Introduction
Pulmonary sarcomatoid carcinomas (PSCs) are rare
tumours, accounting for <1% of all lung cancers. PSCs include
spindle cell carcinoma, giant cell carcinoma, pleomorphic
carcinoma, carcinosarcoma and pulmonary blastoma, with pleomorphic
carcinoma being the most frequent subtype. No major changes in the
terminology or diagnostic criteria have been made since the 2004
World Health Organization (WHO) classification (1). These tumours have a strong
association with tobacco exposure. The clinical behaviour of PSCs
is extremely aggressive, and patient prognosis is extremely poor
(2,3), since these tumours have a high rate
of distant metastasis. The effect of conventional chemotherapy for
PSCs remains controversial due to their low incidence (4); thus, progress on tumour treatments is
moderate.
PSCs are typically biphasic neoplasms, including
both epithelial and fusiform components (5). The fusiform components of PSCs may
originate from the epithelial-mesenchymal transition (EMT) of
epithelial cancer cells (6,7). EMT
plays a pivotal role in cancer aggressiveness, metastasis and
resistance to therapy (7-10). Manzotti et al (5) provided a formal validation of EMT in
the development of PSCs and functional insights into the mechanisms
through which EMT occurs during PSC evolution. Previous studies
(11,12) have demonstrated that non-small cell
lung cancer (NSCLC) cell lines treated with transforming growth
factor ß1 (TGFß1) underwent significant EMT-related morphological
changes. The cells exhibited mesenchymal features, including
morphology and increased Vimentin and reduced E-Cadherin protein
expression following treatment with TGFß1. TGF ß1 induces EMT to
promote lung cancer cell proliferation, invasion and metastasis
(11,12).
Vasculogenic mimicry (VM) represents a specific
tumour blood supply pattern (13).
VM provides an advantage for rapidly growing tumours that require a
blood supply. Previous studies have demonstrated that VM occurs in
highly invasive tumours and is associated with a high histological
grade, invasion, metastasis and a short survival in patients with
malignant tumours (14,15). Previous studies by the authors of
this study and others (16-19)
have demonstrated that VM channel formation in highly aggressive
human tumour cells has a close association with the EMT process.
Therefore, both EMT and VM are synonymous with tumour plasticity,
the transdifferentiation of epithelial cells to a mesenchymal
phenotype, tumour aggressiveness and metastasis.
EMT transcription factors contribute to the
development of resistance against cancer therapy, and they may be
targeted as novel therapeutic approaches for the treatment of
cancer (9). Twist1, a
transcription factor of the basic helix-loop-helix class, was
originally reported as a master regulator of embryonic
morphogenesis. Twist1 is known to induce EMT in a variety of
tumours (20-22). Previous studies by the authors have
revealed that Twist1-induced EMT enhances the invasive, metastatic
and VM formation abilities of hepatocellular carcinoma cells
(23,24). Yochum et al (25) demonstrated that Twist1 functions to
suppress oncogene-induced senescence and apoptosis in multiple
oncogene-driver dependent settings, including tumours with EGFR
mutations. The genetic or pharmacologic inhibition of Twist1
induces EGFR-mutant NSCLC cell growth inhibition and apoptosis, and
it contributes to restoring tumour cell sensitivity to the
chemotherapeutic agent, erlotinib.
In this study, the EMT phenotype, EMT transcription
factor expression and VM were examined in 4l PSC and 79 pulmonary
squamous carcinoma (PSCC) samples. The association of Twist1
expression with clinicopathologic parameters was explored. The
prognostic role of Twist1 in PSCs was evaluated using Cox
regression and Kaplan-Meier analysis. Furthermore, PSCC cells were
treated with TGFpl in vitro to mimic PSC cells and to
demonstrate the biological behaviour of PSCs and the function of
Twist1 in PSCs.
Materials and methods
Patient samples
Human lung cancer tissue collection and analysis in
this study were consented to by the patients and were approved by
the Ethical Committee of Tianjin Medical University. Specimens from
4l cases of PSC and 79 cases of PSCC that were fixed with formalin
and paraffin-embedded from l995 to 20l0 were selected. The
specimens of patients who had not undergone chemotherapy or
radiotherapy prior to surgery were exclusively employed. The
pathological diagnosis was reviewed by two senior pathologists
based on haematoxylin and eosin-stained sections according to the
2015 WHO classification of lung tumours. All the
clinicopathological parameters, including sex, age, metastasis
status, histological grade, tumour size and TNM stage, were
obtained from the records.
All of the patients were followed-up by a clinical
interview or phone call. The overall survival (OS) time was
calculated as the duration from the date of surgery to the date of
death.
Immunohistochemical and histochemical
double-staining methods
Tissue sections (4-5-μm-thick) were
deparaffinized and hydrated utilizing standard procedures.
Immunostaining was performed using a super-sensitivity S-P IHC kit.
Following immersion in 3% H2O2 for l0 min to
eliminate endogenous peroxidase, the sections were microwaved for
antigen retrieval in Tris/EDTA pH 9.0 or sodium citrate pH 6.0 for
l5 min. After blocking in l0% goat serum for 30 min and incubation
with primary antibodies (Twist1: l:l00, sc-l5393, Santa Cruz
Biotechnology; Slug: l:l50, LS-Cl75l6l, LifeSpan Biosciences;
Snail: 1:100, NBPl-19529, Novus Biologicals; p63: ready to use,
ZM-0406, Zhongshan Goldenbridge Biotechnology; CK5/6: ready to use,
ZM-0313, Zhongshan Goldenbridge Biotechnology; CD31: ready to use,
ZA-0568, Zhongshan Goldenbridge Biotechnology; Vimentin: 1:400,
2707-1, Epitomics; EPH receptor A2 (EphA2): 1:100, sc-924, Santa
Cruz Biotechnology; VE-cadherin: 1:100, ab33168, Abcam; MMP2:
1:100, ab37150, Abcam; E-cadherin: 1:200, sc-8426, Santa Cruz
Biotechnology) in commercialized antibody diluent at 4°C overnight,
the tissue sections were incubated with appropriate secondary
antibodies (ready to use, PV-6001 and PV-6002, Zhongshan
Goldenbridge Biotechnology) for 1 h at room temperature, and
positive signals were developed in 3,3-diaminobenzidine
tetrahydrochloride (DAB) solution. After counterstaining with
haematoxylin for 5 min or Periodic acid-Schiff (PAS) (Zhongshan
Goldenbridge Biotechnology) at room temperature, the slides were
ready for microscopic examination. All sections with PAS staining
were oxidized in 0.5% periodic acid solution for 5 min, rinsed in
distilled water, placed in Schiff reagent for 15 min and washed in
tap water for 5 min.
VM channel quantification was assessed by light
microscopy (CX23, Olympus) analysis of the tumour areas. A total of
50 non-overlapping high-power fields (×400 magnification) were
randomly selected per case. VM quantification was scored as
follows: 0 (no VM channels were found), 1 [1 VM channel/50
high-power field (HPF)], 2 (2-4 VM channels/50 HPF), 3 (5-7 VM
channels/50 HPF) and 4 (>8 VM channels/50 HPF). The cases with a
score >1 were considered VM-positive.
Staining was defined as positive for significant
nuclear and cytoplasmic (Twist1, Slug Snail and p63), cytoplasmic
[CK5/6, Vimentin, CD31, EphA2, VE-cadherin and matrix
metalloproteinase (MMP)2] or membranous (E-cadherin)
immunoreactivity in neoplastic cells. Protein expression levels
were quantified according to a previous standard (26) with minor modifications. The
percentage of stained cells ('P') was scored as follows: 0
(staining of <5% of cells), l (5-10% of cells), 2 (10-30%), 3
(30-50%) and 4 (>50%). Staining intensity ('I') was graded as
follows: 0 (no staining), 1 (weak staining), 2 (moderate staining)
and 3 (intense staining). Samples were evaluated for both factors,
i.e., 'P' multiplied by 'I'. Ten high-power fields were randomly
selected per case. The scoring of each case was a mean value of
selected high-power fields. Cases with a score >3 were
considered positive.
Western blot analysis
Cells (please see below) were lysed by using lysis
buffer for western blotting (P00l3, Beyotime) and loaded onto 10%
sodium dodecyl sulphate-polyacrylamide gels and were then
transferred onto polyvinylidene difluoride membranes (Millipore).
The quantification of total protein was performed using a Pierce
BCA Protein Assay kit (Thermo Fisher Scientific), and equal amounts
of protein (30 μg) were used for analysis. Blots were
blocked with 5% milk/TBST and incubated with primary antibodies
(Twist1: 1:500, sc-15393, Santa Cruz Biotechnology; Slug: 1:1,000,
6591, Cell Signaling Technology; Snail: 1:1,000, ab53519, Abcam;
Vimentin: 1:1,000, 2707-1, Epitomis; EphA2: 1:500, sc-924, Santa
Cruz Biotechnology; VE-cadherin: 1:500, ab33168, Abcam; MMP2:
1:500, ab37150, Abcam; E-cadherin: 1:200, SC-8426, Santa Cruz
Biotechnology; p-smad2/3: 1:500, sc-11769, Santa Cruz
Biotechnology) at 4°C overnight, followed by incubation with
secondary antibodies (goat anti-mouse IgG-HRP and goat anti-rabbit
IgG-HRP, 1:2,000; sc-2005 and sc-2030; Santa Cruz Biotechnology)
for 2 h at room temperature. Blots were developed using an enhanced
chemiluminescence detection kit (Amersham Pharmacia Biotech). For
protein loading analyses, p-actin antibody (1:1,000, P30002,
Abmart) or GAPDH (1:2,000, ab9485, Abcam) was used.
Immunofluorescence
Cells cultured on glass slides were washed with PBS
after discarding the medium, and they were then fixed with cold
methanol at -20°C for 15 min. The cells were permeabilized with
0.1% Triton X-100 in PBS for 20 min and blocked with 5% FBS in PBS
at room temperature for 30 min. The cells were then incubated with
primary antibodies (E-cadherin: 1:100, sc-8426, Santa Cruz
Biotechnology; Vimentin: 1:100, 2707-1, Epitomics) for 1 h at 37°C.
The cells were then incubated for 1 h with secondary antibodies
conjugated to Alexa 488 (A32723, Invitrogen; Thermo Fisher
Scientific) or Alexa 568 (A-11011, Invitrogen; Thermo Fisher
Scientific), and then they were washed with PBS. Nuclear staining
with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma) for 10 min at
room temperature was then performed. Slides were viewed under a
fluorescence microscope (Nikon).
Cell culture, treatment and plasmid
transfection
The SK-MES-1 cells (American Type Culture
Collection) were cultured in minimum essential medium (MEM)
supplemented with 10% foetal bovine serum (FBS) (HyClone). The
H1299 and H460 cells (Cell Resource Center, Institute of Basic
Medical Sciences, Peking Union Medical College) were cultured in
RPMI-1640 with 10% FBS. The cells were incubated in media with 10
ng/ml human recombinant TGFβ1 (R&D Systems) in a humidified 5%
CO2 incubator at 37°C for 15 days to induce EMT.
The pGP-Twist1-shRNA plasmid was purchased from
GenePharma. The target sequence [AAGCTGAGCAAG ATTCAGACC (siTwist1
nucleotides 505-525)] was used to downregulate Twist1. A
non-silencing siRNA sequence (target sequence 5′-A ATT CTCCGA ACGT
GT CACGT-3′), was used as a negative control. Plasmid vectors were
transfected into the cells with polyethylenimine (PEI) (Cat. no.
23966, PolyScience, Inc.).
Wound-healing assay
The cells were seeded in 6-well plates. When the
cells reached 100% confluency, a wound was created using a
100-μl sterile pipette tip. The wound was then photographed
by using inverted phase contrast microscope (TS2, Nikon) (0 h). The
rate of gap closure was measured at 24 h. To prevent apoptosis and
cell detachment, 1% FBS medium was used for 24 h. The presence of
serum in the culture medium may permit cell proliferation,
influencing the results of the assay; however, the low percentage
of FBS should sufficiently inhibit proliferation such that the gap
closure was mainly due to cell migration. Each experiment was
performed in triplicate.
Cell migration assay and invasion
assay
Transwell inserts were used in 24-well plates, and
cells (1×105) were added to the upper chamber with
serum-free medium; and MEM with 10% FBS was added to the bottom
chamber. Following 24 h of incubation at 37°C and 5%
CO2, cells that remained on the upper side of the insert
were removed with a cotton swab. The migratory and invasive cells
were fixed with methanol and stained with crystal violet (Sigma)
for 20 min at room temperature. Invasion assays were performed as
with the migration assays, with the exception that the Transwell
chambers were coated with Matrigel before the cells were seeded in
the upper chamber. These cells were counted using an inverted light
microscope (Nikon). Each experiment was performed in
triplicate.
3D Matrigel culture
Tumour cells were mixed with Matrigel (BD
Biosciences) and were seeded to allow for polymerization. The
addition of regular medium was performed during the incubation at
37°C, 5% CO2 for 1 week. Cells were incubated until
tubular structures were formed and were photographed using a phase
contrast microscope.
Statistical analysis
All data in this study were evaluated using SPSS17.0
software (SPSS, Inc.). The correlation between E-cadherin and
Vimentin expression and VM formation was analysed using the
Spearman's rank test. E-cadherin, Vimentin, VM, EphA2, VE-cadherin,
MMP2, Twist1, Slug and Snail immunohistochemical staining score
data were transformed into categorical data by setting up a score
of >3 as positive and a score of <3 as negative. Therefore,
the comparison of the number of patients with positive or negative
expression of these factors between PSCs and PSCCs [i.e., the
comparison of positive rate (the number of patients with positive
expression divided by the total number of patients with PSC or
PSCC)] was performed using Chi-square test. Twist1, Slug and Snail
expression in groups with different clinicopathologic parameters
was analysed using the Chi-square test. Survival curves were
estimated using the Kaplan-Meier method and were compared by the
log rank test. Multivariate analysis of prognostic factors was
tested for using Cox regression analysis. The data analysis of
wound closure, invasive and migratory cell numbers was performed an
using independent-samples t-test. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
PSCs display an EMTphenotype and VM
formation
In this study, 41 PSC and 79 PSCC specimens were
collected. Positive CK5/6 or p63 staining was found in all 79 PSCCs
(data not shown). For the 41 sections of PSCs, there were 30
pleomorphic carcinomas, 7 carcinosarcomas, and 4 spindle cell
carcinomas. The 30 pleomorphic carcinomas were composed of squamous
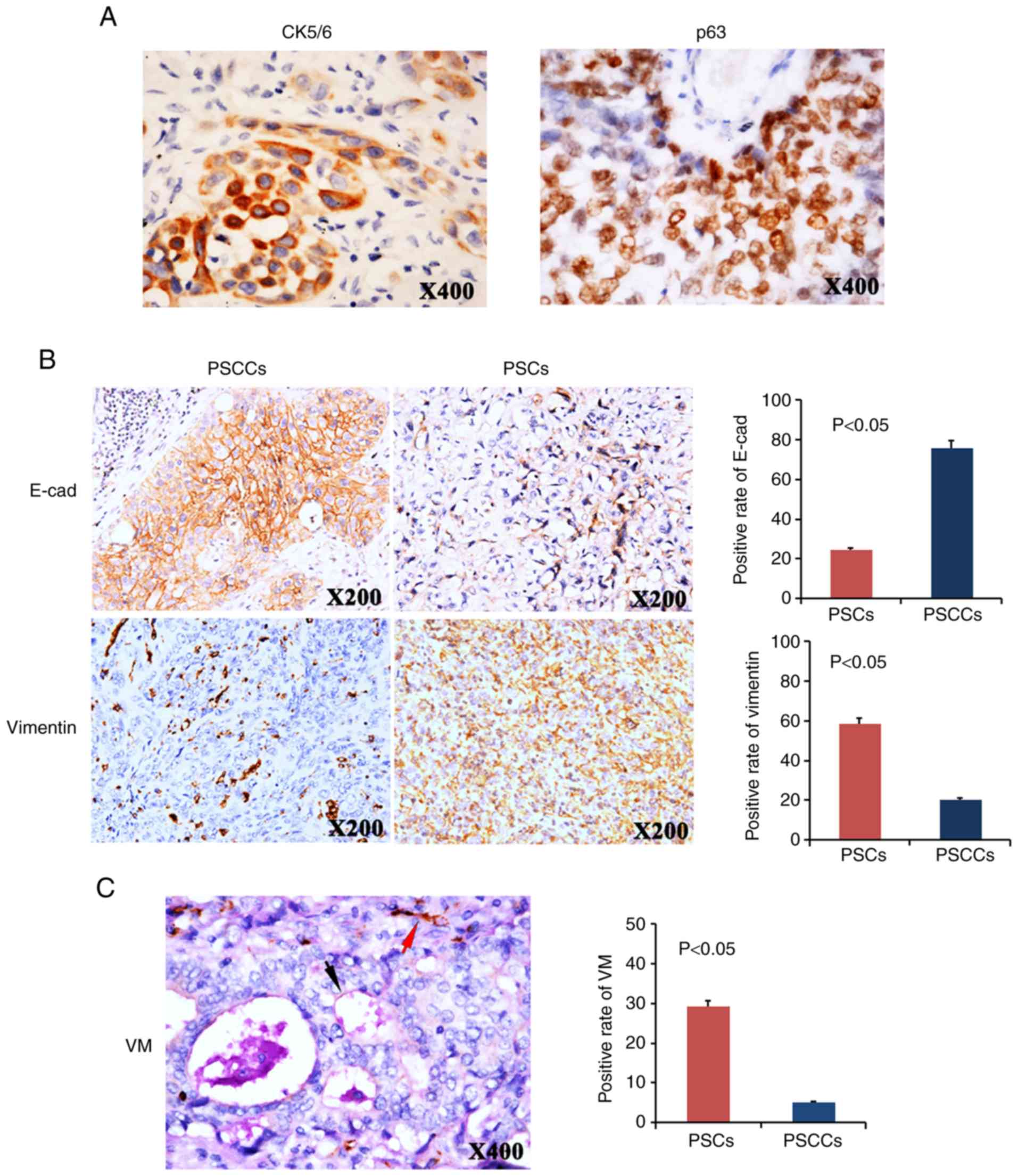
cell carcinoma with positive CK5/6 or p63 expression (Fig. 1A), and they contained at least 10%
spindle and/or giant cells (data not shown). This finding suggested
that these pleomorphic carcinomas may originate from monoclonal
malignant transformed squamous epithelium that they partially
differentiate and exhibit mesenchymal characteristics. In the 7
carcinosarcomas, positive CK5/6 or p63 expression was found in 5
cases, and CK8/18 was found in 2 cases. Positive panCK staining was
found in 4 spindle cell carcinomas, which demonstrated their
epithelial origin (data not shown).
Compared with the PSCCs, the PSCs exhibited an EMT
phenotype. E-cadherin expression was higher in the PSCC group
(positive rate, 75.9%) than it was in the PSC group (positive rate,
24.4%). By contrast, Vimentin expression was higher in the PSC
group (58.5%) than it was in the PSCC group (20.3%) (Fig. 1B).
Since the EMT phenotype represents tumour cell
plasticity, which can contribute to VM formation, the VM channels
were analysed in the PSCs and PSCCs. Using CD31/PAS double-staining
(Fig. 1C), the tubular channels
lined with tumour cells were considered VM formation. These tumour
cells were negative for CD31 staining, demonstrating that they were
not endothelial cells. The membrane-like matrix around the VM
structure was positive for PAS. Necrosis and infiltration of
inflammatory cells in the periphery of the channels were not
observed, and red blood cells were found inside the channels. The
positive VM rate differed markedly between the PSCs and PSCCs. VM
was found in 12 out of 41 PSC samples (29.3%) and in 4 out of 79
PSCC samples (5.1%, P<0.05, Fig.
1C).
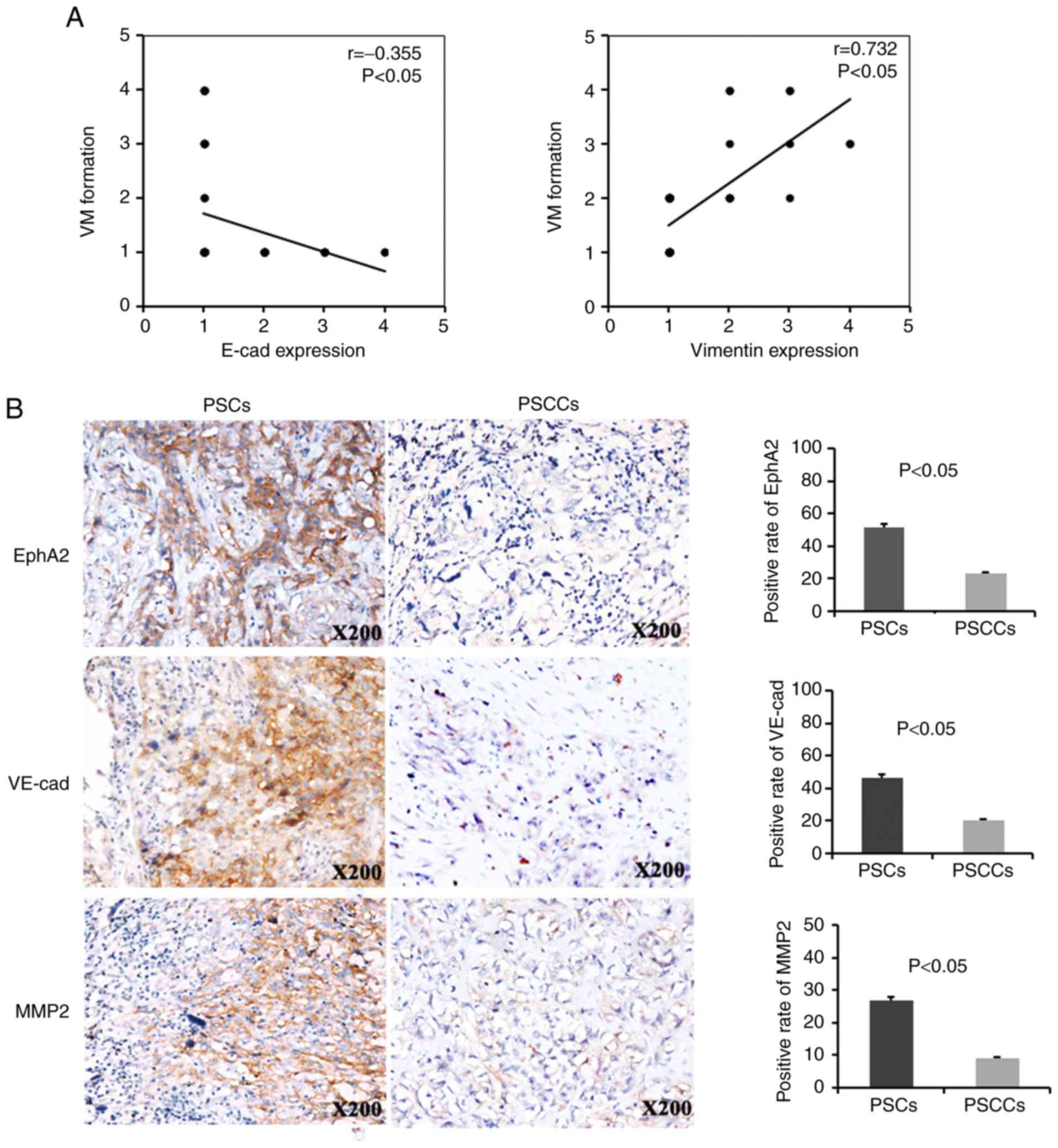
In the PSCs, VM formation exhibited a negative
correlation with E-cadherin expression and a positive correlation
with Vimentin expression (r=−0.355, P<0.05; r=0.732, P<0.05,
respectively) (Fig. 2A). Of the 41
PSCs analysed, 21 (21/41, 51.2%), 19 (19/41, 46.3%) and 11 (11/41,
26.8%) were positive for EphA2, VE-cadherin and MMP2, respectively.
Of the 79 PSCCs analysed, 18 (18/79, 22.8%), 16 (16/79, 20.3%) and
7 (7/79, 8.9%) were positive for EphA2, VE-cadherin and MMP2,
respectively. Therefore, the expression of the VM markers, EphA2,
VE-cadherin and MMP2, was higher in the PSCs compared with the
PSCCs (Fig. 2B).
Twist1 expression indicates a poor
survival of patients with PSC
In previous studies, the EMT regulators Twist1, Slug
and Snail were found to play a role in EMT and VM in hepatocellular
carcinoma and breast cancer (19,24,27).
Therefore, this study examined Twist1, Slug and Snail expression in
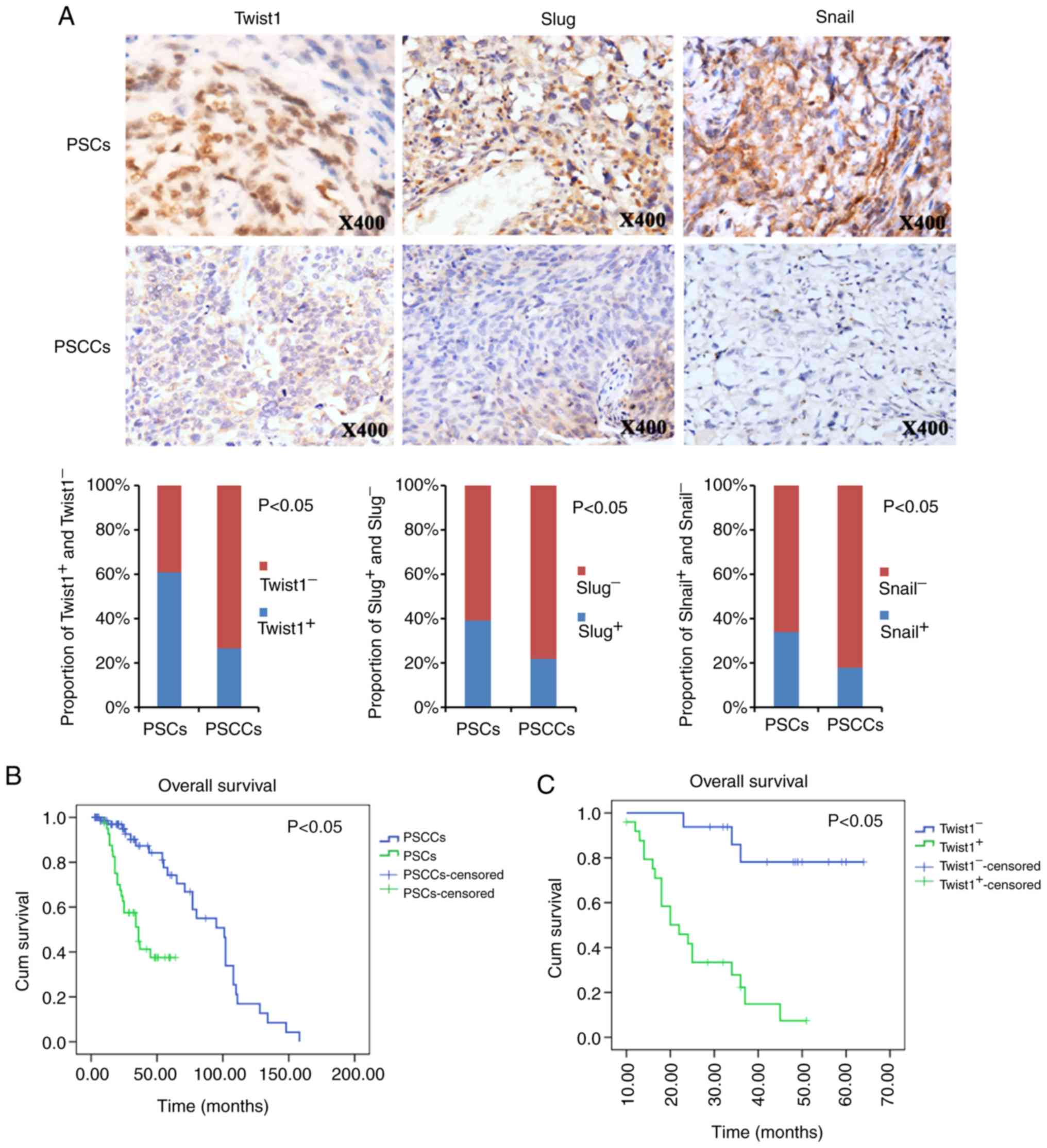
PSCs and PSCCs (Fig. 3A).
Correspondingly, Twist1, Slug and Snail exhibited a significantly
increased expression in the PSCs (61.0, 39.0 and 34.1%,
respectively) over what was observed in the PSCCs (26.6, 21.5 and
17.7%, respectively) (Fig.
3A).
The association of Twist1, Slug and Snail expression
with the patient clinicopathological variables was analysed in the
PSCs and PSCCs. A positive Twist1 expression, but not that of Slug
or Snail, was not only associated with metastasis (including lymph
node metastasis, haematogenous metastasis and pleural implantation
metastasis) (P<0.05), but also with TNM stage (P<0.05) in the
PSCs (Table I). In the PSCCs,
Snail expression, but not that of Twist1 or Slug, was associated
with the histological grade (P<0.05) (Table II).
 | Table ITwist1, Slug and Snail expression in
patients with PSC with different clinicopathologic parameters. |
Table I
Twist1, Slug and Snail expression in
patients with PSC with different clinicopathologic parameters.
| Characteristic | Twist1
| Slug
| Snail
|
|---|
| + | − | P-value | + | − | P-value | + | − | P-value |
|---|
| Sex | | | 0.072 | | | 0.160 | | | 0.153 |
| Male | 15 | 5 | | 10 | 10 | | 9 | 11 | |
| Female | 10 | 11 | | 6 | 15 | | 5 | 16 | |
| Age, years | | | 0.087 | | | 0.288 | | | 0.228 |
| ≤60 | 13 | 4 | | 5 | 12 | | 4 | 13 | |
| >60 | 12 | 12 | | 11 | 13 | | 10 | 14 | |
| Metastasis | | | 0.005a | | | 0.960 | | | 1.000 |
| Yes | 12 | 1 | | 5 | 8 | | 4 | 9 | |
| No | 13 | 15 | | 11 | 17 | | 10 | 18 | |
| Tumour size | | | 0.444 | | | 0.248 | | | 0.062 |
| ≥5 cm | 11 | 9 | | 6 | 14 | | 4 | 16 | |
| <5 cm | 14 | 7 | | 10 | 11 | | 10 | 11 | |
| Stage | | | 0.002a | | | 0.848 | | | 0.706 |
| I | 4 | 11 | | 5 | 10 | | 4 | 11 | |
| II | 9 | 3 | | 5 | 7 | | 5 | 7 | |
| III | 12 | 2 | | 6 | 8 | | 5 | 9 | |
 | Table IITwist1, Slug and Snail expression in
groups with different clinicopathologic parameters in PSCCs. |
Table II
Twist1, Slug and Snail expression in
groups with different clinicopathologic parameters in PSCCs.
| Characteristic | Twist1
| Slug
| Snail
|
|---|
| + | − | P-value | + | − | P-value | + | − | P-value |
|---|
| Sex | | | 0.300 | | | 0.729 | | | 0.186 |
| Male | 16 | 37 | | 12 | 41 | | 12 | 41 | |
| Female | 5 | 21 | | 5 | 21 | | 2 | 24 | |
| Age, years | | | 0.793 | | | 0.621 | | | 0.425 |
| ≤60 | 13 | 34 | | 11 | 36 | | 7 | 40 | |
| >60 | 8 | 24 | | 6 | 26 | | 7 | 25 | |
| Metastasis | | | 0.591 | | | 0.759 | | | 0.160 |
| Yes | 9 | 21 | | 7 | 23 | | 3 | 27 | |
| No | 12 | 37 | | 10 | 39 | | 11 | 38 | |
| Tumour size | | | 0.670 | | | 0.264 | | | 0.149 |
| ≥5 cm | 9 | 28 | | 10 | 27 | | 9 | 28 | |
| <5 cm | 12 | 30 | | 7 | 35 | | 5 | 37 | |
| Grade | | | 0.714 | | | 0.506 | | | <0.001a |
| I | 3 | 13 | | 4 | 12 | | 2 | 14 | |
| II | 13 | 33 | | 11 | 35 | | 1 | 45 | |
| III | 5 | 12 | | 2 | 15 | | 11 | 6 | |
| Stage | | | 0.556 | | | 0.278 | | | 0.851 |
| I | 6 | 23 | | 6 | 23 | | 6 | 23 | |
| II | 9 | 24 | | 5 | 28 | | 5 | 28 | |
| III | 6 | 11 | | 6 | 11 | | 3 | 14 | |
Kaplan-Meier survival analysis revealed that
patients with PSC had a worse OS than the patients with PSCC
(Fig. 3B). The mean (95% CI) OS
times were 39.2 (32.5-45.8) months for the patients with PSC and
88.0 (75.3-100.8) months for patients with PSCC (P<0.05).
Additional survival analysis revealed that the
Twist1-positive patients with PSC exhibited a poorer prognosis for
OS than patients with Twist1-negative expression (Fig. 3C). The mean (95% CI) OS times were
25.7 (20.7-30.7) months for Twist1-positive patients, and 56.9
(49.7-64.1) months for Twist1-negative patients (P<0.05).
However, there was no difference in survival time between the
Slug-positive and -negative patients with PSC or between the
Snail-positive and -negative patients with PSC (Fig. Sl). Twist1, Slug and Snail
expression did not exhibit any prognostic significance for OS in
the patients with PSCC (Fig.
S2).
In addition, multivariate Cox regression analysis
was performed (Table SI), and
Twist1 positivity and metastasis were identified as independent
markers of a poor prognosis for OS in patients with PSC. However,
TNM stage was identified as an independent marker of a poor
prognosis for OS in patients with PSCC (Table SI).
EMT transition of lung cancer cell lines
by TGFβ1 addition
To the best of our knowledge, no PSC-derived cell
lines are currently available. Thus, TGFpl was used to induce the
EMT transition of the NSCLC cell lines, SK-MES-1 (28), H1299 and H460, in order to obtain
an in vitro PSC cell line analogue.
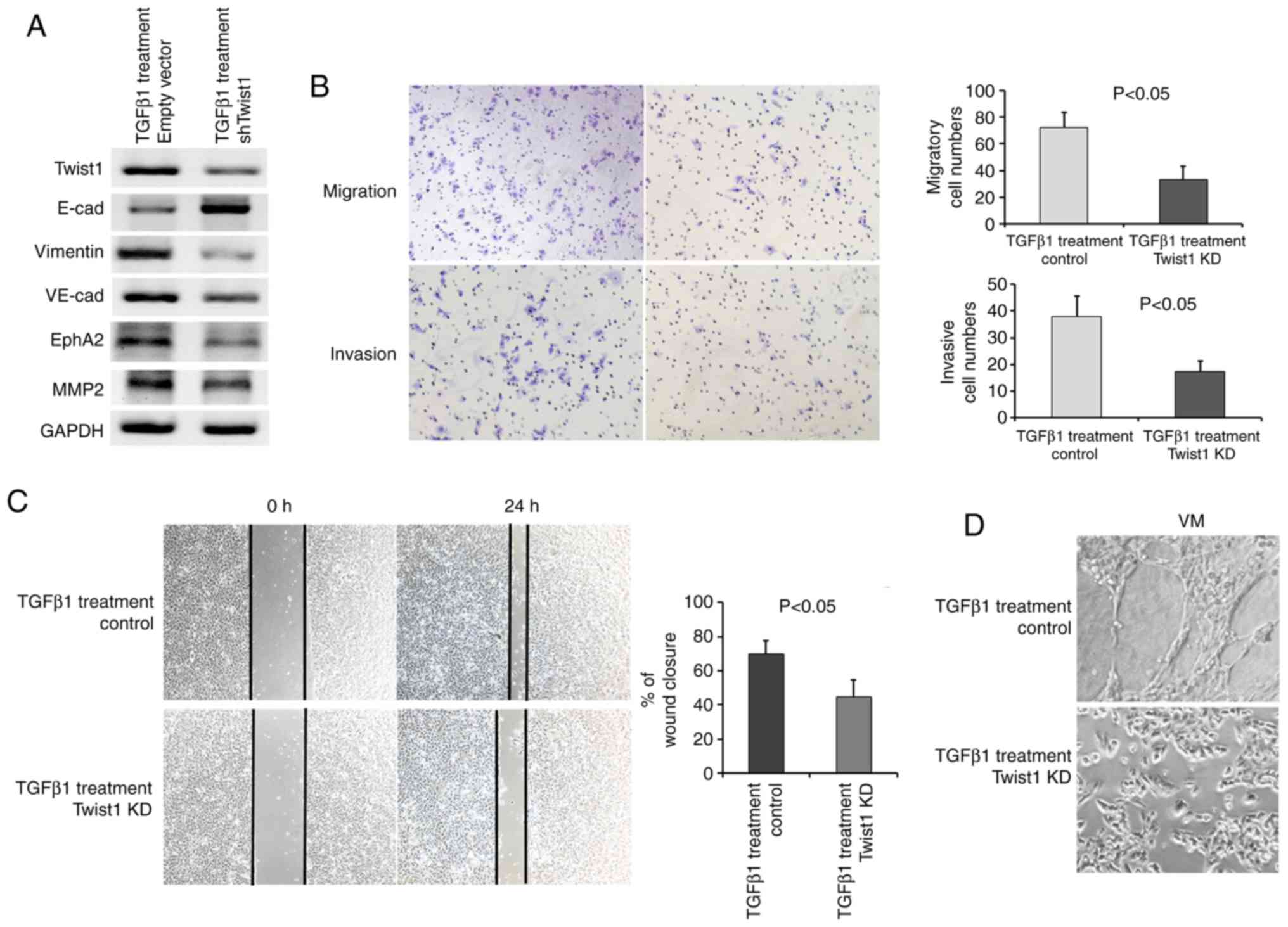
Consistently, all cells exhibited a decreased
E-cadherin and increased Vimentin expression following the addition
of TGFpl, as assessed by immunofluorescence and western blot
analysis (Fig. 4A and B). TGFpl
proteins bind to receptors on the cell surface, initiating a
signalling cascade that leads to phosphorylation of Smad2 and
Smad3. Phosphorylated Smad then translocates to the nucleus and
regulates the transcription of its target genes. Therefore,
phosphorylated Smad2/3 (p-Smad2/3) was detected as a surrogate
marker for TGFpl signalling activity, and p-Smad2/3 expression
exhibited a marked increase in the SK-MES-1 cells following
treatment with TGFpl (Fig. 4A).
Importantly, the expression of Twist1 exhibited a tendency towards
an increased expression, although the expression of Slug and Snail
did not exhibit a similar trend (Fig.
4A). These results suggest that the downstream targets of TGFβ1
signalling, as well as Twist1, may be useful markers for the
evaluation of the occurrence of EMT, which converts epithelial
PSCCs into PSCs with biphasic differentiation containing epithelial
and mesenchymal features. In addition, the PSC cell line analogue
exhibited VM formation in 3D Matrigel culture following the
occurrence of EMT (Fig. 4B).
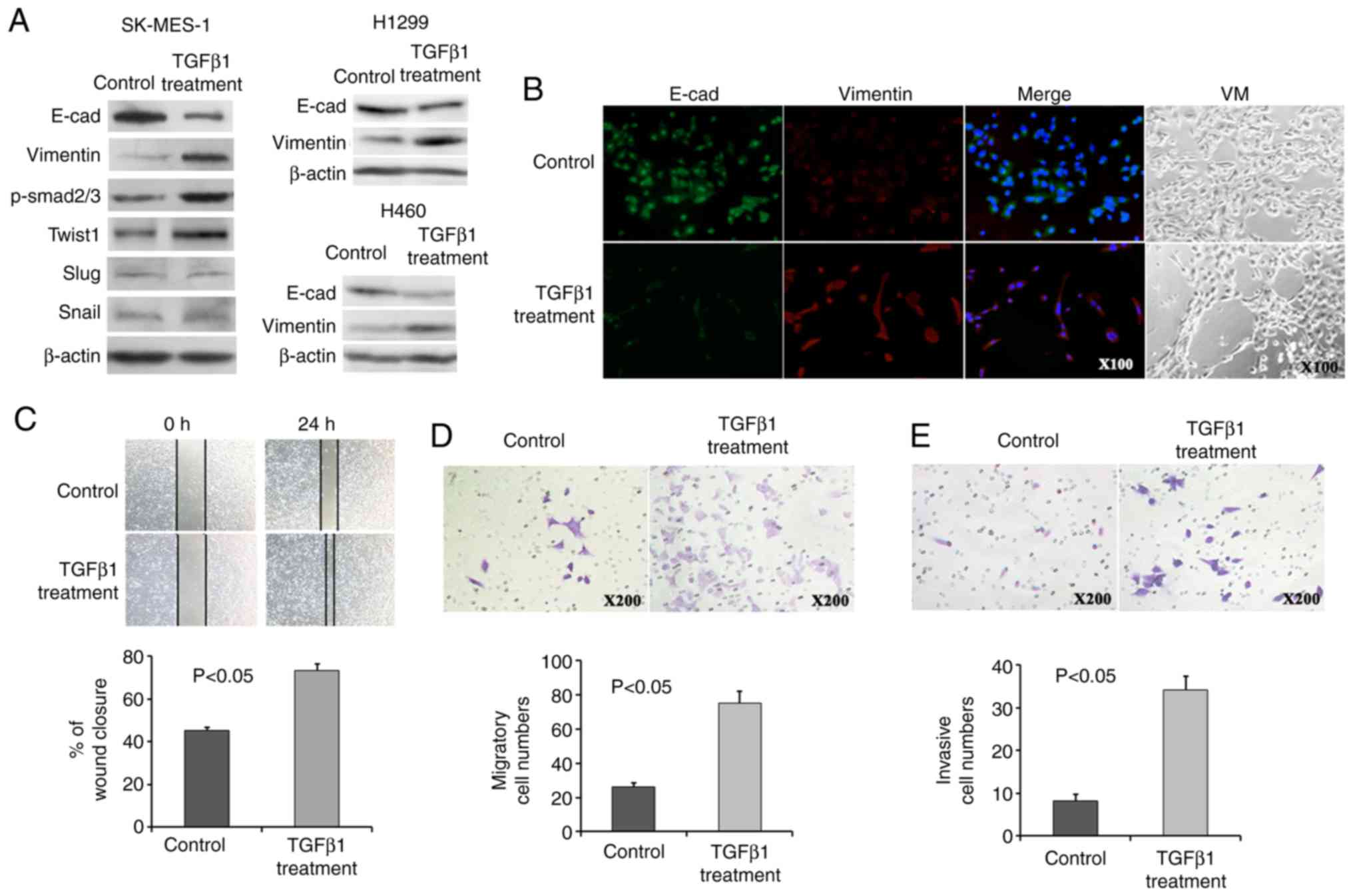 | Figure 4The PSC cell line analogue exhibited
an increased Twist1 expression, EMT phenotype, VM formation and
migratory and invasive ability. (A) NSCLC cells treated with TGFßl
exhibited a decreased E-cadherin and increased Vimentin expression.
Twist1 and p-Smad2/3 expression was induced, while that of Slug and
Snail expression was not altered following TGFßl treatment in
SK-MES-1 cells. (B) Immunofluorescence assays revealed a decreased
E-cadherin expression and an increased Vimentin expression, and 3D
Matrigel culture revealed VM formation following TGFßl treatment.
(C-E) Increased wound closure (C), migratory (D), and invasive (E)
ability was observed following TGFßl treatment. PSC, pulmonary
sarcomatoid carcinoma; EMT, epithelial-mesenchymal transition;
PSCC, pulmonary squamous carcinoma; VM, vasculogenic mimicry. |
Twist1 knockdown (KD) inhibits the
migration and invasion of the PSC cell line analogue
Quantitative analyses of the wound-healing assay
suggested a significant difference in the speed of wound healing
between the PSC cell line analogue and control SK-MES-l cells. The
PSC cell line analogue displayed a faster speed of wound healing
(Fig. 4C). An increased migratory
and invasive ability following treatment with TGFpl was observed in
the PSC cell line analogue by Transwell assays (Fig. 4D and E). Twist1 downregulation in
SK-MES-l cells under standard conditions or TGFpl treatment was
observed following transfection with shTwist1 vector (Figs. 5A and S3). Importantly, the EMT phenotype was
reversed (Fig. 5A), VM marker
expression was decreased (Fig.
5A), the migratory and invasive abilities were decreased
(Fig. 5B), and wound closure
(Fig. 5C) and VM formation
(Fig. 5D) were also decreased in
the PSC cell line analogue following Twist1 knockdown.
Discussion
NSCLC with spindle and/or giant cells or sarcomas
that contain heterologous elements (rhabdomyosarcoma,
chondrosarcoma, and osteosarcoma) are unified under the umbrella
term sarcomatoid carcinoma. Molecular evidence indicates that these
tumours are essentially carcinomas with varying degrees of
divergent differentiation (supporting tumour metaplasia, rather
than the collision 'polyclone hypothesis') (29-31).
In pulmonary sarcomatoid carcinoma, the sarcomatous-like and
carcinomatous components have the same molecular pedigree and are
composed of the same pattern of acquired allelic absence (30), p53 mutations and X chromosome
inactivation (31). In this study,
the expression of CK5/6 or p63 was observed in both carcinomatous
and spindled components of the sarcomatoid carcinoma, indicating
that sarcomatoid carcinoma may be histogenetically converted from
lung squamous cell carcinoma. Demonstrating the gene expression
differences existing between PSCs and PSCCs should aid in the
understanding of the mechanisms through which this conversion
occurs, providing new insight into the biology of PSCs and paving
the way to the definition of novel therapies.
As a transcription factor, Twist1 can exert multiple
biological effects through various downstream pathways by
regulating the expression of target genes. The most critical
pathological function of Twist1 in cancer is facilitating tumour
invasion and metastasis by promoting EMT. EMT is a cellular
plasticity process (32), which
involves the loss of epithelial characteristics by epithelial
cells, such as decreased cell-cell contact and the downregulation
of E-cadherin, with the simultaneous acquisition of mesenchymal
properties, including a spindle-like shape, increased cell
motility, and the upregulation of mesenchymal markers such, as
vimentin. Twist1 interacts with several components of the
Mi2/nucleosome remodelling and deacetylase (Mi2/NuRD) complex
(MTA2, RbAp46, Mi2, HDAC2) and recruits the factors to the proximal
regions of the E-cadherin promoter to downregulate promoter
activity and repress E-cadherin gene expression (33). As the downstream target of Twist1,
in this study, the reduced expression of E-cadherin and the
increased expression of vimentin were more frequently detected in
the PSC group than the PSCC group, and they were more frequent in
SK-MES-1 cells treated with TGFpl than in the control cells,
suggesting that Twist1-induced E-cadherin repression and the EMT
phenotype may be characteristics of PSCs.
The authors previously demonstrated that tumour
cells with EMT characteristics can form VM, and the occurrence of
both EMT and VM indicates that tumour cells harbour high plasticity
(19,24). In this study, there was a
significant difference between the positive rate of VM in PSCs and
PSCCs, which is consistent with the occurrence of EMT in PSCs. The
tumour cells of the VM channel wall can fall off, flow into the
blood and arrive at other organs to grow as a metastatic tumour.
This may explain the shorter survival period observed in patients
with PSC with a higher VM formation ability compared to that of
patients with PSCC. In the present study, it was also demonstrated
that the expression of the VM markers, EphA2, VE-cadherin and MMP2,
was increased in PSCs. Remodelling of the extracellular matrix is
one of the factors that governs VM channel formation, and MMP2,
which can degrade various extracellular matrix proteins and
facilitate tumour invasion and metastasis, is positively correlated
with VM formation (34).
VE-cadherin and EphA2 were also found to be co-localized in
cell-cell adhesion junctions both in vitro and in
vivo. It has been previously demonstrated that EphA2 and
VE-cadherin interact directly and/or indirectly during VM (35,36).
Previous research has shown that EphA2 is a factor upstream of
phosphatidylinositol 3-kinase (PI3K) and that phosphorylated EphA2
can activate PI3K and then regulate the expression of MMP-2
(37). An increased EphA2,
VE-cadherin and MMP2 expression are hallmarks of VM that were more
commonly present in PSCs than they were in PSCCs in this study,
indicating that PSCs develop a more malignant phenotype.
To demonstrate the role of EMT in the occurrence of
PSC in vitro, NSCLC cells were used and were treated with
TGFβ1 to mimic PSC cells, since TGFβ1 is a well-known EMT inducer.
All NSCLC cells exhibited a decreased E-cadherin and an increased
vimentin expression following the addition of TGFβ1. The cells
undergoing EMT cells exhibited an increased VE-cadherin, MMP2 and
EphA2 expression and VM formation, suggesting that these cells may
possess the biological characteristics of PSCs. The migratory and
invasive ability was elevated in these cells, suggesting aggressive
biological behaviour in the PSC cell line analogue. Importantly,
these cells exhibited an increased Twist1 expression, and Twist1
knockdown reversed the EMT phenotype, reduced the migratory and
invasive ability and the expression of VM markers. In addition, in
this study, the expression of Twist1, Slug and Snail was found to
be higher in the PSC group than in the PSCC group. It has been
reported that Twist1, Slug and Snail can stimulate carcinoma
metastasis by mediating E-cadherin repression (19,38,39).
Twist1 had been found not only to function in EMT, but also to
function in VM in previous studies by our group and others
(24,38). VE-cadherin expression is
upregulated following the upregulation of Twist1 expression in the
3D Matrigel culture system (24).
Of note, in this study, only Twist1 expression, not Slug or Snail,
was associated with metastasis and TNM stage in patients with PSC,
suggesting an important role for Twist1 in PSC metastasis by
promoting and maintaining the EMT phenotype and VM formation.
Consistently, it was found that patients with PSC
with Twist1-positive expression exhibited a poorer OS than
Twist1-negative patients, as indicated by Kaplan-Meier analyses.
There was no difference in survival time between Slug-positive and
-negative or between Snail-positive and -negative PSCs. Cox
multivariate analysis demonstrated that a Twist1-positive
expression was an independent prognostic factor for OS in patients
with PSC. These results indicated that Twist1-positive expression
predicted a worse survival and may serve as the key molecular
prognostic indicator for PSCs. Mechanistically, Twist1 expression
in PSCs promoted tumour cell plasticity, which contributed to the
EMT phenotype through the suppression of E-cadherin and the
upregulation of Vimentin, and it contributed to VM formation by the
upregulation of VE-cadherin; thus, Twist1 plays a role in the
aggressive behaviour of PSCs. The association between
Twist1-positive expression and poor survival suggests a feasible
therapeutic strategy for targeting Twist1 in PSCs.
To the best of our knowledge, this study is the
first report on the expression of Twist1, which is closely
associated with tumour cell plasticity, EMT and VM, and may
regulate the molecular mechanisms of aggressiveness in PSCs.
Therefore, the findings of this study may prove to be useful in the
identification of novel therapeutic targets for the inhibition of
PSC angiogenesis and metastasis.
Supplementary Data
Funding
This study was partly supported by a grant from The
National Natural Science Foundation of China (no. 81572872 to XiZ
and no. 81672870 to TL), and the Key project of the National
Natural Science Foundation of China (no. 81230050 to BS).
Availability of data and materials
The datasets and data used and/or analysed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
BS, TL, XiZ, XuZ and YZ conceived and carried out
experiments. TL and XuZ conceived the experiments and analysed the
data. XuZ, XiZ, YZ, XD, NZ and SL carried out immunochemistry and
cell culture experiments. All authors were involved in the writing
of the manuscript and gave the final approval of the submitted and
published versions.
Ethics approval and consent to
participate
Tissue collection and analysis in this study were
approved by the Ethics Committee of Tianjin Medical University,
China. Written consent had been signed by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Mengoli MC, Longo FR, Fraggetta F, Cavazza
A, Dubini A, Ali G, Guddo F, Gilioli E, Bogina G, Nannini N, et al:
The 2015 world health organization classification of lung tumors:
New entities since the 2004 classification. Pathologica. 110:39–67.
2018.PubMed/NCBI
|
|
2
|
Mignard X, Ruppert AM, Antoine M, Vasseur
J, Girard N, Mazières J, Moro-Sibilot D, Fallet V, Rabbe N,
Thivolet-Bejui F, et al: c-MET overexpression as a poor predictor
of MET amplifications or exon 14 mutations in lung sarcomatoid
carcinomas. J Thorac Oncol. 13:1962–1967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ali G, Bruno R, Poma AM, Affinito O,
Monticelli A, Piaggi P, Ricciardi S, Lucchi M, Melfi F, Chella A,
et al: Whole transcriptome targeted gene quantification provides
new insights on pulmonary sarcomatoid carcinomas. Sci Rep.
9:35362019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roesel C, Kambartel K, Kopeika U, Berzins
A, Voshaar T and Krbek T: Lazarus-type tumour response to therapy
with nivolumab for sarcomatoid carcinomas of the lung. Curr Oncol.
26:e270–e273. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manzotti G, Torricelli F, Benedetta D,
Lococo F, Sancisi V, Rossi G, Piana S and Ciarrocchi A: An
epithelial-to-mesenchymal transcriptional switch triggers evolution
of pulmonary sarcomatoid carcinoma (PSC) and identifies dasatinib
as new therapeutic option. Clin Cancer Res. 25:2348–2360. 2019.
View Article : Google Scholar
|
|
6
|
Tamaki T, Shimizu T, Niki M, Shimizu M,
Nishizawa T and Nomura S: Immunohistochemical analysis of NANOG
expression and epithelial-mesenchymal transition in pulmonary
sarcomatoid carcinoma. Oncol Lett. 13:3695–3702. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aiello NM and Kang Y: Context-dependent
EMT programs in cancer metastasis. J Exp Med. 216:1016–1026. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Campbell K: Contribution of
epithelial-mesenchymal transitions to organogenesis and cancer
metastasis. Curr Opin Cell Biol. 55:30–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Staalduinen J, Baker D, Ten Dijke P
and van Dam H: Epithelial-mesenchymal-transition-inducing
transcription factors: New targets for tackling chemoresistance in
cancer? Oncogene. 37:6195–6211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kalluri R: EMT: When epithelial cells
decide to become mesenchymal-like cells. J Clin Invest.
119:1417–1419. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan L, Ye L, Zhuang L, Zou X, Liu S,
Zhang Y, Zhang L, Jin C and Huang Y: VEGFC/VEGFR3 axis mediates
TGFß1-induced epithelial-to-mesenchymal transition in non-small
cell lung cancer cells. PLoS One. 13:e02004522018. View Article : Google Scholar
|
|
12
|
Zhang F, Li T, Han L, Qin P, Wu Z, Xu B,
Gao Q and Song Y: TGFß1-induced down-regulation of microRNA-138
contributes to epithelial-mesenchymal transition in primary lung
cancer cells. Biochem Biophys Res Commun. 496:1169–1175. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Delgado-Bellido D, Serrano-Saenz S,
Fernandez-Cortés M and Oliver FJ: Vasculogenic mimicry signaling
revisited: Focus on non-vascular VE-cadherin. Mol Cancer.
16:652017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo Q, Yuan Y, Jin Z, Xu T, Gao Y, Wei H,
Li C, Hou W and Hua B: Association between tumor vasculogenic
mimicry and the poor prognosis of gastric cancer in China: An
updated systematic review and meta-analysis. Biomed Res Int.
2016:24086452016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu
D, Yu X and Tian Y: Advanced research on vasculogenic mimicry in
cancer. J Cell Mol Med. 19:315–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang HF, Wang SS, Zheng M, Dai LL, Wang K,
Gao XL, Cao MX, Yu XH, Pang X, Zhang M, et al: Hypoxia promotes
vasculogenic mimicry formation by vascular endothelial growth
factor A mediating epithelial-mesenchymal transition in salivary
adenoid cystic carcinoma. Cell Prolif. 52:e126002019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ou H, Chen Z, Xiang L, Fang Y, Xu Y, Liu
Q, Hu Z, Li X, Huang Y and Yang D: Frizzled 2-induced
epithelial-mesenchymal transition correlates with vasculogenic
mimicry, stemness, and Hippo signaling in hepatocellular carcinoma.
Cancer Sci. 110:1169–1182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu T, Sun B, Zhao X, Li Y, Zhao X, Liu Y,
Yao Z, Gu Q, Dong X, Shao B, et al: USP44+ cancer stem cell
subclones contribute to breast cancer aggressiveness by promoting
vasculogenic mimicry. Mol Cancer Ther. 14:2121–2131. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun D, Sun B, Liu T, Zhao X, Che N, Gu Q,
Dong X, Yao Z, Li R, Li J, et al: Slug promoted vasculogenic
mimicry in hepatocellular carcinoma. J Cell Mol Med. 17:1038–1047.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding X, Li F and Zhang L: Knockdown of
delta-like 3 restricts lipopolysaccharide-induced inflammation,
migration and invasion of A2058 melanoma cells via blocking
Twist1-mediated epithelial-mesenchymal transition. Life Sci.
226:149–155. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren H, Du P, Ge Z, Jin Y, Ding D, Liu X
and Zou Q: TWIST1 and BMI1 in cancer metastasis and
chemoresistance. J Cancer. 7:1074–1080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu QQ, Ma C, Wang Q, Song Y and Lv T: The
role of TWIST1 in epithelial-mesenchymal transition and cancers.
Tumour Biol. 37:185–197. 2016. View Article : Google Scholar
|
|
23
|
Sun T, Sun BC, Zhao XL, Zhao N, Dong XY,
Che N, Yao Z, Ma YM, Gu Q, Zong WK and Liu ZY: Promotion of tumor
cell metastasis and vasculogenic mimicry by way of transcription
coactivation by Bcl-2 and Twist1: A study of hepatocellular
carcinoma. Hepatology. 54:1690–1706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW,
Che N, Wang XH, Du J, Liu YX and Sun BC: Expression and functional
significance of Twist1 in hepatocellular carcinoma: Its role in
vasculogenic mimicry. Hepatology. 51:545–556. 2010. View Article : Google Scholar
|
|
25
|
Yochum ZA, Cades J, Wang H, Chatterjee S,
Simons BW, O'Brien JP, Khetarpal SK, Lemtiri-Chlieh G, Myers KV,
Huang EH, et al: Targeting the EMT transcription factor TWIST1
overcomes resistance to EGFR inhibitors in EGFR-mutant
non-small-cell lung cancer. Oncogene. 38:656–670. 2019. View Article : Google Scholar
|
|
26
|
Sun H, Liu T, Zhu D, Dong X, Liu F, Liang
X, Chen C, Shao B, Wang M and Wang Y: HnRNPM and CD44s expression
affects tumor aggressiveness and predicts poor prognosis in breast
cancer with axillary lymph node metastases. Genes Chromosomes
Cancer. 56:598–607. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Sun H, Zhang D, Fan D, Zhang Y,
Dong X, Liu S, Yang Z, Ni C, Li Y, et al: TP53INP1 inhibits
hypoxia-induced vasculogenic mimicry formation via the ROS/snail
signalling axis in breast cancer. J Cell Mol Med. 22:3475–3488.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Chen R, Yang S, Liu W, Li K,
Zhang H, Zhu X and Chen B: Lobaplatin for the treatment of sk-mes-1
lung squamous cell line in vitro and in vivo. OncoTargets Ther.
9:4215–4224. 2016. View Article : Google Scholar
|
|
29
|
Franks TJ and Galvin JR: Sarcomatoid
carcinoma of the lung: Histologic criteria and common lesions in
the differential diagnosis. Arch Pathol Lab Med. 134:49–54.
2010.PubMed/NCBI
|
|
30
|
Dacic S, Finkelstein SD, Sasatomi E,
Swalsky PA and Yousem SA: Molecular pathogenesis of pulmonary
carcinosarcoma as determined by microdissection-based allelotyping.
Am J Surg Pathol. 26:510–516. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Holst VA, Finkelstein S, Colby TV, Myers
JL and Yousem SA: p53 and K-ras mutational genotyping in pulmonary
carcinosarcoma, spindle cell carcinoma, and pulmonary blastoma:
Implications for histogenesis. Am J Surg Pathol. 21:801–811. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chaffer CL, San Juan BP, Lim E and
Weinberg RA: EMT, cell plasticity and metastasis. Cancer Metastasis
Rev. 35:645–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao Z, Rahman MA, Chen ZG and Shin DM:
Multiple biological functions of Twist1 in various cancers.
Oncotarget. 8:20380–20393. 2017.PubMed/NCBI
|
|
34
|
Li Y, Sun B, Zhao X, Wang X, Zhang D, Gu Q
and Liu T: MMP-2 and MMP-13 affect vasculogenic mimicry formation
in large cell lung cancer. J Cell Mol Med. 21:3741–3751. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hess AR, Seftor EA, Gruman LM, Kinch MS,
Seftor RE and Hendrix MJ: VE-cadherin regulates EphA2 in aggressive
melanoma cells through a novel signaling pathway: Implications for
vasculogenic mimicry. Cancer Biol Ther. 5:228–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo JQ, Zheng QH, Chen H, Chen L, Xu JB,
Chen MY, Lu D, Wang ZH, Tong HF and Lin S: Ginsenoside Rg3
inhibition of vasculogenic mimicry in pancreatic cancer through
downregulation of VEcadherin/EphA2/MMP9/MMP2 expression. Int J
Oncol. 45:1065–1072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen LX, He YJ, Zhao SZ, Wu JG, Wang JT,
Zhu LM, Lin TT, Sun BC and Li XR: Inhibition of tumor growth and
vasculogenic mimicry by curcumin through downregulation of the
EphA2/PI3K/MMP pathway in a murine choroidal melanoma model. Cancer
Biol Ther. 11:229–235. 2011. View Article : Google Scholar
|
|
38
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fan XJ, Wan XB, Yang ZL, Fu XH, Huang Y,
Chen DK, Song SX, Liu Q, Xiao HY, Wang L and Wang JP: Snail
promotes lymph node metastasis and Twist enhances tumor deposit
formation through epithelial-mesenchymal transition in colorectal
cancer. Hum Pathol. 44:173–180. 2013. View Article : Google Scholar
|