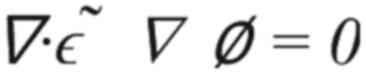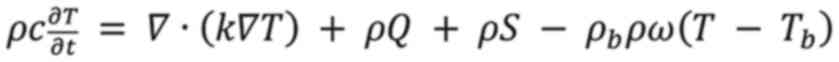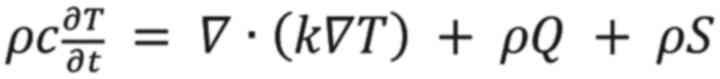Introduction
Tumor-treating fields (TTFields) is an emerging
cancer treatment modality that uses alternating, low intensity (1-3
V/cm) electric fields at intermediate frequencies (100-300 KHz) to
disrupt cancer cell proliferation (1-3).
In initial in vitro experiments conducted in the early
2000s, the application of TTFields to various types of tumor cell
lines was found to exert inhibitory effects on growth and induced
cell cycle arrest and apoptosis (1). The anticancer efficacy of TTFields
was further demonstrated by clinical studies, and the TTFields
system rapidly received FDA approval for newly diagnosed and
recurrent glioblastoma (GB) (4,5).
Clinical studies have demonstrated that the use of TTFields in
combination with chemotherapy improves progression-free, overall
and long-term survival compared with chemotherapy alone (4-6).
The combination of TTFields and immunotherapy may exert a
synergistic effect as cellular stress signals induced by TTFields
facilitate immune activation and immunogen-induced cell death
(7). However, mechanistic
investigations are required to optimize the use of TTFields in
combination with additional modalities, including radiation
therapy.
Although the underlying mechanisms are under debate,
TTFields reportedly target mitosis and cytokinesis (8-10).
By inhibiting mitosis and cytokinesis, TTFields rapidly target
proliferating cells only, which results in tumor specificity.
Therefore, they are considered to cause more significant damage to
cancer cells than normal cells. TTFields align proteins possessing
large dipole moments essential for cell division, such as tubulin
dimers (8) and septins (9), which interferes with spindle
alignment. Consequently, TTFields induce mitotic catastrophe,
leading to cell cycle arrest at the G2/M phase and culminating in
cell death (1,10). In addition, cells dividing
parallel to the externally applied field are more affected by the
field than cells dividing in other directions (11). Other biological mechanisms
considered to be involved in the effects of TTFields include
apoptosis, autophagy, DNA repair and immunogenic cell death
(11,12). Moreover, computational studies
have reported that the dielectrophoretic forces are not sufficient
to exert a significant effect on tubulin and septins, while
possibly affecting cellular molecules (13,14). This contradiction suggests that
there remains a need for in-depth biological studies to elucidate
the mechanisms of action of TTFields.
Although monolayer cell cultures are frequently used
in mechanism studies for TTFields, two-dimensional (2D) cell
cultures are oversimplified versions of tumors and do not
recapitulate in vivo cellular organization and interactions
(15,16). The microenvironment of tumor
spheroids resembles that of tumors more closely and may thus be a
more suitable in vitro cancer model than monolayer cultures.
These properties of tumor spheroids confer anticancer drug
resistance and radiation resistance to tumor spheroids, as observed
in human cancers. The present study, to the best of our knowledge,
is the first to apply TTFields to three-dimensional (3D) glioma
spheroids.
The use of 3D cell models in 3D culture environments
based on induced pluripotent stem cells (iPSCs) has enabled the
study of organs in vitro. Brain organoid formation relies on
the self-organization ability of iPSCs, which develop into
organized structures that resemble distinct regions of the brain,
maintaining hallmarks of key developmental processes involved in
brain formation (17,18). Thus, 3D brain organoids may be a
realistic 3D model which can be used to minimize the gap between 2D
cell cultures and animal models. The present study used brain
organoids as a novel platform to evaluate the effects of TTFields
on normal brain cells.
As the patient is required to wear the TTFields
device for long periods of time, the main design concern for
improving usability for the patient is that it can be worn more
comfortably. Increased compliance with TTFields therapy has been
reported to be an independent prognostic factor for improved
survival in GB (19,20). In general, the longer the duration
of the application of TTFields, the more prominent the therapeutic
effect; therefore, patients are advised to wear the device for
impractically long periods of time (23 h/day with 100% duty cycle),
without any parametric evidence. A previous clinical study revealed
the longer survival of patients with recurrent GB multiforme (GBM)
treated with TTFields for 18 h/day compared with those treated for
<18 h/day (19). In addition,
patients with a compliance of >20 h/day have been shown to
exhibit extended survival rates (20). As the TTFields device requires a
high compliance rate, it is designed to be wearable and portable,
with minimal impact on daily activities. Thus, a further
improvement in the usability of the system, such as device
miniaturization and operation time extension, is imperative to
benefit a greater number of patients. The battery determines the
size and weight of the device. Therefore, a method for efficient
power management for the TTFields system is required (21).
The present study noted that energy efficiency can
be further improved through the regulation of the duty cycle. The
duty cycle is the ratio of time a load or circuit is 'on' compared
to the time the load or circuit is 'off' per minute, which is the
typical definition for the duty cycle of any electronic device.
Previous studies (19,20) have investigated the number of
hours per day of wearing the device, but not the duty cycle that
was explored herein. TTFields with tumor-treating effects are
advantageous even if the duty cycle is lowered. First, energy
consumption can be reduced by adjusting the duty cycle, which in
turn is associated with a smaller and lighter battery, and prolongs
the device operation time without requiring recharging.
Furthermore, it will help advance the TTFields device into more
suitable forms, such as rechargeable implantable systems. These
forms would maximize the usability as patients wish to disguise the
worn device. Currently, patients often use wigs and hats; however,
the cables connecting the power supply and field generator to the
transducer array are intrusive and noticeable (22). However, there is a lack of
agreement on the duty cycle, which may be the key for this form
factor evolution. Therefore, the present study attempted to
establish an association between the duty cycle and treatment
effect of TTFields as the first step towards the further
enhancement of the TTFields system.
The present study aimed to evaluate the treatment
efficacy and safety of TTFields using three glioma cell lines and
normal brain organoids. In addition, the present study evaluated
whether the duty cycles of TTFields affected their therapeutic
efficacy. To the best of our knowledge, there is no study available
to date on the duty cycle of TTFields. Herein, experiments were
conducted on three duty cycles and a control group, and all
experiments were conducted for 72 h, which is the typical duration
for TTFields experiments (23).
For the in vitro experiment, a system was designed that
generates an electric field suitable for TTFields in a cell culture
environment based on numerical electromagnetic simulations and a
simple TTFields system was fabricated that can modulate the duty
cycle freely. In the present study, details of TTF-induced cell
damage to tumor spheroid cells and normal brain organoids were
analyzed and compared according to the duty cycle.
Materials and methods
TTFields system design
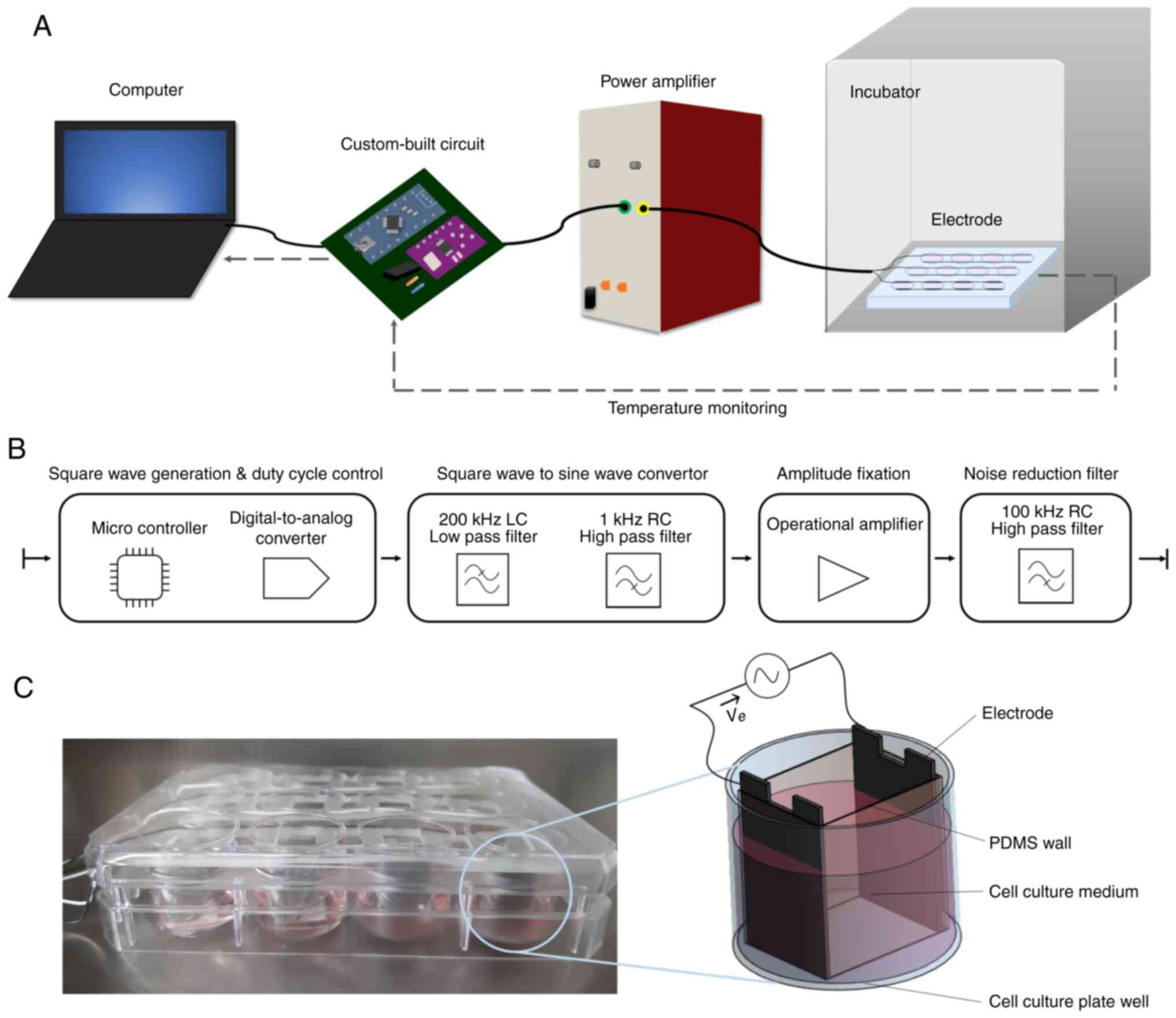
The customized stimulation system is illustrated in
Fig. 1A. This system can be
easily constructed in a laboratory environment as all components
are readily available. Commands were delivered to the
microcontroller included in a custom-built circuit to adjust the
TTFields parameters and the temperature was monitored using a
computer. The custom-built circuit generated a 200 KHz sine wave
and a 2.5 Peak-to-peak voltage (Vpp) amplitude was fixed to the
electrode plate. A system stability test of 7 days (well beyond the
in vitro testing period) revealed no interference errors in
the circuit and output signal. During the experimental period, the
cell plate was kept in an incubator at 37°C with 5% CO2.
The gap between the cell culture plate and the lid was sealed with
Bemis parafilm to prevent the evaporation of the cell medium. The
wire connected to the electrode came out through a small gap in the
incubator door. The detailed configuration of the custom-built
circuit is illustrated in Fig.
1B. The control software was developed using Arduino IDE
software version 1.8.13 (Arduino). Square-wave generation and duty
cycle control were performed using a microcontroller unit (Arduino
Nano V3.0; Arduino). A digital-to-analog control microprocessor
(CJMCU-9833; Shenzhen Bixinda Technology Co., Ltd.) was used to
create a 200 KHz square wave and control the duty from the
microcontroller unit signal. Square wave to sine wave conversion
was performed using our custom built 1 KHz RC high-pass filter and
200 KHz LC low-pass filter. The RC high-pass filter removes the
direct current offset; the LC low-pass filter converts a square
wave to a sine wave. For amplitude control and buffer, a
high-frequency response was required. Amplitude fixation was
performed using an operational amplifier (MC34074; ON Semiconductor
Corp.) as the amplifier and buffer. The noise reduction filter used
a 100 KHz RC high-pass filter and was placed at the end of the
generator immediately before the power amp input. The custom-built
200 KHz sine wave generator circuit is portable, measuring
105×150×55 mm, and weighs 260 g.
The TTFields electrodes were fabricated using a
12-mm-wide, 19-mm-long and 0.5-mm-thick stainless-steel plate, and
the distance between the pair of electrodes per well was 15 mm. The
upper part of the electrode was fixed to a holder made using a 3D
printer (Fig. 1C), and four pairs
of electrodes, corresponding to one row of a 12-well plate, were
connected in parallel. Each pair of electrodes were wrapped using
polydimethylsiloxane (PDMS) to prevent spheroid cells from leaking
out from the uniform field zone.
Heating should be minimized to avoid the confounding
effects of temperature rise and TTFields on cancer cells. To
evaluate the level of heating induced by TTFields on the cell
culture medium, temperature was recorded in the culture medium
during the application of TTFields at the 100% duty cycle as the
'worst-case scenario'. A digital thermometer (DS18B20; Maxim
Integrated) was used to monitor the temperature of the well once
every minute. Little to no change in temperature was induced by
TTFields (Ve=2.5 Vpp) for 72 h, indicating that the confounding
effect of heating on the cell culture medium is unlikely to be
observed during the in vitro testing.
Computational simulation
Numerical computations of electric field
distribution and the resulting temperature increment in the cell
culture medium were performed using Sim4Life ver. 6.0 (ZMT Zurich
MedTech AG). To identify the electric field, a quasi-static
electromagnetic solver (included with the aforementioned software)
was used to calculate low-frequency electromagnetic problems
(24) using the following
equation (Equation 1):
where 'ε~' is the complex electric permittivity
and 'ϕ' is a scalar potential. The electromagnetic field was
recorded for use as a heat source for thermal simulations.
The temperature change in the tissues during thermal
ablation was calculated using the Pennes bioheat equation (Equation 2), which has been used to
solve computational
bioelectromagnetic problems since its formulation (
25): where 'ρ' is material density, 'c'
is specific heat capacity, 'T' is tissue temperature, 'k' is
thermal conductivity, 'Q' is metabolic heat generation rate, 'S' is
the specific absorption rate and 'ω' is the perfusion rate. The
term 'ρbcbρw' is sometimes referred to as the
heat transfer rate by blood perfusion. As the present study did not
need to consider the effect of blood in Equation (
2), this was thus simplified to Equation
(
3).
For simulation, the conductivities of PDMS,
stainless steel and cell culture plate wall were assigned values of
2.5×10-14, 1.1×106 and 5×10-4 S/m,
respectively. The conductivity of the cell culture medium was
determined at 1.8 S/m using a conductivity meter (CP-50N; Isteck,
Inc.) and the conductivity of the spheroid tumor cell was specified
as 0.24 S/m (26). For properties
other than conductivity, the values provided by the software were
used.
Cell preparation: Tumor spheroid cell
culture
U87 (glioblastoma of unknown origin) were purchased
from ATCC (Lot. no. 60173414) and U373 MG ATCC were purchased from
the Korean Cell line Bank (Lot no. 22741). All cell lines had been
authenticated using short tandem repeat profiling. They were
transduced with a lentiviral construct containing the Firefly
luciferase gene as previously described (27). The U251 cell line was purchased
was originally obtained from Sigma-Aldrich; Merck KGaA. The cells
were cultured in suspension at 100 cells/well in 6-well plates for
14 days in a neural stem cell (NSC) medium, consisting of
Neurobasal (Thermo Fisher Scientific, Inc.) and DMEM/F12 media
(HyClone; Cytiva) (1:1) supplemented with 1 × B27, 1 × N2, basic
fibroblast growth factor (bFGF, 20 ng/ml), and epidermal growth
factor (EGF, 20 ng/ml). The number of neurospheres per well was
determined by counting the neurospheres in five wells on days 7 and
14. The cell density was adjusted to 1×105 cells/ml cell
culture medium using a cell counter and 200 μl of the cell
culture was plated into the wells of the sterile 96-well cell
culture plate. Neurospheres with diameters >50 μm were
counted. Bioluminescence was detected using an in vivo
imaging system (IVIS 200; Xenogen Corporation).
Generation of human brain organoids
CMC-hiPSC-011 cells (28) were kindly provided by the
laboratory of Dr Joo (the Catholic University of Korea, https://nih.go.kr/contents.es?mid=a50401110300).
Organoids were generated using the STEMdiff Cerebral Organoid kit
(08570; STEMCELL Technologies) following the manufacturer's
instructions. On day 0, CMC-hiPSC-011 cells at 90% confluency were
dissociated into single cells using Accutase (A1110501, Gibco;
Thermo Fisher Scientific, Inc.) for 5 min at 37°C. Following
centrifugation at 1,000 × g for 5 min at room temperature, the
iPSCs were resuspended in embryoid body (EB) formation medium
(05893, STEMCELL Technologies) with 10 μM Y27632 (Y503;
Sigma-Aldrich; Merck KGaA), a Rho-associated kinase (ROCK)
inhibitor, Y-27632, and diluted to a concentration of
9×103 cells/ml. Subsequently, 100 μl cell
suspension were distributed into each well of a low-attachment
96-well U-bottom plate (Corning, Inc.) to form single EBs, and the
medium was changed every 2 days. On days 5-6, one-half of the
medium was replaced with an induction medium. On day 7, organoids
were harvested, embedded in Matrigel (Corning, Inc.), and grown in
expansion medium in suspension culture in ultra-low attachment
6-well plates (Corning, Inc.). The embedded organoids were
maintained for 3 days and cultured in maturation medium, and the
plates were transferred to a shaker for continuous culturing. The
medium was changed every 3 days.
TTFields treatment and evaluation:
TTFields application
The present study developed an in-house TTFields
system that allows for the accurate adjustment of the duty cycle,
while maintaining a constant voltage of 2.5 Vpp between electrode
plates. TTFields of three duty cycles were applied to the cells. At
this time, the duty cycle was adjusted based on 1 min (e.g., 30 sec
'on' and 30 sec 'off' in the case of 50% duty). The cells were
placed within the PDMS wall and exposed to the electric field
generated between the electrodes. Three to five glioma spheroids
were placed in each well. Three brain organoids matured for 40 days
were placed per well. TTFields were applied for 3 days without
changing the culture medium.
Cell cycle analysis
Following 72 h of TTFields treatment, floating cells
from the medium were harvested by centrifugation at 230 × g for 30
sec at room temperature, and adherent cells were dissolved through
trypsinization (R001100, Gibco; Thermo Fisher Scientific, Inc.).
Both cell populations were washed once with 5 ml ice cold PBS,
combined and resuspended in 500 μl PBS. For cell cycle
analysis, the cells were fixed in 4 ml ice-cold 70% ethanol (J.T.
Baker) and the cell pellet was re-suspended in 150 μl
propidium iodide (Sigma-Aldrich; Merck KGaA) staining solution. The
stained sample was incubated in a 37°C incubator for 40 min. The
DNA content was analyzed using a flow cytometer (BD FACS Canto 2.0,
BD Biosciences) and evaluated using Flowing Software 2.5.1
(University of Turku, Turku, Finland).
H&E staining
The sections were fixed with 4% paraformaldehyde in
0.1 M phosphoric acid buffer (PB, pH 7.4). Samples containing
paraffin were cut into tubular 8-μm-thick sections using a
microtome and mounted on the APS coating slide. The sections were
then deparaffinized and stained with hematoxylin and eosin
(MilliporeSigma). The stained areas were dried using an ethanol
series, washed with xylene and covered. Images were obtained using
an upright optical microscope equipped with a digital camera (DP70,
Olympus Corporation).
Immunofluorescence staining
The human brain organoids were fixed with 4% (w/v)
PFA, OCT-embedded, and cut into 8-μm-thick sections using a
freezing microtome (Leica Microsystems GmbH). The sections were
blocked with 1% (w/v) normal goat serum (Jackson ImmunoResearch
Laboratories, Inc.) and incubated first at room temperature for 2 h
with primary anti-β-III tubulin (1:500, 801201, BioLegend, Inc.),
anti-Nestin (1:500, sc-23927, Santa Cruz Biotechnology Inc.),
anti-glial fibrillary acidic protein (GFAP; 1:500, AB5804, Merck
Millipore), anti-NeuN (1:200, ABN78, Merck Millipore) and anti-SOX2
(1:500, 3579, Cell Signaling, Inc.) antibodies, and subsequently
with goat anti-mouse or rabbit Alexa Fluor 488 or 546 antibodies
(1:1,000; Molecular Probes; Thermo Fisher Scientific, Inc.). Nuclei
were labeled with DAPI (1:1,000, Sigma-Aldrich; Merck KGaA) at room
temperature for 10 min, and fluorescence was observed using a Zeiss
LSM510 confocal microscope (Zeiss AG).
Statistical analysis
All data are expressed as the mean±SD or mean ± SEM.
Data were analyzed using an unpaired Student's t-test when
comparing two groups. ANOVA with a post hoc Tukey's multiple
comparisons test was used to determine whether differences between
groups were statistically significant. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Electromagnetic and thermal
simulation
The computed electric field distribution and
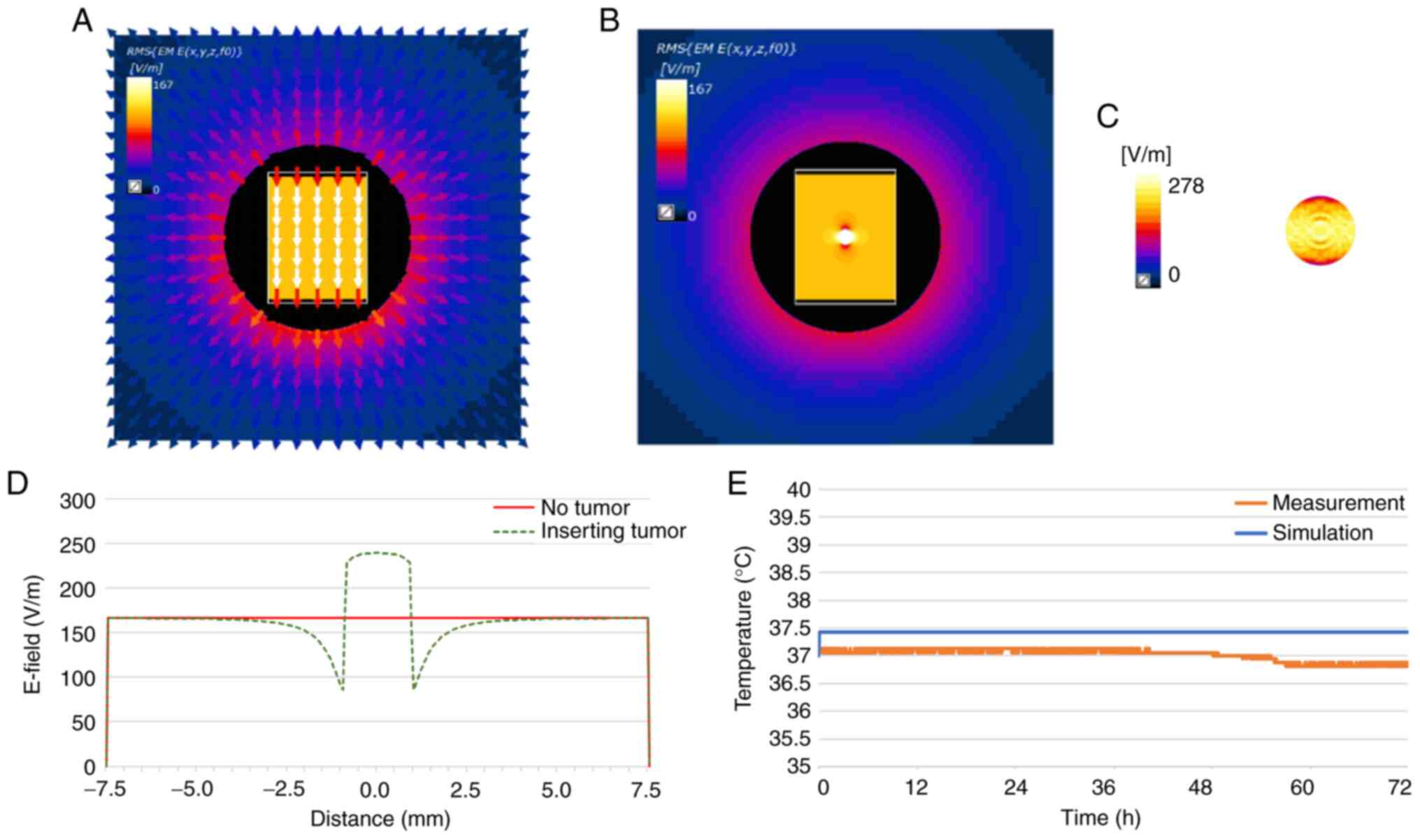
measured temperature rise in a well is illustrated in Fig. 2. These results confirm that the
current system generates a homogeneous electric field between the
electrodes, and the induced temperature increase resulting from
this electric field is negligible (Fig. 2). The designed electrode generated
a homogeneous electric field of 167 V/m between the electrodes
(Fig. 2A and D). When tumor cells
were inserted, the electric field was concentrated in the tumor
cells, increasing up to 272 V/m (Fig.
2B and D). The electric field formed on the surface of the
tumor cell was examined (Fig.
2C). The temperature increment was verified using two methods:
Through simulation and experimental measurements (Fig. 2E). While the temperature
calculated for the cell culture medium revealed saturation after
increasing by 0.4°C, the measured temperature only fluctuated
within the accuracy of the thermal sensor (DS18B20; Maxim
Integrated) of ±0.5°C (29).
These results confirm that the system used herein does not induce
heating during the in vitro experiments.
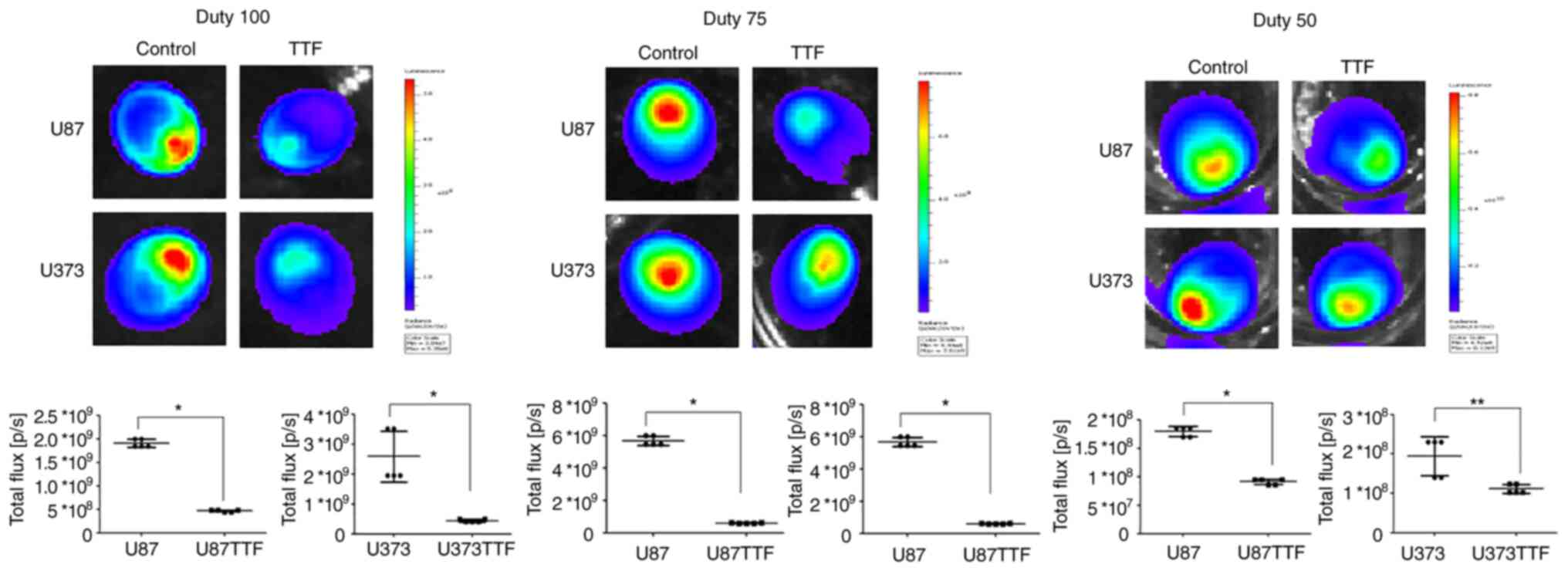
TTFields application on cancer cells
The bioluminescence intensity of glioma spheroids
(U87-Luc and U373-Luc) was evaluated after applying TTFields. The
bioluminescence intensity in the U87 and U373 cells treated with
the TTFields was significantly lower than that in the control
(Fig. 3 and Table SI). At 100% duty, the mean
intensities in the U87 and U373 cells were 0.469E+09 and 0.442E+09
compared with the control at 1.90E+09 and 2.58E+09, respectively.
At 75% duty, the mean intensities in the U87 and U373 cells were
0.595E+09 and 1.07E+09 compared with the control at 5.67E+09 and
5.92E+09, respectively. At 50% duty, mean intensities in U87 and
U373 were 0.913E+08 and 1.11E+08, compared with the control at
1.80E+08 and 1.95E+08, respectively.
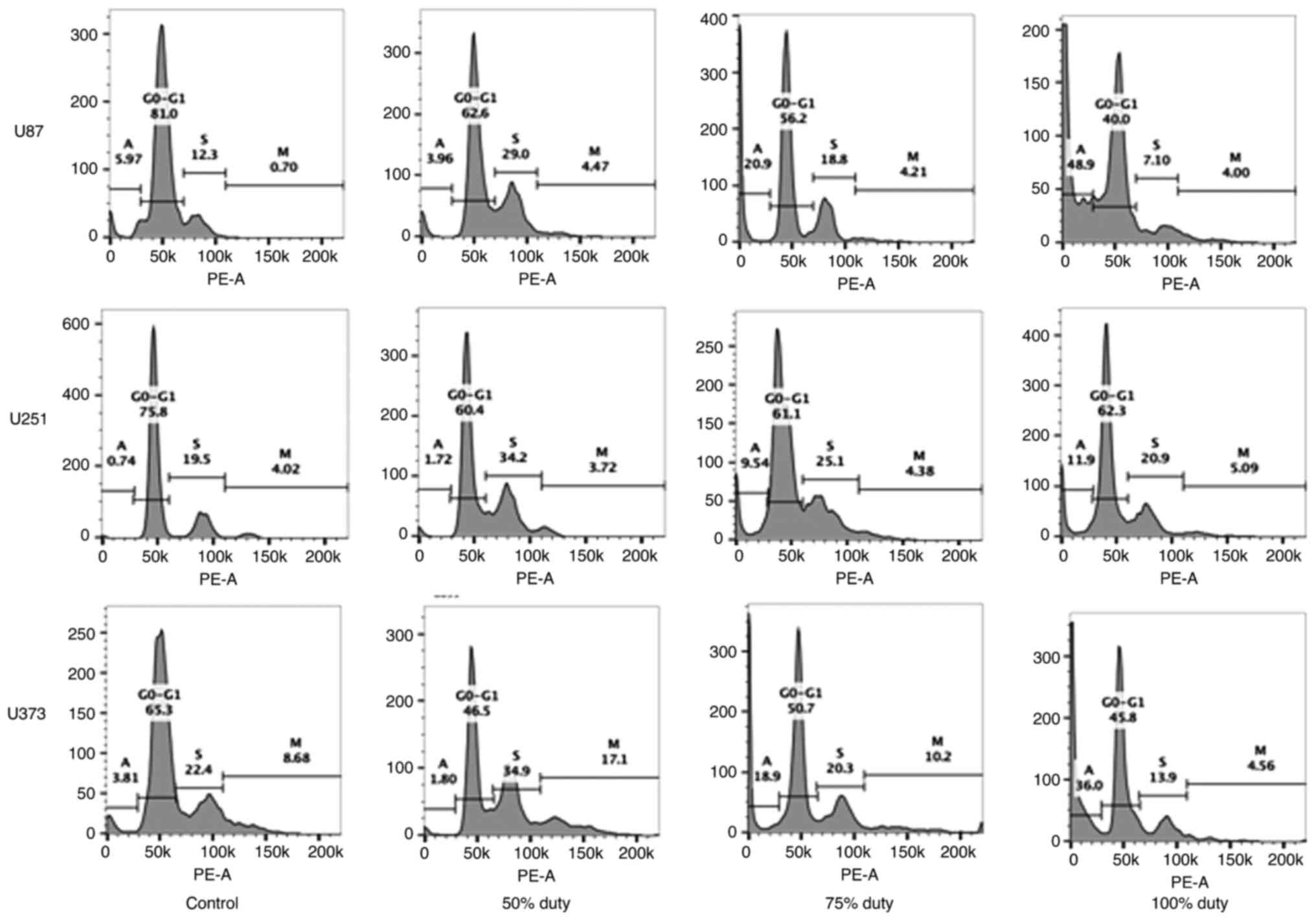
Characterization of the cell cycle by FACS analyses
revealed that the higher the duty cycle, the higher the number of
sub-G1 cells detected (Figs. 4
and S1). Sub-G1 cells are dead
cells, and they indicate apoptosis. For the U87 cells, 48.9% of the
cells were in the sub-G1 phase when treated with the 100% duty
TTFields application compared with 20.9 and 3.96% cells in this
phase when treated with the 75 and 50% duty TTFields application,
respectively. For the U251 cells, 11.9% cells were in the sub-G1
phase when treated with the 100% duty TTFields application compared
with 9.54 and 1.72% cells when treated with the 75 and 50% duty
TTFields application, respectively. For the U373 cells, 36.0% cells
were in the sub-G1 phase when treated with the 100% duty TTFields
application compared with 18.9 and 1.80% cells when treated with
the 75 and 50% duty TTFields application, respectively.
TTFields application on normal brain
organoids
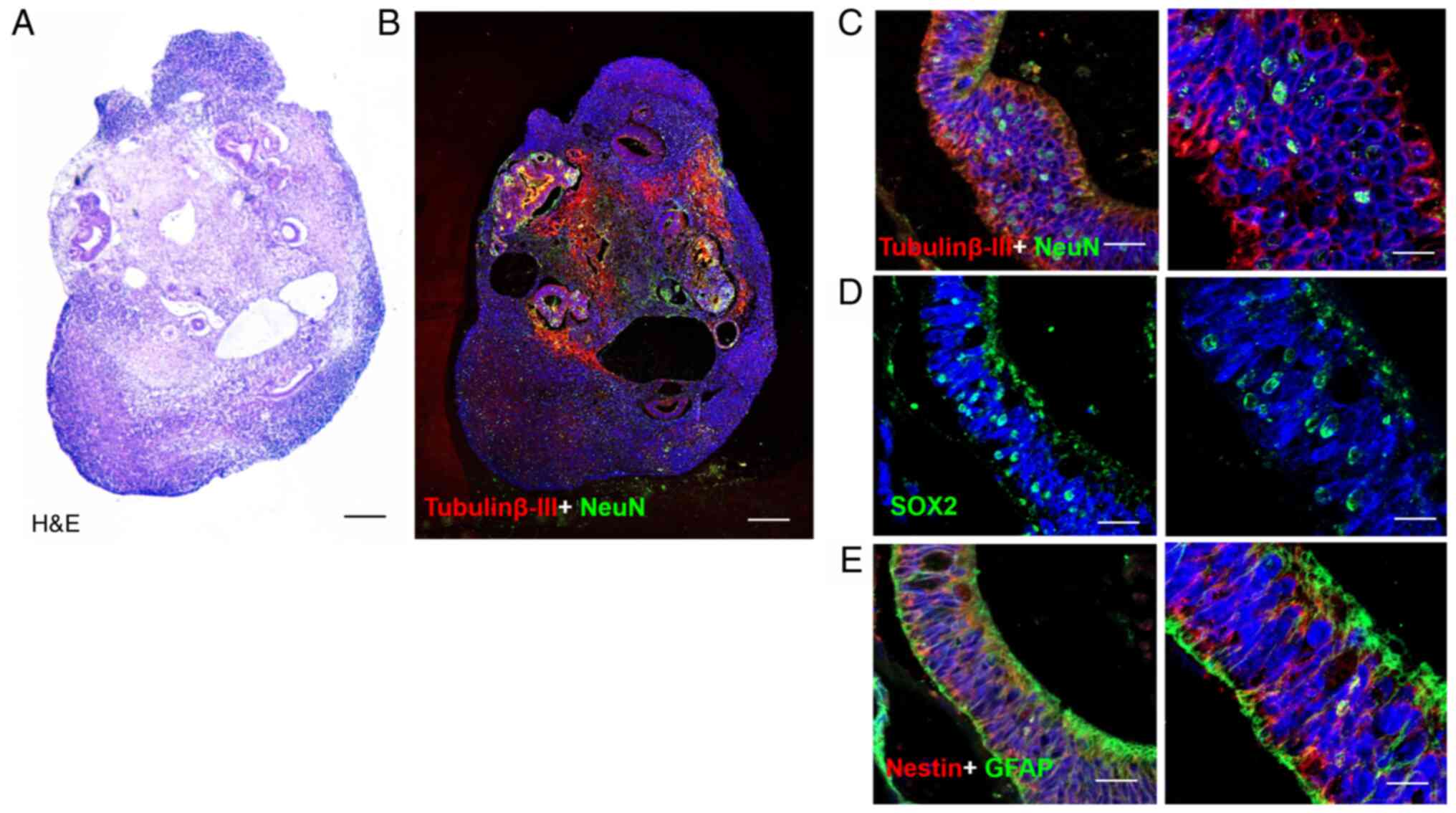
Brain organoids were developed by culturing iPSCs
under conditions that promote 3D neuroectoderm differentiation for
40 days. Brain organoids formed rosettes that were morphologically
similar to ventricles. Neuroepithelium-like structures expressed
the neural precursor marker, Nestin, the progenitor marker, SOX2,
the neuronal markers, β-III tubulin and NeuN, and the astrocyte
marker, GFAP (Figs. 5 and
S2). In the control organoids,
multiple rosettes were observed in the cortex of the organoid. In
organoids treated with the TTFields at the 50 or 75% duty cycles,
the cavity of rosettes expanded and loosened. The organoids treated
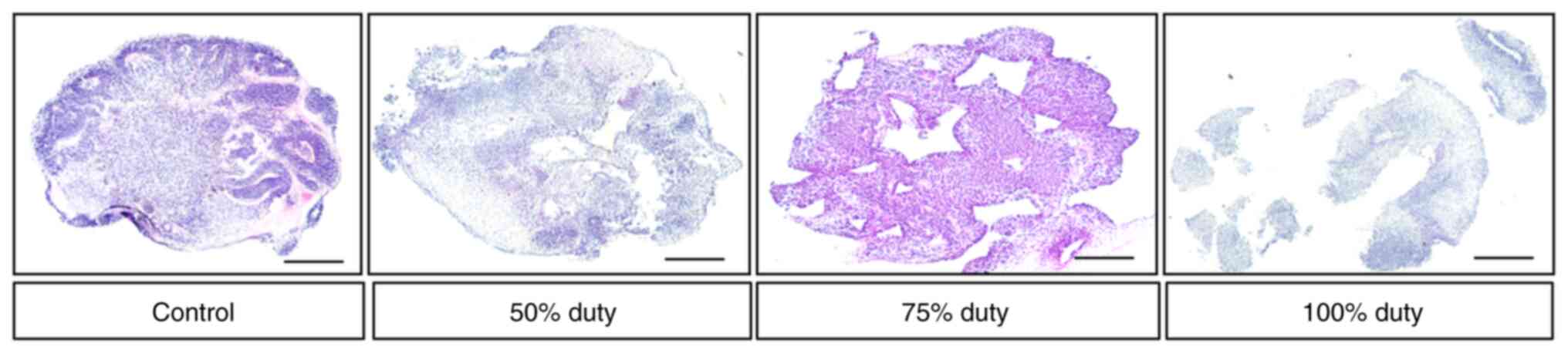
with TTFields at 100% duty were disorganized (Figs. 6 and S3).
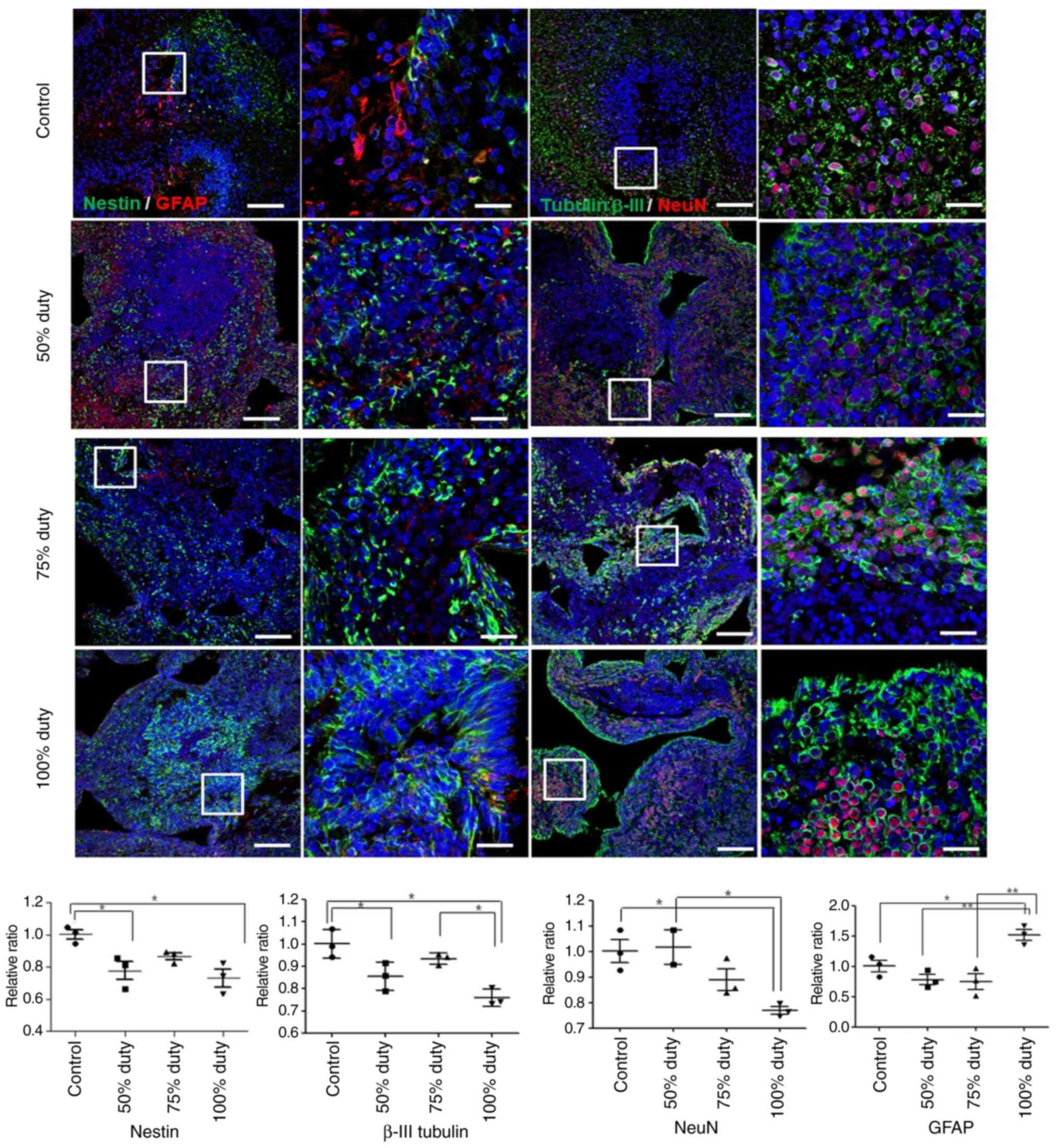
The present study then compared the expression of
neuronal markers in brain organoids treated with 50, 75 or 100%
duty cycles for 3 days. NSC proliferation markers (Nestin) and
neuronal markers (β-III tubulin and NeuN), which indicate the
differentiation and maturation of newly formed neurons, were used.
At 50% duty, the expression of Nestin and β-III tubulin decreased
compared with the control; however, NeuN expression was similar to
that in the control. At 75% duty, the expression of Nestin and
β-III tubulin was similar to that in the control. However, the
expression of Nestin, β-III tubulin and NeuN was significantly
decreased in the brain organoids treated with TTFields at 100% duty
compared with the control. By contrast, the expression of GFAP was
significantly increased in the brain organoids treated with the
TTFields at 100% duty. The expression of GFAP was similar in the
organoids treated at the 50 and 75% duty compared with the control
(Fig. 7 and Table SI).
Discussion
TTFields represent an emerging treatment modality
for various tumors, including high-grade gliomas. This technology
has received considerable interest as it functions based on
mechanisms different from conventional cancer treatments, and thus,
can be used solely or in conjunction with other therapeutic
procedures. TTFields is a portable, home-usable device that can be
managed via telemedicine; thus, it can be safely used in patients
with GBM, even during the COVID-19 pandemic (30).
Although clinical results have demonstrated that
TTFields inhibit tumor growth and improve overall survival
(4-7), the underlying mechanisms are not
fully understood. Studies have conducted in vitro
experiments to reveal the mechanisms of action of TTFields;
however, conventional 2D-cultured cells may not represent the
essential cellular environment observed in vivo. One
strategy with whch to reduce the gap between in vitro and
live tissue is to use well-suited 3D-cultured cells. Spheroids are
organized structures composed of several layers. The external
layers are accessible to nutrients and oxygen and contain
proliferative cells; the intermediate layers are composed of
senescent cells; the core of the spheroid is mainly necrotic
(31). The present study
evaluated the effects of TTFields using three tumor spheroid cells
(U87, U251 and U373) as individual GBs may respond differently to
electric field exposure.
Furthermore, brain organoids were used to examine
the safety of TTFields in normal cells. The safety profile of
TTFields in healthy animals has also been investigated. As
previously demonstrated, following a 1-month follow-up period, all
animals were euthanized, and samples of the major organs were
examined by a pathologist; no treatment-related toxicities were
recorded in any animal (10).
According to the global post-marketing safety surveillance
described above (32), 52
pediatric patients (aged <18 years) were treated with TTFields
therapy. The incidence of all adverse events was lowest in
pediatric patients, most likely due to the smaller sample size.
Although safety is the most important issue in pediatric clinical
trials, the effects of TTF on the normal brain in pediatric
patients have rarely been investigated. Cerebral organoids can
serve as an informative tool for studying human neural development
(33). A number of advantages of
non-organoid-based in vitro models of cortical development
are also applicable to brain organoids, including the ability to
generate large numbers of cells, perform genetic manipulations and
perform assays at multiple time points. However, knowledge of the
effects of TTFields on normal brain organoids is limited. TTFields
has been shown to interfere with mitosis in rapidly dividing cancer
cells, thereby impairing cancer proliferation (7,9).
It has been reported that TTFields result in two forms of cell
stress, namely, shear stress and extensile stress, that affect the
cell membrane. Cancer cells are more deformable compared to
non-cancer cells due to their altered membrane composition.
Therefore, cancer cells' responsiveness to stress is different from
that of non-cancer cells (34).
To the best of our knowledge, the present study was first to
address the effect of TTF on neural cells of brain organoids. The
findings presented herein suggest that TTFields with the
conventional parameters can lead to the apoptosis of normal brain
organoids and thus merit attention for further development and
clinical application. Recently, the authors used oligomeric Aβ1-42
to induce neuronal cell death in human brain organoid cultures and
found that there was greater apoptotic cell death in brain
organoids cultured with oligomeric Aβ1-42 compared to
brain organoids cultured in the absence of Aβ1-42 (unpublished
data). The present study examined whether neurotoxicity was
observed in brain organoids treated with TTFs at different duty
cycles.
For conducting in vitro experiments, a
TTFields system, including a custom-built sine wave generator was
developed, which maintains constant voltage regardless of the
change in cell culture medium volume and allows easy adjustment of
the duty cycle. The entire system was built using readily available
components with easy-to-fabricate electrodes. For system
verification, computational simulations were performed, which
confirmed that the experimental setup generated a uniform electric
field distribution between the electrodes. Calibration testing
confirmed that the system did not induce significant heating when
the highest duty cycle (100%) was applied; the temperature increase
in the cell culture medium was <0.5°C and was maintained at
<41°C, which is below the temperature for thermal injury
(22). The treatment effects at
100, 75 and 50% duty were compared with those of the control. To
the best of our knowledge, this is the first study to control the
duty cycle of TTFields. Although clinical studies have demonstrated
that patients wearing the device >18 h/day experienced
therapeutic effects, this is not equivalent to a prescribed duty
cycle, but simply a measure of the therapeutic effect based on the
wearing period; it is not a controlled study for defined duty
cycles (20,21). Reducing the duty cycle has
advantages, such as increasing energy efficiency, thereby reducing
the size and weight of the battery, and these advantages increase
the possibility of transforming the device type to an insertion
type or one with wireless recharging.
The results of the present study confirmed that high
duty cycles of TTFields effectively induced the death of cancer
cells. The evaluation of bioluminescence intensity (Fig. 3 and Table SI) revealed that the TTFields
application at 75% duty inhibited cell proliferation (average 85%
decrease compared to the control), which was very similar to that
achieved using 100% stimulation (average 78.5% decrease compared to
control), although slightly more effective. By contrast, when
applying 50% TTFields, the average reduction rate was only 43%
compared to the control. The difference in the treatment effect
depending on the duty cycle was more pronounced in the evaluation
of the cell cycle (Fig. 4). When
comparing the apoptosis phase of the TTFields group and the control
group, it was found that apoptosis conspicuously increased in the
75 and 100% groups, although no significant change was observed in
the 50% group. There was also a difference depending on the type of
glioma spheroids, which we speculate is because of the gradients of
proliferation, oxygen, nutrients, and pH observed from the external
layer to the inner part of the spheroid (35,36). However, the possible effect of the
stem-cell like population in GB was not considered herein. Recent
cancer treatment research has aimed to eradicate cancer stem cells
as well as cancer cells, and thus further studies are required to
investigate the effects of TTFields on cancer stem cells.
Of note, it was found that the expression of NSC
markers decreased and that of GFAP, an astrocyte marker (37), increased following TTFields
application at 100% duty. For a negative control, it was confirmed
that GFAP was not expressed in kidney organoids in which
GFAP-positive cells are not present (Fig. S4). In the central nervous system,
astrocytes are involved in the regulation of neurodevelopment,
neurotransmission, cerebral metabolism and blood flow (38,39). In neuronal injury, astrocytes
protect neurons against oxidative stress and toxicity (40). It was hypothesized that TTFields
at 100% duty would affect the neuronal differentiation of normal
brain organoids, and astrocyte differentiation is accelerated to
escape TTF-induced stimuli. As an additional method for apoptosis
detection, terminal deoxynucleotidyl transferase-mediated dUTP
nick-end labeling (TUNEL) assay was performed for the analyses of
glioma spheroid cells and brain organoids, as described in Data S1.
It was confirmed that the apoptosis was activated at the 100 and
75% duty cycle of the TTFields in glioma spheroid cells (Fig. S1). The portion of TUNEL-positive
cells in brain organoids treated with 50% and 75% duty cycle of
TTFields was higher than that without TTFields exposure and brain
organoids treated with 100% duty cycle of TTFields were severely
disorganized (Fig. S3). There
are certain limitations to the present study, involving the lack of
specific markers of apoptosis. Collectively, the expression of
Nestin, β-III tubulin and NeuN was decreased, and apoptosis
occurred in the brain organoids treated with TTFields. With these
findings, it was concluded that the proliferative neural cell
phenotype was affected by the TTFields via the apoptotic pathway.
The long-term use of 100% duty may be associated with neurotoxicity
in these subjects. Duty cycle adjustment is necessary in terms of
safety, in addition to energy saving. Taken together, research on
neurotoxicity in the context of long-term TTFields application is
required as in in the present study, TTFields exposure lasted for
only 3 days.
Considering these results, it was concluded that the
TTFields application at 75% duty induced cancer cell death at a
similar level as the 100% duty and had less neurotoxicity. It is
considered that this is an important finding for the advancement of
TTFields therapy. TTFields devices have limitations in that they
cause inconvenience to patients due to the size and weight of the
battery, and the presence of exposed cables outside the clothes.
One approach to solving this issue is to evolve the device into an
implantable form using wireless recharging technology. However, to
implement this, energy saving, which can be achieved through duty
cycle reduction, is a key requirement for device miniaturization
and operation time extension. Finally, it is considered that the
present study paves the way for enhancing the usability and safety
of TTFields therapy through parametric optimization. However,
although the in vivo environment was closely simulated using
3D-cultured cells, there is a limitation in that it cannot
perfectly reproduce the complex brain tissues composed of both
cancer cells and normal brain cells. To compare the cycle of GBM
spheroids and brain organoids, brain organoids and U87 cells were
co-cultured under the same culture conditions (Fig. S5). From the additional
experiments with co-cultured cells, it was found that the TTFields
gave rise to the apoptosis of both GBM spheroids and brain
organoids (Figs. 4, 6, S1 and
S3). The higher the duty cycle of TTFields, the more prominent
was the cell apoptosis. However, as illustrated in Figs. 6 and S3, normal brain organoids treated with
TTFields were also damaged and shrunken; thus, the quantitative
comparison of the cell cycle is difficult with the shape of the
co-cultured cell clusters completely changing after the TTFields
application. Apoptosis was observed in cell clusters not treated
with TTFields, as well as with in those treated with TTFields. For
the cause of this observation, it was hypothesized that cancer
cells hindered the survival of brain organoids cells regardless of
the effects of TTFields. In addition, it may be the result of an
unsuitable cell culture medium. Since the cell culture medium is a
crucial factor for cellular growth, optimizing suitable conditions
for co-cultured cell clusters requires many parameter studies.
Therefore, due to the confounding effects of TTFields and
co-culturing, this as a limitation of the present in vitro
study. The authors aim to design a TTFields system for animal
experiments to investigate the effect of TTFields on cancer cells
and the behavior of the animal models in a future study.
In conclusion, the present study constructed a
simple TTFields system and cultured three types of cancer spheroid
cells and brain organoids. Using the cancer spheroid cells, it was
confirmed that the TTFields exhibited a significant anti-cancer
effects even when duty cycle was decreased to 75%. By contrast,
morphological abnormalities appeared in brain organoids exposed to
100% TTFields. These findings suggest that further in-depth studies
are warranted to optimize the duty cycle to enhance the safety of
this emerging therapeutic modality. It is considered that the
current portable, wearable form factor is suboptimal for the
effectiveness and usability of TTFields therapy. Duty cycle
optimization may be the first evolutionary step for TTFields
systems, and the current results may serve as scientific evidence
for this improvement.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
All authors contributed intellectually to the
research. EY, SHY and SMP designed the experiments. EY and JEL
performed the experiments and analyzed the data. The custom-built
circuit was designed by YSL. The manuscript was prepared by EY and
JEL. SHY and SMP reviewed the manuscript critically for important
intellectual content. SHY and JEL confirm the authenticity of all
the raw data. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea government (no.
2021R1C1C201046911) and funded by the Ministry of Education (grant
no. 2020R1A6A1A03047902), and in part by the Po-Ca Networking
Groups, funded by the Postech-Catholic Biomedical Engineering
Institute (PCBMI) under grant no. 5-2021-B0001-00301.
Abbreviations:
|
2D
|
two-dimensional
|
|
3D
|
three-dimensional
|
|
bFGF
|
basic fibroblast growth factor
|
|
EB
|
embryoid body
|
|
EGF
|
epidermal growth factor
|
|
GB
|
glioblastoma
|
|
iPSCs
|
induced pluripotent stem cells
|
|
NSC
|
neural stem cell
|
|
PDMS
|
polydimethylsiloxane
|
|
ROCK
|
Rho-associated kinase
|
|
TTFields
|
tumor-treating fields
|
References
|
1
|
Kirson ED, Gurvich Z, Schneiderman R,
Dekel E, Itzhaki A, Wasserman Y, Schatzberger R and Palti Y:
Disruption of cancer cell replication by alternating electric
fields. Cancer Res. 64:3288–3295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neuhaus E, Zirjacks L, Ganser K, Klumpp L,
Schüler U, Zips D, Eckert F and Huber SM: Alternating electric
fields (TTFields) activate Cav 1.2 channels in human
glioblastoma cells. Cancers. 11:1102019. View Article : Google Scholar
|
|
3
|
Trusheim J, Dunbar E, Battiste J, Iwamoto
F, Mohile N, Damek D, Bota DA and Connelly J: A state-of-the-art
review and guidelines for tumor treating fields treatment planning
and patient follow-up in glioblastoma. CNS Oncol. 6:29–43. 2017.
View Article : Google Scholar
|
|
4
|
Stupp R, Wong ET, Kanner AA, Steinberg D,
Engelhard H, Heidecke V, Kirson ED, Taillibert S, Liebermann F,
Dbalý V, et al: NovoTTF-100A versus physician's choice chemotherapy
in recurrent glioblastoma: A randomised phase III trial of a novel
treatment modality. Eur J Cancer. 48:2192–2202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Taillibert S, Kanner A, Read W,
Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K,
et al: Effect of tumor-treating fields plus maintenance
temozolomide vs maintenance temozolomide alone on survival in
patients with glioblastoma: A randomized clinical trial. JAMA.
318:2306–2316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stupp R, Taillibert S, Kanner AA, Kesari
S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink
KL, et al: Maintenance therapy with tumor-treating fields plus
temozolomide vs temozolomide alone for glioblastoma: A randomized
clinical trial. JAMA. 314:2535–2543. 2015. View Article : Google Scholar
|
|
7
|
Giladi M, Schneiderman RS, Voloshin T,
Porat Y, Munster M, Blat R, Sherbo S, Bomzon Z, Urman N, Itzhaki A,
et al: Mitotic spindle disruption by alternating electric fields
leads to improper chromosome segregation and mitotic catastrophe in
cancer cells. Sci Rep. 5:180462015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hottinger AF, Pacheco P and Stupp R: Tumor
treating fields: A novel treatment modality and its use in brain
tumors. Neuro Oncol. 18:1338–1349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gera N, Yang A, Holtzman TS, Lee SX, Wong
ET and Swanson KD: Tumor treating fields perturb the localization
of septins and cause aberrant mitotic exit. PLoS One.
10:e01252692015. View Article : Google Scholar :
|
|
10
|
Kirson ED, Dbalý V, Tovaryš F, Vymazal J,
Soustiel JF, Itzhaki A, Mordechovich D, Steinberg-Shapira S,
Gurvich Z, Schneiderman R, et al: Alternating electric fields
arrest cell proliferation in animal tumor models and human brain
tumors. Proc Natl Acad Sci USA. 104:10152–10157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giladi M, Voloshin T, Shteingauz A,
Munster M, Blat R, Porat Y, Schneiderman RS, Cahal S, Itzhaki A,
Kirson E, et al: Alternating electric fields (TTFields) induce
immunogenic cell death resulting in enhanced antitumor efficacy
when combined with anti-PD-1 therapy. J Immunol. 196(Suppl 1):
75.262016.
|
|
12
|
Silginer M, Weller M, Stupp R and Roth P:
Biological activity of tumor treating fields in preclinical glioma
models. Cell Death Dis. 8:e27532017. View Article : Google Scholar
|
|
13
|
Tuszynski JA, Wenger C, Friesen DE and
Preto J: An overview of sub-cellular mechanisms involved in the
action of TTFields. Int J Environ Res Public Health. 13:11282016.
View Article : Google Scholar :
|
|
14
|
Wenger C, Miranda PC, Salvador R,
Thielscher A, Bomzon Z, Giladi M, Mrugala MM and Korshoej AR: A
review on tumor-treating fields (TTFields): Clinical implications
inferred from computational modeling. IEEE Rev Biomed Eng.
11:195–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pampaloni F, Reynaud EG and Stelzer EH:
The third dimension bridges the gap between cell culture and live
tissue. Nat Rev Mol Cell Biol. 8:839–845. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vinci M, Gowan S, Boxall F, Patterson L,
Zimmermann M, Lomas C, Mendiola M, Hardisson D and Eccles SA:
Advances in establishment and analysis of three-dimensional tumor
spheroid-based functional assays for target validation and drug
evaluation. BMC Biol. 10:292012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lancaster MA, Renner M, Martin CA, Wenzel
D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP and
Knoblich JA: Cerebral organoids model human brain development and
microcephaly. Nature. 501:373–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Lullo E and Kriegstein AR: The use of
brain organoids to investigate neural development and disease. Nat
Rev Neurosci. 18:573–584. 2017. View Article : Google Scholar :
|
|
19
|
Kanner AA, Wong ET, Villano JL and Ram Z;
EF-11 Investigators: Post-hoc analyses of intention-to-treat
population in phase III comparison of NovoTTF-100A™ system versus
best physician's choice chemotherapy. Semin Oncol. 41(Suppl 6):
S25–S34. 2014. View Article : Google Scholar
|
|
20
|
Benson L: Tumor treating fields
technology: Alternating electric field therapy for the treatment of
solid tumors. Semin Oncol Nurs. 34:137–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lacouture ME, Davis ME, Elzinga G,
Butowski N, Tran D, Villano JL, DiMeglio L, Davies AM and Wong ET:
Characterization and management of dermatologic adverse events with
the NovoTTF-100A System, a novel anti-mitotic electric field device
for the treatment of recurrent glioblastoma. Semin Oncol. 41(Suppl
4): S1–S14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Toms SA, Kim CY, Nicholas G and Ram Z:
Increased compliance with tumor treating fields therapy is
prognostic for improved survival in the treatment of glioblastoma:
A subgroup analysis of the EF-14 phase III trial. J Neurooncol.
141:467–473. 2019. View Article : Google Scholar :
|
|
23
|
Berkelmann L, Bader A, Meshksar S, Dierks
A, Majernik GH, Krauss JK, Schwabe K, Manteuffel D and Ngezahayo A:
Tumour-treating fields (TTFields): Investigations on the mechanism
of action by electromagnetic exposure of cells in
telophase/cytokinesis. Sci Rep. 9:76322019. View Article : Google Scholar
|
|
24
|
Plonsey R and Heppner DB: Considerations
of quasistationarity in electrophysiological systems. Bull Math
Biophys. 29:657–664. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pennes HH: Analysis of tissue and arterial
blood temperatures in the resting human forearm. J Appl Physiol
(1985). 85:5–34. 1998. View Article : Google Scholar
|
|
26
|
Korshoej AR, Hansen FL, Thielscher A, von
Oettingen GB and Sørensen JC: Impact of tumor position,
conductivity distribution and tissue homogeneity on the
distribution of tumor treating fields in a human brain: A computer
modeling study. PLoS One. 12:e01792142017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang SH, Hong YK, Jeun SS, Kim IS, Hong
JT, Sung JH, Son BC, Lee SW, Kim MC and Lee KS: Assessment of
cetuximab efficacy by bioluminescence monitoring of intracranial
glioblastoma xenograft in mouse. J Neurooncol. 95:23–28. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim Y, Park N, Rim YA, Nam Y, Jung H, Lee
K and Ju JH: Establishment of a complex skin structure via layered
co-culture of keratinocytes and fibroblasts derived from induced
pluripotent stem cells. Stem Cell Res Ther. 9:2172018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maxim Integrated Programmable Resolution
1-Wire Digital Thermometer. Maxim Integrated Products, Inc; San
Jose, CA: 2019, https://datasheets.maximintegrated.com/en/ds/DS18B20.pdf.
|
|
30
|
Gatson NT, Barnholtz-Sloan J, Drappatz J,
Henriksson R, Hottinger AF, Hinoul P, Kruchko C, Puduvalli VK, Tran
DD, Wong ET, et al: Tumor treating fields for glioblastoma therapy
during the COVID-19 pandemic. Front Oncol. 11:6797022021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mehta G, Hsiao AY, Ingram M, Luker GD and
Takayama S: Opportunities and challenges for use of tumor spheroids
as models to test drug delivery and efficacy. J Control Release.
164:192–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi W, Blumenthal DT, Oberheim Bush NA,
Kebir S, Lukas RV, Muragaki Y, Zhu JJ and Glas M: Global
post-marketing safety surveillance of tumor treating fields
(TTFields) in patients with high-grade glioma in clinical practice.
J Neurooncol. 148:489–500. 2020. View Article : Google Scholar :
|
|
33
|
Lewis EM, Kaushik K, Sandoval LA, Antony
I, Dietmann S and Kroll KL: Epigenetic regulation during human
cortical development: Seqing answers from the brain to the
organoid. Neurochem Int. 147:1050392021. View Article : Google Scholar
|
|
34
|
Aguilar AA, Ho MC, Chang E, Carlson KW,
Natarajan A, Marciano T, Bomzon Z and Patel CB: Permeabilizing cell
membranes with electric fields. Cancers (Basel). 13:22832021.
View Article : Google Scholar
|
|
35
|
Carlsson J and Acker H: Relations between
pH, oxygen partial pressure and growth in cultured cell spheroids.
Int J Cancer. 42:715–720. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khaitan D, Chandna S, Arya MB and
Dwarakanath BS: Establishment and characterization of multicellular
spheroids from a human glioma cell line; Implications for tumor
therapy. J Transl Med. 4:122006. View Article : Google Scholar :
|
|
37
|
Dezonne RS, Sartore RC, Nascimento JM,
Saia-Cereda VM, Romão LF, Alves-Leon SV, Souza JM, Martins-de-Souza
D, Rehen SK and Gomes FC: Derivation of functional human astrocytes
from cerebral organoids. Sci Rep. 7:450912017. View Article : Google Scholar :
|
|
38
|
Siracusa R, Fusco R and Cuzzocrea S:
Astrocytes: Role and functions in brain pathologies. Front
Pharmacol. 10:11142019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khakh BS and Sofroniew MV: Diversity of
astrocyte functions and phenotypes in neural circuits. Nat
Neurosci. 18:942–952. 2015. View Article : Google Scholar
|
|
40
|
Ouyang YB, Xu L, Lu Y, Sun X, Yue S, Xiong
XX and Giffard RG: Astrocyte-enriched miR-29a targets PUMA and
reduces neuronal vulnerability to forebrain ischemia. Glia.
61:1784–1794. 2013. View Article : Google Scholar : PubMed/NCBI
|