Epigenetic alterations have critical roles in
various physiological and pathological processes (1). Histone deacetylases (HDACs) are
among the most important epigenetic regulators, which deacetylate
lysine residues on specific histone and non-histone proteins
(2). According to the homologies
of the respective yeast orthologues, human HDACs have been divided
into four classes (3,4). The human sirtuin (SIRT) family,
belonging to class-III HDACs, is a class of
NAD+-dependent deacetylases and contains seven members
(SIRT1-7) (5). Among them, SIRT1
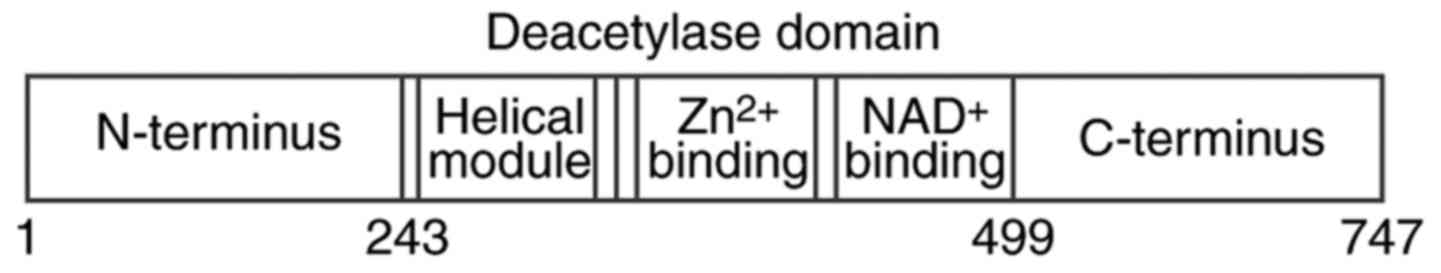
has been most widely studied. The human SIRT1 protein contains 747
amino acids and is composed of three major regions (Fig. 1) (6,7).
The level and activity of SIRT1 is regulated at the transcriptional
and post-transcriptional level (8), with post-translational modifications
including phosphorylation (9),
sumoylation (10), methylation
(11), S-nitrosylation (12) and carboxylation (13). Besides, the activity of SIRT1
distinctly depends on the NAD+/NADH ratio and may be
affected by nucleocytoplasmic shuttling (14).
The SIRT family has been identified to be highly
evolutionarily conserved in various organisms from bacteria to
humans (15). SIRT1, maintaining
the silenced chromatin state and genomic stability (16), has been associated with various
physiological and pathological processes and conditions, including
DNA repair, metabolic regulation, aging, oxidative stress,
angiogenesis, inflammation, neurodegenerative diseases and
cardiovascular dysfunction. Previous reviews have indicated the
major roles of SIRT1 in retinal and ocular aging (17–19). Increasing evidence has
demonstrated that SIRT1 is also involved in other eye diseases,
which are not limited to aging. The present review focuses on the
current understanding and the potential therapeutic value of SIRT1
in ocular disorders.
It has been reported that mice carrying two null
alleles of SIRT1 (also known as SIR2a in lower organisms) are
small, and most of them die shortly after birth. Outbred SIRT1-null
animals usually survived until adulthood, but were sterile
(20). In SIRT1-deficient mice,
eyelids remained closed accompanied by abnormalities of the cornea,
lens and retina (20,21), and eyes were smaller, with the
optic fissure being abnormally closed (22). Furthermore, there were
significantly thinner retinal cell layers and disordered inner and
outer nuclear layers. In addition, it was difficult to identify the
inner and outer segments of photoreceptor cells. These eye defects
occurred in early embryos, which implied that SIRT1 regulated
ocular morphogenesis and retinal development (22). In addition, SIRT1 was reduced in
the retina of mice with knockout of E2fs, which are essential
positive cell cycle regulators, resulting in hyperacetylation of
p53, a pro-apoptotic factor downstream of SIRT1, and increased
retinal progenitor cell apoptosis (23). Treatment of pregnant mice with
resveratrol, an activator of SIRT1, significantly blocked the
apoptosis at P0 (23). Thus, the
pro-survival role of E2f/SIRT1/p53 in retinal development has been
established.
SIRT1 was detected in the cornea, lens, ciliary
body, retinal pigment epithelium (RPE) and neuroretina in mice and
humans, and in human normal conjunctival epithelium (24–26). Jaliffa et al (24) firstly reported that SIRT1 was
predominantly localized in the nuclei of ocular cell, including
corneal epithelial cells, ciliary process cells and ciliary
epithelial cells, the epithelial and fiber cells of the lens, RPE
cells and melanocytes, and exclusively in the nuclei of cells of
the outer nuclear layer (ONL), inner nuclear layer (INL) and
ganglion cell layer (GCL) but never in the cytoplasm. They also
detected SIRT1 in the cytoplasm of corneal epithelial cells and
choroidal vessel endothelial cells (24). Another study reported that SIRT1
was mainly expressed in the cytoplasm in the GCL, inner plexiform
layer (IPL), outer plexiform layer (OPL), and inner segments of
photoreceptors (26). In
addition, SIRT1 was identified to be exclusively expressed in the
cytoplasm of mouse retinal progenitor cells (RPCs), while it was
present in the nuclei and cytoplasm in human RPCs (26). These apparently different SIRT1
distributions in different cell types of different species suggest
that SIRT1 expression may be variable during different periods of
retinal development and cell differentiation.
Under normal conditions, the corneal epithelium is
important for the maintenance of the physiological corneal
function, rendering the cornea highly resistant to microbial
invasion. A high-glucose (HG) environment led to the downregulation
of SIRT1 and upregulation of acetylated p53 (Ac-p53) and
insulin-like growth factor binding protein-3 (IGFPB3) in primary
human corneal epithelial cells as well as corneas from insulin
(Ins)2Akita/+ mice (27). Overexpression of SIRT1 in corneal
epithelial cells and Ins2Akita/+ mice significantly led
to a downregulation of Ac-p53 and IGFPB3, and an upregulation of
the levels of phosphorylated (p)-AKT and IGF-1 receptor precursor.
In addition, SIRT1 overexpression in corneal epithelium promoted
the wound healing process under HG conditions, which may involve
reinforcement of the IGFBP3/IGF-1/AKT pathway with the decrease of
Ac-p53 (27). With the
progression of diabetic dry eye (DE) in a mouse model, SIRT1
expression in the cornea rose in the first stage and then
decreased. Furthermore, the expression of forkhead box O3 (FOXO3),
which ameliorated the response to oxidative stress as a substrate
of SIRT1, and the antioxidant enzyme Mn-superoxide dismutase
(MnSOD) protein had a similar tendency with SIRT1, which suggests a
role of SIRT1 in the resistance to oxidative stress (28). In summary, SIRT1 activation may be
an effective approach for treating diabetic keratopathy.
Recent studies have demonstrated that microRNAs
(miRNAs) may regulate corneal development and diseases. miRNA-204
directly downregulated SIRT1 in the cornea, and overexpression of
miRNA-204 in human corneal epithelial cells inhibited cell cycle
progression, cell proliferation and cell migration during the
healing of wounded corneal epithelium in mice (29,30). Furthermore, miR-204-5p antagomir
promoted the wound healing process via SIRT1 regulation in type 1
diabetic Ins2Akita/+ mice (30). Wang et al (31) reported that miRNA-182 was the
downstream miRNA target of SIRT1 under HG conditions, and protected
against peripheral damage of trigeminal ganglions and keratopathy
in diabetic db/db mice by decreasing the expression of one of its
target genes, NADPH oxidase 4. Therefore, SIRT1 may protect the
cornea through the miRNA-mRNA regulatory network.
Age-associated cataract (ARC) is a condition
characterized by multiple mechanisms and has various risk factors,
including genetic, metabolic, nutritional and environmental
factors, as well as other ocular diseases (32). Previous studies have indicated
that resveratrol is able to protect human lens epithelial cells
from oxidative damage induced by H2O2
(33,34) and suppress experimental cataract
formation in rats (35). The
SIRT1 levels in the lens were identified to be significantly
decreased in individuals aged ≥51 years, and to be negatively
correlated with ARC in humans (36). Of note, SIRT1 was significantly
increased in patients aged >50 years with ARC compared with that
in age-matched subjects without ARC (37), and SIRT1 levels in the aqueous
humor of ARC patients were positively correlated with the severity
of nuclear cataract (38).
Furthermore, while the expression of the downstream components of
SIRT1, FOXO3a and FOXO4, was downregulated with age, it exhibited
relative increases in ARC patients (37). By contrast, the expression of p53
increased with age, but active Ac-p53 was decreased in older
patients with cataract compared with that in old individuals
without cataract (37). These
studies indicate that the increased SIRT1 may function as a
compensation to alleviate ARC formation through inhibiting its
downstream p53 acetylation and activating the FOXO pathway
(37). Another study indicated
that the enhanced interaction between SIRT1 and 8-oxoguanine
(8-oxoG)-DNA glycosylase 1 (OGG1) and/or insufficient interaction
between the histone acetyltransferase p300 and OGG1 may decrease
the acetylation of OGG1 in the lens of patients with ARC, resulting
in abnormal accumulation of 8-oxoG, a biomarker of oxidative
damage, in the lens (39).
Eventually, these changes accelerate the development of ARC,
implying a destructive role of SIRT1 upregulation (39). These divergent results are
possibly attributed to the difference in research methods and
subjects. More comprehensive studies focusing on the precise
mechanisms of SIRT1 in the pathogenesis of ARC are required.
Age-associated macular degeneration (AMD) manifests
as either drusen/geographic atrophy or choroidal neovascularization
(CNV). The pathophysiology and risk factors of AMD are complex
(40). Chen et al
(41) investigated three variants
of the SIRT1 gene associated with AMD in Chinese Han individuals,
and identified that the rs12778366 polymorphism within the promoter
region of SIRT1 was significantly associated with AMD in recessive
and codominant models. Expression of SIRT1 was more frequent in RPE
and vascular endothelial cells (VECs) in human CNV membranes
(42). By contrast, another study
indicated that the expression and self-renewal ability of SIRT1 in
retinal stem cells (RSCs) (43),
human retina and RPE cells (44)
obviously declined with age.
Dysfunction of RPE cells is a major risk factor for
the development of AMD. In aged RPE cells, overexpression of SIRT1
and octamer binding transcription factor 4, a POU-domain
transcription factor, reprogrammed the cells into retinal
progenitor-like cells and enhanced their antioxidant enzymatic
activities (44). The expression
of p53 increased in aged RPE, and in young RPE cells, sirtinol (a
SIRT1 inhibitor) increased p53 acetylation and phosphorylation, but
only had a marginal effect on p53 expression and increased
caspase-3 activation, which contributed to apoptosis of RPE cells
(45). Furthermore, resveratrol
obviously prevented p53 acetylation and phosphorylation, and
eventually alleviated caspase-3-dependent RPE cell apoptosis
(45). Recently, Golestaneh et
al (46) developed an in
vitro disease model of AMD through the generation of induced
pluripotent stem cells (iPSCs) from RPE from patients with AMD and
differentiation of these iPSCs into RPE (AMD RPE-iPSC-RPE), and
observed the downregulation of SIRT1 and peroxisome proliferator
activated receptor-γ co-activator-1α (PGC-1α) in AMD RPE-iPSC-RPE
compared with that in normal RPE-iPSC-RPE. The study indicated that
dysfunctional SIRT1/PGC-1α may decrease mitochondrial activity and
increase reactive oxygen species (ROS) production in AMD
RPE-iPSC-RPE, and contribute to the pathophysiology of AMD
(46).
Oxidative stress accelerates the progression of AMD.
Overexpression of SIRT1 or treatment with resveratrol protected
against oxidative stress-induced RPE cell senescence through
downregulation of p53 K382 acetylation and p21Waf1/Cip1
accumulation (47), and increased
the viability of H2O2-treated rat RSCs
(43). On the contrary, knockdown
of SIRT1 or application of SIRT1 inhibitors including nicotinamide
(48) and sirtinol enhanced the
toxicity of H2O2, making RPE cells
hyper-sensitive to oxidative stress (47). Furthermore, SIRT1 rescued
complement factor H (CFH) expression through increasing recruitment
of signal transducer and activator of transcription 1 and
decreasing the occupancy of the repressor FOXO3 in the CFH promoter
of H2O2-treated ARPE-19 cells, which may
prevent the oxidative stress-induced aging and cell damage and
decrease the risk of AMD (49). A
further experimental study indicated that SIRT1 levels were reduced
in human RPE cells after treatment with amyloid β (Aβ), which is
one of the constituents of drusen (50). Treatment with SRT1720, a potent
SIRT1 agonist that suppresses the nuclear factor (NF)-κB signaling
system, significantly decreased Aβ-mediated upregulation of
inflammatory cytokines in RPE cells, and balanced the morphology
and barrier function of RPE cell monolayers, which was obviously
suppressed by knockdown of SIRT1 (50).
The expression of SIRT1 mRNA exhibited daily
variations under the light-dark cycle conditions in the retina and
was obviously upregulated in the dark phase (51). Considering that retinal cells
consume more energy in the dark, the study linked SIRT1 regulation
with the response to light stimuli and metabolic dysfunction in
age-associated retinal diseases including AMD (51). In an in vitro study,
ultraviolet B activated the phosphoinositide-3
kinase/AKT/extracellular signal-regulated kinase (ERK) pathway by
reducing the expression of SIRT1 in a dose-dependent manner in
ARPE-19 cells and suppressed the growth of the cells (52). In a mouse model of light-induced
retinal degeneration, retinal SIRT1 activity was significantly
reduced (53). Systemic
administration of resveratrol not only significantly recovered
retinal SIRT1 activity, but also restored histological and
functional damage to the retina (53). Likewise, gene transfer of SIRT1
decreased retinal cell loss and improved the light-induced
electroretinographic damage in rat retinas (44). In addition, the levels of
activator protein (AP)-1 subunit c-fos were elevated in the retina
of light-exposed mice and reduced by application of resveratrol
(53). These studies suggest that
SIRT1 activators or overexpression of SIRT1 protect the retina from
light damage through inhibiting AP-1 bioactivity (53) and suppressing AKT and ERK
phosphorylation (52).
CNV formation is a typical characteristic of wet
AMD. Previous studies have indicated the regulative roles of SIRT1
in angiogenesis (54,55). Expression of SIRT1 was higher in
human CNV membranes from AMD patients than in eyes from donors
without AMD (42). In
vitro studies demonstrated that hypoxia-induced upregulation of
SIRT1 levels augmented hypoxia-inducible factor (HIF)-2α expression
in choroidal endothelial cells, which in turn activated and
released vascular endothelial growth factor (VEGF) (56). Thus, SIRT1 may promote CNV
formation. However, other studies indicated that SIRT1 activation
by resveratrol inhibited various inflammatory cytokines,
transforming growth factor (TGF)-β-mediated VEGF secretion and
hypoxia-mediated choroidal VEC proliferation through downregulation
of HIF-1α (57,58). A study by our group indicated that
resveratrol inhibited the HIF-1α/VEGF/VEGF receptor 2 signaling
axis partly through SIRT1 (59).
Khan et al (60)
demonstrated that resveratrol inhibited the proliferation and
migration of VECs and led to severely blunted neovascularization
through activating eukaryotic elongation factor-2 kinase instead of
the SIRT1-dependent pathway. The difference in drugs and
experimental models may produce discrepant results regarding the
function of SIRT1. The mechanisms of the effects of SIRT1 on CNV
formation require more comprehensive elucidation. The role of SIRT1
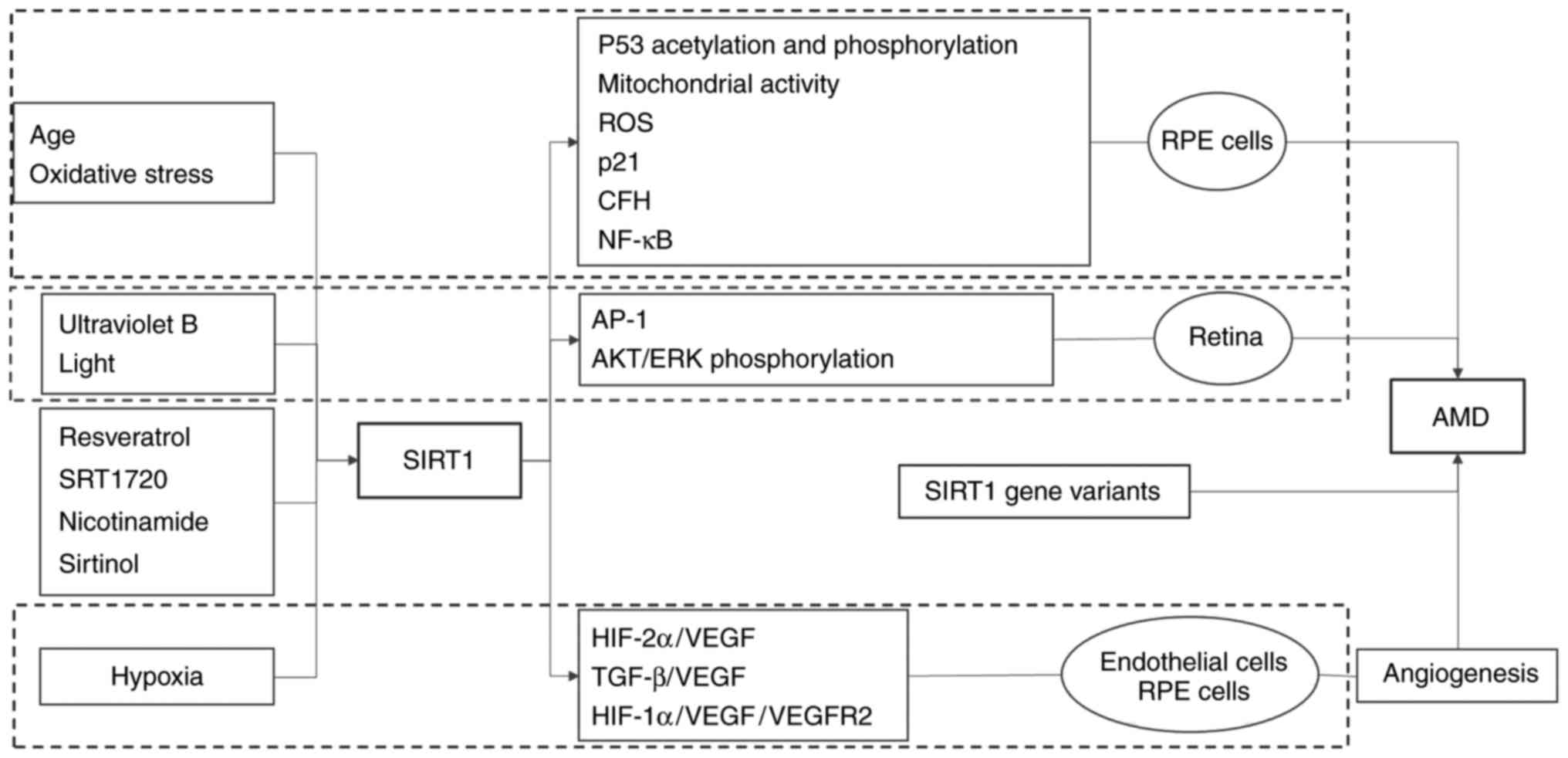
in the pathogenesis of AMD is summarised in Fig. 2.
DR is a severe complication of diabetes mellitus.
Progression of diabetic blood glucose control suggests a 'metabolic
memory' phenomenon (61,62). The expression of SIRT1 was reduced
in the retinas of diabetic mice (63–66). Zheng et al (67) observed a decrease in SIRT1 and an
increase of NF-κB, the pro-apoptotic gene B-cell lymphoma
2-associated X protein (Bax), poly ADP-ribose polymerase (PARP) and
ROS in bovine retinal endothelial cells (RECs) cultured under HG
after glucose normalization. SIRT1 overexpression mediated liver
kinase B1 (LKB1)/AMP-activated protein kinase (AMPK) activity,
which inhibited ROS pathway activation in HG in RECs, resulting in
the suppression of NF-κB, Bax and PARP expression. In addition,
ROS-induced PARP activity, at least in part, led to the
downregulation of SIRT1 expression and amplified an auto-feedback
loop regulating SIRT1 expression. These results implied that SIRT1
mediated a metabolic memory effect induced by HG through the
SIRT1/LKB1/AMPK/ROS cascade (67). Another study reported that
transient hyperglycemia caused persistent endothelial cell
senescence through the imbalance between SIRT1, and that P300
induced the upregulation of Ac-p53 and its downstream p21 (68). Recently, Zhao et al
(69) identified that increased
expression of miR-23b-3p directly downregulated SIRT1, which
increased Ac-NF-κB levels in human RECs with a metabolic memory
effect induced by HG. Similar results were obtained in rats with
streptozotocin-induced diabetic retinopathy as a metabolic memory
model, in which vascular permeability was significantly suppressed
by miR-23b-3p inhibitor (69).
Furthermore, metformin, a blood glucose-lowering therapeutic,
fenofibrate, a lipid-lowering therapeutic and resveratrol
suppressed the memory of hyperglycemic stress via the
SIRT1-dependent signaling pathway (67,68,70).
The overproduction of mitochondrial ROS and
cytokines promotes the development of DR (71,72). Reduced SIRT1 in HG-cultured RECs
obviously enhanced the acetylation of NF-κB p65 and AP-1, which
binds to the promoter of matrix metalloproteinase (MMP)-9, which
eventually activates MMP-9 (64,73). SIRT1 overexpression decreased the
transcription of MMP-9 in REC (64,73) and ameliorated NF-κB/Rac1/NADPH
oxidase-mediated mitochondrial damage in diabetic rat retina
(65). SIRT1 overexpression
suppressed the upregulation of endothelin 1, TGF-β1, collagen 1α
and fibronectin, and prevented glucose-induced endothelial
permeability and increases in total ROS/RNS levels in the retina of
diabetic mice (74). In addition,
systemic administration of resveratrol to the diabetic animals
suppressed leukostasis and the upregulation of intercellular
adhesion molecule-1 and VEGF (66). Exendin-4, a glucagon-like peptide
1 analogue, moderated ROS-mediated retinal cell death and recovered
visual function by upregulating SIRT1 and SIRT3 expression in
early-stage diabetic rats (75).
Furthermore, SIRT1 may protect proliferative DR progression by
inhibiting interleukin-17 (76).
Another study indicated that miR-195 antagomir normalised tissue
damage mediated by SIRT1 reduction in a rat model of DR (77).
Mice with oxygen-induced ischemic retinopathy (OIR)
exhibit certain features of neovascularization that are
characteristic of proliferative DR in humans (78). Increased SIRT1 in avascular
retinal neurons of OIR mice mediated physiological
revascularization of ischemic areas through modulating the HIF
signaling pathway and secretion of pro-angiogenic and
neuroprotective factors (79).
However, ectopic overexpression of SIRT1 in mouse retinas or oral
administration of SIRT1 activator did not alter the
vaso-obliteration, pathologic neovascularization or retinal neuron
degeneration in OIR (80). The
protective role of SIRT1 in OIR requires more comprehensive
study.
Glaucoma is a group of chronic eye diseases ascribed
to the irreversible death of retinal ganglion cells (RGCs) and
progressive optic neuropathy, and results in serious vision loss
and blindness. RGCs transmit light signals from the retina along
their axons to the brain. Various types of stimuli, including
trauma, ischemia, increased intraocular pressure, oxidative stress
and inflammation, have been reported to lead to RGC death (81). SIRT1 has been linked with
Alzheimer's and Huntington's disease in respective animal models
and exerted a neuroprotective role in these diseases (82). Further studies have indicated the
neuroprotective effects of SIRT1 on RGCs. For instance, resveratrol
protected RGC-5 cells against serum deprivation-induced apoptosis
by promoting the expression of SIRT1 and facilitating the
translocation of PGC-1α from the cytoplasm to the nucleus (83,84). In addition, oral resveratrol
administration or overexpression of SIRT1 following optic nerve
crush injury in mice reduced RGC loss and ROS accumulation in the
optic nerve (85). However,
resveratrol was unable to prevent RGC loss after optic nerve crush
injury in the eyes of SIRT1-knockout mice (85), which confirmed the necessity of
SIRT1 expression for resveratrol-mediated neuroprotection.
Furthermore, SIRT1 increased the viability of RGCs under hypoxic
conditions through inhibiting stress-activated protein kinase/c-Jun
N-terminal kinase and caspase-3 activation (86), in ischemic mouse retinas,
mangiferin prevented RGC loss via SIRT1, which was suppressed by
sirtinol (87), suggesting a
neuroprotection role of SIRT1 on RGCs under hypoxic condition.
RGC loss also has been demonstrated in several
experimental models of optic neuritis, including experimental
autoimmune encephalomyelitis (EAE), which is an animal model of
multiple sclerosis (MS). SIRT1 activator-associated suppression of
RGCs loss delayed the onset of EAE and attenuated neuronal damage
in EAE mice (88–90). The fact that the protective effect
on RGCs by SIRT1 activators was blocked by sirtinol further
suggests the neuroprotection role of SIRT1 activation (88,90). Pre-treatment with SIRT1
activators, resveratrol and SRTAW04, significantly reduced ROS and
cell death caused by H2O2 in RGC-5 cells
(91). Furthermore, SIRT1
activators induced a significant increase in SOD2 and succinate
dehydrogenase expression in stressed RGC-5 cells and enhanced
deacetylation and activation of PGC-1α (91). Similar protective mechanisms were
observed in a mouse hepatitis virus A59-induced MS model (92). However, administration of SIRT1
activators neither suppressed the gross level of inflammation in
the optic nerve nor attenuated the development of clinical EAE
(88–90,92). During disease remission, EAE
patients retain proper axonal density, suggesting that SIRT1
activator prevents permanent neurological dysfunction and neuronal
damage in MS after acute spinal cord inflammation is resolved
(90). In addition, resveratrol,
through promoting SIRT1 expression and cholesterol synthesis,
restored the number of surviving RGCs in the rats with optic nerve
injury (93). Most importantly,
the neuroprotective effects of SIRT1 activators without
immunosuppression may imply a potential benefit of combining
anti-inflammatory therapies for optic neuritis as well as for
non-inflammatory optic nerve diseases.
Uveitis is characterized by a process of intraocular
inflammation resulting from multiple factors. Corticosteroids and
immunosuppressive drugs are effective for relieving diverse
uveitis, but have severe side effects, which limits their clinical
application (94). Alternative,
novel drugs or treatments are therefore required. Recent studies
have applied SIRT1 activators in the treatment of uveitis animal
models. For instance, oral application of resveratrol to mice with
endotoxin-induced uveitis (EIU) led to inhibition of oxidative
damage and significant increases in SIRT1 activity in the
RPE-choroid, resulting in the suppression of NF-κB-mediated
inflammation in the eye (95).
Resolvin D1, a lipid-derived protein for intravitreal injection,
prevented EIU in rats through increasing SIRT1-mediated
downregulation of Ac-p53 and FOXO1 (96). Furthermore, treatment of mice with
experimental autoimmune uveoretinitis (EAU) with SIRT1 activator
SRT2379 alleviated inflammation through suppressing T cell
proliferation, pro-inflammatory cytokine production and leukocyte
infiltration (97). Gardner et
al (98) reported that tumor
necrosis factor (TNF)-α mediated the cleavage and inactivation of
SIRT1 to drain lymph node effector cells in EAU, and that combined
application of a suboptimal TNF-α blockade and SIRT1 activation had
a synergistic suppressive effect on EAU. In addition, in an in
vitro model of antibody-mediated autoimmune retinopathy,
resveratrol treatment led to an upregulation of SIRT1 and Ku70 in
retinal cells, blocked the influx of intracellular calcium and the
entry of pro-apoptotic Bax from the cytoplasm to the mitochondria,
to subsequently prevent caspase-3 activation and protect cells from
apoptotic death induced by antibodies against recoverin and
α-enolase (99). These studies
demonstrated that activation of SIRT1 may be a potential treatment
option for ocular uveitis.
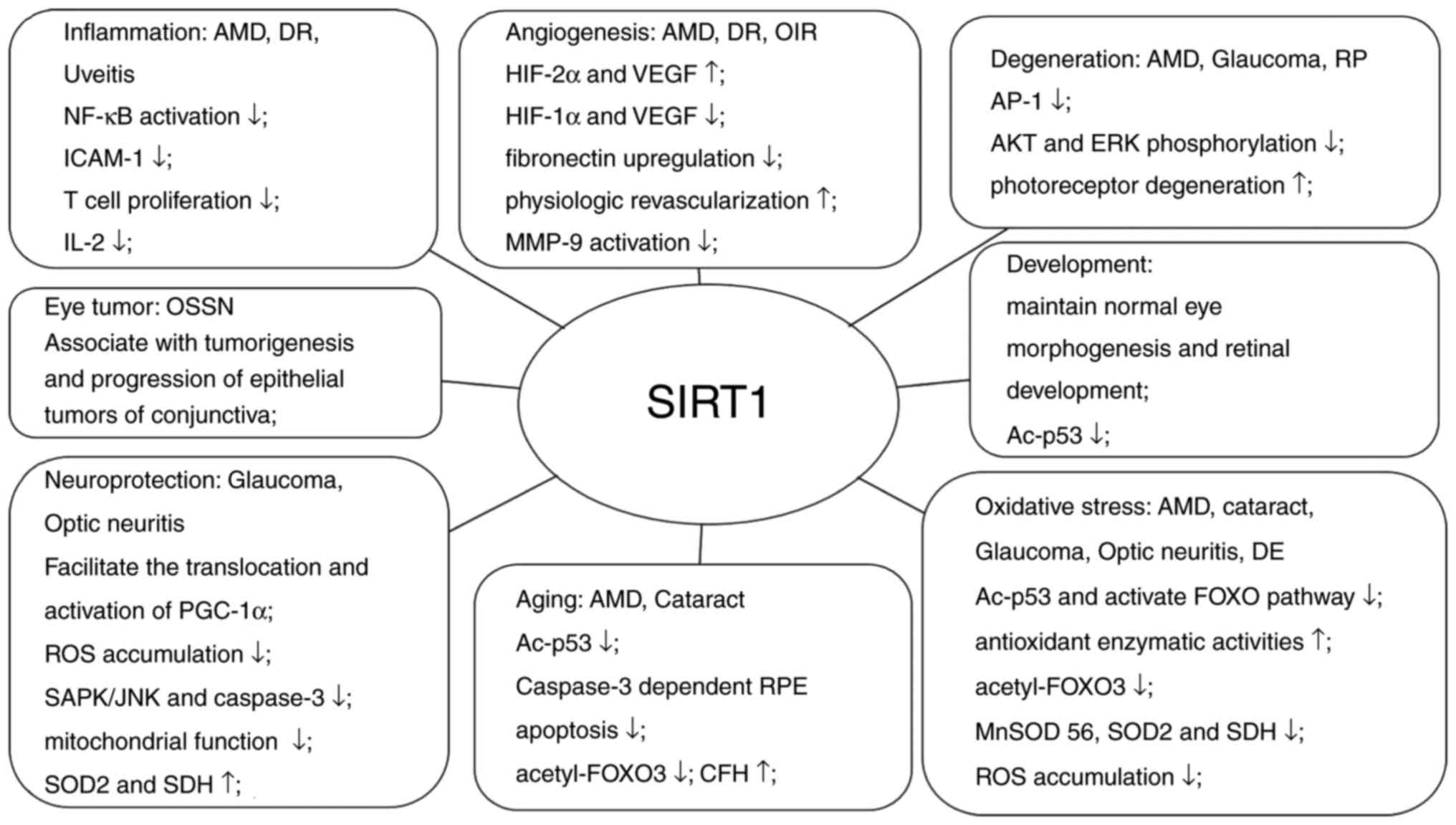
The present review mainly focused on the emerging
evidence of the association between SIRT1 and the eyes. As
summarized in Fig. 3, SIRT1
serves a significant role in ocular diseases by influencing various
physiological and pathological processes such as inflammation,
angiogenesis, aging, oxidative stress, neuroprotection. Although
the potential protective role of SIRT1 has been demonstrated in
numerous in vitro and in vivo models of ocular
diseases, further studies are necessary to confirm the accurate
mechanism and the most effective administration of SIRT1 activators
and inhibitors in these diseases (100). In addition, it is required to
determine whether the data obtained using animal models are
applicable to human ocular diseases. In summary, SIRT1 may be
considered as a valuable therapeutic target in ocular diseases.
The authors would like to thank Dr Yedi Zhou and Dr
Lusi Zhang for their important advice regarding the preparation of
the manuscript.
|
1
|
Portela A and Esteller M: Epigenetic
modifications and human disease. Nat Biotechnol. 28:1057–1068.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glozak MA, Sengupta N, Zhang X and Seto E:
Acetylation and deacetylation of non-histone proteins. Gene.
363:15–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gray SG and Ekström TJ: The human histone
deacetylase family. Exp Cell Res. 262:75–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao L, Cueto MA, Asselbergs F and Atadja
P: Cloning and functional characterization of HDAC11, a novel
member of the human histone deacetylase family. J Biol Chem.
277:25748–25755. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feldman JL, Dittenhafer-Reed KE and Denu
JM: Sirtuin catalysis and regulation. J Biol Chem. 287:42419–42427.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sauve AA, Wolberger C, Schramm VL and
Boeke JD: The biochemistry of sirtuins. Annu Rev Biochem.
75:435–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davenport AM, Huber FM and Hoelz A:
Structural and functional analysis of human SIRT1. J Mol Biol.
426:526–541. 2014. View Article : Google Scholar :
|
|
8
|
Yamakuchi M: MicroRNA regulation of SIRT1.
Front Physiol. 3:682012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sasaki T, Maier B, Koclega KD, Chruszcz M,
Gluba W, Stukenberg PT, Minor W and Scrable H: Phosphorylation
regulates SIRT1 function. PLoS One. 3:e40202008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Fu W, Chen J, Olashaw N, Zhang X,
Nicosia SV, Bhalla K and Bai W: SIRT1 sumoylation regulates its
deacetylase activity and cellular response to genotoxic stress. Nat
Cell Biol. 9:1253–1262. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Wang D, Zhao Y, Tu B, Zheng Z, Wang
L, Wang H, Gu W, Roeder RG and Zhu WG: Methyltransferase Set7/9
regulates p53 activity by interacting with Sirtuin 1 (SIRT1). Proc
Natl Acad Sci USA. 108:1925–1930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kornberg MD, Sen N, Hara MR, Juluri KR,
Nguyen JV, Snowman AM, Law L, Hester LD and Snyder SH: GAPDH
mediates nitrosylation of nuclear proteins. Nat Cell Biol.
12:1094–1100. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caito S, Rajendrasozhan S, Cook S, Chung
S, Yao H, Friedman AE, Brookes PS and Rahman I: SIRT1 is a
redox-sensitive deacetylase that is post-translationally modified
by oxidants and carbonyl stress. FASEB J. 24:3145–3159. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanno M, Sakamoto J, Miura T, Shimamoto K
and Horio Y: Nucleocytoplasmic shuttling of the
NAD+-dependent histone deacetylase SIRT1. J Biol Chem.
282:6823–6832. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brachmann CB, Sherman JM, Devine SE,
Cameron EE, Pillus L and Boeke JD: The SIR2 gene family, conserved
from bacteria to humans, functions in silencing, cell cycle
progression, and chromosome stability. Genes Dev. 9:2888–2902.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guarente L: Sir2 links chromatin
silencing, metabolism, and aging. Genes Dev. 14:1021–1026.
2000.PubMed/NCBI
|
|
17
|
Ozawa Y, Kubota S, Narimatsu T, Yuki K,
Koto T, Sasaki M and Tsubota K: Retinal aging and sirtuins.
Ophthalmic Res. 44:199–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mimura T, Kaji Y, Noma H, Funatsu H and
Okamoto S: The role of SIRT1 in ocular aging. Exp Eye Res.
116:17–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Balaiya S, Abu-Amero KK, Kondkar AA and
Chalam KV: Sirtuins expression and their role in retinal diseases.
Oxid Med Cell Longev. 2017:31875942017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McBurney MW, Yang X, Jardine K, Hixon M,
Boekelheide K, Webb JR, Lansdorp PM and Lemieux M: The mammalian
SIR2alpha protein has a role in embryogenesis and gametogenesis.
Mol Cell Biol. 23:38–54. 2003. View Article : Google Scholar :
|
|
21
|
Kamel C, Abrol M, Jardine K, He X and
McBurney MW: SirT1 fails to affect p53-mediated biological
functions. Aging Cell. 5:81–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng HL, Mostoslavsky R, Saito S, Manis
JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW and Chua KF:
Developmental defects and p53 hyperacetylation in Sir2 homolog
(SIRT1)-deficient mice. Proc Natl Acad Sci USA. 100:10794–10799.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen D, Pacal M, Wenzel P, Knoepfler PS,
Leone G and Bremner R: Division and apoptosis of E2f-deficient
retinal progenitors. Nature. 462:925–929. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jaliffa C, Ameqrane I, Dansault A, Leemput
J, Vieira V, Lacassagne E, Provost A, Bigot K, Masson C, Menasche M
and Abitbol M: Sirt1 involvement in rd10 mouse retinal
degeneration. Invest Ophthalmol Vis Sci. 50:3562–3572. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alves LF, Fernandes BF, Burnier JV,
Mansure JJ, Maloney S, Odashiro AN, Antecka E, De Souza DF and
Burnier MN Jr: Expression of SIRT1 in ocular surface squamous
neoplasia. Cornea. 31:817–819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maloney SC, Antecka E, Odashiro AN,
Fernandes BF, Doyle M, Lim LA, Katib YA and Miguel NB Jr:
Expression of SIRT1 and DBC1 in developing and adult retinas. Stem
Cells Int. 2012:9081832012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Zhao X, Shi D, Chen P, Yu Y, Yang
L and Xie L: Overexpression of SIRT1 promotes high
glucose-attenuated corneal epithelial wound healing via p53
regulation of the IGFBP3/IGF-1R/AKT pathway. Invest Ophthalmol Vis
Sci. 54:3806–3814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Sheng M, Liu Y, Wang P, Chen Y,
Chen L, Wang W and Li B: Expression of SIRT1 and oxidative stress
in diabetic dry eye. Int J Clin Exp Pathol. 8:7644–7653.
2015.PubMed/NCBI
|
|
29
|
An J, Chen X, Chen W, Liang R, Reinach PS,
Yan D and Tu L: MicroRNA expression profile and the Role of miR-204
in corneal wound healing. Invest Ophthalmol Vis Sci. 56:3673–3683.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao J, Wang Y, Zhao X, Chen P and Xie L:
MicroRNA-204-5p-mediated regulation of SIRT1 contributes to the
delay of epithelial cell cycle traversal in diabetic corneas.
Invest Ophthalmol Vis Sci. 56:1493–1504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Zhao X, Wu X, Dai Y, Chen P and
Xie L: microRNA-182 mediates Sirt1-induced diabetic corneal nerve
regeneration. Diabetes. 65:2020–2031. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hodge WG, Whitcher JP and Satariano W:
Risk factors for age-related cataracts. Epidemiol Rev. 17:336–346.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng Y, Liu Y, Ge J, Wang X, Liu L, Bu Z
and Liu P: Resveratrol protects human lens epithelial cells against
H2O2-induced oxidative stress by increasing catalase, SOD-1, and
HO-1 expression. Mol Vis. 16:1467–1474. 2010.PubMed/NCBI
|
|
34
|
Zheng T and Lu Y: SIRT1 protects human
lens epithelial cells against oxidative stress by Inhibiting
p53-dependent apoptosis. Curr Eye Res. 41:1068–1075. 2016.
View Article : Google Scholar
|
|
35
|
Doganay S, Borazan M, Iraz M and Cigremis
Y: The effect of resveratrol in experimental cataract model formed
by sodium selenite. Curr Eye Res. 31:147–153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin TJ, Peng CH, Chiou SH, Liu JH,
Lin-Chung-Woung, Tsai CY, Chuang JH and Chen SJ: Severity of lens
opacity, age, and correlation of the level of silent information
regulator T1 expression in age-related cataract. J Cataract Refract
Surg. 37:1270–1274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng T and Lu Y: Changes in SIRT1
expression and its downstream pathways in age-related cataract in
humans. Curr Eye Res. 36:449–455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kondo A, Goto M, Mimura T and Matsubara M:
Silent information regulator T1 in aqueous humor of patients with
cataract. Clin Ophthalmol. 10:307–312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang L, Zhao W, Zhang G, Wu J and Guan H:
Acetylated 8-oxoguanine DNA glycosylase 1 and its relationship with
p300 and SIRT1 in lens epithelium cells from age-related cataract.
Exp Eye Res. 135:102–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
van Lookeren Campagne M, LeCouter J,
Yaspan BL and Ye W: Mechanisms of age-related macular degeneration
and therapeutic opportunities. J Pathol. 232:151–164. 2014.
View Article : Google Scholar
|
|
41
|
Chen Z, Zhai Y, Zhang W, Teng Y and Yao K:
Single nucleotide polymorphisms of the sirtuin 1 (SIRT1) gene are
associated with age-related macular degeneration in Chinese han
individuals: A case-control pilot study. Medicine (Baltimore).
94:e22382015. View Article : Google Scholar
|
|
42
|
Maloney SC, Antecka E, Granner T,
Fernandes B, Lim LA, Orellana ME and Burnier MN Jr: Expression of
SIRT1 in choroidal neovascular membranes. Retina. 33:862–866. 2013.
View Article : Google Scholar
|
|
43
|
Peng CH, Chang YL, Kao CL, Tseng LM, Wu
CC, Chen YC, Tsai CY, Woung LC, Liu JH, Chiou SH and Chen SJ:
SirT1-a sensor for monitoring self-renewal and aging process in
retinal stem cells. Sensors. 10:6172–6194. 2010. View Article : Google Scholar
|
|
44
|
Peng CH, Cherng JY, Chiou GY, Chen YC,
Chien CH, Kao CL, Chang YL, Chien Y, Chen LK, Liu JH, et al:
Delivery of Oct4 and SirT1 with cationic polyurethanes-short branch
PEI to aged retinal pigment epithelium. Biomaterials. 32:9077–9088.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bhattacharya S, Chaum E, Johnson DA and
Johnson LR: Age-related susceptibility to apoptosis in human
retinal pigment epithelial cells is triggered by disruption of
p53-Mdm2 association. Invest Ophthalmol Vis Sci. 53:8350–8366.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Golestaneh N, Chu Y, Cheng SK, Cao H,
Poliakov E and Berinstein DM: Repressed SIRT1/PGC-1α pathway and
mitochondrial disintegration in iPSC-derived RPE disease model of
age-related macular degeneration. J Transl Med. 14:3442016.
View Article : Google Scholar
|
|
47
|
Zhuge CC, Xu JY, Zhang J, Li W, Li P, Li
Z, Chen L, Liu X, Shang P, Xu H, et al: Fullerenol protects retinal
pigment epithelial cells from oxidative stress-induced premature
senescence via activating SIRT1. Invest Ophthalmol Vis Sci.
55:4628–4638. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jackson MD, Schmidt MT, Oppenheimer NJ and
Denu JM: Mechanism of nicotinamide inhibition and
transglycosidation by Sir2 histone/protein deacetylases. J Biol
Chem. 278:50985–50998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu Z, Lauer TW, Sick A, Hackett SF and
Campochiaro PA: Oxidative stress modulates complement factor H
expression in retinal pigmented epithelial cells by acetylation of
FOXO3. J Biol Chem. 282:22414–22425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cao L, Liu C, Wang F and Wang H: SIRT1
negatively regulates amyloid-beta-induced inflammation via the
NF-κB pathway. Braz J Med Biol Res. 46:659–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ban N, Ozawa Y, Inaba T, Miyake S,
Watanabe M, Shinmura K and Tsubota K: Light-dark condition
regulates sirtuin mRNA levels in the retina. Exp Gerontol.
48:1212–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chou WW, Chen KC, Wang YS, Wang JY, Liang
CL and Juo SH: The role of SIRT1/AKT/ERK pathway in ultraviolet B
induced damage on human retinal pigment epithelial cells. Toxicol
In Vitro. 27:1728–1736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kubota S, Kurihara T, Ebinuma M, Kubota M,
Yuki K, Sasaki M, Noda K, Ozawa Y, Oike Y, Ishida S and Tsubota K:
Resveratrol prevents light-induced retinal degeneration via
suppressing activator protein-1 activation. Am J Pathol.
177:1725–1731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Potente M, Ghaeni L, Baldessari D,
Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana
E, Alt FW, et al: SIRT1 controls endothelial angiogenic functions
during vascular growth. Genes Dev. 21:2644–2658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Potente M and Dimmeler S: Emerging roles
of SIRT1 in vascular endothelial homeostasis. Cell Cycle.
7:2117–2122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Balaiya S, Khetpal V and Chalam KV:
Hypoxia initiates sirtuin1-mediated vascular endothelial growth
factor activation in choroidal endothelial cells through hypoxia
inducible factor-2α. Mol Vis. 18:114–120. 2012.
|
|
57
|
Nagineni CN, Raju R, Nagineni KK,
Kommineni VK, Cherukuri A, Kutty RK, Hooks JJ and Detrick B:
Resveratrol suppresses expression of VEGF by human retinal pigment
epithelial cells: Potential nutraceutical for age-related macular
degeneration. Aging Dis. 5:88–100. 2014.PubMed/NCBI
|
|
58
|
Balaiya S, Murthy RK and Chalam KV:
Resveratrol inhibits proliferation of hypoxic choroidal vascular
endothelial cells. Mol Vis. 19:2385–2392. 2013.PubMed/NCBI
|
|
59
|
Zhang H, He S, Spee C, Ishikawa K and
Hinton DR: SIRT1 mediated inhibition of VEGF/VEGFR2 signaling by
resveratrol and its relevance to choroidal neovascularization.
Cytokine. 76:549–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Khan AA, Dace DS, Ryazanov AG, Kelly J and
Apte RS: Resveratrol regulates pathologic angiogenesis by a
eukaryotic elongation factor-2 kinase-regulated pathway. Am J
Pathol. 177:481–492. 2010. View Article : Google Scholar :
|
|
61
|
Diabetes Control and Complications Trial
Research Group; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford
O, Davis M, Rand L and Siebert C: The effect of intensive treatment
of diabetes on the development and progression of long-term
complications in insulin-dependent diabetes mellitus. N Engl J Med.
329:977–986. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nathan DM, Cleary PA, Backlund JY, Genuth
SM, Lachin JM, Orchard TJ, Raskin P and Zinman B; Diabetes Control
and Complications Trial/Epidemiology of Diabetes Interventions and
Complications (DCCT/EDIC) Study Research Group: Intensive diabetes
treatment and cardiovascular disease in patients with type 1
diabetes. N Engl J Med. 353:2643–2653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mortuza R, Chen S, Feng B, Sen S and
Chakrabarti S: High glucose induced alteration of SIRTs in
endothelial cells causes rapid aging in a p300 and FOXO regulated
pathway. PLoS One. 8:e545142013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kowluru RA, Santos JM and Zhong Q: Sirt1,
a negative regulator of matrix metalloproteinase-9 in diabetic
retinopathy. Invest Ophthalmol Vis Sci. 55:5653–5660. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kowluru RA, Mishra M and Kumar B: Diabetic
retinopathy and transcriptional regulation of a small molecular
weight G-Protein, Rac1. Exp Eye Res. 147:72–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kubota S, Ozawa Y, Kurihara T, Sasaki M,
Yuki K, Miyake S, Noda K, Ishida S and Tsubota K: Roles of
AMP-activated protein kinase in diabetes-induced retinal
inflammation. Invest Ophthalmol Vis Sci. 52:9142–9148. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zheng Z, Chen H, Li J, Li T, Zheng B,
Zheng Y, Jin H, He Y, Gu Q and Xu X: Sirtuin 1-mediated cellular
metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and
therapeutic effects of metformin. Diabetes. 61:217–228. 2012.
View Article : Google Scholar
|
|
68
|
Zhang E, Guo Q, Gao H, Xu R, Teng S and Wu
Y: Metformin and resveratrol inhibited high glucose-induced
metabolic memory of endothelial senescence through
SIRT1/p300/p53/p21 pathway. PLoS One. 10:e01438142015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhao S, Li T, Li J, Lu Q, Han C, Wang N,
Qiu Q, Cao H, Xu X, Chen H and Zheng Z: miR-23b-3p induces the
cellular metabolic memory of high glucose in diabetic retinopathy
through a SIRT1-dependent signalling pathway. Diabetologia.
59:644–654. 2016. View Article : Google Scholar
|
|
70
|
Zhao S, Li J, Wang N, Zheng B, Li T, Gu Q,
Xu X and Zheng Z: Fenofibrate suppresses cellular metabolic memory
of high glucose in diabetic retinopathy via a sirtuin 1-dependent
signalling pathway. Mol Med Rep. 12:6112–6118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Simó R and Hernández C: Novel approaches
for treating diabetic retinopathy based on recent pathogenic
evidence. Prog Retin Eye Res. 48:160–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Vujosevic S and Simó R: Local and systemic
inflammatory biomarkers of diabetic retinopathy: An integrative
approach. Invest Ophthalmol Vis Sci. 58:BIO68–BIO75. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mishra M, Flaga J and Kowluru RA:
Molecular mechanism of transcriptional regulation of matrix
metalloproteinase-9 in diabetic retinopathy. J Cell Physiol.
231:1709–1718. 2016. View Article : Google Scholar
|
|
74
|
Mortuza R, Feng B and Chakrabarti S: SIRT1
reduction causes renal and retinal injury in diabetes through
endothelin 1 and transforming growth factor β1. J Cell Mol Med.
19:1857–1867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zeng Y, Yang K, Wang F, Zhou L, Hu Y, Tang
M, Zhang S, Jin S, Zhang J, Wang J, et al: The glucagon like
peptide 1 analogue, exendin-4, attenuates oxidative stress-induced
retinal cell death in early diabetic rats through promoting Sirt1
and Sirt3 expression. Exp Eye Res. 151:203–211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu S, Lin YU and Liu XIN: Protective
effects of SIRT1 in patients with proliferative diabetic
retinopathy via the inhibition of IL-17 expression. Exp Ther Med.
11:257–262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mortuza R, Feng B and Chakrabarti S:
miR-195 regulates SIRT1-mediated changes in diabetic retinopathy.
Diabetologia. 57:1037–1046. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen J and Smith LE: Retinopathy of
prematurity. Angiogenesis. 10:133–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen J, Michan S, Juan AM, Hurst CG,
Hatton CJ, Pei DT, Joyal JS, Evans LP, Cui Z, Stahl A, et al:
Neuronal sirtuin1 mediates retinal vascular regeneration in
oxygen-induced ischemic retinopathy. Angiogenesis. 16:985–992.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Michan S, Juan AM, Hurst CG, Cui Z, Evans
LP, Hatton CJ, Pei DT, Ju M, Sinclair DA, Smith LE and Chen J:
Sirtuin1 over-expression does not impact retinal vascular and
neuronal degeneration in a mouse model of oxygen-induced
retinopathy. PLoS One. 9:e850312014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fischer D and Leibinger M: Promoting optic
nerve regeneration. Prog Retin Eye Res. 31:688–701. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tang BL and Chua CE: SIRT1 and neuronal
diseases. Mol Aspects Med. 29:187–200. 2008. View Article : Google Scholar
|
|
83
|
Kim SH, Park JH, Kim YJ and Park KH: The
neuroprotective effect of resveratrol on retinal ganglion cells
after optic nerve transection. Mol Vis. 19:1667–1676.
2013.PubMed/NCBI
|
|
84
|
Chen S, Fan Q, Li A, Liao D, Ge J, Laties
AM and Zhang X: Dynamic mobilization of PGC-1α mediates
mitochondrial biogenesis for the protection of RGC-5 cells by
resveratrol during serum deprivation. Apoptosis. 18:786–799. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zuo L, Khan RS, Lee V, Dine K, Wu W and
Shindler KS: SIRT1 promotes RGC survival and delays loss of
function following optic nerve crush. Invest Ophthalmol Vis Sci.
54:5097–5102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Balaiya S, Ferguson LR and Chalam KV:
Evaluation of sirtuin role in neuroprotection of retinal ganglion
cells in hypoxia. Invest Ophthalmol Vis Sci. 53:4315–4322. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kim SJ, Sung MS, Heo H, Lee JH and Park
SW: Mangiferin protects retinal ganglion cells in ischemic mouse
retina via SIRT1. Curr Eye Res. 41:844–855. 2016.
|
|
88
|
Shindler KS, Ventura E, Rex TS, Elliott P
and Rostami A: SIRT1 activation confers neuroprotection in
experimental optic neuritis. Invest Ophthalmol Vis Sci.
48:3602–3609. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Fonseca-Kelly Z, Nassrallah M, Uribe J,
Khan RS, Dine K, Dutt M and Shindler KS: Resveratrol
neuroprotection in a chronic mouse model of multiple sclerosis.
Front Neurol. 3:842012. View Article : Google Scholar :
|
|
90
|
Shindler KS, Ventura E, Dutt M, Elliott P,
Fitzgerald DC and Rostami A: Oral resveratrol reduces neuronal
damage in a model of multiple sclerosis. J Neuroophthalmol.
30:328–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Khan RS, Fonseca-Kelly Z, Callinan C, Zuo
L, Sachdeva MM and Shindler KS: SIRT1 activating compounds reduce
oxidative stress and prevent cell death in neuronal cells. Front
Cell Neurosci. 6:632012. View Article : Google Scholar
|
|
92
|
Khan RS, Dine K, Das Sarma J and Shindler
KS: SIRT1 activating compounds reduce oxidative stress mediated
neuronal loss in viral induced CNS demyelinating disease. Acta
Neuropathol Commun. 2:32014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang Y, Li H, Cao Y, Zhang M and Wei S:
Sirtuin 1 regulates lipid metabolism associated with optic nerve
regeneration. Mol Med Rep. 12:6962–6968. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lin P, Suhler EB and Rosenbaum JT: The
future of uveitis treatment. Ophthalmology. 121:365–376. 2014.
View Article : Google Scholar :
|
|
95
|
Kubota S, Kurihara T, Mochimaru H,
Satofuka S, Noda K, Ozawa Y, Oike Y, Ishida S and Tsubota K:
Prevention of ocular inflammation in endotoxin-induced uveitis with
resveratrol by inhibiting oxidative damage and nuclear
factor-kappaB activation. Invest Ophthalmol Vis Sci. 50:3512–3519.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Rossi S, Di Filippo C, Gesualdo C, Testa
F, Trotta MC, Maisto R, Ferraro B, Ferraraccio F, Accardo M,
Simonelli F and D'Amico M: Interplay between Intravitreal RvD1 and
Local Endogenous Sirtuin-1 in the protection from endotoxin-induced
uveitis in rats. Mediators Inflamm. 2015:1264082015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Gardner PJ, Joshi L, Lee RW, Dick AD,
Adamson P and Calder VL: SIRT1 activation protects against
autoimmune T cell-driven retinal disease in mice via inhibition of
IL-2/Stat5 signaling. J Autoimmun. 42:117–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Gardner PJ, Yazid S, Chu CJ, Copland DA,
Adamson P, Dick AD and Calder VL: TNFα regulates SIRT1 cleavage
during ocular autoimmune disease. Am J Pathol. 185:1324–1333. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Anekonda TS and Adamus G: Resveratrol
prevents antibody-induced apoptotic death of retinal cells through
upregulation of Sirt1 and Ku70. BMC Res Notes. 1:1222008.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bola C, Bartlett H and Eperjesi F:
Resveratrol and the eye: Activity and molecular mechanisms. Graefes
Arch Clin Exp Ophthalmol. 252:699–713. 2014. View Article : Google Scholar : PubMed/NCBI
|