Introduction
The induction of cancer cell-specific apoptosis via
the activation of tumour necrosis factor-related apoptosis-inducing
ligand (TRAIL) signalling has become an important focus of cancer
research. TRAIL triggers apoptosis in cancer cells with no toxicity
toward normal tissues (1,2). Endogenous TRAIL plays an important
role in immunosurveillance and defence against tumour cells.
TRAIL-mediated apoptosis of pre-malignant or malignant cells
represents an immune preventive mechanism against tumour
initiation, formation and progression. TRAIL is expressed on the
surface of T lymphocytes, natural killer cells, dendritic cells,
neutrophils, monocytes or macrophages and can be cleaved into a
soluble, secreted form (1–3). This death ligand induces apoptosis in
cancer cells via a receptor-mediated pathway involving interactions
with TRAIL-R1/death receptor (DR)4 and/or TRAIL-R2/DR5. Stimulation
of the death receptor system by TRAIL results in the recruitment of
the adaptor molecule, Fas-associated death domain (FADD), to form
the death inducing signalling complex (DISC), which subsequently
activates caspase-8. Crosstalk between the extrinsic
(receptor-dependent) and intrinsic (mitochondrial-dependent)
apoptotic pathways is linked by caspase-8. The activation of
caspase-8 directly causes the activation of the caspase cascade and
cell death. Simultaneously, caspase-8 leads indirectly to the
activation of effector caspases through the cleavage of the
BH3-interacting domain death agonist (Bid), along with the release
of cytochrome c and mitochondrial membrane potential (ΔΨm)
disruption (1–6). However, some cancer cells are
resistant to TRAIL-induced cytotoxicity (7–11).
This failure to undergo apoptosis has been implicated in the
resistance of cancer cells to TRAIL surveillance and, therefore, in
tumour development (1–3,6). The
expression of the death receptors and pro-apoptotic or
anti-apoptotic proteins in cancer cells is involved in TRAIL
resistance (1,2,6).
TRAIL-resistant prostate cancer cells can be sensitized to
TRAIL-mediated apoptosis by certain phenolic compounds (6,8,12–20).
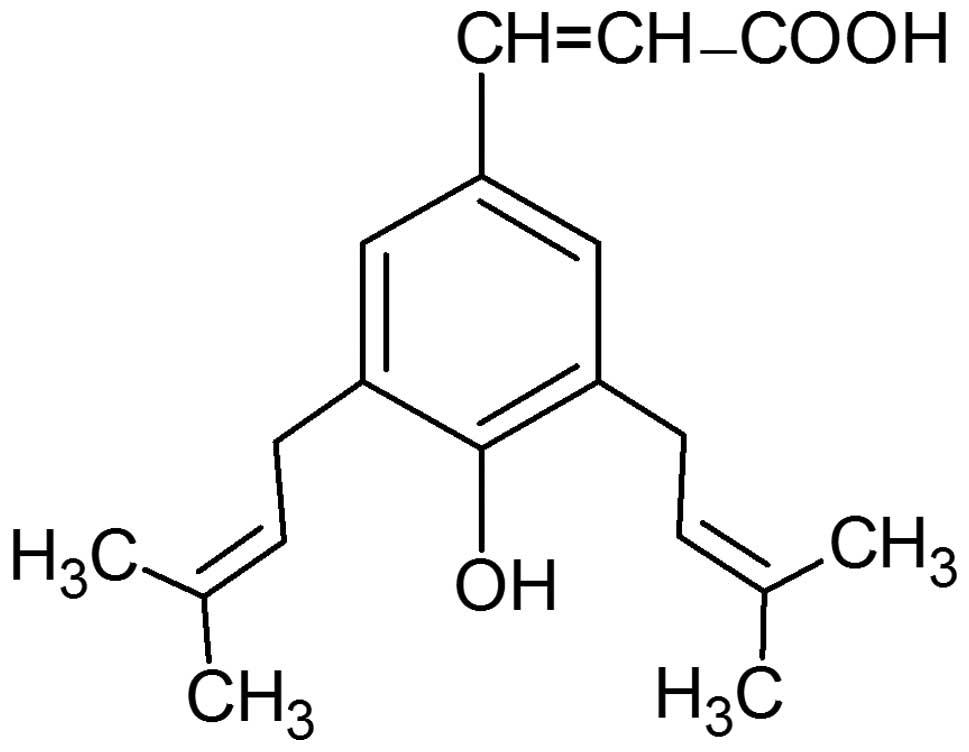
Artepillin C (3,5-diprenyl-4-hydroxycinnamic acid)
is the major biologically active phenolic component found in green
propolis, which is collected from the plant Baccharis
dracunculiforia in Southeastern Brazil (21–23).
Artepillin C possesses antioxidant, antimicrobial,
anti-inflammatory, antigenotoxic, anti-angiogenic and anticancer
properties (24–36). The structure of this
hydroxycinnamic acid derivative is presented in Fig. 1. Artepillin C exerts direct
antiproliferative, cytotoxic and apoptotic effects on gastric,
colon or lung cancer cells and inhibits the growth of transplanted
solid human or mouse tumours in athymic and thymic mice,
respectively (25). The role of
natural phenolic compounds in cancer prevention has been confirmed
in numerous laboratory and epidemiological studies (37–43).
Prostate cancer is one of the most commonly
diagnosed cancers in men, the third leading cause of cancer-related
mortality in Europe, and the second in the United States (16,44).
Chemopreventive intervention using dietary phenolics is an
attractive option in prostate cancer due to its incidence,
prevalence and disease-related morbidity and mortality (45).
Previous findings have demonstrated that the
ethanolic extract of Brazilian green propolis (EEP) and its
constituent, artepillin C, can help cells overcome TRAIL resistance
and significantly augments the apoptotic activity of TRAIL in LNCaP
prostate cancer cells (17).
Artepillin C sensitizes prostate cancer cells to TRAIL-mediated
immunoprevention, which confirms the potential role of this
prenylated hydroxycinnamic acid derivative as a chemopreventive
agent in prostate carcinogenesis. The aim of this study was to
evaluate the mechanisms by which artepillin C affects the
TRAIL-induced death signalling pathway in prostate cancer
cells.
Materials and methods
Prostate cancer cell culture
The LNCaP human androgen-dependent prostate cancer
cell line was obtained from the German Collection of Microorganisms
and Cell Cultures (DSMZ, Braunschweig, Germany). The cells were
grown in monolayer cultures at 37°C in a 5% CO2
humidified incubator and were maintained in RPMI-1640 medium
supplemented with 10% heat-inactivated fetal bovine serum (FBS), 4
mM L-glutamine, 100 U/ml penicillin and 100 μg/ml
streptomycin (7,8,16,17).
Reagents for cell culture were purchased from the PAA Cell Culture
Company (Pasching, Austria).
Reagents
Artepillin C was provided by Wako Pure Chemicals
(Osaka, Japan) as a natural component isolated from Brazilian green
propolis. Artepillin C was dissolved in dimethyl sulphoxide (DMSO)
to obtain the working concentrations. Soluble recombinant human
TRAIL was purchased from PeproTech Inc. (Rocky Hill, NJ, USA).
Human recombinant TRAIL-R1/Fc and TRAIL-R2/Fc chimera proteins, the
general caspase inhibitor, Z-VAD-FMK, the caspase-8 inhibitor,
Z-IETD-FMK, and the caspase-3 inhibitor, Z-DEVD-FMK, were obtained
from R&D Systems (Minneapolis, MN, USA).
Cytotoxicity assay
Cytotoxicity was measured by the 3-(4,5-
dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay
(46,47). The MTT assay is based on the
cleavage of the tetrazolium salt MTT to a blue formazan dye by
viable cells. LNCaP cells (2×105/ml) were seeded in a
96-well plate for 24 h before the experiments. The cells were then
incubated with TRAIL (50–200 ng/ml) and/or artepillin C (50–100
μM). After 24 h, 20 μl of MTT solution (5 mg/ml) were
added to each well for 4 h. The resulting blue formazan crystals
were dissolved in DMSO. These reagents were purchased from Sigma
Chemical Company (St. Louis, MO, USA). The controls included native
cells and medium alone. Spectrophotometric absorbance was measured
at a 550-nm wavelength using a microplate reader (ELx 800, Bio-Tek
Instruments Inc., Winooski, VT, USA). The percentage cytotoxicity
was calculated by the following formula: percentage cytotoxicity
(cell death) = [1−(absorbance value of experimental
wells/absorbance value of control wells)]) ×100%.
Lactate dehydrogenase (LDH) release
assay
LDH is a stable cytosolic enzyme that is released
upon membrane damage in necrotic cells. LDH activity was measured
using a cytotoxicity assay kit (Roche Diagnostics GmbH, Mannheim,
Germany) (14,48). LNCaP cells were treated with TRAIL
(50–200 ng/ml) and/or artepillin C (50–100 μM) for the
indicated period of time. LDH released into the culture
supernatants was detected with a coupled enzymatic assay, that
results in the conversion of a tetrazolium salt into a red formazan
product. Spectrophotometric absorbance was measured at a 490-nm
wavelength using a microplate reader. Maximal release of LDH was
obtained after treating the control cells with 1% Triton X-100
(Sigma) for 10 min at room tempe rature. The percentage of necrotic
cells was expressed using the following formula: (sample
value/maximal release) ×100%.
Detection of apoptosis by flow
cytometry
Apoptosis was determined by flow cytometry using the
Apoptest-FITC kit with Annexin V (Dako, Glostrup, Denmark). LNCaP
cells (2×105/ml) were seeded in 24-well plates for 24 h
prior to experimentation and then exposed to TRAIL (100 ng/ml)
and/or artepillin C (50–100 μM) for 24 h. After this
incubation, the cells were washed twice with phosphate-buffered
saline (PBS) solution and resuspended in 1 ml of binding buffer.
The cell suspension (500 μl) was then incubated with 5
μl of Annexin V-FITC and 10 μl of propidium iodide
(PI) for 10 min at room temperature in the dark. The population of
Annexin V-positive cells was evaluated by flow cytometry (BD
FACScan; Becton-Dickinson Immunocytometry Systems, San Jose, CA,
USA) (15,49).
Detection of apoptosis by fluorescence
microscopy
Apoptotic cells were quantified using the
fluorescence microscopy method of the Apoptotic & Necrotic
& Healthy Cells Quantification kit from Biotium, Inc. (Hayward,
CA, USA) (15,17). LNCaP cells (2×105/ml)
were seeded in a 24-well plate for 24 h before the experiments.
TRAIL (100 ng/ml) and/or artepillin C (50 and 100 μM) were
added to the cells, and 24 h later, the cells were washed with PBS
and trypsinised. The cells were then centrifuged to discard the
supernatant, washed with PBS and resuspended in binding buffer (100
μl/sample). A combination of 5 μl of Annexin V-FITC,
5 μl of ethidium homodimer III and 5 μl of Hoechst
33342 solution was added to each tube. The samples were incubated
at room temperature for 15 min in the dark, and then the cells were
washed with binding buffer, placed on a glass slide and covered
with a glass cover slip. The stained cells were observed with an
IX51 fluorescence inverted microscope (Olympus, Tokyo, Japan) using
filter sets for FITC, TRITC and DAPI. The cells were counted, and
the number of apoptotic cells is expressed as a percentage of the
total number of cells.
Flow cytometric analysis of death
receptor expression on the cell surface
The cell surface expression of the death receptors,
TRAIL-R1 and TRAIL-R2, was determined by flow cytometry (BD
FACSCanto, Becton-Dickinson Immunocytometry Systems). LNCaP cells
(2×105/ml) were seeded in 24-well plates for 24 h and
exposed to artepillin C (50 and 100 μM) for 24 h. The cells
were then harvested using a solution of trypsin and
ethylenediaminetetra acetic acid (EDTA), washed twice in PBS and
resuspended in PBS containing 0.5% bovine serum albumin (BSA).
LNCaP cells were incubated with 10 μl
phycoerythrin-conjugated anti-TRAIL-R1 or anti-TRAIL-R2 monoclonal
antibody (R&D Systems) at 4°C for 45 min. After staining, the
cells were washed with PBS and analyzed using flow cytometry
(17–20,50).
The control sample consisted of cells in a separate tube treated
with phycoerythrin-labelled mouse IgG1 or mouse
IgG2B (R&D Systems).
Caspase activity assays
Caspase-3 and caspase-8 activities were assessed
using colorimetric protease assay kits (R&D Systems). The tests
are based on the spectrophotometric detection of the chromophore,
p-nitroaniline (pNA), after cleavage from the caspase
substrate (caspase-specific peptide conjugated to pNA). LNCaP cells
(1×106/ml) were seeded in Petri dishes 24 h before the
experiments. TRAIL (100 ng/ml) and/or artepillin C (50–100
μM) were added to the cells, after 24 h the cells were
centrifuged, the supernatant was discarded and the cells were
treated with lysis buffer. The cell lysates were tested for
protease activity by the addition of a labelled caspase substrate,
DEVD-pNA for caspase-3 activity or IETD-pNA for caspase-8 activity.
pNA absorbance was quantified using a V-630 spectrophotometer
(Jasko International Co., Tokyo, Japan) at a 405-nm wavelength
(20).
Evaluation of ΔΨm by DePsipher
staining
The DePsipher kit (R&D Systems) was used to
measure the ΔΨm using fluorescence microscopy (17–20).
LNCaP cells (2×105/ml) were seeded in a 24-well plate 24
h prior to the experiments. TRAIL (100 ng/ml) and/or artepillin C
(50–100 μM) were added, and 24 h later, the cells were
washed with PBS and harvested by trypsinisation. The cells were
incubated in the dark with DePsipher
(5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl
carbocyanin iodide) solution at a concentration of 5 μg/ml
for 30 min at 37°C, washed with reaction buffer with stabiliser,
placed on a glass slide and covered with a glass cover slip. The
stained cells were observed with a fluorescence inverted microscope
using filter sets for FITC and TRITC. DePsipher staining exhibits
potential-dependent accumulation in the mitochondria, which is
indicated by a fluorescence emission shift from red (590 nm) to
green (530 nm).
The activity of NF-κB
NF-κB activity was measured using the ELISA-based
TransAM NF-κB kit (Active Motif Europe, Rixensart, Belgium) on
nuclear extracts. LNCaP cells (1×106/ml) were seeded in
Petri dishes and allowed to attach for 24 h before the experiments.
Artepillin C (50–100 μM) with or without TRAIL (100 ng/ml)
was added to the cells for 24 h. The commercially available Nuclear
Extract kit was obtained from Active Motif Europe for the
preparation of the LNCaP cell nuclear extracts. The TransAM
NF-assay for NF-κB (p65) activity was performed according to the
manufacturer’s instructions (17,19,20).
NF-κB DNA-binding activity was assessed using the ELISA kit for the
transcription factor, p65. Oligonucleotides containing the NF-κB
consensus binding site (5′-GGGACTTCC-3′) were immobilised on a
96-well plate. The active forms of NF-κB in the nuclear extracts
were bound to the oligonucleotides on the plate and detected
colorimetrically. The samples were read at an absorbance of 450 nm
on a spectrophotometer with a reference wavelength of 650 nm. The
detection limit for the TransAM NF-κB kit is <0.4 ng/ml purified
p65.
Statistical analysis
The results are expressed as the means ± SD obtained
from three independent experiments performed in quadruplicate
(n=12) or duplicate (n=6). Statistical significance was evaluated
using the Levene test or Bartlett χ2 test followed by
analysis of variance (ANOVA) and post-hoc test. A p-value <0.05
was considered significant.
Results
Cytotoxic and apoptotic effects of TRAIL
on LNCaP cells
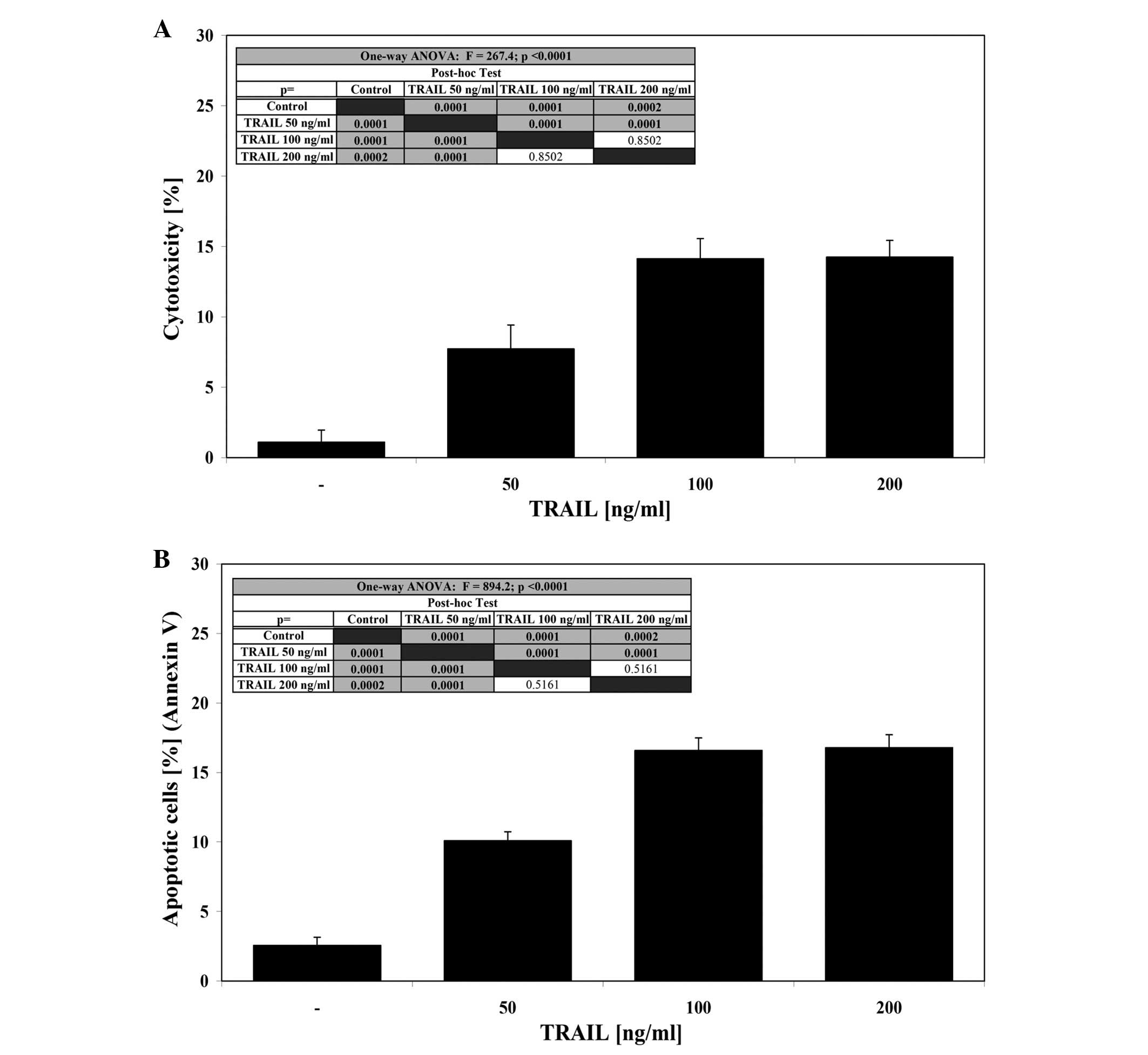
The cytotoxic effect of TRAIL at concentrations of
50–100 ng/ml after a 24-h incubation was 7.7±1.7–14.1±1.4% cell
death (Fig. 2A). At the same
concentrations TRAIL induced 10.1±0.6–16.6±0.9% apoptosis in a
dose-dependent manner in LNCaP cells (Fig. 2B). TRAIL concentrations higher than
100 ng/ml resulted in no significant increase in cytotoxic or
apoptotic activity. These data confirm that the LNCaP cell line is
resistant to TRAIL-mediated apoptosis.
Cytotoxic and apoptotic effects of TRAIL
in combination with artepillin C on LNCaP cells
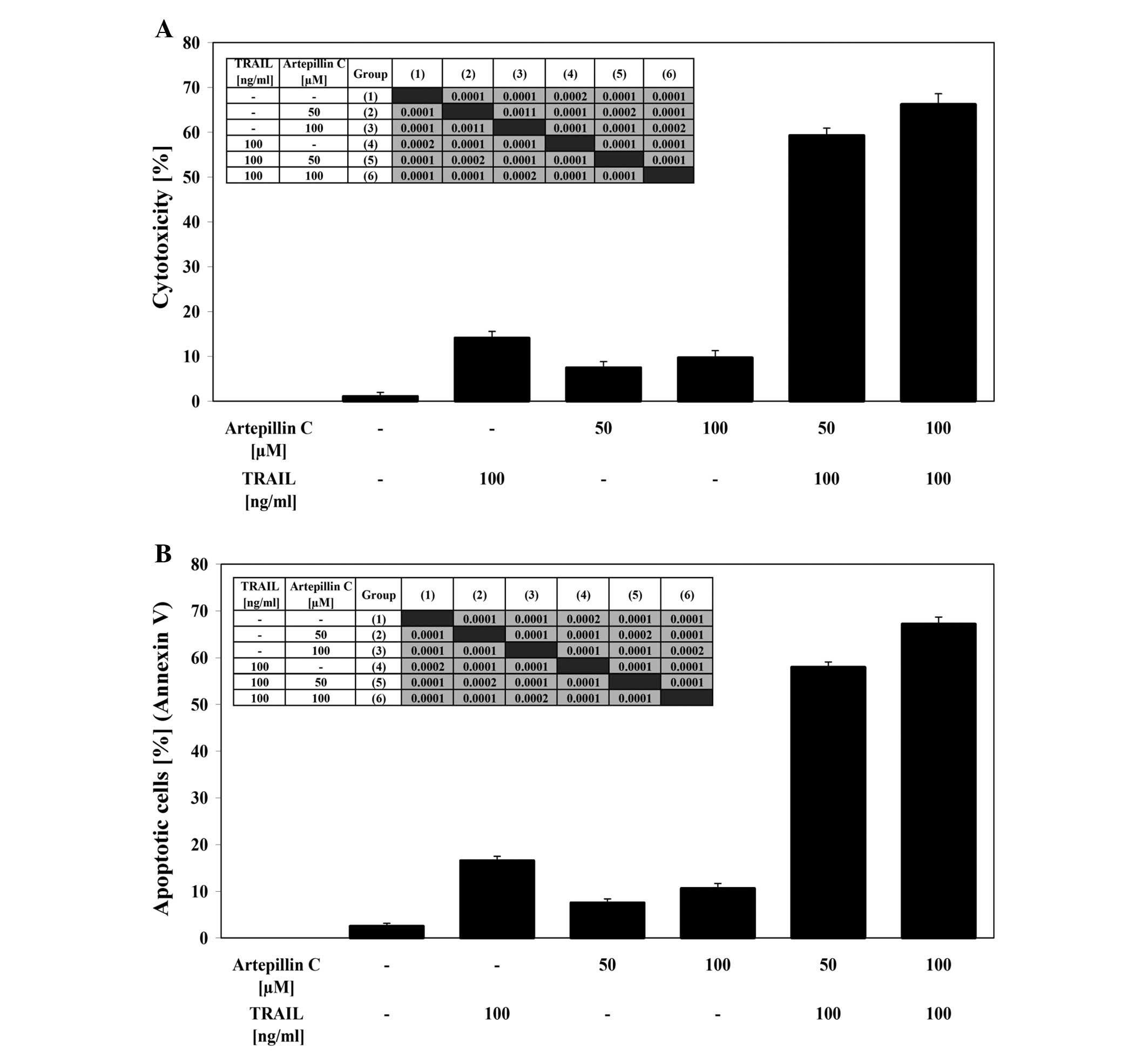
After co-treatment of LNCaP cells with 100 ng/ml
TRAIL and 50–100 μM artepillin C for 24 h the cytotoxicity
ranged from 59.3±1.6 to 66.3±2.3%. The cytotoxicity measured by MTT
assay is shown in Fig. 3A.
Artepillin C cooperated with TRAIL to induce apoptosis in the
prostate cancer cells. When the cells were treated with the same
concentration of TRAIL and artepillin C for 24 h, the percentage of
apoptotic cells was elevated to 58.0±1.1–67.2±1.5% as determined by
Annexin V-FITC staining using flow cytometry (Fig. 3B). Artepillin C sensitized the
TRAIL-resistant LNCaP cells to TRAIL-mediated apoptosis. The
necrotic cell death percentage of LNCaP cells examined by
Apoptest-FITC and LDH assay was near zero. The Annexin V-FITC
staining, visualised by fluorescence microscopy, supports the hypo
thesis that the apoptotic activity of TRAIL was augmented by
artepillin C in LNCaP cells (Fig.
3C).
Effects of artepillin C on death receptor
expression in LNCaP cells
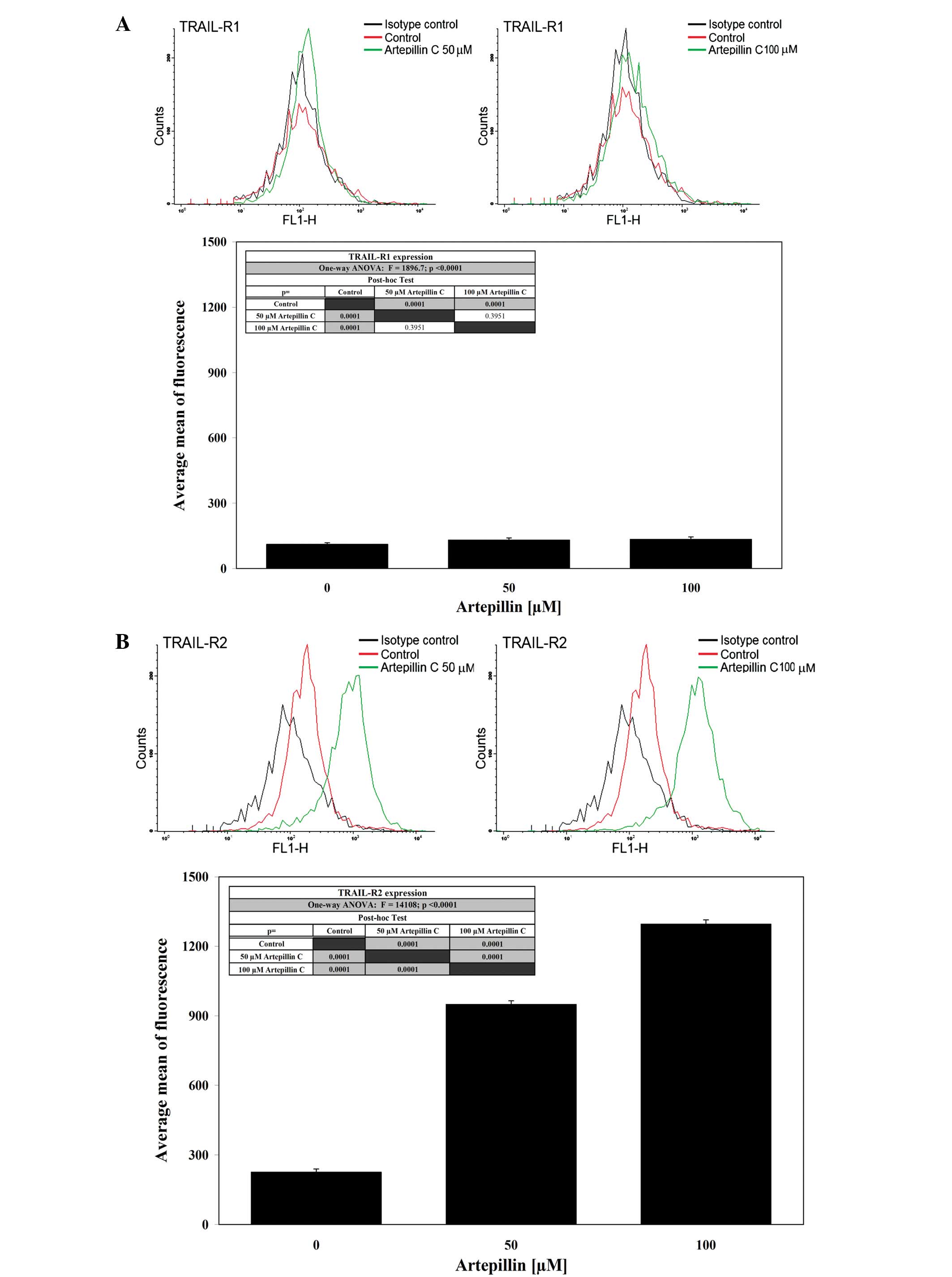
The activation of death receptors on the cell
surface is critical for TRAIL-mediated apoptosis. Therefore, we
analyzed the expression of TRAIL-R1 and TRAIL-R2 in LNCaP cells
after a 24-h treatment with 50–100 μM artepillin C by flow
cytometry (Fig. 4). Treatment with
artepillin C significantly increased the expression of TRAIL-R2,
but did not alter TRAIL-R1 expression on the cell surface.
Artepillin C sensitized the prostate cancer cells through the
extrinsic apoptotic pathway. To show that the induction of
apoptosis caused by the co-treatment of TRAIL and artepillin C was
mediated through TRAIL-R2, we used the TRAIL-R2/Fc chimera protein,
which acts as a dominant-negative against endogenous TRAIL-R2. The
chimeric protein efficiently blocked apoptosis when the cells were
treated with TRAIL and artepillin C.
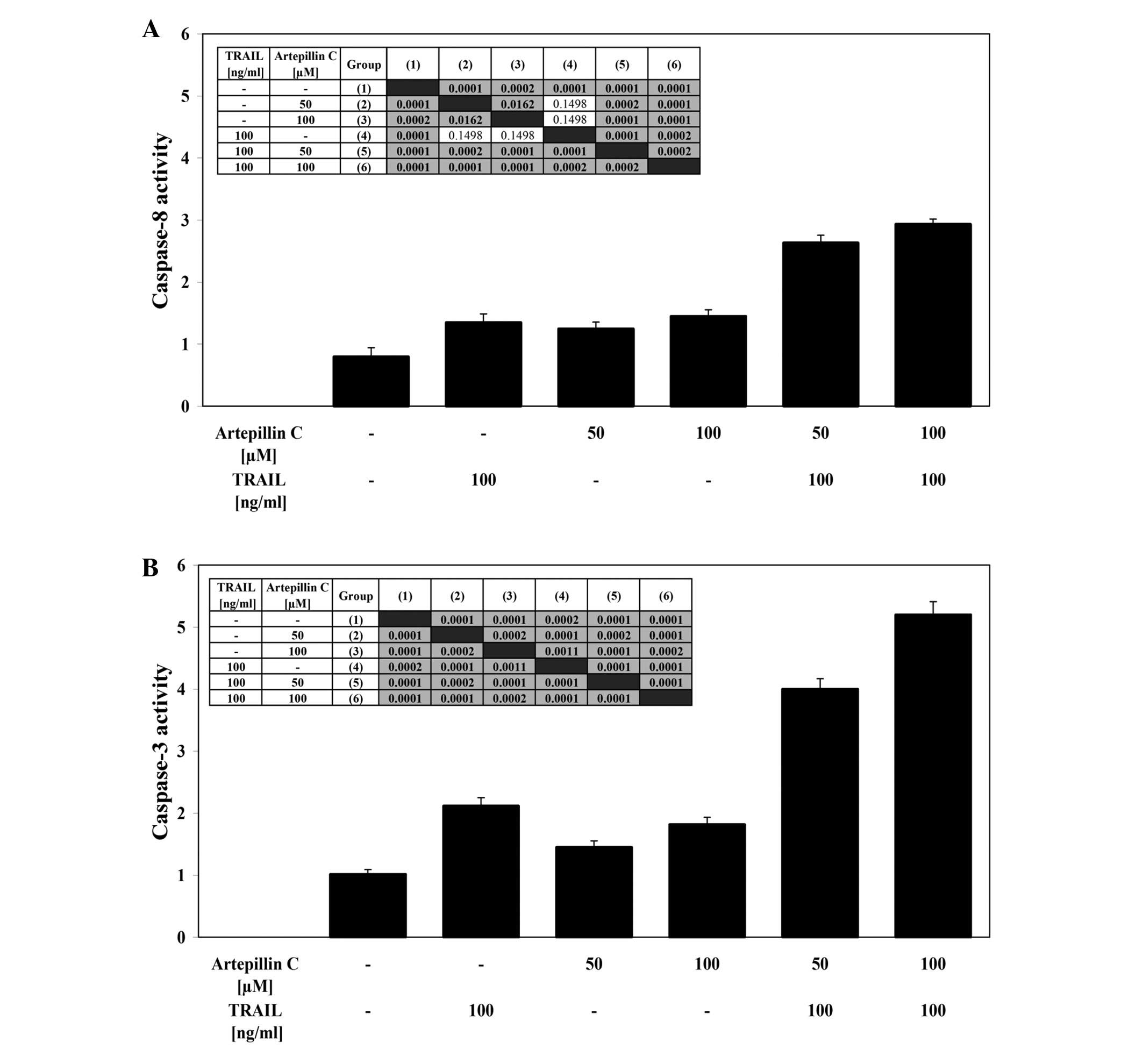
Effects of TRAIL and artepillin C on
caspase-8 and caspase-3 activities in LNCaP cells
LNCaP cells were treated with 100 ng/ml TRAIL and/or
50–100 μM artepillin C for the indicated period of time. The
stimulation of death receptors induces DISC formation, resulting in
the recruitment and activation of caspase-8. TRAIL and artepillin C
alone weakly activated caspase-8 in cancer cells. The simultaneous
incubation of LNCaP cells with TRAIL and artepillin C significantly
increased the caspase-8 activity (Fig.
5A). Caspase-3 is an effector caspase that plays a central role
in apoptosis. Co-treatment of LNCaP cells with TRAIL and artepillin
C markedly enhanced caspase-3 activity compared to treatment with
TRAIL or artepillin C alone (Fig.
5B). The use of the pan-caspase inhibitor, Z-VAD-FMK, the
caspase-8 inhibitor, Z-IETD-FMK, or the caspase-3 inhibitor,
Z-DEVD-FMK, completely blocked the subsequent cell death induced by
TRAIL in combination with artepillin C. These results suggest that
artepillin C promotes TRAIL-mediated apoptosis through a caspase
cascade.
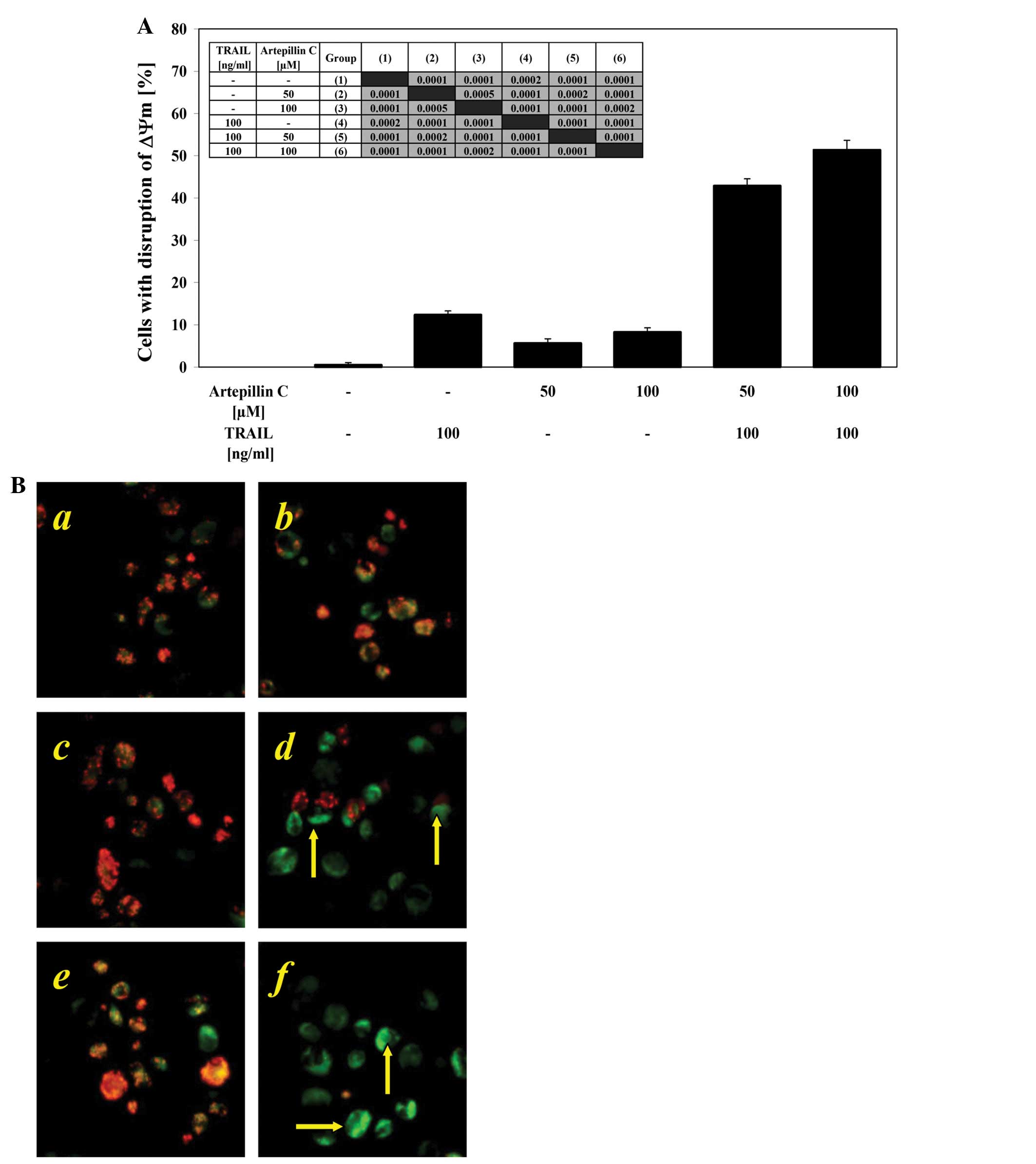
Effects of TRAIL and artepillin C on ΔΨm
in LNCaP cells
Mitochondrial membrane depolarization is one of the
first intracellular changes that occur after the onset of
apoptosis. We evaluated whether artepillin C sensitizes cancer
cells to TRAIL-induced mitochondrial dysfunction. When the LNCaP
cells were treated with 100 ng/ml TRAIL or 50–100 μM
artepillin C alone, there was little effect on ΔΨm (12.4±0.9% and
5.6±1.1–8.3±1.0%, respectively). The combination treatment of TRAIL
with artepillin C enhanced the loss of ΔΨm in a large percentage of
the cells (42.9±1.6–51.4±2.3%) and induced a significant disruption
of the ΔΨm (Fig. 6A and B). These
results demonstrate that the intrinsic apoptotic pathway is engaged
in LNCaP cells treated with both TRAIL and artepillin C.
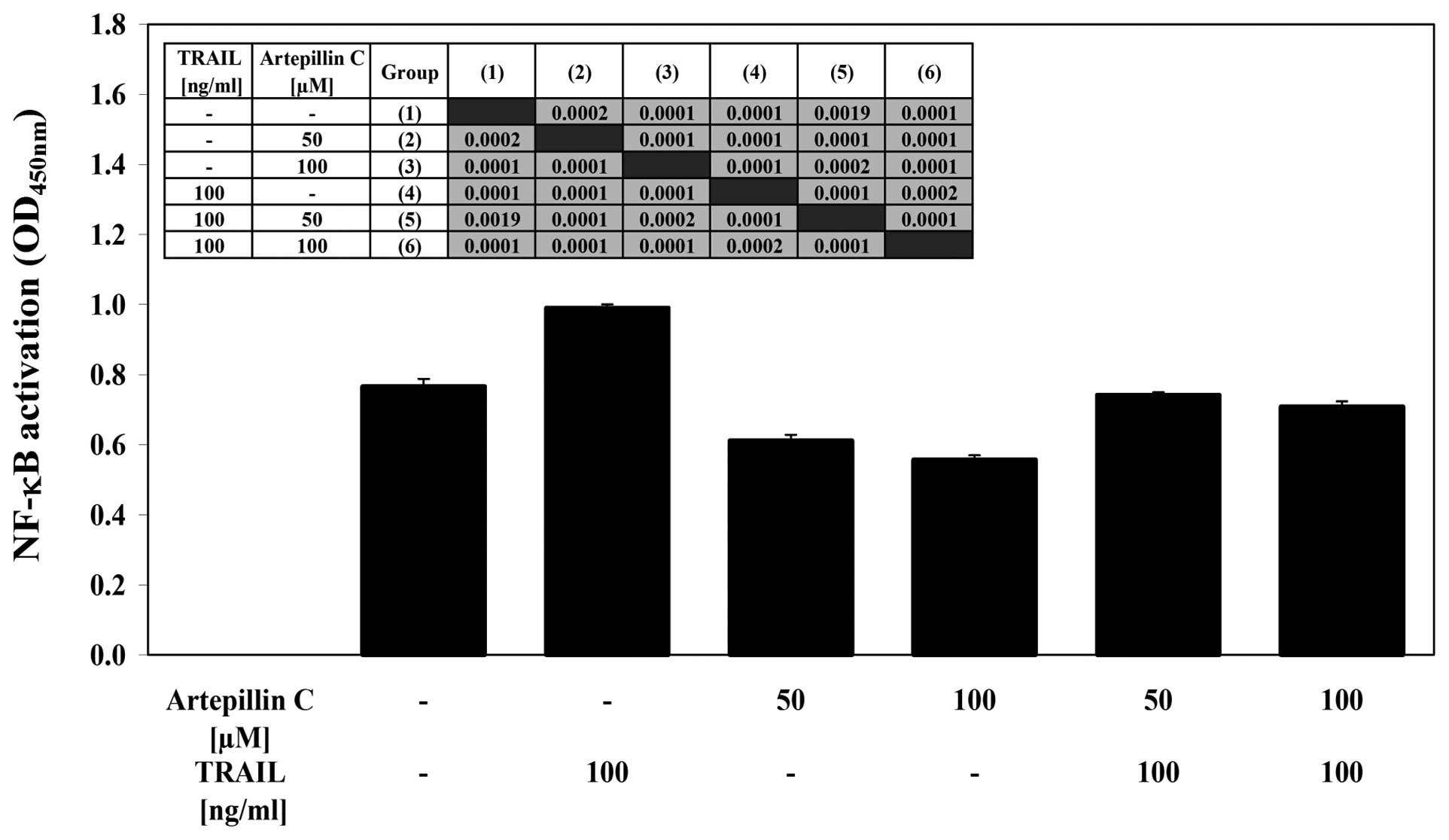
Effects of artepillin C and TRAIL on
NF-κB activity in LNCaP cells
We examined the effect of artepillin C and/or TRAIL
on NF-κB activation in LNCaP cells (Fig. 7). Using the ELISA-based TransAM
NF-κB test, we determined the binding activity of the p65 subunit
in LNCaP nuclear extracts. Treatment with artepillin C decreased
the activity of NF-κB compared with the control cells. By contrast,
TRAIL induced the activation of NF-κB in the LNCaP cells. The
co-treatment of artepillin C with TRAIL also significantly
decreased the NF-κB activity. This shows that artepillin C can help
cells overcome TRAIL resistance in LNCaP cells by blocking the
NF-κB activation induced by TRAIL.
Discussion
Propolis contains various phenolic compounds and
exhibits a broad spectrum of biological activities (16,36,47).
The composition of propolis is complex and largely depends on the
geographical origin and specific flora at the site of its
collection (17,47). Baccharis dracunculiforia is
the main botanical source of resins for the green propolis rich in
artepillin C (21–23). Phenolic ingredients contribute to
the overall cancer preventive and antitumour effects of propolis
(16,36). Therefore, propolis is a promising
raw mixture of natural compounds that should be studied to discover
new pharmaceutical products with chemopreventive properties.
Artepillin C has shown a marked activity against
different tumour cells in vitro, and it affects cancer cells
by inhibiting cell growth and inducing apoptosis (24–26).
Akao et al demonstrated the suppression of tumour cell
growth by artepillin C and two other cinnamic acid derivatives
detected in propolis, drupanin and baccharin (51). In our study, artepillin C exerted
low direct cytotoxic and apoptotic effects on LNCaP cells. Numerous
tests have confirmed that the LNCaP prostate cancer cell line is
resistant to TRAIL-mediated apoptosis (15–20).
Inactivation of the TRAIL pathway and escape from the
TRAIL-mediated immunosurveillance may play important roles in
tumour onset and progression (2).
Previous studies have shown that TRAIL in combination with propolis
extracts or phenolic compounds identified in propolis results in
the synergistic induction of cancer cell death (16,17,47).
We then treated the TRAIL-resistant LNCaP cells with a combination
of TRAIL and artepillin C. The tested compound significantly
augmented TRAIL-induced death in the prostate cancer cells. These
results suggest that artepillin C exhibits mainly indirect
antitumour action by stimulating the TRAIL-mediated apoptotic
pathway.
TRAIL triggers a pro-apoptotic signal by binding to
the death receptors. The inactivation of TRAIL-R2 (also known as
DR5) significantly increases tumour growth in vitro and
in vivo. Thus, the expression of TRAIL-R2 may contribute to
the tumour selective induction of apoptosis mediated by TRAIL
(1–5). We found that the artepillin
C-mediated sensitization of LNCaP cells to TRAIL-induced apoptosis
is associated with the upregulation of TRAIL-R2 expression. Indeed,
other phenolic compounds isolated from propolis can evoke similar
increases in the cell surface expression of TRAIL-R2. Apigenin,
kaempferol and quercetin, all reverse TRAIL resistance in cancer
cells by influencing TRAIL-R2 levels (12,13,52).
Caspases are crucial players in the induction of
apoptosis. TRAIL-mediated apoptosis is primarily executed by the
extrinsic death receptor pathway. This pathway involves caspase-8
as the initiator and caspase-3 as the executor (2,19).
We observed that co-treatment with TRAIL and artepillin C resulted
in the significant activation of caspase-8 and caspase-3 in LNCaP
cells, whereas treatment with TRAIL or artepillin C alone weakly
activated both caspases. Previous studies have reported that TRAIL
induces caspase cleavage when combined with propolis constituents
(apigenin, chrysin, kaempferol and quercetin) in cancer cells
(12,13,52,53).
The present study confirms that the sensitization of prostate
cancer cells to TRAIL by artepillin C is accomplished through an
extrinsic, receptor-and caspase-dependent pathway.
Mitochondrial dysfunction is considered a hallmark
of apoptosis (14,18,19).
Further analysis of the ΔΨm showed that the TRAIL and artepillin C
co-treatment affected the intrinsic pathway in LNCaP cells via a
significant reduction in ΔΨm compared to treatment with TRAIL or
artepillin C alone.
In addition to defects in the extrinsic and
intrinsic apoptotic pathways, the NF-κB survival pathway in tumour
cells may be responsible for the failure to undergo apoptosis
(19,20,54).
The activation of NF-κB in LNCaP cells leads to TRAIL resistance,
while the downregulation of NF-κB by artepillin C sensitizes cancer
cells to TRAIL-mediated death.
To our knowledge, in this study, we show for the
first time the mechanisms by which artepillin C helps cancer cells
to overcome TRAIL resistance. Artepillin C achieves this through
the upregulation of the TRAIL-R2 receptor, activation of caspase-8
and caspase-3, the loss of ΔΨm and the downregulation of NF-κB.
Artepillin C as the main phenolic compound isolated from Brazilian
green propolis sensitizes prostate cancer cells to TRAIL-induced
apoptosis, engaging similar cellular targets in LNCaP cells, such
as those induced by EEP (17). The
evidence confirms that of all the phenolic compounds in Brazilian
green propolis, artepillin C is predominantly responsible for the
action of propolis on TRAIL-mediated apoptosis in cancer cells.
These results define the cancer chemopreventive
action of artepillin C through the modulation of TRAIL-mediated
apoptotic signalling pathways. We hypothesize that artepillin C
supports TRAIL-mediated immune defense against cancer cells and may
therefore represent a prostate cancer immunochemopreventive
agent.
Acknowledgements
This project was supported by a
research grant (KNW-1-017/P/1/0) from the Medical University of
Silesia in Katowice (Poland).
References
|
1
|
Lee JY, Huerta-Yepez S, Vega M, Baritaki
S, Spandidos DA and Bonavida B: The NO TRAIL to YES TRAIL in cancer
therapy (Review). Int J Oncol. 31:685–691. 2007.PubMed/NCBI
|
|
2
|
Voelkel-Johnson C: TRAIL-mediated
signaling in prostate, bladder and renal cancer. Nat Rev Urol.
8:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baritaki S, Katsman A, Chatterjee D, Yeung
KC, Spandidos DA and Bonavida B: Regulation of tumor cell
sensitivity to TRAIL-induced apoptosis by the metastatic suppressor
Raf kinase inhibitor protein via Yin Yang 1 inhibition and death
receptor 5 up-regulation. J Immunol. 179:5441–5453. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baritaki S, Huerta-Yepez S, Sakai T,
Spandidos DA and Bonavida B: Chemotherapeutic drugs sensitize
cancer cells to TRAIL-mediated apoptosis: up-regulation of DR5 and
inhibition of Yin Yang 1. Mol Cancer Ther. 6:1387–1399. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baritaki S, Suzuki E, Umezawa K, Spandidos
DA, Berenson J, Daniels TR, Penichet ML, Jazirehi AR, Palladino M
and Bonavida B: Inhibition of Yin Yang 1-dependent repressor
activity of DR5 transcription and expression by the novel
proteasome inhibitor NPI-0052 contributes to its TRAIL-enhanced
apoptosis in cancer cells. J Immunol. 180:6199–6210. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szliszka E and Krol W: The role of dietary
polyphenols in tumor necrosis factor-related apoptosis inducing
ligand (TRAIL)-induced apoptosis for cancer chemoprevention. Eur J
Cancer Prev. 20:63–69. 2011. View Article : Google Scholar
|
|
7
|
Szliszka E, Bronikowska J, Majcher A,
Miszkiewicz J and Krol W: Enhanced sensitivity of
hormone-refractory prostate cancer cells to tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) mediated
cytotoxicity by taxanes. CEJ Urol. 62:29–34. 2009.
|
|
8
|
Szliszka E, Bronikowska J, Czuba ZP and
Krol W: Isoflavones augment the effect of tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) on prostate cancer
cells. CEJ Urol. 63:182–186. 2010.
|
|
9
|
Szliszka E, Gebka J, Bronikowska J and
Krol W: Dietary flavones enhance the effect of tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) on bladder cancer
cells. CEJ Urol. 63:138–143. 2010.
|
|
10
|
Lee SJ, Noh HJ, Sung EG, Song IH, Kim JY,
Kwon TK and Lee TJ: Berberine sensitizes TRAIL-induced apoptosis
through proteasome-mediated downregulation of c-FLIP and Mcl-1
proteins. Int J Oncol. 38:485–492. 2011.PubMed/NCBI
|
|
11
|
Fujiwara J, Sowa Y, Horinaka M, Koyama M,
Wakada M, Miki T and Sakai T: The anti-obesity drug orlistat
promotes sensitivity to TRAIL by two different pathways in
hormone-refractory prostate cancer cells. Int J Oncol.
40:1483–1491. 2012.PubMed/NCBI
|
|
12
|
Horinaka M, Yoshida T, Shiraishi T, Nakata
S, Wakada M and Sakai T: The dietary flavonoid apigenin sensitizes
malignant tumor cells to tumor necrosis factor-related
apoptosis-inducing ligand. Mol Cancer Ther. 5:945–951. 2006.
View Article : Google Scholar
|
|
13
|
Jung YH, Heo J, Lee YJ, Kwon TK and Kim
YH: Quercetin enhances TRAIL-mediated apoptosis in prostate cancer
cells via increased protein stability of death receptor 5. Life
Sci. 86:351–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szliszka E, Czuba ZP, Mazur B, Paradysz A
and Krol W: Chalcones and dihydrochalcones augment TRAIL-mediated
apoptosis in prostate cancer cells. Molecules. 15:5336–5353. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Szliszka E, Czuba ZP, Sedek L, Paradysz A
and Krol W: Enhanced TRAIL-mediated apoptosis in prostate cancer
cells by the bioactive compounds neobavaisoflavone and psoralidin
isolated from Psoralea corylifolia. Pharmacol Rep.
63:139–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szliszka E, Czuba ZP, Bronikowska J,
Mertas A, Paradysz A and Krol W: Ethanolic extract of propolis
(EEP) augments TRAIL-induced apoptotic death in prostate cancer
cells. Evid Based Complement Alternat Med. 2011:5351722011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szliszka E, Zydowicz G, Janoszka B, Dobosz
C, Kowalczyk-Ziomek G and Krol W: Ethanolic extract of Brazilian
green propolis sensitizes prostate cancer cells to TRAIL-induced
apoptosis. Int J Oncol. 38:941–953. 2011.PubMed/NCBI
|
|
18
|
Szliszka E and Krol W: Soy isoflavones
augment the effect of TRAIL-mediated apoptotic death in prostate
cancer cells. Oncol Rep. 26:533–541. 2011.PubMed/NCBI
|
|
19
|
Szliszka E, Helewski KJ, Mizgala E and
Krol W: The dietary flavonol fisetin enhances the
apoptosis-inducing potential of TRAIL in prostate cancer cells. Int
J Oncol. 39:771–779. 2011.PubMed/NCBI
|
|
20
|
Szliszka E, Czuba ZP, Mertas A, Paradysz A
and Krol W: The dietary isoflavone biochanin-A sensitizes prostate
cancer cells to TRAIL-induced apoptosis. Urol Oncol. http://dx.doi.org/10.1016/j.urolonc.2011.01.019.
2011, PubMed/NCBI
|
|
21
|
Park YK, Paredes-Guzman JF, Aguiar CL,
Alencar SM and Fujiwara FY: Chemical constituents in Baccharis
dracunculifolia as the main botanical origin of southeastern
Brazilian propolis. J Agric Food Chem. 52:1100–1113. 2004.
|
|
22
|
Matsuda AH and de Almeida-Muradian LB:
Validated method for the quantification of artepillin C in
Brazilian propolis. Phytochem Anal. 19:179–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Sousa JP, Leite MF, Jorge RF, Resende
DO, da Silva Filho AA, Furtado NA, Soares AE, Spadaro AC, de
Magalhaes PM and Bastos JK: Seasonality role on the phenolics from
cultivated Baccharis dracunculifolia. Evid Based Complement
Alternat Med. 2011:4642892011.PubMed/NCBI
|
|
24
|
Matsuno T, Jung SK, Matsumoto Y, Saito M
and Morikawa J: Preferential cytotoxicity to tumor cells of
3,5-diprenyl-4-hydroxycinnamic acid (artepillin C) isolated from
propolis. Anticancer Res. 17:3565–3568. 1997.PubMed/NCBI
|
|
25
|
Kimoto T, Arai S, Kohguchi M, Aga M,
Nomura Y, Micallef MJ, Kurimoto M and Mito K: Apoptosis and
suppression of tumor growth by artepillin C extracted from
Brazilian propolis. Cancer Detect Prev. 22:506–515. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kimoto T, Aga M, Hino K, Koya-Miyata S,
Yamamoto Y, Micallef MJ, Hanaya T, Arai S, Ikeda M and Kurimoto M:
Apoptosis of human leukemia cells induced by artepillin C, an
active ingredient of Brazilian propolis. Anticancer Res.
21:221–228. 2001.PubMed/NCBI
|
|
27
|
Ahn MR, Kunimasa K, Ohta T, Kumazawa S,
Kamihira M, Kaji K, Uto Y, Hori H, Nagasawa H and Nakayama T:
Suppression of tumor-induced angiogenesis by Brazilian propolis:
major component artepillin C inhibits in vitro tube formation and
endothelial cell proliferation. Cancer Lett. 252:235–243. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paulino N, Abreu SR, Uto Y, Koyama D,
Nagasawa H, Hori H, Dirsch VM, Vollmar AM, Scremin A and Bretz WA:
Anti-inflammatory effects of a bioavailable compound, Artepillin C,
in Brazilian propolis. Eur J Pharmacol. 587:296–301. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahn MR, Kunimasa K, Kumazawa S, Nakayama
T, Kaji K, Uto Y, Hori H, Nagasawa H and Ohta T: Correlation
between antiangiogenic activity and antioxidant activity of various
components from propolis. Mol Nutr Food Res. 53:643–651. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Izuta H, Narahara Y, Shimazawa M, Mishima
S, Kondo S and Hara H: 1,1-diphenyl-2-picrylhydrazyl radical
scavenging activity of bee products and their constituents
determined by ESR. Biol Pharm Bull. 32:1947–1951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Messerli SM, Ahn MR, Kunimasa K,
Yanagihara M, Tatefuji T, Hashimoto K, Mautner V, Uto Y, Hori H,
Kumazawa S, Kaji K, Ohta T and Maruta H: Artepillin C (ARC) in
Brazilian green propolis selectively blocks oncogenic PAK1
signaling and suppresses the growth of NF tumors in mice. Phytother
Res. 23:423–427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simoes-Ambrosio LM, Gregorio LE, Sousa JP,
Figueiredo-Rinhel AS, Azzolini AE, Bastos JK and Lucisano-Valim YM:
The role of seasonality on the inhibitory effect of Brazilian green
propolis on the oxidative metabolism of neutrophils. Fitoterapia.
81:1102–1108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Azevedo Bentes Monteiro Neto M, de
Souza Lima IM, Furtado RA, Bastos JK, da Silva Filho AA and Tavares
DC: Antigenotoxicity of artepillin C in vivo evaluated by the
micronucleus and comet assays. J Appl Toxicol. 31:714–719.
2011.PubMed/NCBI
|
|
34
|
Fonseca YM, Marquele-Oliveira F, Vicentini
FT, Furtado NA, Sousa JP, Lucisano-Valim YM and Fonseca MJ:
Evaluation of the potential of Brazilian propolis against
UV-induced oxidative stress. Evid Based Complement Alternat Med.
2011:8639172010.PubMed/NCBI
|
|
35
|
Moura SA, Negri G, Salatino A, Lima LD,
Dourado LP, Mendes JB, Andrade SP, Ferreira MA and Cara DC: Aqueous
extract of Brazilian green propolis: primary components, evaluation
of inflammation and wound healing by using subcutaneous implanted
sponges. Evid Based Complement Alternat Med. 2011:7482832011.
View Article : Google Scholar
|
|
36
|
Watanabe MA, Amarante MK, Conti BJ and
Sforcin JM: Cytotoxic constituents of propolis inducing anticancer
effects: a review. J Pharm Pharmacol. 63:1378–1386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Androutsopoulos VP, Ruparelia K, Arroo RR,
Tsatsakis AM and Spandidos DA: CYP1-mediated antiproliferative
activity of dietary flavonoids in MDA-MB-468 breast cancer cells.
Toxicology. 264:162–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Androutsopoulos VP, Papakyriakou A,
Vourloumis D, Tsatsakis AM and Spandidos DA: Dietary flavonoids in
cancer therapy and prevention: substrates and inhibitors of
cytochrome P450 CYP1 enzymes. Pharmacol Ther. 126:9–20. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Androutsopoulos VP, Papakyriakou A,
Vourloumis D and Spandidos DA: Comparative CYP1A1 and CYP1B1
substrate and inhibitor profile of dietary flavonoids. Bioorg Med
Chem. 19:2842–2849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Davalli P, Rizzi F, Caldara GF, Davoli S,
Corti A, Silva A, Astancolle S, Vitale M, Bettuzzi S, Arcari M and
Azzali G: Chronic administration of green tea extract to TRAMP mice
induces the collapse of Golgi apparatus in prostate secretory cells
and results in alterations of protein post-translational
processing. Int J Oncol. 39:1521–1527. 2011.PubMed/NCBI
|
|
41
|
Onoda C, Kuribayashi K, Nirasawa S, Tsuji
N, Tanaka M, Kobayashi D and Watanabe N:
(−)-Epigallocatechin-3-gallate induces apoptosis in gastric cancer
cell lines by down-regulating survivin expression. Int J Oncol.
38:1403–1408. 2011.
|
|
42
|
Teiten MH, Gaascht F, Cronauer M, Henry E,
Dicato M and Diederich M: Anti-proliferative potential of curcumin
in androgen-dependent prostate cancer cells occurs through
modulation of the Wingless signaling pathway. Int J Oncol.
38:603–611. 2011.
|
|
43
|
Miki H, Uehara N, Kimura A, Sasaki T, Yuri
T, Yoshizawa K and Tsubura A: Resveratrol induces apoptosis via
ROS-triggered autophagy in human colon cancer cells. Int J Oncol.
40:1020–1028. 2012.PubMed/NCBI
|
|
44
|
Marech I, Vacca A, Ranieri G, Gnoni A and
Dammacco F: Novel strategies in the treatment of
castration-resistant prostate cancer (Review). Int J Oncol.
40:1313–1320. 2012.PubMed/NCBI
|
|
45
|
Venkateswaran V and Klotz LH: Diet and
prostate cancer: mechanisms of action and implications for
chemoprevention. Nat Rev Urol. 7:442–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bronikowska J, Szliszka E, Czuba ZP,
Zwolinski D, Szmydki B and Krol W: The combination of TRAIL and
isoflavones enhances apoptosis in cancer cells. Molecules.
15:2000–2015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Szliszka E, Czuba ZP, Domino M, Mazur B,
Zydowicz G and Krol W: Ethanolic extract of propolis (EEP) enhances
the apoptosis-inducing potential of TRAIL in cancer cells.
Molecules. 14:738–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Szliszka E, Skaba D, Czuba ZP and Krol W:
Inhibition of inflammatory mediators by neobavaisoflavone in
activated RAW264.7 macrophages. Molecules. 16:3701–3712. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Szliszka E, Czuba ZP, Kawczyk-Krupka A,
Sieron-Stoltny A, Sieron A and Krol W: Chlorin-based photodynamic
therapy enhances the effect of tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) in bladder cancer cells. Med Sci
Monit. 18:BR47–BR53. 2012. View Article : Google Scholar
|
|
50
|
Szliszka E, Mazur B, Zydowicz G, Czuba ZP
and Krol W: TRAIL-induced apoptosis and expression of death
receptor TRAIL-R1 and TRAIL-R2 in bladder cancer cells. Folia
Histochem Cytobiol. 47:579–585. 2009.PubMed/NCBI
|
|
51
|
Akao Y, Maruyama H, Matsumoto K, Ohguchi
K, Nishizawa K, Sakamoto T, Araki Y, Mishima S and Nozawa Y: Cell
growth inhibitory effect of cinnamic acid derivatives from propolis
on human tumor cell lines. Biol Pharm Bull. 26:1057–1059. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yoshida T, Konishi M, Horinaka M, Yasuda
T, Goda AE, Taniguchi H, Yano K, Wakada M and Sakai T: Kaempferol
sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem
Biophys Res Commun. 375:129–133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li X, Wang JN, Huang JM, Xiong XK, Chen
MF, Ong CN, Shen HM and Yang XF: Chrysin promotes tumor necrosis
factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced
apoptosis in human cancer cell lines. Toxicol In Vitro. 25:630–635.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Baritaki S, Militello L, Malaponte G,
Spandidos DA, Salcedo M and Bonavida B: The anti-CD20 mAb LFB-R603
interrupts the dysregulated NF-κB/Snail/RKIP/PTEN resistance loop
in B-NHL cells: Role in sensitization to TRAIL apoptosis. Int J
Oncol. 38:1683–1694. 2011.PubMed/NCBI
|