The glutathione-S transferase (GST) family consists
of a group of isoenzymes involved in phase II detoxification of
xenobiotics by glutathione conjugation (1,2). It
is widely found in nematodes, fruit flies, yeast, and the cytoplasm
of higher vertebrates. Studies have shown that soluble GST accounts
for 4% of total soluble protein in human and rodent livers
(3). Three major protein
subfamilies have been reported to exhibit glutathione transferase
activity: Cytoplasmic, mitochondrial and microsomal GSTs (4,5).
Microsomal GSTs are membrane-associated proteins in eicosanoid and
glutathione metabolism (6,7). Cytoplasmic GSTs are the largest
subfamily of these transferases and have unique activities. They
catalyze the thiolysis of 4-nitrophenyl acetate, exhibit thiol
transferase activity, reduce trinitroglycerin, dehydroascorbic acid
and monomethyl decanoic acid, and catalyze ethyl maleate and 5-3
isomerization of ketosteroids (8).
According to the similarity in amino acid sequences,
different structures of genes, and immunological cross-reactivity,
GSTs are divided into seven subtypes (9) as follows: Alpha (α), pi (π), mu (μ),
theta (θ), omega (ω), sigma (σ), and zeta (Table I). Among those, μ, θ and π are the
most widely studied GST subtypes in mammals (10). It is well known that μ-class
glutathione S-transferase 1 (GSTM1) plays an important role in the
toxicity and effectiveness of medical drugs. GSTM10 is the most
common polymorphism, resulting in loss of enzymatic activity
(11,12). Glutathione S-transferase θ 1
(GSTT1) plays a role in human carcinogenesis (13). GSTM1-null and GSTT1-null may
contribute to the clinical course of patients with type 2 diabetes
mellitus (T2DM) (14). GSTs
interact with several factors, such as regulatory kinases, and
modulate numerous pathways involved in cell proliferation,
differentiation and death. Previous studies have demonstrated that
GST plays a major role in cancer cell proliferation and death via
its cytoprotective and regulatory functions (15,16).
GST enzymes also play an important role in detoxifying chemotherapy
drugs (17). They may be used to
detoxify oxidized or alkylated drugs directly by combining active
compounds or drugs (18). In
addition to their well-characterized catalytic activity, there is
evidence that GST isoenzymes also participate in regulating the
expression of mitogen-activated protein kinases, and promote
S-glutathionylation of cysteine residues in target proteins
(19). In addition, genetic
variants of GST have been reported to be involved in various
fluorouracil- and platinum-based chemotherapies for the treatment
of metastatic advanced cancers, such as acute myeloid leukemia
(AML), gastrointestinal tumors, non-small cell lung cancer (NSCLC)
and prostate cancer (PCa) (20,21).
Therefore, it is clear that members of the GST family have a wide
range of applications in detoxification and drug treatment.
GSTP1 has a wide range of physiological functions:
It is involved in metabolism, detoxification and elimination of
potentially genotoxic foreign complexes, metabolizes a variety of
carcinogenic compounds, and protects cells against DNA damage and
canceration. In the GST family, early studies demonstrated that the
GSTP1 gene plays an important role in several cellular processes,
including catalysis and deoxylation of electrophilic compounds,
oxidative stress regulation, cell signaling and carcinogenesis
(25,26). GSTP1 actively protects cells from
carcinogens and electrophilic compounds (27,28).
It has been suggested that GSTP1 also protects cells from oxidants
and electrophilic-mediated genomic damage (29). GSTP1 is involved in apoptosis
resistance and metabolism of several chemotherapeutic agents.
Platinum-based drugs have been found to be metabolized by GSTP1,
allowing GSTP1 to be expressed in ovarian tumors. Therefore, GSTP1
may be used as a target gene and candidate response biomarker for
platinum-based chemotherapy. In addition, GSTP1 plays a major role
in the metabolism of cisplatin and carboplatin in ovarian cancer
cells (30,31). Differences in expression of GSTP1
may affect the response of patients with ovarian cancer to
platinum-based chemotherapy (32).
Taken together, the studies on changes in GSTP1 expression of tumor
cells may contribute to the development of antitumor drugs. GSTP1
appears hold promise in drug development, and the GSTP1 gene is
involved in the regulation of activator proteins. It plays an
important role in the regulation of tumor necrosis factor. Both
activator protein 1 and nuclear factor (NF)-κB mediate regulation
of GSTP through a redox process (33). A chimeric inhibitor that binds the
affinity recognition moiety to a chelated transition metal has been
explored to develop metal-mediated affinity reagents or drugs for
hGSTP1-1 (34). GSTP1 is also a
key regulator of hepatocyte proliferation during the initial stages
of liver regeneration (35). The
-323/-314 sequence located in the GSTP1 promoter binds to
NF-κBp50/65 and p65/p65 dimers, and is involved in the regulation
of this gene by tumor necrosis factor α (36-38).
The GSTP1 gene (OMIM 134660) encoding the π-GST partial GSTP1-1
protein is widely expressed in most tissues, particularly in the
lungs, esophagus and placenta (39). Ubiquitous epigenetic silencing of
GSTP1 in PCa leads to increased survival and accumulation of
potential priming DNA conjugates after exposure to long-term
oxidative damage, suggesting that GSTP1 has protective and
antitumor functions (40). As
mentioned above, GSTP1 has important physiological functions in the
detoxification and antioxidation of metabolites.
GSTP1 not only has important physiological
functions, but also major pathological functions. GSTP1 is closely
associated with exposure to low doses of ionizing radiation, heavy
metals, and other chemicals (Table
II). Manganese (Mn) has been shown to be a naturally occurring
trace element that is essential for human health and development,
but is neurotoxic at high concentrations (41,42).
Studies have shown a possible synergistic effect between the blood
Mn concentration and GSTP1 in autism spectrum disorder (43). GSTP1 is induced by lead and may be
used as a biomarker for lead exposure. It is only involved in the
changes during the later stages of lead poisoning (44,45).
Previous studies have found that arsenic compounds are useful as
drugs, but have toxic effects, and GSTP1 is a major factor in
resistance to these drugs (46,47).
GSTP1 detoxifies arsenic-based drugs by isolating the active site
and dimer interface, reacting with cysteine in the presence of
sufficient GSH under low GSH conditions (48,49).
GSTP1 also reduces the retention time of
As2O3 in the cells. Catabolism of
H2O2 reduces the amount of
H2O2 in the cells, thereby blocking apoptosis
of lymphoma cells induced by As2O3 (50). In addition, GSTP1 may participate
in the elimination of carcinogens in tobacco and participates in
the occurrence of smoking-related lung adenocarcinoma. GSTP1 plays
a role in the elimination of toxic substances from cigarette smoke
in both normal lung and cancer cells (51). GSTP1 is also involved in the
detoxification process of benzo(a)pyrene (BaP), which excretes the
conjugates of BaP metabolism and detoxification (52). It is also involved in the
protection of cells against 222Rn-induced DNA damage
(53). GSTP1 also blocks
lipopolysaccharide (LPS) -induced overproduction of proinflammatory
factors and has anti-inflammatory effects on the LPS response
(54). Therefore, as a detoxifying
enzyme, GSTP1 plays an important role in the detoxification of
heavy metals and may facilitate exploring metal-mediated affinity
drugs. GSTP1 is also closely associated with radiation damage. Our
previous studies indicated that GSTP1 is involved in the
radiation-induced stress response of liver tissue in C57BL/6J mice
and it may be used as a biomarker of low-dose radiation for early
identification of radiation contamination. Therefore, the mechanism
of GSTP1 in the radiation-induced stress response is worthy of
further investigation (55,56).
Of note, the involvement of GSTP1 in the development of diseases is
a complex process involving multiple steps and factors. GSTP1 is a
key regulator in the occurrence and development of multiple cancer
types.
There is a close association between GSTP1
expression and tumor development. In several tumor tissues, >90%
of active GSTs is GSTP1 (57).
Therefore, the difference in expression of GSTP1 in diseases such
as tumors has attracted significant attention. Compared with normal
tissues, the difference in GSTP1 expression is associated with
multiple diseases (Table III).
GSTP1 is highly expressed in various types of cancer and
preneoplastic legions, such as colorectal, esophageal, lung,
bladder, thyroid and breast cancer (58,59).
GSTP1 is overexpressed at various stages of colorectal cancer, from
abnormal crypt foci to advanced cancer (8,60).
GSTP1 may be used as a clinically useful target for anti-colon
cancer drugs (61). High
expression of GSTP1 in esophageal cancer tissues may reduce the
chemosensitivity of cancer cells (62,63).
Increased expression of GSTP1 in bladder transitional cell
carcinoma is associated with altered apoptotic pathways (64). Upregulation of GSTP1 expression
contributes to an increase in the antioxidant capacity of bladder
transitional cell carcinoma cells (65,66).
High expression of GSTP1 plays a role in tumor growth and
carcinogenesis of papillary thyroid cancer (67). Moreover, high expression of GSTP1
confers resistance of breast cancer cells to adriamycin by
promoting autophagy (68). In
addition, overexpression of GSTP1 inhibits the proliferation of
HepG2 and Huh7 liver cancer (69).
GSTP1 and multidrug resistance protein 1 (MRP1) are overexpressed
in malignant melanoma. GSTP1 acts together with MRP1 to protect
melanoma cells against the toxic effects of etoposide (70).
It is interesting that miRNAs regulate GSTP1,
particularly in relation to human diseases. miR-133b overexpression
contributes to the suppression of malignant growth and
aggressiveness of cisplatin-resistant NSCLC cells by targeting
GSTP1 (71). miRNA-130b may be
involved in the development of drug resistance of ovarian cancer by
regulating the expression level of the GSTP1 protein (72). Similarly, miR-133a directly
regulates the GSTP1 gene in bladder cancer (BC) and mediates
GSTP1-mediated anti-apoptotic effects by downregulation of miR-133a
in human BC (73). There are also
data suggesting that miR-133α in head and neck squamous cell
carcinoma (HNSCC) regulates the carcinogenic effects of GSTP1,
thereby providing new insights into the mechanisms underlying HNSCC
carcinogenesis (74).
Downregulation of GSTP1 may facilitate the function of miR-124 in
doxorubicin resistance and enable the development of new treatments
to overcome chemoresistance in colorectal cancer (CRC) patients
(75). miR-513a-3p sensitizes
human lung adenocarcinoma cells to cisplatin by regulating GSTP1
(76). Therefore, the regulation
of GSTP1 by miRNA is crucial in several human diseases.
The expression of GSTP1 in tumors is low; for
example, its expression in PCa is low, and downregulation of GSTP1
expression may play an important role in the progression of PCa
(77). Downregulation of GSTP1
expression in PCa may be a useful biomarker for early detection and
prognosis (78). Loss of GSTP1
expression in human PCa cells increases their susceptibility to
oxidative stress-induced DNA damage and may be an important target
for primary prevention of PCa (79). In certain diseases, the expression
of GSTP1 may also play a regulatory and predictive role. GSTP1
expression may predict the pathological response to
5-fluorouracil/epirubicin/cyclophosphamide in estrogen receptor
(ER)-negative tumors (80). The
difference in the expression of GSTP1 is associated with multiple
diseases and plays an important role in prediction and
treatment.
The promoter region of the GSTP1 gene is usually
affected by methylation, and changes in methylation status suppress
normal gene expression, which may lead to weakening or loss of its
detoxification and antioxidant functions. In several cancer types,
the GSTP1 gene is affected by hypermethylation. GSTP1 is a major
tissue biomarker that performs well in several types of
malignancies, such as PCa, breast and lung cancer, and
hepatocellular carcinoma (HCC) (81). GSTP1 methylation has been found to
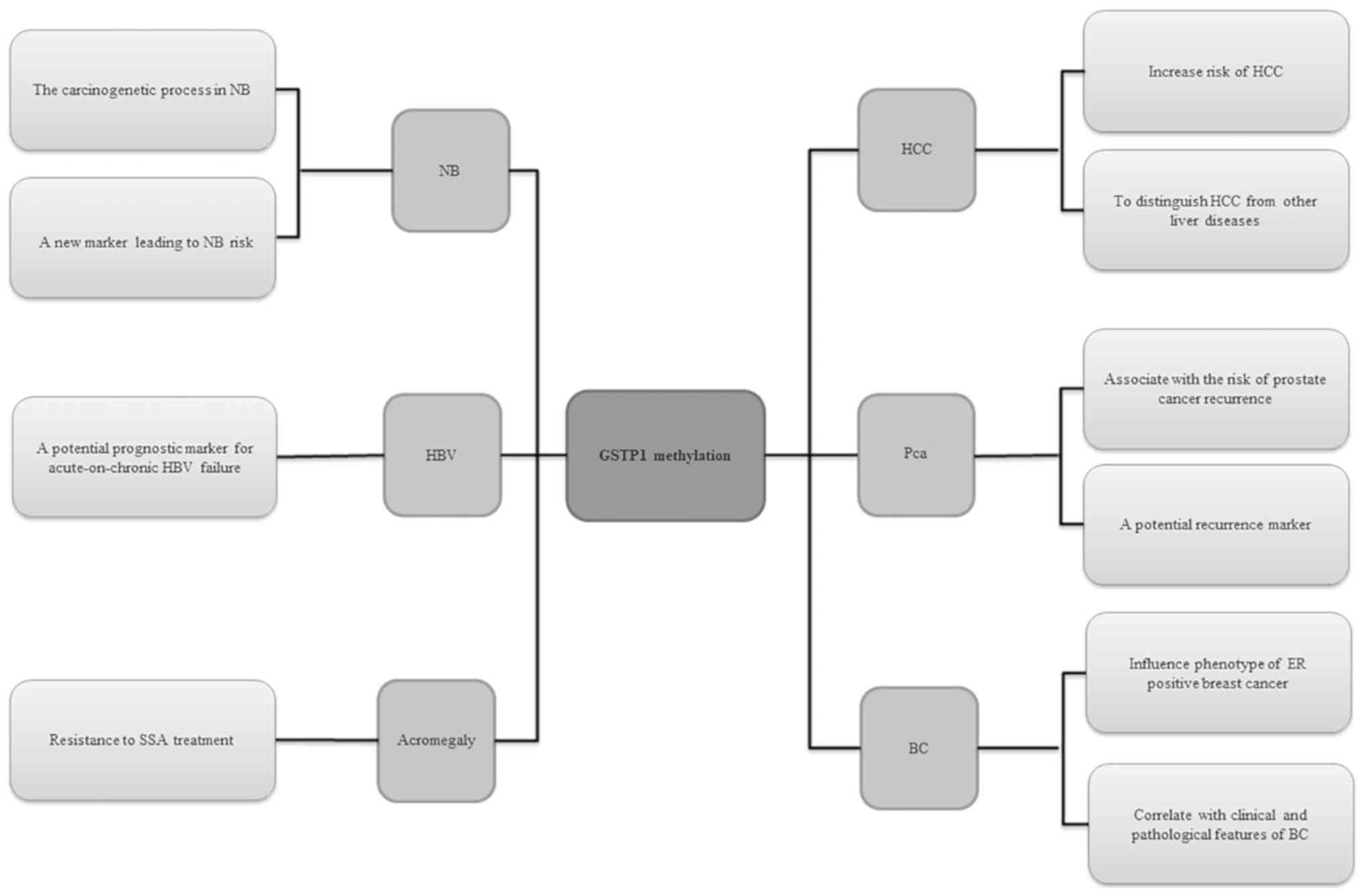
be associated with the development of several diseases (Fig. 1). Recent research has confirmed
that hypermethylation of GSTP1 inactivates the GSTP1 gene and plays
a major role in liver cancer. It may increase the risk of HCC, and
is also significantly associated with a poor prognosis of patients
with HCC (82,83). It has been reported that
hypermethylation of the GSTP1 gene promoter region may be a
potential biomarker for distinguishing HCC from other liver
diseases (84). Of note,
methylation of the GSTP1 gene promoter may be associated with the
invasiveness of HCC. Chronic hepatitis B virus infection may be
responsible for inactivation of p16 induced by GSTP1 methylation
(85). GSTP1 methylation is
associated with oxidative stress-induced liver injury in
acute-chronic hepatitis B liver failure. Abnormal methylation of
the GSTP1 promoter is also present in acute-chronic hepatitis B
liver failure and may have a high predictive value for short-term
mortality. Therefore, GSTP1 may be a potential prognostic indicator
of acute hepatitis B-related acute liver failure (86). GSTP1 methylation has a strong
influence on liver-related diseases and may play a role in the
treatment of liver-related diseases. GSTP1 methylation is
associated with the recurrence and prognosis of PCa, and may be a
potential epigenetic marker (87-92).
Similar to PCa, GSTP1 hypermethylation also occurs in early events
of breast cancer (93). The
heterogenous DNA methylation pattern in the GSTP1 promoter is a
major obstacle for DNA methylation analysis of the GSTP1 gene,
which explains some of the contradictory differences in the role of
GSTP1 promoter methylation in breast cancer (94). Although previous studies have shown
no clear correlation between the GSTP1 status and the
clinicopathological characteristics of PCa, GSTP1 methylation is
associated with a more aggressive ER-positive breast cancer
phenotype (95). Furthermore,
GSTP1 methylation is associated with ER positivity (96-98).
GSTP1 methylation was found to be correlated with the
clinicopathological characteristics of breast cancer (99). Therefore, GSTP1 methylation is
important for breast cancer research. The frequency of GSTP1
methylation in cancer tissues of patients with NSCLC ranges from 0
to 25%, while lower or no methylation is observed in adjacent
benign tissues (100-105). Abnormal methylation of GSTP1 may
contribute to the carcinogenesis of neuroblastoma and may be used
as a new marker (106). In
addition, in acromegaly, methylation of the GSTP1 gene is
associated with resistance to treatment with somatostatin analogues
(107). Therefore, GSTP1
methylation appears to play a key role in numerous diseases.
It has been demonstrated that GSTP1 enzymatic
activity is strongly dependent on a single-nucleotide polymorphism,
the A313 G polymorphism, which replaces isoleucine (Ile) with
valine (Val) at the 105 amino acid position (Ile105Val) (IE),
producing three GSTP1 genotypes: Ile/Ile homozygous wildtype,
Ile/Val heterozygotes, and Val/Val homozygous variants (108,109). Genetic polymorphism of GSTP1 is
associated with several cancer types. The genetic polymorphism of
GSTP1 may be associated with the detoxification of polycyclic
aromatic hydrocarbons in cigarette smoke and exhibits the highest
expression in lung tissue (110,111). In the Chinese population, the
GSTP1 Ile105Val polymorphism may increase the risk of lung cancer
(112-114). Furthermore, GSTP1 exon 5
polymorphism is associated with lung cancer susceptibility.
Stratified analysis has revealed a correlation between the GSTP1
exon 5 gene polymorphism and the risk of lung squamous cell
carcinoma (115). GSTP1
genotyping may help identify patients at higher risk of developing
anti-tuberculosis treatment-related hepatotoxicity (114,116). GSTP1 gene polymorphism may also
be used as an independent prognostic marker for patients with HCC
(117). GSTP1 Ile105Val may be
associated with an increased risk of CRC (118,116). The Ile105Val polymorphism of the
GSTP1 gene may also have a genetic effect on the occurrence of skin
cancer (119,120). The GSTP1 polymorphism has been
shown to be a potential biomarker for PCa risk (121,122). The GSTP1 Ile105Val polymorphism
may also be associated with the risk of gastric cancer (123,124), and the GSTP1 Val allele appears
to reduce the risk of premalignant lesions (125,126). An interesting finding is that the
GSTP1 *C variant may exert protective effects against pancreatic
cancer in the elderly (127). It
has been demonstrated that GSTP1 is associated with response to
chemotherapy, PFS and OS in patients with osteosarcoma, and GSTP1
polymorphism may help design individualized treatments (128,129). GSTP1 gene polymorphism may also
play an important role in the prognosis of osteosarcoma patients
treated with chemotherapy (130,131). In addition, GSTP1 gene
polymorphism is associated with risk of oral SCC (132). The GSTP1 codon 105 polymorphism
may play a major role in leukemia by altering the protein function
and reducing its ability to detoxify certain mutagens and
carcinogens, which may result in increased DNA damage and mutations
that increase cancer risk (133).
An individual with at least one Val allele at codon 105 of the
GSTP1 enzyme may be susceptible to cancer. Following cytotoxic
chemotherapy, the Val allele at GSTP1 codon 105 may result in
treatment-related AML (134). It
has been reported that GSTP1 polymorphism is important for the
development of AML and the formation of AML-specific chromosomal
abnormalities (135,136). Variant genotypes of the GSTP1
Ile105Val gene polymorphism may contribute to the risk of chronic
myelogenous leukemia (137-139). Therefore, GSTP1 gene polymorphism
may contribute to the treatment of leukemia.
GSTP1 gene polymorphism is not only involved in the
development of cancer, but is also associated with a number of
other diseases (Table IV).
Diabetic neuropathy is a common complication of T2DM, and GSTP1
gene polymorphism may contribute to the development of T2DM
(140,141). There is no clinical proof that
GSTP1 IIe105Val polymorphisms affect the risk of gestational
diabetes mellitus in a Chinese population (142). A significant correlation has been
found between the GSTP1 (105)
Ile/(105)Ile genotype and the
development of grade ≥2 docetaxel (taxotere)-induced peripheral
neuropathy (143). It has been
demonstrated that the presence of the GSTP1 A114V rather than the
I105V variant increases the risk of motor neuron disease (MND).
Moreover, the combination of GSTP1 polymorphisms in codons 105 and
114 may result in protective reduction in the toxicity of
electrophilic compounds to organic and inorganic hydrogen peroxides
in MND patients (144). GSTP1
polymorphism plays a role in the incidence of chronic kidney
disease and is associated with higher numbers of micronuclei
(145,146). The GSTP1 Ile105Val genotype may
affect the excretion and metabolism of inorganic arsenic (147). Furthermore, changes in GSTP1 may
affect the risk of non-photo-induced drug eruptions (148). It has been found that the GSTP1
Ile105Val polymorphism is also associated with inter-individual
variations in urinary and blood arsenic levels (132). Thus, GSTP1 gene polymorphism is
closely associated with various diseases and may prove to be
helpful for the development of therapeutic drugs.
GSTP1 may mediate the storage and transport
mechanisms of gases and react with some compounds. Nitric oxide
(NO) plays an important role in cell signaling, blood pressure,
coagulation, and tumor cell killing. A novel GSTP1 and multidrug
resistance protein 1-mediated NO storage and transport mechanism
(MRP1/ABCC1) protects an M1-macrophage model from the effect of NO
(149-153). GSTP1 also reduces the inducible
NO synthase (iNOS) protein level. GSTP1 regulates iNOS by affecting
S-nitrosylation, dimerization and stability (154). It is also associated with some
chemicals in the human body, and 1-octyl-3-methylimidazolium
bromide upregulates GSTP1 (155).
The compound 4b ('p-cyano-PABA/NO') is more favorable for product
distribution in the presence of GSTP1 (156). GSTP1 is also involved in
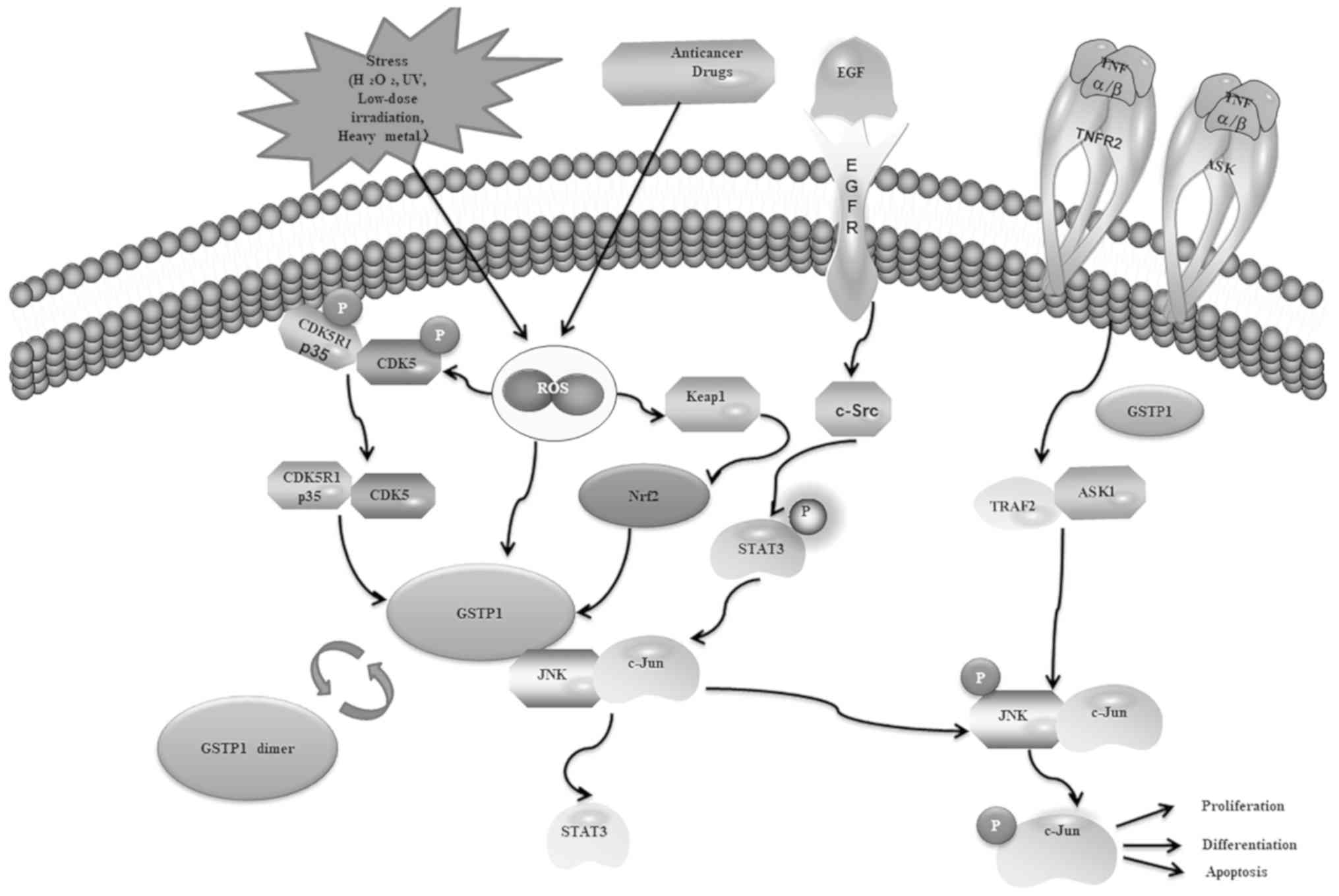
endogenous regulation of cellular signaling pathways (Fig. 2). The c-Jun N-terminal kinase
(JNK)-mediated cell signaling pathway is endogenously regulated by
protein-protein interactions with GSTP1 (157). It is believed that there is a
direct interaction between the C-terminus of JNK and GSTP1, and
GSTP1 is considered to act as a key ligand-binding protein that
regulates the kinase pathway (158). GSTP1 binds to mitogen-activated
protein kinase JNK and inhibits JNK downstream signaling (159). Epidermal growth factor receptor
phosphorylation of GSTP1 enhances JNK signaling and provides a
survival advantage for tumors (160). GSTP1 acts as a direct inhibitor
of JNK in vivo to regulate constitutive expression of
specific downstream molecular targets of the JNK signaling pathway
(161). The mechanism of GSTP1
protection against serum depletion-induced cell death is mediated
through an apoptosis signal-regulating kinase 1 (ASK1) pathway,
ASK1-MKK7-JNK (162). There may
also be a novel non-enzymatic effect of GSTP, which plays an
important role in the regulation of the classical ERα signaling
pathway by modification of transcriptional cofactors, such as
receptor interacting protein 140 (163). Increased levels of GSTP1 may be
another mechanism regulating cyclin-dependent kinase-5 signaling,
eliminating oxidative stress, and preventing neurodegeneration
(164). Transcriptional
activation of the GSTP1 gene is also regulated by the Nrf2 pathway
(165). GSTP1 plays an important
role in the regulation of signal transduction. Therefore, GSTP1 may
be involved in complex processes mediated by multiple factors and
multiple signals.
As an important phase II detoxification enzyme,
GSTP1 is involved in the development and progression of various
types of cancer. The expression, methylation and genetic
polymorphisms of GSTP1 are closely associated with cancer. GSTP1
plays an important regulatory role in the metabolism,
detoxification and elimination of potentially genotoxic foreign
complexes. Therefore, GSTP1 may act as a critical regulator in the
occurrence and development of multiple cancer types.
The present study was supported by grants from the
Hunan Natural Science Foundation (no. 2019JJ40238), the Defense
Industrial Technology Development Program (no. JCKY2016403C001),
the National Natural Science Foundation of China (no. 81400117),
the China Postdoctoral Science Foundation (no. 2014 M562115) and
the Research Initiation Funding of University of South China for
the Returned Scholars From Abroad (no. 2014XQD46) and Hunan
Province University Students Study and Innovation Pilot Project
(no. S201910555122).
Not applicable.
JC made substantial contributions to conception and
design of the study, acquisition, analysis and interpretation of
data, revised the manuscript critically for important intellectual
content, and was a major contributor to writing the manuscript. GL,
JY, LL, YT, HW, BL, LD, JT and YC were involved in drafting the
manuscript. LY made substantial contributions to the conception and
design of the study, acquisition, analysis and interpretation of
data, and gave final approval of the version to be published.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Townsend DM and Tew KD: The role of
glutathione-S-transferase in anti-cancer drug resistance. Oncogene.
22:7369–7375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chatterjee A and Gupta S: The multifaceted
role of glutathione S-transferases in cancer. Cancer Lett.
433:33–42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eaton DL and Bammler TK: Concise review of
the glutathione S-transferases and their significance to
toxicology. Toxicol Sci. 49:156–164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ladner JE, Parsons JF, Rife CL, Gilliland
GL and Armstrong RN: Parallel evolutionary pathways for glutathione
transferases: Structure and mechanism of the mitochondrial class
kappa enzyme rGSTK1-1. Biochemistry. 43:352–361. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayes JD, Flanagan JU and Jowsey IR:
Glutathione transferases. Annu Rev Pharmacol Toxicol. 45:51–88.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jakobsson PJ, Morgenstern R, Mancini J,
Ford-Hutchinson A and Persson B: Common structural features of
MAPEG - a widespread superfamily of membrane associated proteins
with highly divergent functions in eicosanoid and glutathione
metabolism. Protein Sci. 8:689–692. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Board PG, Coggan M, Chelvanayagam G,
Easteal S, Jermiin LS, Schulte GK, Danley DE, Hoth LR, Griffor MC,
Kamath AV, et al: Identification, characterization, and crystal
structure of the Omega class glutathione transferases. J Biol Chem.
275:24798–24806. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyanishi K, Takayama T, Ohi M, Hayashi T,
Nobuoka A, Nakajima T, Takimoto R, Kogawa K, Kato J, Sakamaki S, et
al: Glutathione S-transferase-pi overexpression is closely
associated with K-ras mutation during human colon carcinogenesis.
Gastroenterology. 121:865–874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morel F, Rauch C, Petit E, Piton A, Theret
N, Coles B and Guillouzo A: Gene and protein characterization of
the human glutathione S-transferase kappa and evidence for a
peroxisomal localization. J Biol Chem. 279:16246–16253. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nordgard SH, Ritchie MD, Jensrud SD,
Motsinger AA, Alnaes GI, Lemmon G, Berg M, Geisler S, Moore JH,
Lønning PE, et al: ABCB1 and GST polymorphisms associated with TP53
status in breast cancer. Pharmacogenet Genomics. 17:127–136. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vasieva O: The many faces of glutathione
transferase pi. Curr Mol Med. 11:129–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hollman AL, Tchounwou PB and Huang HC: The
Association between Gene-Environment Interactions and Diseases
Involving the Human GST Superfamily with SNP Variants. Int J
Environ Res Public Health. 13:3792016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bolt HM and Thier R: Relevance of the
deletion polymorphisms of the glutathione S-transferases GSTT1 and
GSTM1 in pharmacology and toxicology. Curr Drug Metab. 7:613–628.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pinheiro DS, Rocha Filho CR, Mundim CA,
Júnior PM, Ulhoa CJ, Reis AA and Ghedini PC: Evaluation of
glutathione S-transferase GSTM1 and GSTT1 deletion polymorphisms on
type-2 diabetes mellitus risk. PLoS One. 8:e762622013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin Z, Ivanov VN, Habelhah H, Tew K and
Ronai Z: Glutathione S-transferase p elicits protection against
H2O2-induced cell death via coordinated regulation of stress
kinases. Cancer Res. 60:4053–4057. 2000.PubMed/NCBI
|
|
16
|
Singh S: Cytoprotective and regulatory
functions of glutathione S-transferases in cancer cell
proliferation and cell death. Cancer Chemother Pharmacol. 75:1–15.
2015. View Article : Google Scholar
|
|
17
|
Salinas AE and Wong MG: Glutathione
S-transferases - a review. Curr Med Chem. 6:279–309.
1999.PubMed/NCBI
|
|
18
|
Hayes JD and Pulford DJ: The glutathione
S-transferase supergene family: Regulation of GST and the
contribution of the isoenzymes to cancer chemoprotection and drug
resistance. Crit Rev Biochem Mol Biol. 30:445–600. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McIlwain CC, Townsend DM and Tew KD:
Glutathione S-transferase polymorphisms: Cancer incidence and
therapy. Oncogene. 25:1639–1648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hung RJ, van der Hel O, Tavtigian SV,
Brennan P, Boffetta P and Hashibe M: Perspectives on the molecular
epidemiology of aerodigestive tract cancers. Mutat Res.
592:102–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schnekenburger M, Karius T and Diederich
M: Regulation of epigenetic traits of the glutathione S-transferase
P1 gene: From detoxification toward cancer prevention and
diagnosis. Front Pharmacol. 5:1702014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brockmöller J, Cascorbi I, Kerb R and
Roots I: Combined analysis of inherited polymorphisms in arylamine
N-acetyltransferase 2, glutathione S-transferases M1 and T1,
microsomal epoxide hydrolase, and cytochrome P450 enzymes as
modulators of bladder cancer risk. Cancer Res. 56:3915–3925.
1996.PubMed/NCBI
|
|
23
|
Cowell IG, Dixon KH, Pemble SE, Ketterer B
and Taylor JB: The structure of the human glutathione S-transferase
pi gene. Biochem J. 255:79–83. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laborde E: Glutathione transferases as
mediators of signaling pathways involved in cell proliferation and
cell death. Cell Death Differ. 17:1373–1380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moyer AM, Salavaggione OE, Wu TY, Moon I,
Eckloff BW, Hildebrandt MA, Schaid DJ, Wieben ED and Weinshilboum
RM: Glutathione s-transferase p1: Gene sequence variation and
functional genomic studies. Cancer Res. 68:4791–4801. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sau A, Pellizzari Tregno F, Valentino F,
Federici G and Caccuri AM: Glutathione transferases and development
of new principles to overcome drug resistance. Arch Biochem
Biophys. 500:116–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Henderson CJ, McLaren AW, Moffat GJ, Bacon
EJ and Wolf CR: Pi-class glutathione S-transferase: Regulation and
function. Chem Biol Interact. 111-112:69–82. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nelson WG, De Marzo AM, Deweese TL, Lin X,
Brooks JD, Putzi MJ, Nelson CP, Groopman JD and Kensler TW:
Preneoplastic prostate lesions: An opportunity for prostate cancer
prevention. Ann N Y Acad Sci. 952:135–144. 2001. View Article : Google Scholar
|
|
29
|
Nelson WG, DeWeese TL and DeMarzo AM: The
diet, prostate inflammation, and the development of prostate
cancer. Cancer Metastasis Rev. 21:3–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sawers L, Ferguson MJ, Ihrig BR, Young HC,
Chakravarty P, Wolf CR and Smith G: Glutathione S-transferase P1
(GSTP1) directly influences platinum drug chemosensitivity in
ovarian tumour cell lines. Br J Cancer. 111:1150–1158. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hagrman D, Goodisman J and Souid AK:
Kinetic study on the reactions of platinum drugs with glutathione.
J Pharmacol Exp Ther. 308:658–666. 2004. View Article : Google Scholar
|
|
32
|
Zhang J, Wu Y, Hu X, Wang B, Wang L, Zhang
S, Cao J and Wang Z: GSTT1, GSTP1, and GSTM1 genetic variants are
associated with survival in previously untreated metastatic breast
cancer. Oncotarget. 8:105905–105914. 2017.PubMed/NCBI
|
|
33
|
Duvoix A, Schnekenburger M, Delhalle S,
Blasius R, Borde-Chiché P, Morceau F, Dicato M and Diederich M:
Expression of glutathione S-transferase P1-1 in leukemic cells is
regulated by inducible AP-1 binding. Cancer Lett. 216:207–219.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alqarni MH, Muharram MM and Labrou NE:
Ligand-induced glutathione transferase degradation as a therapeutic
modality: Investigation of a new metal-mediated affinity cleavage
strategy for human GSTP1-1. Int J Biol Macromol. 116:84–90. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pajaud J, Ribault C, Ben Mosbah I, Rauch
C, Henderson C, Bellaud P, Aninat C, Loyer P, Morel F and Corlu A:
Glutathione transferases P1/P2 regulate the timing of signaling
pathway activations and cell cycle progression during mouse liver
regeneration. Cell Death Dis. 6:e15982015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morceau F, Duvoix A, Delhalle S,
Schnekenburger M, Dicato M and Diederich M: Regulation of
glutathione S-transferase P1-1 gene expression by NF-kappaB in
tumor necrosis factor alpha-treated K562 leukemia cells. Biochem
Pharmacol. 67:1227–1238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Duvoix A, Morceau F, Delhalle S, Schmitz
M, Schnekenburger M, Galteau MM, Dicato M and Diederich M:
Induction of apoptosis by curcumin: Mediation by glutathione
S-transferase P1-1 inhibition. Biochem Pharmacol. 66:1475–1483.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Duvoix A, Delhalle S, Blasius R,
Schnekenburger M, Morceau F, Fougère M, Henry E, Galteau MM, Dicato
M and Diederich M: Effect of chemopreventive agents on glutathione
S-transferase P1-1 gene expression mechanisms via activating
protein 1 and nuclear factor kappaB inhibition. Biochem Pharmacol.
68:1101–1111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moscow JA, Fairchild CR, Madden MJ, Ransom
DT, Wieand HS, O'Brien EE, Poplack DG, Cossman J, Myers CE and
Cowan KH: Expression of anionic glutathione-S-transferase and
P-glycoprotein genes in human tissues and tumors. Cancer Res.
49:1422–1428. 1989.PubMed/NCBI
|
|
40
|
Mian OY, Khattab MH, Hedayati M, Coulter
J, Abubaker-Sharif B, Schwaninger JM, Veeraswamy RK, Brooks JD,
Hopkins L, Shinohara DB, et al: GSTP1 Loss results in accumulation
of oxidative DNA base damage and promotes prostate cancer cell
survival following exposure to protracted oxidative stress.
Prostate. 76:199–206. 2016. View Article : Google Scholar :
|
|
41
|
Rahbar MH, Samms-Vaughan M, Ma J, Bressler
J, Dickerson AS, Hessabi M, Loveland KA, Grove ML,
Shakespeare-Pellington S, Beecher C, et al: Synergic effect of
GSTP1 and blood manganese concentrations in Autism Spectrum
Disorder. Res Autism Spectr Disord. 18:73–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bhang SY, Cho SC, Kim JW, Hong YC, Shin
MS, Yoo HJ, Cho IH, Kim Y and Kim BN: Relationship between blood
manganese levels and children's attention, cognition, behavior, and
academic performance--a nationwide cross-sectional study. Environ
Res. 126:9–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rahbar MH, Samms-Vaughan M, Lee M,
Christian MA, Bressler J, Hessabi M, Grove ML,
Shakespeare-Pellington S, Desai CC, Reece JA, et al: Interaction
between manganese and GSTP1 in relation to autism spectrum disorder
while controlling for exposure to mixture of lead, mercury,
arsenic, and cadmium. Res Autism Spectr Disord. 55:50–63. 2018.
View Article : Google Scholar
|
|
44
|
Wright LS, Kornguth SE, Oberley TD and
Siegel FL: Effects of lead on glutathione S-transferase expression
in rat kidney: A dose-response study. Toxicol Sci. 46:254–259.
1998.
|
|
45
|
Li C, Yang X, Xu M, Zhang J and Sun N:
Association between GSTP1 CpG methylation and the early phase of
lead exposure. Toxicol Mech Methods. 24:111–115. 2014. View Article : Google Scholar
|
|
46
|
Kwong YL and Todd D: Delicious poison:
Arsenic trioxide for the treatment of leukemia. Blood.
89:3487–3488. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu J, Chen H, Miller DS, Saavedra JE,
Keefer LK, Johnson DR, Klaassen CD and Waalkes MP: Overexpression
of glutathione S-transferase II and multidrug resistance transport
proteins is associated with acquired tolerance to inorganic
arsenic. Mol Pharmacol. 60:302–309. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Parker LJ, Bocedi A, Ascher DB, Aitken JB,
Harris HH, Lo Bello M, Ricci G, Morton CJ and Parker MW:
Glutathione transferase P1-1 as an arsenic drug-sequestering
enzyme. Protein Sci. 26:317–326. 2017. View Article : Google Scholar
|
|
49
|
González-Martínez F, Sánchez-Rodas D,
Cáceres DD, Martínez MF, Quiñones LA and Johnson-Restrepo B:
Arsenic exposure, profiles of urinary arsenic species, and
polymorphism effects of glutathione-s-transferase and
metallothioneins. Chemosphere. 212:927–936. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou L, Jing Y, Styblo M, Chen Z and
Waxman S: Glutathione-S-transferase pi inhibits
As2O3-induced apoptosis in lymphoma cells:
Involvement of hydrogen peroxide catabolism. Blood. 105:1198–1203.
2005. View Article : Google Scholar
|
|
51
|
Van Dyck E, Nazarov PV, Muller A, Nicot N,
Bosseler M, Pierson S, Van Moer K, Palissot V, Mascaux C, Knolle U,
et al: Bronchial airway gene expression in smokers with lung or
head and neck cancer. Cancer Med. 3:322–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Krais AM, Mühlbauer KR, Kucab JE, Chinbuah
H, Cornelius MG, Wei QX, Hollstein M, Phillips DH, Arlt VM and
Schmeiser HH: Comparison of the metabolic activation of
environmental carcinogens in mouse embryonic stem cells and mouse
embryonic fibroblasts. Toxicol In Vitro. 29:34–43. 2015. View Article : Google Scholar
|
|
53
|
Gilliland FD, Harms HJ, Crowell RE, Li YF,
Willink R and Belinsky SA: Glutathione S-transferase P1 and NADPH
quinone oxidoreductase polymorphisms are associated with aberrant
promoter methylation of P16(INK4a) and O(6)-methylguanine-DNA
methyltransferase in sputum. Cancer Res. 62:2248–2252.
2002.PubMed/NCBI
|
|
54
|
Xue B, Wu Y, Yin Z, Zhang H, Sun S, Yi T
and Luo L: Regulation of lipopolysaccharide-induced inflammatory
response by glutathione S-transferase P1 in RAW264.7 cells. FEBS
Lett. 579:4081–4087. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yi L, Li L, Yin J, Hu N, Li G and Ding D:
Proteomics analysis of liver tissues from C57BL/6J mice receiving
low-dose 137Cs radiation. Environ Sci Pollut Res Int. 23:2549–2556.
2016. View Article : Google Scholar
|
|
56
|
Yi L, Hu N, Yin J, Sun J, Mu H, Dai K and
Ding D: Up-regulation of calreticulin in mouse liver tissues after
long-term irradiation with low-dose-rate gamma rays. PLoS One.
12:e01826712017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lu M, Xia L, Luo D, Waxman S and Jing Y:
Dual effects of glutathione-S-transferase pi on
As2O3 action in prostate cancer cells:
Enhancement of growth inhibition and inhibition of apoptosis.
Oncogene. 23:3945–3952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fujikawa Y, Nampo T, Mori M, Kikkawa M and
Inoue H: Fluorescein diacetate (FDA) and its analogue as substrates
for Pi-class glutathione S-transferase (GSTP1) and their biological
application. Talanta. 179:845–852. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang J, Ren JH, Lin B, Jing YK and Cheng
MS: Advances of studies on glutathione-S-transferase π. Zhongguo
Yaowu Huaxue Zazhi. 15:251–256. 2005.
|
|
60
|
Ranganathan S and Tew KD:
Immunohistochemical localization of glutathione S-transferases
alpha, mu, and pi in normal tissue and carcinomas from human colon.
Carcinogenesis. 12:2383–2387. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang R, Kang KA, Piao MJ, Kim KC, Zheng
J, Yao CW, Cha JW, Maeng YH, Chang WY, Moon PG, et al: Epigenetic
alterations are involved in the overexpression of glutathione
S-transferase π-1 in human colorectal cancers. Int J Oncol.
45:1275–1283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ding XQ, Bi JH, Ma ZG, Jiang ZX, Xi JF,
Liu PZ and Zhang B: Correlation of ERCC1 and GSTP1 expression in
esophageal cancer tissue with platinum-based chemotherapy
sensitivity as well as apoptosis and proliferation gene expression.
Hainan Yixueyuan Xuebao. 23:103–107. 2017.
|
|
63
|
Song Y, Du Y, Zhou Q, Ma J, Yu J, Tao X
and Zhang F: Association of GSTP1 Ile105Val polymorphism with risk
of esophageal cancer: A meta-analysis of 21 case-control studies.
Int J Clin Exp Med. 7:3215–3224. 2014.PubMed/NCBI
|
|
64
|
Pljesa-Ercegovac M, Savic-Radojevic A,
Dragicevic D, Mimic-Oka J, Matic M, Sasic T, Pekmezovic T,
Vuksanovic A and Simic T: Enhanced GSTP1 expression in transitional
cell carcinoma of urinary bladder is associated with altered
apoptotic pathways. Urol Oncol. 29:70–77. 2011. View Article : Google Scholar
|
|
65
|
Savic-Radojevic A, Mimic-Oka J,
Pljesa-Ercegovac M, Opacic M, Dragicevic D, Kravic T, Djokic M,
Micic S and Simic T: Glutathione S-transferase-P1 expression
correlates with increased antioxidant capacity in transitional cell
carcinoma of the urinary bladder. Eur Urol. 52:470–477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gao L, Fang YQ, Zhang TY, Ge B, Xu B,
Huang JF, Zhang ZF and Tan N: GSTP1 arrests bladder cancer T24
cells in G0/G1 phase and up-regulates p21 expression. Int J Clin
Exp Med. 7:2984–2991. 2014.PubMed/NCBI
|
|
67
|
Oguztuzun Serpil, Ergün Duygu, Kilic
Murat, Bozer Busra, Simsek Gulcin Güler and Bulus Hakan: The
expression of GST and CYP isoenzymes in thyroid nodular hyperplasia
and papillary thyroid cancer tissue: Correlation with clinical
parameters. Entomol Appl Sci Lett. 5:97–103. 2016.
|
|
68
|
Dong X, Yang Y, Zhou Y, Bi X, Zhao N,
Zhang Z, Li L, Hang Q, Zhang R, Chen D, et al: Glutathione
S-transferases P1 protects breast cancer cell from
adriamycin-induced cell death through promoting autophagy. Cell
Death Differ. 26:2086–2099. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu X, Tan N, Liao H, Pan G, Xu Q, Zhu R,
Zou L, He S and Zhu H: High GSTP1 inhibits cell proliferation by
reducing Akt phosphorylation and is associated with a better
prognosis in hepatocellular carcinoma. Oncotarget. 9:8957–8971.
2017.
|
|
70
|
Depeille P, Cuq P, Passagne I, Evrard A
and Vian L: Combined effects of GSTP1 and MRP1 in melanoma drug
resistance. Br J Cancer. 93:216–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lin C, Xie L, Lu Y, Hu Z and Chang J:
miR-133b reverses cisplatin resistance by targeting GSTP1 in
cisplatin-resistant lung cancer cells. Int J Mol Med. 41:2050–2058.
2018.PubMed/NCBI
|
|
72
|
Zong C, Wang J and Shi TM: MicroRNA 130b
enhances drug resistance in human ovarian cancer cells. Tumour
Biol. 35:12151–12156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Uchida Y, Chiyomaru T, Enokida H, Kawakami
K, Tatarano S, Kawahara K, Nishiyama K, Seki N and Nakagawa M:
MiR-133a induces apoptosis through direct regulation of GSTP1 in
bladder cancer cell lines. Urol Oncol. 31:115–123. 2013. View Article : Google Scholar
|
|
74
|
Mutallip M, Nohata N, Hanazawa T, Kikkawa
N, Horiguchi S, Fujimura L, Kawakami K, Chiyomaru T, Enokida H,
Nakagawa M, et al: Glutathione S-transferase P1 (GSTP1) suppresses
cell apoptosis and its regulation by miR-133α in head and neck
squamous cell carcinoma (HNSCC). Int J Mol Med. 27:345–352.
2011.
|
|
75
|
Zhu J, Zhang R, Yang D, Li J, Yan X, Jin
K, Li W, Liu X, Zhao J, Shang W, et al: Knockdown of Long
Non-Coding RNA XIST Inhibited Doxorubicin Resistance in Colorectal
Cancer by Upregulation of miR-124 and Downregulation of SGK1. Cell
Physiol Biochem. 51:113–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang X, Zhu J, Xing R, Tie Y, Fu H, Zheng
X and Yu B: miR-513a-3p sensitizes human lung adenocarcinoma cells
to chemotherapy by targeting GSTP1. Lung Cancer. 77:488–494. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Song X, Wang R, Xiao F, Zhang SX, Gong M,
Wang XL, Zhang Y and Huang JY: Expressions of glutathione
S-transferase P1 and 4-hydroxynonenal and the progression of
prostate cancer. Zhonghua Nan Ke Xue. 23:412–416. 2017.In
Chinese.
|
|
78
|
Singh S, Shukla GC and Gupta S: MicroRNA
Regulating Glutathione S-Transferase P1 in Prostate Cancer. Curr
Pharmacol Rep. 1:79–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kanwal R, Pandey M, Bhaskaran N, Maclennan
GT, Fu P, Ponsky LE and Gupta S: Protection against oxidative DNA
damage and stress in human prostate by glutathione S-transferase
P1. Mol Carcinog. 53:8–18. 2014. View Article : Google Scholar :
|
|
80
|
Miyake T, Nakayama T, Naoi Y, Yamamoto N,
Otani Y, Kim SJ, Shimazu K, Shimomura A, Maruyama N, Tamaki Y, et
al: GSTP1 expression predicts poor pathological complete response
to neoadjuvant chemotherapy in ER-negative breast cancer. Cancer
Sci. 103:913–920. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gurioli G, Martignano F, Salvi S,
Costantini M, Gunelli R and Casadio V: GSTP1 methylation in cancer:
A liquid biopsy biomarker? Clin Chem Lab Med. 56:702–717. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li Y, Cai Y, Chen H and Mao L: Clinical
significance and association of GSTP1 hypermethylation with
hepatocellular carcinoma: A meta-analysis. J Cancer Res Ther.
14(Suppl): S486–S489. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li QF, Li QY, Gao AR and Shi QF:
Correlation between promoter methylation in the GSTP1 gene and
hepatocellular carcinoma development: A meta-analysis. Genet Mol
Res. 14:6762–6772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Jain S, Chen S, Chang KC, Lin YJ, Hu CT,
Boldbaatar B, Hamilton JP, Lin SY, Chang TT, Chen SH, et al: Impact
of the location of CpG methylation within the GSTP1 gene on its
specificity as a DNA marker for hepatocellular carcinoma. PLoS One.
7:e357892012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Qu Z, Jiang Y, Li H, Yu DC and Ding YT:
Detecting abnormal methylation of tumor suppressor genes GSTP1,
P16, RIZ1, and RASSF1A in hepatocellular carcinoma and its clinical
significance. Oncol Lett. 10:2553–2558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gao S, Sun FK, Fan YC, Shi CH, Zhang ZH,
Wang LY and Wang K: Aberrant GSTP1 promoter methylation predicts
short-term prognosis in acute-on-chronic hepatitis B liver failure.
Aliment Pharmacol Ther. 42:319–329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Costa VL, Henrique R and Jerónimo C:
Epigenetic markers for molecular detection of prostate cancer. Dis
Markers. 23:31–41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Goering W, Kloth M and Schulz WA: DNA
methylation changes in prostate cancer. Methods Mol Biol.
863:47–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Maldonado L, Brait M, Loyo M, Sullenberger
L, Wang K, Peskoe SB, Rosenbaum E, Howard R, Toubaji A, Albadine R,
et al: GSTP1 promoter methylation is associated with recurrence in
early stage prostate cancer. J Urol. 192:1542–1548. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zelic R, Fiano V, Zugna D, Grasso C,
Delsedime L, Daniele L, Galliano D, Pettersson A, Gillio-Tos A,
Merletti F, et al: Global Hypomethylation (LINE-1) and
Gene-Specific Hypermethylation (GSTP1) on Initial Negative Prostate
Biopsy as Markers of Prostate Cancer on a Rebiopsy. Clin Cancer
Res. 22:984–992. 2016. View Article : Google Scholar
|
|
91
|
Lee WH, Morton RA, Epstein JI, Brooks JD,
Campbell PA, Bova GS, Hsieh WS, Isaacs WB and Nelson WG: Cytidine
methylation of regulatory sequences near the pi-class glutathione
S-transferase gene accompanies human prostatic carcinogenesis. Proc
Natl Acad Sci USA. 91:11733–11737. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Haluskova J, Lachvac L and Nagy V: The
investigation of GSTP1, APC and RASSF1 gene promoter
hypermethylation in urine DNA of prostate-diseased patients.
Bratisl Lek Listy. 116:79–82. 2015.PubMed/NCBI
|
|
93
|
Lee JS: GSTP1 promoter hypermethylation is
an early event in breast carcinogenesis. Virchows Arch.
450:637–642. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Alnaes GI, Ronneberg JA, Kristensen VN and
Tost J: Heterogeneous DNA Methylation Patterns in the GSTP1
Promoter Lead to Discordant Results between Assay Technologies and
Impede Its Implementation as Epigenetic Biomarkers in Breast
Cancer. Genes (Basel). 6:878–900. 2015. View Article : Google Scholar
|
|
95
|
Miyake T, Nakayama T, Kagara N, Yamamoto
N, Nakamura Y, Otani Y, Uji K, Naoi Y, Shimoda M, Maruyama N, et
al: Association of GSTP1 methylation with aggressive phenotype in
ER-positive breast cancer. Anticancer Res. 33:5617–5623.
2013.PubMed/NCBI
|
|
96
|
Muggerud AA, Rønneberg JA, Wärnberg F,
Botling J, Busato F, Jovanovic J, Solvang H, Bukholm I,
Børresen-Dale AL, Kristensen VN, et al: Frequent aberrant DNA
methylation of ABCB1, FOXC1, PPP2R2B and PTEN in ductal carcinoma
in situ and early invasive breast cancer. Breast Cancer Res.
12:R32010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Feng W, Shen L, Wen S, Rosen DG, Jelinek
J, Hu X, Huan S, Huang M, Liu J, Sahin AA, et al: Correlation
between CpG methylation profiles and hormone receptor status in
breast cancers. Breast Cancer Res. 9:R572007. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sunami E, Shinozaki M, Sim MS, Nguyen SL,
Vu AT, Giuliano AE and Hoon DS: Estrogen receptor and HER2/neu
status affect epigenetic differences of tumor-related genes in
primary breast tumors. Breast Cancer Res. 10:R462008. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Song B, Wang L, Zhang Y, Li N, Dai H, Xu
H, Cai H and Yan J: Combined Detection of HER2, Ki67, and GSTP1
Genes on the Diagnosis and Prognosis of Breast Cancer. Cancer
Biother Radiopharm. 34:85–90. 2019. View Article : Google Scholar
|
|
100
|
Yanagawa N, Tamura G, Oizumi H, Takahashi
N, Shimazaki Y and Motoyama T: Promoter hypermethylation of tumor
suppressor and tumor-related genes in non-small cell lung cancers.
Cancer Sci. 94:589–592. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Russo AL, Thiagalingam A, Pan H, Califano
J, Cheng KH, Ponte JF, Chinnappan D, Nemani P, Sidransky D and
Thiagalingam S: Differential DNA hypermethylation of critical genes
mediates the stage-specific tobacco smoke-induced neoplastic
progression of lung cancer. Clin Cancer Res. 11:2466–2470. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Vaissière T, Hung RJ, Zaridze D, Moukeria
A, Cuenin C, Fasolo V, Ferro G, Paliwal A, Hainaut P, Brennan P, et
al: Quantitative analysis of DNA methylation profiles in lung
cancer identifies aberrant DNA methylation of specific genes and
its association with gender and cancer risk factors. Cancer Res.
69:243–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Haroun RA, Zakhary NI, Mohamed MR,
Abdelrahman AM, Kandil EI and Shalaby KA: Assessment of the
prognostic value of methylation status and expression levels of
FHIT, GSTP1 and p16 in non-small cell lung cancer in Egyptian
patients. Asian Pac J Cancer Prev. 15:4281–4287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Drilon A, Sugita H, Sima CS, Zauderer M,
Rudin CM, Kris MG, Rusch VW and Azzoli CG: A prospective study of
tumor suppressor gene methylation as a prognostic biomarker in
surgically resected stage I to IIIA non-small-cell lung cancers. J
Thorac Oncol. 9:1272–1277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kim DS, Cha SI, Lee JH, Lee YM, Choi JE,
Kim MJ, Lim JS, Lee EB, Kim CH, Park TI, et al: Aberrant DNA
methylation profiles of non-small cell lung cancers in a Korean
population. Lung Cancer. 58:1–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gumy-Pause F, Pardo B, Khoshbeen-Boudal M,
Ansari M, Gayet-Ageron A, Sappino AP, Attiyeh EF and Ozsahin H:
GSTP1 hypermethylation is associated with reduced protein
expression, aggressive disease and prognosis in neuroblastoma.
Genes Chromosomes Cancer. 51:174–185. 2012. View Article : Google Scholar
|
|
107
|
Ferraù F, Romeo PD, Puglisi S, Ragonese M,
Spagnolo F, Salpietro C, Ientile R, Currò M, Visalli G, Alibrandi
A, et al: GSTP1 gene methylation and AHR rs2066853 variant predict
resistance to first generation somatostatin analogs in patients
with acromegaly. J Endocrinol Invest. 2018.
|
|
108
|
Ali-Osman F, Akande O, Antoun G, Mao JX
and Buolamwini J: Molecular cloning, characterization, and
expression in Escherichia coli of full-length cDNAs of three human
glutathione S-transferase Pi gene variants. Evidence for
differential catalytic activity of the encoded proteins. J Biol
Chem. 272:10004–10012. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Cote ML, Chen W, Smith DW, Benhamou S,
Bouchardy C, Butkiewicz D, Fong KM, Gené M, Hirvonen A, Kiyohara C,
et al: Meta- and pooled analysis of GSTP1 polymorphism and lung
cancer: A HuGE-GSEC review. Am J Epidemiol. 169:802–814. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Pérez-Ramírez C, Cañadas-Garre M, Alnatsha
A, Villar E, Delgado JR, Calleja-Hernández MÁ and Faus-Dáder MJ:
Impact of DNA repair, folate and glutathione gene polymorphisms on
risk of non small cell lung cancer. Pathol Res Pract. 214:44–52.
2018. View Article : Google Scholar
|
|
111
|
Saarikoski ST, Voho A, Reinikainen M,
Anttila S, Karjalainen A, Malaveille C, Vainio H,
Husgafvel-Pursiainen K and Hirvonen A: Combined effect of
polymorphic GST genes on individual susceptibility to lung cancer.
Int J Cancer. 77:516–521. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li XM, Yu XW, Yuan Y, Pu MZ, Zhang HX,
Wang KJ and Han XD: Glutathione S-transferase P1, gene-gene
interaction, and lung cancer susceptibility in the Chinese
population: An updated meta-analysis and review. J Cancer Res Ther.
11:565–570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Tarek F: Role of glutathione S-transferase
P-1 (GSTP-1) gene polymorphism in COPD patients. Egypt J Chest Dis
Tuberc. 65:739–744. 2016. View Article : Google Scholar
|
|
114
|
Lakhdar R, Denden S, Knani J, Leban N,
Daimi H, Hassine M, Lefranc G, Ben Chibani J and Haj Khelil A:
Relationship between glutathione S-transferase P1 polymorphisms and
chronic obstructive pulmonary disease in a Tunisian population.
Genet Mol Res. 9:897–907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wang Y, Ren BU, Zhang L and Guo Z:
Correlation between metabolic enzyme GSTP1 polymorphisms and
susceptibility to lung cancer. Exp Ther Med. 10:1521–1527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
He L, Gao L, Shi Z, Li Y, Zhu L, Li S,
Zhang P, Zheng G, Ren Q, Li Y, et al: Involvement of cytochrome
P450 1A1 and glutathione S-transferase P1 polymorphisms and
promoter hypermethylation in the progression of anti-tuberculosis
drug-induced liver injury: A case-control study. PLoS One.
10:e01194812015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Qu K, Liu SS, Wang ZX, Huang ZC, Liu SN,
Chang HL, Xu XS, Lin T, Dong YF and Liu C: Polymorphisms of
glutathione S-transferase genes and survival of resected
hepatocellular carcinoma patients. World J Gastroenterol.
21:4310–4322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Song QB, Wang Q and Hu WG: A systemic
review of glutathione S-transferase P1 Ile105Val polymorphism and
colorectal cancer risk. Chin J Cancer Res. 26:255–267.
2014.PubMed/NCBI
|
|
119
|
Lei Z, Liu T, Li X, Xu X and Fan D:
Contribution of glutathione S-transferase gene polymorphisms to
development of skin cancer. Int J Clin Exp Med. 8:377–386.
2015.PubMed/NCBI
|
|
120
|
Lira MG, Provezza L, Malerba G, Naldi L,
Remuzzi G, Boschiero L, Forni A, Rugiu C, Piaserico S, Alaibac M,
et al: Glutathione S-transferase and CYP1A1 gene polymorphisms and
non-melanoma skin cancer risk in Italian transplanted patients. Exp
Dermatol. 15:958–965. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Gsur A, Haidinger G, Hinteregger S,
Bernhofer G, Schatzl G, Madersbacher S, Marberger M, Vutuc C and
Micksche M: Polymorphisms of glutathione-S-transferase genes
(GSTP1, GSTM1 and GSTT1) and prostate-cancer risk. Int J Cancer.
95:152–155. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Harries LW, Stubbins MJ, Forman D, Howard
GC and Wolf CR: Identification of genetic polymorphisms at the
glutathione S-transferase Pi locus and association with
susceptibility to bladder, testicular and prostate cancer.
Carcinogenesis. 18:641–644. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Chen ZH, Xian JF and Luo LP: Association
between GSTM1, GSTT1, and GSTP1 polymorphisms and gastric cancer
risk, and their interactions with environmental factors. Genet Mol
Res. 16:gmr160188772017. View Article : Google Scholar
|
|
124
|
Nguyen TV, Janssen MJ, van Oijen MG,
Bergevoet SM, te Morsche RH, van Asten H, Laheij RJ, Peters WH and
Jansent JB: Genetic polymorphisms in GSTA1, GSTP1, GSTT1, and GSTM1
and gastric cancer risk in a Vietnamese population. Oncol Res.
18:349–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Negovan A, Iancu M, Moldovan V, Mocan S
and Banescu C: The Interaction between GSTT1, GSTM1, and GSTP1
Ile105Val Gene Polymorphisms and Environmental Risk Factors in
Premalignant Gastric Lesions Risk. BioMed Res Int.
2017:73650802017. View Article : Google Scholar :
|
|
126
|
Tripathi S, Ghoshal U, Ghoshal UC, Mittal
B, Krishnani N, Chourasia D, Agarwal AK and Singh K: Gastric
carcinogenesis: Possible role of polymorphisms of GSTM1, GSTT1, and
GSTP1 genes. Scand J Gastroenterol. 43:431–439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Jiao L, Bondy ML, Hassan MM, Chang DZ,
Abbruzzese JL, Evans DB, Smolensky MH and Li D: Glutathione
S-transferase gene polymorphisms and risk and survival of
pancreatic cancer. Cancer. 109:840–848. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Liu S, Yi Z, Ling M, Shi J, Qiu Y and Yang
S: Predictive potential of ABCB1, ABCC3, and GSTP1 gene
polymorphisms on osteosarcoma survival after chemotherapy. Tumour
Biol. 35:9897–9904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wei L, Song XR, Wang XW, Li M and Zuo WS:
Expression of MDR1 and GST-pi in osteosarcoma and soft tissue
sarcoma and their correlation with chemotherapy resistance.
Zhonghua Zhong Liu Za Zhi. 28:445–448. 2006.In Chinese. PubMed/NCBI
|
|
130
|
Zhang SL, Mao NF, Sun JY, Shi ZC, Wang B
and Sun YJ: Predictive potential of glutathione S-transferase
polymorphisms for prognosis of osteosarcoma patients on
chemotherapy. Asian Pac J Cancer Prev. 13:2705–2709. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Yang LM, Li XH and Bao CF: Glutathione
S-transferase P1 and DNA polymorphisms influence response to
chemotherapy and prognosis of bone tumors. Asian Pac J Cancer Prev.
13:5883–5886. 2012. View Article : Google Scholar
|
|
132
|
Ben Ami T, Sarig O, Sprecher E and
Goldberg I: Glutathione S-transferase polymorphisms in patients
with photosensitive and non-photosensitive drug eruptions.
Photodermatol Photoimmunol Photomed. 35:214–220. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Rajesh D, Balakrishna S, Azeem Mohiyuddin
SM, Suryanarayana R and Kutty AVM: Novel association of oral
squamous cell carcinoma with GSTP1 Arg187Trp gene polymorphism. J
Cell Biochem. 120:5906–5912. 2019. View Article : Google Scholar
|
|
134
|
Dunna NR, Vuree S, Kagita S, Surekha D,
Digumarti R, Rajappa S and Satti V: Association of GSTP1 gene
(I105V) polymorphism with acute leukaemia. J Genet. 91:e60–e63.
2012.PubMed/NCBI
|
|
135
|
Allan JM, Wild CP, Rollinson S, Willett
EV, Moorman AV, Dovey GJ, Roddam PL, Roman E, Cartwright RA and
Morgan GJ: Polymorphism in glutathione S-transferase P1 is
associated with susceptibility to chemotherapy-induced leukemia.
Proc Natl Acad Sci USA. 98:11592–11597. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Daraki A, Zachaki S, Rosmaraki F,
Kalomoiraki M, Aleporou-Marinou V, Sambani C, Kollia P and Manola
KN: Association of GSTP1 inactivating polymorphism with acute
myeloid leukemia and its specific chromosomal abnormalities. Leuk
Lymphoma. 58:2505–2507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Bănescu C, Iancu M, Trifa AP, Cândea M,
Benedek Lazar E, Moldovan VG, Duicu C, Tripon F, Crauciuc A and
Dobreanu M: From Six Gene Polymorphisms of the Antioxidant System,
Only GPX Pro198Leu and GSTP1 Ile105Val Modulate the Risk of Acute
Myeloid Leukemia. Oxid Med Cell Longev. 2016:25367052016.
View Article : Google Scholar
|
|
138
|
Bănescu C, Trifa AP, Voidăzan S, Moldovan
VG, Macarie I, Benedek Lazar E, Dima D, Duicu C and Dobreanu M:
CAT, GPX1, MnSOD, GSTM1, GSTT1, and GSTP1 genetic polymorphisms in
chronic myeloid leukemia: A case-control study. Oxid Med Cell
Longev. 2014:8758612014. View Article : Google Scholar
|
|
139
|
He HR, Zhang XX, Sun JY, Hu SS, Ma Y, Dong
YL and Lu J: Glutathione S-transferase gene polymorphisms and
susceptibility to chronic myeloid leukemia. Tumour Biol.
35:6119–6125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Sailaja K, Surekha D, Rao DN, Rao DR and
Vishnupriya S: Association of the GSTP1 gene (Ile105Val)
polymorphism with chronic myeloid leukemia. Asian Pac J Cancer
Prev. 11:461–464. 2010.PubMed/NCBI
|
|
141
|
Kasznicki J, Kosmalski M, Sliwinska A,
Mrowicka M, Stanczyk M, Majsterek I and Drzewoski J: Evaluation of
oxidative stress markers in pathogenesis of diabetic neuropathy.
Mol Biol Rep. 39:8669–8678. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Stoian A, Bănescu C, Bălaşa RI, Moţăţăianu
A, Stoian M, Moldovan VG, Voidăzan S and Dobreanu M: Influence of
GSTM1, GSTT1, and GSTP1 Polymorphisms on Type 2 Diabetes Mellitus
and Diabetic Sensorimotor Peripheral Neuropathy Risk. Dis Markers.
2015:6386932015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Qiu YH, Xu YL and Zhang WH: Effect of
GSTM1, GSTT1, and GSTP1 IIe105Val polymorphisms on susceptiblity to
gestational diabetes mellitus. Genet Mol Res. 15:gmr.150277112016.
View Article : Google Scholar
|
|
144
|
Mir O, Alexandre J, Tran A, Durand JP,
Pons G, Treluyer JM and Goldwasser F: Relationship between GSTP1
Ile(105)Val polymorphism and docetaxel-induced peripheral
neuropathy: Clinical evidence of a role of oxidative stress in
taxane toxicity. Ann Oncol. 20:736–740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Gajewska B, Kaźmierczak B,
Kuźma-Kozakiewicz M, Jamrozik Z and Barańczyk-Kuźma A: GSTP1
Polymorphisms and their Association with Glutathione Transferase
and Peroxidase Activities in Patients with Motor Neuron Disease.
CNS Neurol Disord Drug Targets. 14:1328–1333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Pastor S, Rodríguez-Ribera L, Corredor Z,
da Silva Filho MI, Hemminki K, Coll E, Försti A and Marcos R:
Levels of DNA damage (Micronuclei) in patients suffering from
chronic kidney diseaseRole of GST polymorphisms. Mutat Res Genet
Toxicol Environ Mutagen. 836(Pt A): 41–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Agusa T, Kunito T, Tue NM, Lan VT,
Fujihara J, Takeshita H, Minh TB, Trang PT, Takahashi S, Viet PH,
et al: Individual variations in arsenic metabolism in Vietnamese:
The association with arsenic exposure and GSTP1 genetic
polymorphism. Metallomics. 4:91–100. 2012. View Article : Google Scholar
|
|
148
|
Stoyanova E, Pastor S, Coll E, Azqueta A,
Collins AR and Marcos R: Base excision repair capacity in chronic
renal failure patients undergoing hemodialysis treatment. Cell
Biochem Funct. 32:177–182. 2014. View Article : Google Scholar
|
|
149
|
Lok HC, Suryo Rahmanto Y, Hawkins CL,
Kalinowski DS, Morrow CS, Townsend AJ, Ponka P and Richardson DR:
Nitric oxide storage and transport in cells are mediated by
glutathione S-transferase P1-1 and multidrug resistance protein 1
via dinitrosyl iron complexes. J Biol Chem. 287:607–618. 2012.
View Article : Google Scholar :
|
|
150
|
Suryo Rahmanto Y, Kalinowski DS, Lane DJ,
Lok HC, Richardson V and Richardson DR: Nitrogen monoxide (NO)
storage and transport by dinitrosyl-dithiol-iron complexes:
Long-lived NO that is trafficked by interacting proteins. J Biol
Chem. 287:6960–6968. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Lok HC, Sahni S, Richardson V, Kalinowski
DS, Kovacevic Z, Lane DJ and Richardson DR: Glutathione
S-transferase and MRP1 form an integrated system involved in the
storage and transport of dinitrosyldithiolato iron complexes in
cells. Free Radic Biol Med. 75:14–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Lok HC, Sahni S, Jansson PJ, Kovacevic Z,
Hawkins CL and Richardson DR: A Nitric Oxide Storage and Transport
System That Protects Activated Macrophages from Endogenous Nitric
Oxide Cytotoxicity. J Biol Chem. 291:27042–27061. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Kovacevic Z, Sahni S, Lok H, Davies MJ,
Wink DA and Richardson DR: Regulation and control of nitric oxide
(NO) in macrophages: Protecting the "professional killer cell" from
its own cytotoxic arsenal via MRP1 and GSTP1. Biochim Biophys Acta,
Gen Subj. 1861(5 Pt A): 995–999. 2017. View Article : Google Scholar
|
|
154
|
Cao X, Kong X, Zhou Y, Lan L, Luo L and
Yin Z: Glutathione S-transferase P1 suppresses iNOS protein
stability in RAW264.7 macrophage-like cells after LPS stimulation.
Free Radic Res. 49:1438–1448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Zhang B, Jing C, Li X and Wang J: Effect
of 1-octyl-3-methyl-imidazolium bromide on the expressions of
CYP1A1, CYP1A2, CYP3A4, and GSTP1, and the receptors AhR, ARNT, and
PXR in HepG2 cells. J Toxicol Toxin Rev. 34:72015. View Article : Google Scholar
|
|
156
|
Kim Y, Maciag AE, Cao Z, Deschamps JR,
Saavedra JE, Keefer LK and Holland RJ: PABA/NO lead optimization:
Improved targeting of cytotoxicity to glutathione S-transferase
P1-overexpressing cancer cells. Bioorg Med Chem. 23:4980–4988.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Adler V, Yin Z, Fuchs SY, Benezra M,
Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, et
al: Regulation of JNK signaling by GSTp. EMBO J. 18:1321–1334.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Wang T, Arifoglu P, Ronai Z and Tew KD:
Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal
kinase (JNK1) signaling through interaction with the C terminus. J
Biol Chem. 276:20999–21003. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Tew KD and Townsend DM: Regulatory
functions of glutathione S-transferase P1-1 unrelated to
detoxification. Drug Metab Rev. 43:179–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Okamura T, Antoun G, Keir ST, Friedman H,
Bigner DD and Ali-Osman F: Phosphorylation of Glutathione
S-Transferase P1 (GSTP1) by Epidermal Growth Factor Receptor (EGFR)
Promotes Formation of the GSTP1-c-Jun N-terminal kinase (JNK)
Complex and Suppresses JNK Downstream Signaling and Apoptosis in
Brain Tumor Cells. J Biol Chem. 290:30866–30878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Elsby R, Kitteringham NR, Goldring CE,
Lovatt CA, Chamberlain M, Henderson CJ, Wolf CR and Park BK:
Increased constitutive c-Jun N-terminal kinase signaling in mice
lacking glutathione S-transferase Pi. J Biol Chem. 278:22243–22249.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Yin ZM, Liu AH and Jiang Y: Glutathione
S-transferase pi Protects Serum Depletion-induced Cell Death by
Inhibiting ASK1-MKK7-JNK Pathway in the 293 Cells. Sheng Wu Hua Xue
Yu Sheng Wu Wu Li Xue Bao (Shanghai). 33:185–190. 2001.
|
|
163
|
Liu X, An BH, Kim MJ, Park JH, Kang YS and
Chang M: Human glutathione S-transferase P1-1 functions as an
estrogen receptor α signaling modulator. Biochem Biophys Res
Commun. 452:840–844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Sun KH, Chang KH, Clawson S, Ghosh S,
Mirzaei H, Regnier F and Shah K: Glutathione-S-transferase P1 is a
critical regulator of Cdk5 kinase activity. J Neurochem.
118:902–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Wan Hasan WN, Kwak MK, Makpol S, Wan Ngah
WZ and Mohd Yusof YA: Piper betle induces phase I & II genes
through Nrf2/ARE signaling pathway in mouse embryonic fibroblasts
derived from wild type and Nrf2 knockout cells. BMC Complement
Altern Med. 14:722014. View Article : Google Scholar :
|