Introduction
Periodontal disease has become a global public
health concern, with a high prevalence (1). Periodontal disease is defined as the
chronic inflammation of the periodontal supporting tissue, caused
by chronic infection with bacteria, including Porphyromonas
gingivalis (P. gingivalis) (2). Lipopolysaccharide (LPS) derived from
Porphyromonas gingivalis (P. gingivalis LPS) is
responsible for a substantial proportion of its systemic effects.
When a host is invaded by a periodontal pathogen, the LPS released
is recognized by the immune system, leading to a robust
inflammatory response, and this can cause alveolar bone resorption
(3). In addition, the inflammation
may extend from the gingiva into the periodontal membrane, alveolar
bone and cementum, leading to periodontitis. Chronic periodontal
inflammation is also associated with the entry of host and
bacterially-derived factors into the circulation (4). In addition, periodontal bacteria may
colonize the gut via the oral route (5,6).
Thus, periodontal bacteria can cause or affect systemic
disease.
Epidemiological research has demonstrated an
association between obesity and periodontal disease (7). In addition, a number of previous
studies have demonstrated a link between periodontal inflammation
and obesity (4,8-10).
Obesity is associated with a higher incidence of tooth loss over a
period of 5 years, and the periodontal conditions of individuals
with obesity are significantly worse following periodontal
treatment than those of individuals without obesity (11). Furthermore, the periodontal
inflamed surface area index is positively associated with body mass
index (BMI) (4).
Periodontal disease may affect glucose metabolism
via low-grade inflammation (12).
Accordingly, diabetes mellitus (DM) has been identified as a risk
factor for the progression of periodontal disease (13,14).
Furthermore, obesity predisposes towards type 2 DM (4). Host pro-inflammatory factors released
by immune cells activated by bacterial products may reach the
adipose tissue via the circulation in patients with periodontal
inflammation. Therefore, local inflammation may have widespread
effects on the body through effects on adipose tissue (4,15).
However, the effects of periodontitis on obesity remain
unclear.
Brown adipocytes are thermogenic, helping to
maintain body temperature by increasing basal metabolism in cold
environments. Thermogenesis in brown adipocytes is induced by the
uncoupling of mitochondrial oxidative from phosphorylation by
uncoupling protein 1 (UCP1) (4),
and this has been shown to protect against obesity and
obesity-related disease (16).
Periodontopathic bacteria affect the development of obesity,
glucose intolerance and hepatic steatosis, and also alter lipid
metabolism and the thermogenesis of brown adipose tissue (BAT)
(17,18). In addition, P. gingivalis
administration has been shown to modify gene expression in the BAT
of pregnant mice (17).
Long non-coding RNAs (lncRNAs) are RNA transcripts
of >200 nucleotides in length that do not encode proteins and
exhibit poor sequence conservation (19,20).
lncRNAs play roles in a number of physiological and pathological
processes, including development and differentiation. They regulate
gene expression by functioning as microRNA sponges and by affecting
transcription, splicing, and translation (20). Recent research has also
demonstrated that lncRNAs are involved in brown adipogenesis, the
browning of white adipose tissue, and brown adipose thermogenesis
(21). These lncRNAs include
lncRNA-BATE1, lncRNA-BATE10, AK079912, Blnc1, H19, Lnc-Uc.417 and
Lnc-dPrdm16(21).
To date, research into the effects of periodontitis
on obesity has mainly focused on P. gingivalis-induced
endotoxemia; however, it remains unclear whether there are direct
effects of P. gingivalis LPS on brown adipocytes, and
whether these are mediated by lncRNAs. Therefore, the present study
aimed to determine the effects of P. gingivalis LPS on
BAT.
Materials and methods
Mice
C57BL/6J mice (n=10, male, 6-8 weeks old, weighing
20-22 g) were purchased from Shanghai Laboratory Animal Center,
housed under standard environmental conditions at a temperature of
22±2˚C and 55-60% humidity, with free access to food and water and
a 12-h light/dark cycle and were allocated into two groups as
follows: The first was administered a sonicated P.
gingivalis suspension in PBS buffer (P. gingivalis
group, n=5) via the tail vein, and the second was administered PBS
alone (control group, n=5). According to a previous study (18), after 18 h, the mice were
euthanized, and samples of BAT were collected for use in reverse
transcription-quantitative PCR (RT-qPCR). The mice were monitored
before and 18 h after the P. gingivalis injection. All mice
were euthanized using 30% vol/min CO2 inhalation. Death
was verified by confirming the following: The cessation of
respiratory and cardiovascular movements by observation at room air
for at least 10 min. The experimental protocols were approved by
the Institutional Animal Care and Use Committee of Zhejiang
University (Approval no. ZJU20170237,2017-02-24).
Culture of P. gingivalis
Porphyromonas gingivalis [donated by Dr
Peihui Ding (22)] was cultured on
trypticase soy agar (Qiangdao Hope Bio-Technology Co., Ltd.),
containing 10% defibrinated horse blood, hemin and menadione
(Qiangdao Hope Bio-Technology Co., Ltd.), under anaerobic
conditions at 37˚C. The bacteria were collected in PBS buffer (pH
7.4) (Shandong Victoryx Biotechnology Co., Ltd.; http://www.vxbiotech.com/en/) and 109
CFU/ml of the bacterial suspension was sonicated at 20 kHz for 5
min on ice using a Vibra cell sonicator (Sonics & Materials,
Inc.).
Brown adipocyte culture in vitro
Preadipocytes obtained from the BAT of mice
according to a previously described method (23) [donated by Professor Zhuoxian Meng
(24)] were cultured in
high-glucose Dulbecco's modified Eagle medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc.) containing 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.). To induce the adipogenic
differentiation of the preadipocytes, they were cultured in
induction medium containing 20 nM insulin (cat. no. I5500,
MilliporeSigma), 1 µM dexamethasone (cat. no. D1756,
MilliporeSigma), 0.5 mM 3-isobutyl-1-methylxanthine (cat. no.
I-5879, MilliporeSigma), 1 nM triiodothyronine (T3) (cat. no.
T2877, MilliporeSigma), 125 µM indomethacin (cat. no. I-7378,
MilliporeSigma) and 10% fetal bovine serum (FBS, Gibco, Thermo
Fisher Scientific, Inc.) for 2 days and then in differentiation
medium containing 20 nM insulin, T3, and 10% FBS for an additional
2 days. Subsequently, the differentiation medium was replaced every
2 days until day 7. P. gingivalis LPS (cat. no. tlrl-pglps,
InvivoGen) or LPS from Escherichia coli (E. coli LPS)
(cat. no. L4391, MilliporeSigma) was added to the induction and
differentiation media.
Transfection with small interfering
RNA (siRNA)
50 nM LncRNA-BATE10-siRNA or scramble siRNA
[negative control (NC)] were provided by Biomics Biotechnologies
Co., Ltd. and mixed with transfection reagent (INVI DNA RNA, 20
µM/µl; Invigentech) and added to the preadipocytes; the mix of
siRNA and the transfection reagent were kept at room temperature
for 15 min before transfection (50 nM siRNA) into the cells, and
then after 48 h, the cells were induced to differentiate. The siRNA
duplex sequences were as follows: lncRNA-BATE10,
5'-GAGUACUGAUCAUCAUUAAdTdT-3' (sense) and
5'-UUAAUGAUGAUCAGUACUCdTdT-3' (antisense); and NC,
5'-UUCUCCGAACGUGUCACGUdTdT-3' (sense) and
5'-ACGUGACACGUUCGGAGAAdTdT-3' (antisense).
RT-qPCR
RNA was extracted using TRIzol reagent (cat. no.
15596026; Invitrogen; Thermo Fisher Scientific, Inc.) from BAT
following the manufacturer's instructions. cDNA was synthesized
using the WCGENE mRNA cDNA kit (cat. no. WC-SJH0001; WCGENE
Biotech), at 37˚C for 15 min and 85˚C for 5 sec. qPCR (WcGene mRNA
qPCR mix; cat. no. WC-SJH0002, WCGENE Biotech) was performed using
the Bio-Rad CFX96 Touch Real-Time PCR Detection System (Bio-Rad
Laboratories, Inc.) and StepOnePlus™ Real-Time PCR Detection System
(Thermo Fisher Scientific, Inc.), using the following primers
synthesized by Sangon Biotech (Shanghai) Co., Ltd.: Mouse UCP1
forward, 5'-GGCATTCAGAGGCAAATCAGCT-3' and reverse,
5'-CAATGAACACTGCCACACCTC-3'; mouse Actb forward,
5'-CGTTGACATCCGTAAAGACC-3' and reverse, 5'-AACAGTCCGCCTAGAAGCAC-3';
lncRNA-BATE10 forward, 5'-AAGCAGCAGAGCCAGAACTC-3' and reverse,
5'-CCATGCAGACCTCCTTGGTT-3'. The following PCR conditions were used:
1 cycle at 95˚C for 30 sec, then 40 cycles at 95˚C for 5 sec and
60˚C for 34 sec.
For the analysis of the mRNA expression data,
relative quantification was used (25). Relative quantification relates the
PCR signal of the target transcript in a treatment group to that of
another sample such as an untreated control. The analysis used the
2-ΔΔCq method. ΔCq=the target
CT-the average of the reference (β-actin) Cq
ΔΔCq=treated ΔCq-untreated (or other
reference group) ΔCq. Relative expressive was calculated
as the 2-ΔΔCq values of the treat groups/mean of the
2-ΔΔCq value of the reference group (as ‘1.0’).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Difference between groups were compared using ANOVA and
Tukey's multiple comparisons test (for more than two groups) or the
Student's t-test (for two groups) using Prism software (GraphPad
Software, Inc.). P-values ≤0.05 were considered to indicate
statistically significant differences.
Results
P. gingivalis LPS reduces UCP1
expression and oil droplet formation in preadipocytes during their
differentiation
The present study first examined the effects of
P. gingivalis LPS on differentiating brown adipocytes. The
expression of UCP1 decreased with the increasing
concentration of P. gingivalis LPS (Fig. 1A). In addition, the accumulation of
lipid droplets decreased as the concentration of P.
gingivalis LPS increased (Fig.
1B). These results suggested that P. gingivalis LPS
exerted a negative effect on the differentiation of preadipocytes
into brown adipocytes. E. coli LPS exerted a similar effect
on UCP1 expression during brown preadipocyte differentiation
(Fig. 1D). In addition, the
present study examined the effects of an intravenous injection of
108 CFU P. gingivalis suspension in 100 µl saline
or 100 µl PBS on the BAT UCP1 expression of mice, and it was
found that the bacterial administration reduced UCP1
expression (Fig. 1C).
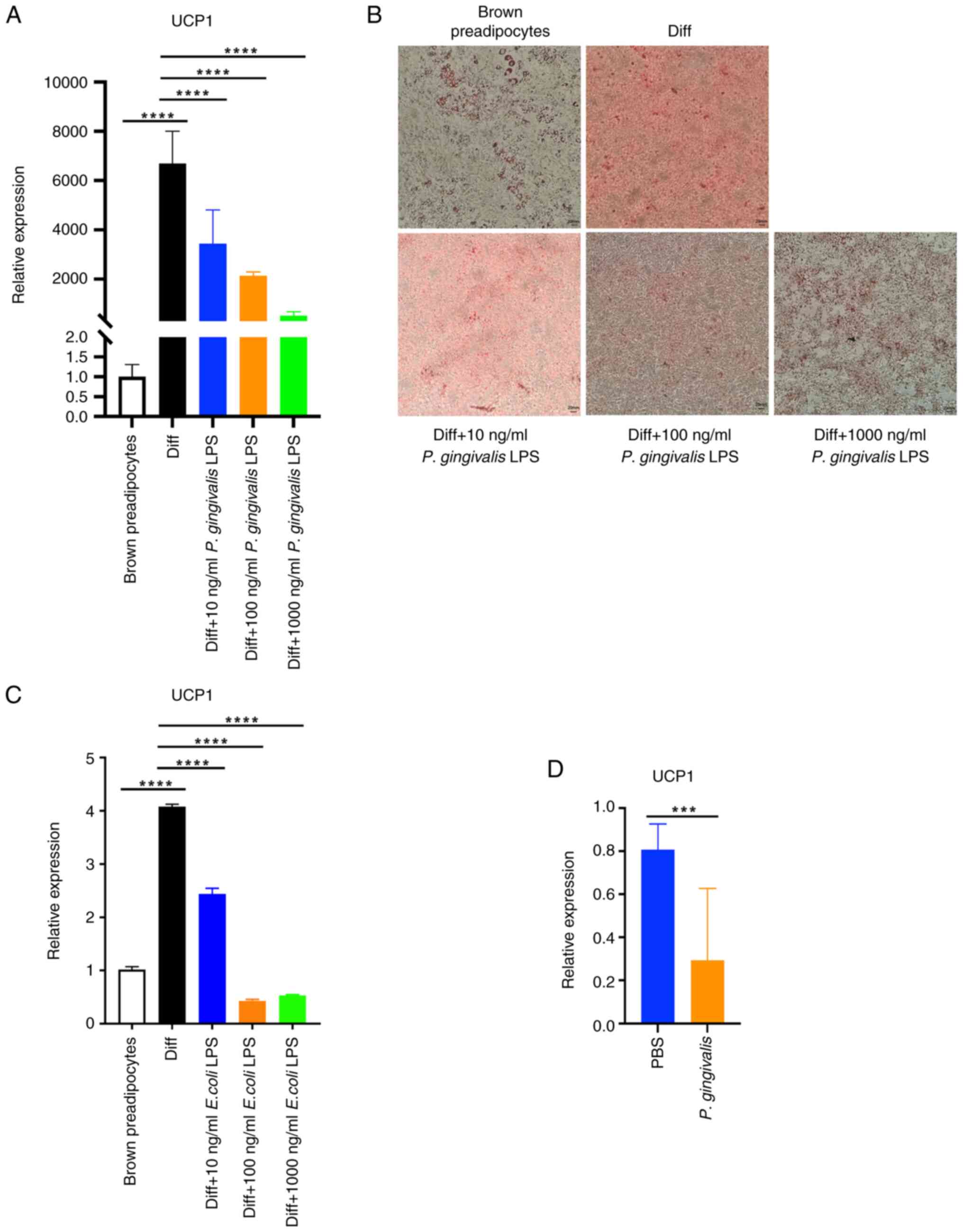 | Figure 1P. gingivalis LPS reduces
UCP1 expression and lipid droplet formation in
differentiating brown adipocytes. Preadipocytes were induced to
differentiate into brown adipocytes, during which P.
gingivalis LPS was added to the medium. (A) UCP1 mRNA
expression in brown adipocytes (Diff) and preadipocytes. The brown
preadipocyte group was used as the reference group and relative
expression was calculated as the 2-ΔΔCq values of the
other groups/mean of 2-ΔΔCq value of the brown
preadipocyte group (as ‘1.0’). UCP1: P<0.0001 for ANOVA.
Diff. vs. brown preadipocytes, P<0.0001; Diff vs. Diff + 10
ng/ml P. gingivalis LPS, P<0.0001; Diff vs. Diff + 100
ng/ml P. gingivalis LPS, P<0.0001; Diff vs. Diff + 1,000
ng/ml P. gingivalis LPS, P<0.0001. (B) Oil Red O-stained
brown adipocytes (Diff) or preadipocytes. (C) Preadipocytes were
induced to differentiate into brown adipocytes, during which E.
coli LPS was present in the medium. UCP1 mRNA expression
was measured in brown adipocytes (Diff) and preadipocytes. The
brown preadipocyte group was used as the reference group and
relative expression was calculated as the 2-ΔΔCq values
of the other groups/mean of 2-ΔΔCq value of the brown
preadipocyte group (as ‘1.0’). UCP1: P<0.0001 for ANOVA.
Diff vs. brown preadipocytes, P<0.0001; Diff vs. Diff + 10 ng/ml
E. coli LPS, P<0.0001; Diff vs. Diff + 100 ng/ml E.
coli LPS, P<0.0001; Diff vs. Diff + 1,000 ng/ml E.
coli LPS, P<0.0001. (D) UCP1 mRNA expression in the
brown adipose tissue of mice injected with P. gingivalis 100
µl (108 CFU) or PBS 18 h previously. The PBS group was
used as the reference group and relative expression was calculated
as the 2-ΔΔCq values of the other groups/mean of
2-ΔΔCq value of the PBS group (as ‘1.0’). UCP1:
PBS vs. P. gingivalis, P=0.0005). ***P<0.001
and ****P<0.0001. P. gingivalis,
Porphyromonas gingivalis; P. gingivalis LPS,
lipopolysaccharide derived from Porphyromonas gingivalis;
LPS, lipopolysaccharide; UCP1, uncoupling protein 1; E. coli
LPS, LPS derived from Escherichia coli. |
P. gingivalis LPS reduces
lncRNA-BATE10 expression in differentiating brown adipocytes and
differentiated BAT in mice
In a previous study, it was shown that lncRNA-BATE10
may be involved in brown adipocyte thermogenesis (26). Therefore, the present study
measured the expression of lncRNA-BATE10 in differentiating brown
adipocytes treated with P. gingivalis LPS and BAT from mice
administered P. gingivalis. As the concentration of P.
gingivalis LPS increased, lncRNA-BATE10 expression decreased
during brown adipocyte differentiation (Fig. 2A). E. coli LPS exerted a
similar effect on lncRNA-BATE10 expression during brown
preadipocyte differentiation (Fig.
2B). Consistent with P. gingivalis LPS, lncRNA-BATE10
expression was lower in the BAT of mice administered P.
gingivalis (Fig. 2C).
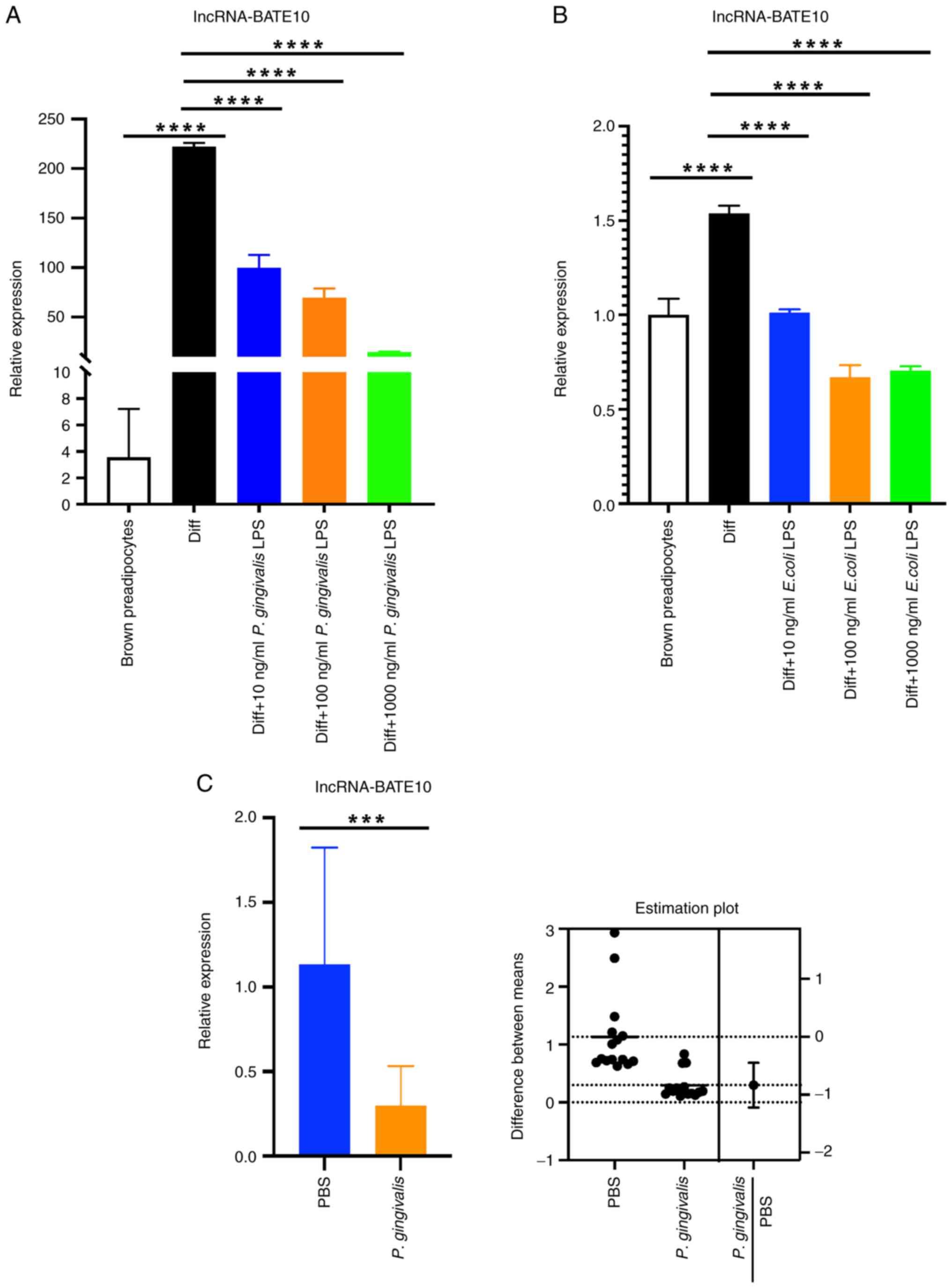 | Figure 2P. gingivalis LPS reduces
lncRNA-BATE10 expression in differentiating brown adipocytes and
P. gingivalis reduces lncRNA-BATE10 expression in the BAT of
mice. (A) Preadipocytes were induced to differentiate into brown
adipocytes, during which P. gingivalis LPS was added to the
medium. lncRNA-BATE10 expression was examined in brown adipocytes
(Diff) and preadipocytes. The brown preadipocyte group was used as
the reference group and relative expressive was calculated as the
2-ΔΔCq values of the other groups/mean of
2-ΔΔCq value of the brown preadipocyte group (as ‘1.0’).
P<0.0001 for ANOVA. Diff vs. preadipocytes, P<0.0001; Diff
vs. Diff + 10 ng/ml P. gingivalis LPS, P<0.0001; Diff vs.
Diff + 100 ng/ml P. gingivalis LPS, P<0.0001; Diff vs.
Diff + 1,000 ng/ml P. gingivalis LPS, P<0.0001. (B)
Preadipocytes were induced to differentiate into brown adipocytes,
during which E. coli LPS was present in the medium, then
lncRNA-BATE10 expression in brown adipocytes (Diff) and
preadipocytes was measured. The brown preadipocyte group was used
as the reference group and relative expressive was calculated as
the 2-ΔΔCq values of the other groups/mean of
2-ΔΔCq value of the brown preadipocyte group (as ‘1.0’).
P<0.0001 for ANOVA. Diff vs. preadipocytes, P<0.0001.; Diff
vs. Diff + 10 ng/ml E. coli LPS, P<0.0001; Diff vs. Diff
+ 100 ng/ml E. coli LPS, P<0.0001; Diff vs. Diff + 1,000
ng/ml E. coli LPS, P<0.0001. (C, left panel)
lncRNA-BATE10 expression in the BAT of mice intravenously
administered P. gingivalis 100 µl (108 CFU) or
PBS 18 h earlier. The PBS group was used as the reference group and
relative expressive was calculated as the 2-ΔΔCq values
of the other groups/mean of 2-ΔΔCq value of the PBS
group (as ‘1.0’). PBS vs. P. gingivalis, P=0.0001. (C, right
panel) Estimation plot displaying the raw data and the confidence
interval for the difference between the means.
***P<0.001 and ****P<0.0001. P.
gingivalis, Porphyromonas gingivalis; P.
gingivalis LPS, lipopolysaccharide derived from
Porphyromonas gingivalis; LPS, lipopolysaccharide; E.
coli LPS, LPS derived from Escherichia coli. |
lncRNA-BATE10 is involved in the
differentiation of brown adipocytes
To better understand the role of lncRNA-BATE10 in
brown adipocyte differentiation, the effects of siRNA targeting
this lncRNA on UCP1 expression were assessed. lncRNA-BATE10
siRNA (Fig. 3A) was added to brown
preadipocytes, differentiation was induced and UCP1
expression was then measured. It was found that UCP1
expression was decreased following the knockdown of lncRNA-BATE10
expression (Fig. 3B). Thus,
lncRNA-BATE10 may be involved in brown adipocyte differentiation.
In addition, after lncRNA-BATE10 was knocked down using siRNA, the
effects of P. gingivalis LPS on UCP1 expression
during the differentiation of brown adipocytes were less pronounced
(Fig. 3C). A comparison of the
ratios of UCP1 expression in differentiating brown
adipocytes transfected with negative control siRNA ± P.
gingivalis LPS treatment with that of the expression in cells
transfected with lncRNA-BATE10 siRNA ± P. gingivalis LPS
also revealed that the inhibition of brown adipocyte
differentiation by P. gingivalis LPS was suppressed by
lncRNA-BATE10 knockdown (Fig. 3D).
Thus, lncRNA-BATE10 may be involved in the effects of P.
gingivalis LPS on brown adipocyte differentiation.
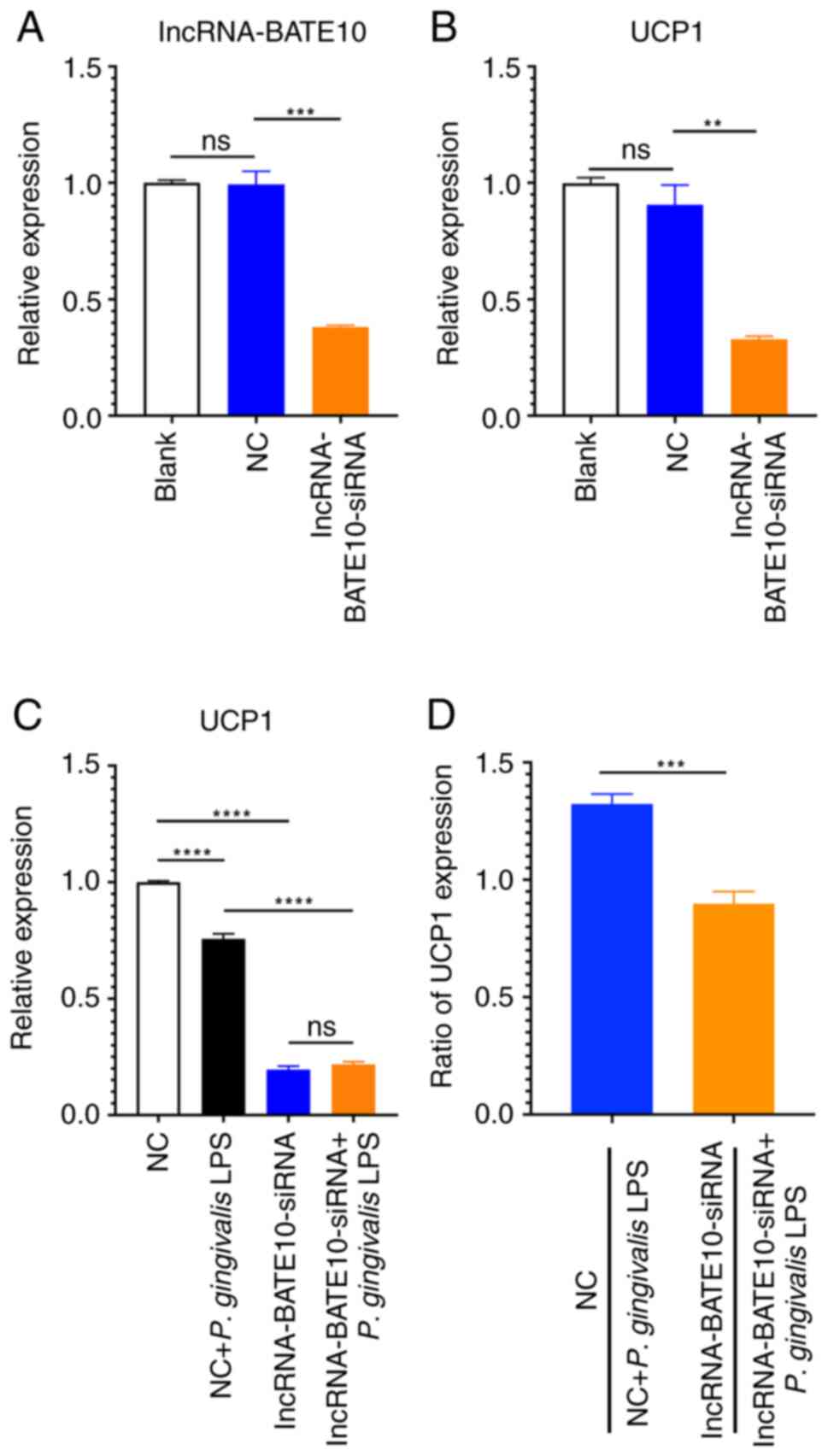 | Figure 3lncRNA-BATE10 is involved in the
differentiation of brown adipocytes. lncRNA-BATE10-siRNA or
scramble siRNA (NC) 50 nM were added to the culture medium of
preadipocytes, and 48 h later, the cells were induced to
differentiate. (A) lncRNA-BATE10 and (B) UCP1 expression
during the differentiation of brown adipocytes. lncRNA-BATE10:
P<0.0001 for ANOVA. NC vs. blank, P=0.9774; lncRNA-BATE10-siRNA
vs. blank, P=0.0008; UCP1, P<0.0001 for ANOVA. NC vs.
blank, P=0.3112; lncRNA-BATE10-siRNA vs. NC, P=0.0032. (C)
Following lncRNA-BATE10 knockdown, the cells were induced to
differentiate in the presence or absence of P. gingivalis
LPS, and UCP1 expression was measured using reverse
transcription-quantitative PCR. The NC group was used as the
reference group and relative expressive was calculated as the
2-ΔΔCq values of the other groups/mean of
2-ΔΔCq value of the NC group (as ‘1.0’). UCP1,
P<0.0001 for ANOVA. NC vs. NC + P. gingivalis LPS,
P<0.0001; NC vs. lncRNA-BATE10-siRNA, P<0.0001; NC + P.
gingivalis LPS vs. lncRNA-BATE10-siRNA + P. gingivalis
LPS, P<0.0001; lncRNA-BATE10-siRNA vs. lncRNA-BATE10-siRNA +
P. gingivalis LPS, P=0.3282. (D) Ratio of UCP1 expression
between the NC and NC + P. gingivalis LPS,
lncRNA-BATE10-siRNA or lncRNA-BATE10-siRNA + P. gingivalis
LPS groups. For the comparison between the ratios of NC to NC +
P. gingivalis LPS and lncRNA-BATE10-siRNA to
lncRNA-BATE10-siRNA + P. gingivalis LPS, P=0.0004.
**P<0.01, ***P<0.001 and
****P<0.0001. ns, no significant; NC, negative
control; P. gingivalis, Porphyromonas gingivalis;
P. gingivalis LPS, lipopolysaccharide derived from
Porphyromonas gingivalis; LPS, lipopolysaccharide; UCP1,
uncoupling protein 1. |
Discussion
The present study examined the effects of P.
gingivalis and P. gingivalis LPS on brown adipocytes and
mouse BAT. It was found that P. gingivalis decreased
UCP1 expression and lncRNA-BATE10 expression in BAT, and
that P. gingivalis LPS decreased the expression of
UCP1 and lncRNA-BATE10 in differentiating brown adipocytes.
In addition, the present study provided evidence that lncRNA-BATE10
may be involved in the effects of P. gingivalis LPS on brown
adipocyte differentiation.
Periodontitis is a local form of inflammation that
may have a systemic effect on obesity. Immune cells are activated
in the adipose tissue of individuals with obesity. In addition,
certain bacterial products, such as LPS, danger associated
molecular patterns, bacterial flagellar protein, etc., activate
immune cells (4,27,28),
which may be transported to the adipose tissue via the circulation.
Thus, local inflammation may have whole-body effects through
effects on obese adipose tissue. Thus, obesity may be associated
with periodontal disease and the presence of periodontal disease
may also exacerbate the inflammation that characterizes obesity
(4,15).
In mice with diet-induced obesity, P.
gingivalis has been shown to exacerbate weight gain and the
expansion of adipose tissue (29).
Endotoxemia associated with P. gingivalis also affects BAT
function. The administration of P. gingivalis has been shown
to increase the expression of inflammation-related genes and to
reduce that of UCP1 and Cidea, as well as that of the
genes related to lipolysis, Lipe and Pnpla2, in BAT (18). Notably, the expression of
Pparg and Adipoq has been found to be lower in BAT,
but not in white adipose tissue from P. gingivalis-treated
mice (18). Periodontal bacteria
have been identified in the gut of patients with inflammatory bowel
disease. They may be transported to ectopically colonize the gut
via the oral route (5,6). Thus, the systemic effects of
periodontal inflammation may be mediated through P.
gingivalis.
lncRNA-BATE10 is BAT-specific and is a member of the
lncRNA-BATE family. lncRNA-BATE10 is transcribed from four exons in
an intergenic region of mouse chromosome 18 and is ~1.7 kb in
length (21). lncRNA-BATE10
expression in white adipose tissue is increased by exposure to
cold, β-adrenergic agonists and intense physical exercise (26). Accordingly, lncRNA-BATE10
expression is increased by exposure to cold in BAT and is lower at
30˚C (26). During the
differentiation of brown preadipocytes, the knockdown of
lncRNA-BATE10 leads to a decrease in the expression levels of
BAT-specific genes, including UCP1 and Pgc1a
(26,30). These findings demonstrate that
lncRNA-BATE10 may play a role in BAT thermogenesis; therefore, it
was hypothesized that P. gingivalis LPS inhibits the
expression of UCP1 during the differentiation of brown adipocytes
by reducing lncRNA-BATE10 expression.
In conclusion, P. gingivalis may have
deleterious effects on BAT that are mediated by LPS. Specifically,
P. gingivalis reduces UCP1 expression, and lncRNA-BATE10
promotes a pro-inflammatory state. The results of the present study
may enhance the current understanding of the association between
periodontal disease and obesity.
Acknowledgements
The authors would like to thank Professor Zhuoxian
Meng (Department of Pathology and Pathophysiology, Key Laboratory
of Disease Proteomics of Zhejiang Province, School of Medicine,
Zhejiang University, Hangzhou, Zhejiang, China) for providing the
brown preadipocytes and Dr Peihui Ding (Stomatology Hospital,
School of Stomatology, Zhejiang University School of Medicine,
Clinical Research Center for Oral Diseases of Zhejiang Province,
Key Laboratory of Oral Biomedical Research of Zhejiang Province,
Zhejiang, Hangzhou, China) for providing Porphyromonas
gingivalis.
Funding
Funding: The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81700972),
the Cao Guangbiao High Sci-Tech Development Fund of Zhejiang
University (grant no. 2020QN026), and the Pre-Research Fund from
School of Medicine, Zhejiang University (grant no.
519600-I52104/004).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WD conceived and designed the study. FZ, LS, NZ, LL
and JG performed the experiments. FZ, LS, NZ, LL, JG and WD
prepared a draft of the manuscript, and WD and FZ finalized the
manuscript. FZ, LS and WD confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The animal experimental protocols were approved by
the Institutional Animal Care and Use Committee of Zhejiang
University (Approval no. ZJU20170237, 2017-02-24).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nassar H, Kantarci A and van Dyke TE:
Diabetic Periodontitis: A model for activated innate immunity and
impaired resolution of inflammation. Periodontol 2000. 43:233–244.
2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zaric SS, Lappin MJ, Fulton CR, Lundy FT,
Coulter WA and Irwin CR: Sialylation of Porphyromonas
gingivalis LPS and its effect on bacterial-host interactions.
Innate Immun. 23:319–326. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Iwashita M, Hayashi M, Nishimura Y and
Yamashita A: The link between periodontal inflammation and obesity.
Curr Oral Health Rep. 8:76–83. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Atarashi K, Suda W, Luo C, Kawaguchi T,
Motoo I, Narushima S, Kiguchi Y, Yasuma K, Watanabe E, Tanoue T, et
al: Ectopic colonization of oral bacteria in the intestine drives
T(H)1 cell induction and inflammation. Science. 358:359–365.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schirmer M, Denson L, Vlamakis H, Franzosa
EA, Thomas S, Gotman NM, Rufo P, Baker SS, Sauer C, Markowitz J, et
al: Compositional and temporal changes in the gut microbiome of
pediatric ulcerative colitis patients are linked to disease course.
Cell Host Microbe. 24:600–610.e4. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jepsen S, Suvan J and Deschner J: The
association of periodontal diseases with metabolic syndrome and
obesity. Periodontol 2000. 83:125–153. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pamuk F and Kantarci A: Inflammation as a
link between periodontal disease and obesity. Periodontology 2000.
90:186–196. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Aoyama N, Fujii T, Kida S, Nozawa I,
Taniguchi K, Fujiwara M, Iwane T, Tamaki K and Minabe M:
Association of periodontal status, number of teeth, and obesity: A
cross-sectional study in Japan. J Clin Med. 10(208)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Krongbaramee T, Zhu M, Qian Q, Zhang Z,
Eliason S, Shu Y, Qian F, Akkouch A, Su D, Amendt BA, et al:
Plasmid encoding microRNA-200c ameliorates Periodontitis and
systemic inflammation in obese mice. Mol Ther Nucleic Acids.
23:1204–1216. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vallim AC, Gaio EJ, Oppermann RV, Rösing
CK, Albandar JM, Susin C and Haas AN: Obesity as a risk factor for
tooth loss over 5 years: A population-based cohort study. J Clin
Periodontol. 48:14–23. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Borgnakke WS, Ylöstalo PV, Taylor GW and
Genco RJ: Effect of periodontal disease on diabetes: Systematic
review of epidemiologic observational evidence. J Clin Periodontol.
40 (Suppl 14):S135–S152. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Papapanou PN, Sanz M, Buduneli N, Dietrich
T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani
F, et al: Periodontitis: Consensus report of workgroup 2 of the
2017 world workshop on the classification of periodontal and
peri-implant diseases and conditions. J Periodontol. 89 (Suppl
1):S173–S182. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tonetti MS, Greenwell H and Kornman KS:
Staging and grading of Periodontitis: Framework and proposal of a
new classification and case definition. J Clin Periodontol. 45
(Suppl 20):S149–S161. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nakarai H, Yamashita A, Nagayasu S,
Iwashita M, Kumamoto S, Ohyama H, Hata M, Soga Y, Kushiyama A,
Asano T, et al: Adipocyte-macrophage interaction may mediate
LPS-induced low-grade inflammation: Potential link with metabolic
complications. Innate Immun. 18:164–170. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cheng L, Wang J, Dai H, Duan Y, An Y, Shi
L, Lv Y, Li H, Wang C, Ma Q, et al: Brown and beige adipose tissue:
A novel therapeutic strategy for obesity and type 2 diabetes
mellitus. Adipocyte. 10:48–65. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yoshida S, Hatasa M, Ohsugi Y, Tsuchiya Y,
Liu A, Niimi H, Morita K, Shimohira T, Sasaki N, Maekawa S, et al:
Porphyromonas gingivalis administration induces gestational
obesity, alters gene expression in the liver and brown adipose
tissue in pregnant mice, and causes underweight in fetuses. Front
Cell Infect Microbiol. 11(745117)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hatasa M, Ohsugi Y, Katagiri S, Yoshida S,
Niimi H, Morita K, Tsuchiya Y, Shimohira T, Sasaki N, Maekawa S, et
al: Endotoxemia by Porphyromonas gingivalis alters endocrine
functions in brown adipose tissue. Front Cell Infect Microbiol.
10(580577)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu X, Huang CY and Oka SI: LncRNA KCNQ1OT1
promotes Atg12-mediated autophagy via inhibiting miR-26a-5p in
ischemia reperfusion. Int J Cardiol. 339:132–133. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Z, Tang X, Wu X, Yang M, Wang W, Wang
L, Tang D and Wang D: Cardamonin exerts anti-gastric cancer
activity via inhibiting LncRNA-PVT1-STAT3 axis. Biosci Rep.
39(BSR20190357)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lai S, Du K, Shi Y, Li C, Wang G, Hu S,
Jia X, Wang J and Chen S: Long non-coding RNAs in brown adipose
tissue. Diabetes Metab Syndr Obes. 13:3193–3204. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kang S, Dai A, Wang H and Ding PH:
Interaction between autophagy and Porphyromonas gingivalis-induced
inflammation. Front Cell Infect Microbiol.
12(892610)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Klein J, Fasshauer M, Klein HH, Benito M
and Kahn CR: Novel adipocyte lines from brown fat: A model system
for the study of differentiation, energy metabolism, and insulin
action. Bioessays. 24:382–388. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang GX, Zhao XY, Meng ZX, Kern M,
Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, et al: The
brown fat-enriched secreted factor Nrg4 preserves metabolic
homeostasis through attenuation of hepatic lipogenesis. Nat Med.
20:1436–1443. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bai Z, Chai XR, Yoon MJ, Kim HJ, Lo KA,
Zhang ZC, Xu D, Siang DTC, Walet ACE, Xu SH, et al: Dynamic
transcriptome changes during adipose tissue energy expenditure
reveal critical roles for long noncoding RNA regulators. PLoS Biol.
15(e2002176)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Luci C, Vieira E, Perchet T, Gual P and
Golub R: Natural killer cells and type 1 innate lymphoid cells are
new actors in non-alcoholic fatty liver disease. Front Immunol.
10(1192)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cullender TC, Chassaing B, Janzon A, Kumar
K, Muller CE, Werner JJ, Angenent LT, Bell ME, Hay AG, Peterson DA,
et al: Innate and adaptive immunity interact to quench microbiome
flagellar motility in the gut. Cell Host Microbe. 14:571–581.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rojas C, García MP, Polanco AF,
González-Osuna L, Sierra-Cristancho A, Melgar-Rodríguez S,
Cafferata EA and Vernal R: Humanized mouse models for the study of
Periodontitis: An opportunity to elucidate unresolved aspects of
its immunopathogenesis and analyze new immunotherapeutic
strategies. Front Immunol. 12(663328)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cui X, You L, Li Y, Zhu L, Zhang F, Xie K,
Cao Y, Ji C and Guo X: A transcribed ultraconserved noncoding RNA,
uc.417, serves as a negative regulator of brown adipose tissue
thermogenesis. FASEB J. 30:4301–4312. 2016.PubMed/NCBI View Article : Google Scholar
|

















