush S and
Khaki-Khatibi F: Docosahexaenoic acid attenuates the detrimental
effect of palmitic acid on human endothelial cells by modulating
genes from the atherosclerosis signaling pathway. J Cell Biochem.
119:9752–9763. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ku CW, Ho TJ, Huang CY, Chu PM, Ou HC and
Hsieh PL: Cordycepin Attenuates palmitic acid-induced inflammation
and apoptosis of vascular endothelial cells through mediating
PI3K/Akt/eNOS signaling pathway. Am J Chin Med. 49:1703–1722.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tang H, Li K, Zhang S, Lan H, Liang L,
Huang C and Li T: Inhibitory effect of paeonol on apoptosis,
oxidative stress, and inflammatory response in human umbilical vein
endothelial cells induced by high glucose and palmitic acid induced
through regulating SIRT1/FOXO3a/NF-κB pathway. J Interferon
Cytokine Res. 41:111–124. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mury P, Chirico EN, Mura M, Millon A,
Canet-Soulas E and Pialoux V: Oxidative stress and inflammation,
key targets of atherosclerotic plaque progression and
vulnerability: Potential impact of physical activity. Sports Med.
48:2725–2741. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Montezano AC and Touyz RM: Reactive oxygen
species and endothelial function-role of nitric oxide synthase
uncoupling and nox family nicotinamide adenine dinucleotide
phosphate oxidases. Basic Clin Pharmacol Toxicol. 110:87–94.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Toualbi LA, Adnane M, Abderrezak K,
Ballouti W, Arab M, Toualbi C, Chader H, Tahae R and Seba A:
Oxidative stress accelerates the carotid atherosclerosis process in
patients with chronic kidney disease. Arch Med Sci Atheroscler Dis.
5:e245–e254. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gryszczynska B, Formanowicz D, Budzyń M,
Kossowska MW, Pawliczak E, Formanowicz P, Majewski W, Strzyżewski
KW, Kasprzak MP and Iskra M: Advanced oxidation protein products
and carbonylated proteins as biomarkers of oxidative stress in
selected atherosclerosis-mediated diseases. Biomed Res Int.
2017(4975264)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
He F, Li J, Liu Z, Chuang CC, Yang W and
Zuo L: Redox mechanism of reactive oxygen species in exercise.
Front Physiol. 7(486)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ji C, Pan Y, Xu S, Yu C, Ji J, Chen M and
Hu F: Propolis ameliorates restenosis in hypercholesterolemia
rabbits with carotid balloon injury by inhibiting lipid
accumulation, oxidative stress, and TLR4/NF-κB pathway. J Food
Biochem. 45(e13577)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang F, Feng J, Zhang J, Kang X and Qian
D: Quercetin modulates AMPK/SIRT1/NF-κB signaling to inhibit
inflammatory/oxidative stress responses in diabetic high fat
diet-induced atherosclerosis in the rat carotid artery. Exp Ther
Med. 20(280)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li JM and Shah AM: Endothelial cell
superoxide generation: Regulation and relevance for cardiovascular
pathophysiology. Am J Physiol Regul Integr Comp Physiol.
287:R1014–R1030. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Luo S, Wang F, Chen S, Chen A, Wang Z, Gao
X, Kong X, Zuo G, Zhou W, Gu Y, et al: NRP2 promotes
atherosclerosis by upregulating PARP1 expression and enhancing low
shear stress-induced endothelial cell apoptosis. FASEB J.
36(e22079)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bennett MR, Sinha S and Owens GK: Vascular
smooth muscle cells in atherosclerosis. Circ Res. 118:692–702.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kirii H, Niwa T, Yamada Y, Wada H, Saito
K, Iwakura Y, Asano M, Moriwaki H and Seishima M: Lack of
interleukin-1beta decreases the severity of atherosclerosis in
ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 23:656–660.
2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shimokawa H, Ito A, Fukumoto Y, Kadokami
T, Nakaike R, Sakata M, Takayanagi T, Egashira K and Takeshita A:
Chronic treatment with interleukin-1 beta induces coronary intimal
lesions and vasospastic responses in pigs in vivo. The role of
platelet-derived growth factor. J Clin Invest. 97:769–776.
1996.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cagnin S, Biscuola M, Patuzzo C, Trabetti
E, Pasquali A, Laveder P, Faggian G, Iafrancesco M, Mazzucco A,
Pignatti PF and Lanfranchi G: Reconstruction and functional
analysis of altered molecular pathways in human atherosclerotic
arteries. BMC Genomics. 10(13)2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chang TT, Yang HY, Chen C and Chen JW:
CCL4 inhibition in atherosclerosis: Effects on plaque stability,
endothelial cell adhesiveness, and macrophages activation. Int J
Mol Sci. 21(6567)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Montecucco F, Lenglet S, Gayet-Ageron A,
Bertolotto M, Pelli G, Palombo D, Pane B, Spinella G, Steffens S,
Raffaghello L, et al: Systemic and intraplaque mediators of
inflammation are increased in patients symptomatic for ischemic
stroke. Stroke. 41:1394–1404. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Komissarov A, Potashnikova D, Freeman ML,
Gontarenko V, Maytesyan D, Lederman MM, Vasilieva E and Margolis L:
Driving T cells to human atherosclerotic plaques: CCL3/CCR5 and
CX3CL1/CX3CR1 migration axes. Eur J Immunol. 51:1857–1859.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Munjal A and Khandia R: Atherosclerosis:
Orchestrating cells and biomolecules involved in its activation and
inhibition. Adv Protein Chem Struct Biol. 120:85–122.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bai HL, Lu ZF, Zhao JJ, Ma X, Li XH, Xu H,
Wu SG, Kang CM, Lu JB, Xu YJ, et al: Microarray profiling analysis
and validation of novel long noncoding RNAs and mRNAs as potential
biomarkers and their functions in atherosclerosis. Physiol
Genomics. 51:644–656. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Krausgruber T, Blazek K, Smallie T,
Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M and Udalova
IA: IRF5 promotes inflammatory macrophage polarization and TH1-TH17
responses. Nat Immunol. 12:231–238. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Seneviratne AN, Edsfeldt A, Cole JE,
Kassiteridi C, Swart M, Park I, Green P, Khoyratty T, Saliba D,
Goddard ME, et al: Interferon regulatory factor 5 controls necrotic
core formation in atherosclerotic lesions by impairing
efferocytosis. Circulation. 136:1140–1154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Posadas-Sanchez R, Cardoso-Saldaña G,
Fragoso JM and Vargas-Alarcón G: Interferon regulatory factor 5
(IRF5) gene haplotypes are associated with premature coronary
artery disease. Association of the IRF5 polymorphisms with
cardiometabolic parameters. The genetics of atherosclerotic disease
(GEA) Mexican study. Biomolecules. 11(443)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gaubatz JW, Ballantyne CM, Wasserman BA,
He M, Chambless LE, Boerwinkle E and Hoogeveen RC: Association of
circulating matrix metalloproteinases with carotid artery
characteristics: The atherosclerosis risk in communities carotid
MRI study. Arterioscler Thromb Vasc Biol. 30:1034–1042.
2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Polonskaya YV, Kashtanova EV, Murashov IS,
Striukova EV, Kurguzov AV, Stakhneva EM, Shramko VS, Maslatsov NA,
Chernyavsky AM and Ragino YI: Association of matrix
metalloproteinases with coronary artery calcification in patients
with CHD. J Pers Med. 11(506)2021.PubMed/NCBI View Article : Google Scholar
|