Programmed death ligand 1 (PD-L1) is a type I
transmembrane protein encoded by the CD274 gene (1,2).
Immunohistochemical detection has revealed that PD-L1 mRNA and
protein expression is upregulated in various cancer types (3). However, since PD-L1 mRNA is strictly
post-transcriptionally regulated under normal physiological
conditions, PD-L1 protein is scarcely expressed in normal cells
(4). As a key member of the immune
checkpoints, PD-L1, together with its receptor programmed cell
death protein 1 (PD-1), serves an important role in tumor cell
clearance and immune surveillance by mediating signaling processes
that limit autoimmunity and prevent excessive immune responses
(5). In addition, quantification
of PD-L1 expression by immunohistochemistry on different detection
platforms has been used in various clinical trials as a key
determinant of the efficacy of checkpoint immunotherapy (6).
As one of the most promising approaches to activate
the immune system, immune checkpoint blockade has achieved
remarkable efficacy in antitumor therapy in the last decade
(7). In addition, the exploration
of drugs targeting the PD-1/PD-L1 axis has led to the development
of a number of immune checkpoint inhibitors (ICIs), such as
anti-PD-L1 monoclonal antibodies (atezolizumab, durvalumab and
avelumab) and anti-PD-1 monoclonal antibodies (nivolumab,
pembrolizumab and tislelizumab), which have become first-line
therapy for various solid tumors (3,8,9).
These ICIs enhance the immune system surveillance capacity and
generate antitumor immune responses by manipulating the interaction
between PD-L1 and PD-1, leading to improved overall survival (OS)
and progression-free survival (PFS) of patients with cancer
(3,10).
However, due to the diversity and complexity of the
tumor immune microenvironment and the continuous genetic changes in
tumor cells, immunotherapy is ineffective in most patients with
advanced tumors (11,12). In addition, the complex drug
resistance mechanism of cancer cells to immunotherapy influences
the clinical outcomes of patients with cancer (13). Therefore, a combination therapy to
improve the response rate to PD-1/PD-L1 blockade and overcome
resistance to anti-PD-1/PD-L1 therapy is urgently required.
Epigenetic modifications that serve an important role in
interactions between the tumor microenvironment and tumor cells and
in the development of cancer cells represent such opportunities
(14).
Epigenetic modifications are heritable changes in
gene expression caused by environmental, dietary, age and disease
factors that do not include changes in the DNA sequence itself
(15). Epigenetic modifications
can reshape the tumor microenvironment and alter cellular
phenotypes through aberrant histone patterns, non-coding RNAs
levels and DNA methylation at specific promoters, enabling cells to
grow and evade immune surveillance (16). Since epigenetic modifications are
susceptible to external factors and are often reversible, they are
considered to be potential therapeutic targets for various cancer
types (17). Azacitidine, the
first epigenetic drug approved by the Food and Drug Administration
(FDA), marks a breakthrough in epigenetic medicine from theory to
application (18). Tazemetostat, a
small-molecule inhibitor of the histone methyltransferase enhancer
of zeste homolog 2 (EZH2), has recently been approved by the FDA to
treat solid tumors, including relapsed or refractory follicular
lymphoma and locally advanced or metastatic epithelioid sarcoma
(19). In addition, multiple DNA
methyltransferase (DNMT) inhibitors and histone-modifying enzyme
inhibitors have shown promising therapeutic effects in solid tumors
highlighting the potential of epigenetic therapy in the treatment
of solid tumors (20,21).
Detailed descriptions of the resistance mechanisms
of ICIs targeting the PD-1/PD-L1 axis have been provided in several
studies (22,23); therefore, these are only briefly
summarized in the present review. In addition, the latest research
progress and related mechanisms of epigenetic factors interfering
with PD-L1 expression, including histone modifications such as
acetylation and methylation, non-coding RNA regulation and DNA
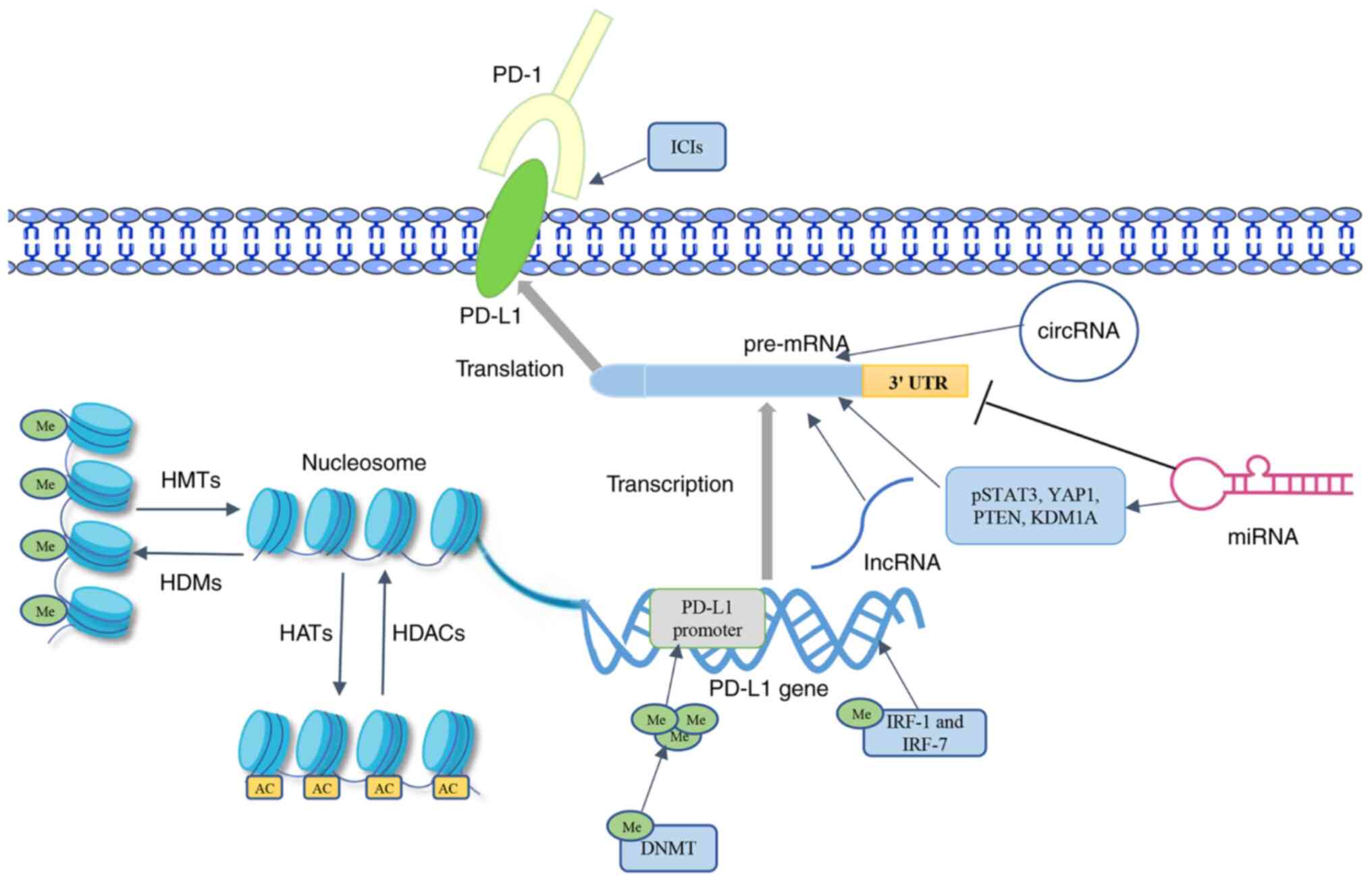
methylation, in solid tumors are summarized and discussed (Fig. 1). Among them (Table I), a variety of chromatin-modifying
enzymes can regulate PD-L1 expression by affecting the
modifications that occur on lysine and arginine residues (24-26).
Noncoding RNAs can inhibit PD-L1 expression by binding to the 3′
UTR or act as upstream regulators of the PD-1/PD-L1 axis (27-29).
The research on DNA methylation mainly focuses on its effect on the
PD-L1 promoter (30). The present
review aims to provide novel insights for further development of
potential combination therapy strategies to improve the response
rate and tolerability of immunotherapy in solid tumors.
However, clinical data have indicated limited ICI
efficacy in a large group of patients with primary resistance
unresponsive to PD-1/PD-L1 blockade or acquired resistance after
initial response (13). To the
best of our knowledge, due to the complexity of antitumor immunity,
the exact mechanism of resistance to ICIs targeting the PD-1/PD-L1
axis has not been fully elucidated or extensively reviewed.
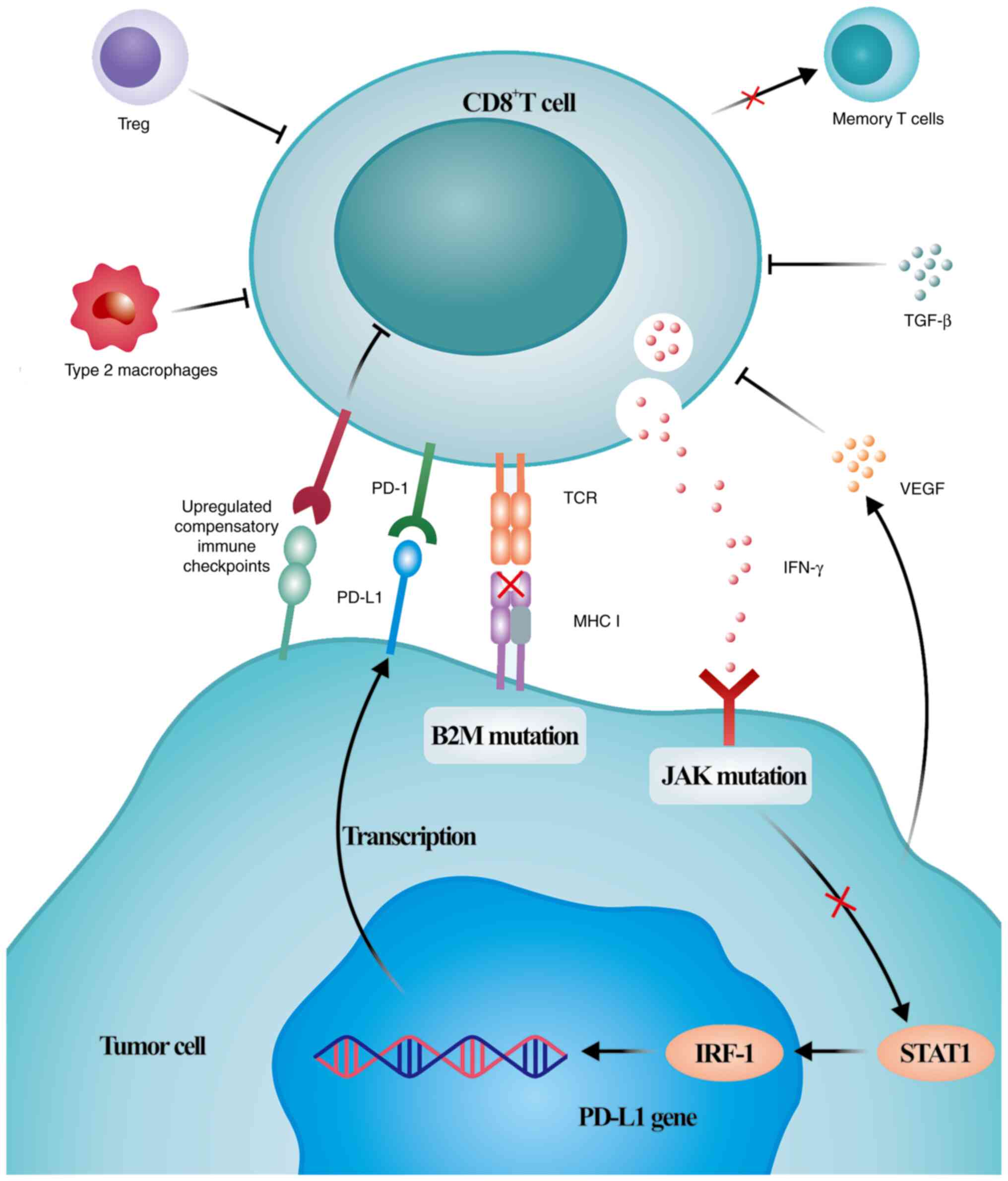
Resistance is triggered by various complex mechanisms (Fig. 2). Mechanisms leading to primary
resistance include insufficient immunogenicity of tumor antigens
formed by non-mutated proteins or mutant proteins that are not
fully tolerated by T cells, irreversible exhaustion of T cells
because of multiple inhibitory axes in the tumor microenvironment,
which prevent tumor-specific T cells from becoming memory T cells,
dysfunction of MHC class I complexes caused by β-2-microglobulin
mutations, resistance to IFN-γ signaling caused by Janus kinase
(JAK)1/2 mutations, and immunosuppression because of
immunosuppressive cells, cytokines and tumor metabolites in the
tumor microenvironment (37-43).
The mechanisms of acquired resistance are primarily associated with
tumor subclones, leading to increased numbers of tumor cells that
can escape antitumor immunity, re-exhaustion of T cells because of
persistently high antigen levels and activation of compensatory
inhibitory signals (44-46). As previously mentioned, the
mechanism of resistance to ICIs targeting the PD-1/PD-L1 axis is a
complex intervention system that is constantly being updated with
the increasing understanding of immunotherapy.
As one of the components of eukaryotic nucleosomes,
histones can acquire a diverse set of post-translational
modifications (47), of which
acetylation and methylation are the most studied ones. In cancer
cells, these modifications can alter the structural attributes of
chromatin, regulate the function of nucleosomes, and affect the
expression of specific genes, such as PD-L1 (48,49).
Furthermore, histone modification, a dynamic and reversible
process, is influenced by a number of chromatin-modifying enzymes
that exist as multicomponent protein complexes (50). These enzymes are divided into
writers, erasers and readers, according to their different
functions (51). In multiple
cancer types, including colon cancer and lung cancer, PD-L1
expression is affected by different chromatin-modifying enzymes,
particularly histone deacetylases (HDAC) and histone
methyltransferases (52,53).
The acetylation of histones at their tail lysine
residue can reduce the affinity of histones for DNA by neutralizing
positive charges, which will facilitate chromatin opening and
transcription (51). Enhancement
of histone H3 acetylation in the PD-L1 promoter is involved in
PD-L1 expression in various drug-resistant cancer cells, including
those of breast cancer, lung cancer and hepatocellular carcinoma
(12). Histone acetylation serves
as a key mediator in the regulation of gene expression, the levels
and states of which are influenced by the balance of factors
opposing HDACs and histone acetyltransferases (HATs) (54). HDACs are involved in regulating the
transcription of PD-L1 by regulating histone acetylation through
removing acetyl groups of lysine residues from histone substrates
(12).
HDAC3 is the key HDAC isoform responsible for
regulating PD-L1 transcription in tumors (55). Inhibition of HDAC3 expression can
increase IFN-γ production and PD-L1 promoter region histone
acetylation, thereby activating PD-L1 transcription in tumor cells
and increasing the levels of PD-L1 in dendritic cells in the tumor
microenvironment (55,56). Furthermore, HDAC3 maintains PD-L1
expression by inhibiting histone H3 acetylation at the PD-L1
promoter in drug-resistant cells of lung cancer, breast cancer and
hepatocellular carcinoma (12). As
an oncogenic transcription factor, STAT3 is activated in various
cancer types, such as pancreatic cancer, breast cancer and
osteosarcoma, and thus, affects the expression and transcription of
genes involved in cellular immune responses, proliferation and
chemoresistance (57). HDAC3
upregulates PD-L1 expression in pancreatic cancer by intervening in
the STAT3 signaling pathway (58).
In primary melanoma, HDAC8 can inhibit PD-L1 expression by
controlling the transcriptional activation of PD-L1 by acting on
STAT3-containing transcriptional complexes (59). HDAC6 upregulates PD-L1 expression
in melanoma and osteosarcoma by recruiting and activating the
transcription factor STAT3 (60,61).
In addition, HDAC6 expression is positively associated with PD-L1
expression in ovarian cancer (62). HDAC10, another member of the class
IIB HDAC family, has been reported to be positively associated with
PD-L1 expression in patients with lung cancer (63).
HDAC1/2, which belong to the class I HDAC family,
can be recruited by tet methylcytosine dioxygenase 2 proteins to
the PD-L1 promoter to deacetylate H3K27 acetylation, thereby
inhibiting the transcription of PD-L1 in breast cancer (24). Additionally, HDAC2 promotes PD-L1
expression by upregulating the phosphorylation of JAK1, JAK2 and
STAT1, as well as translocation of STAT1 to the nucleus and
recruitment of STAT1 to the PD-L1 promoter (64). HDAC1 expression is consistently
upregulated in tumor spheres derived from breast cancer and affects
the epithelial-mesenchymal transition (EMT)-induced upregulation of
PD-L1 expression (65). In
addition, EMT-induced upregulation of PD-L1 expression in breast
cancer is also affected by HATs (65). HATs are involved in histone
acetylation by catalyzing the transfer of acetyl groups (54). HAT1 was the first HAT to be
discovered, HAT1 expression is upregulated in various solid tumors
and HAT1 acts as a transcription factor to regulate the expression
of multiple genes (66,67). In pancreatic cancer, upregulation
of HAT1 expression is not only associated with poor prognosis but
can also enhance PD-L1 transcription by promoting the binding of
bromodomain-containing 4 (BRD4)-containing complex to acetylated
histone H4 (25).
HDAC inhibitors (HDACis) can inhibit HDAC-mediated
deacetylation, leading to the hyperacetylation of histones and
re-expression of epigenetically silenced genes (68). At present, only a few HDACis, such
as vorinostat, romidepsin, belinostat and Panobinostat, have been
approved by the FDA to treat malignancies, while other HDACis are
undergoing various clinical trials as options for the treatment of
malignancies (69). HDACis exert
antitumor effects by inducing cell apoptosis, inhibiting
angiogenesis, and regulating cell autophagy and immune responses;
however, to the best of our knowledge, the mechanisms by which they
regulate PD-L1 have not been well defined (70,71).
Class I HDACis can elevate PD-L1 expression in a
variety of tumors, including chondrosarcoma, Hodgkin's lymphoma,
melanoma, lung cancer, prostate cancer and colorectal cancer
(52,71-77).
Among them, chidamide can upregulate PD-L1 expression in
chondrosarcoma by activating the transcription factor STAT1
(72). In addition, it could
enhance the antigen presentation process in a chondrosarcoma mouse
model to improve therapeutic efficacy (72). When suberoylanilide hydroxamic acid
is used to treat prostate cancer cells, it can increase histone H3
acetylation of the CD274 promoter to induce CD274 transcription,
leading to upregulation of PD-L1 expression (76). As a naturally occurring selective
inhibitor of HDACs 1 and 2, romidepsin increases PD-L1 expression
in colorectal cancer, mainly through the regulation of histone
acetylation and the transcription factor BRD4 (52). Furthermore, HDAC6 inhibitors, as
class II HDACis, can dose-dependently reduce PD-L1 expression in
colorectal, lung and urothelial cancer (78-80).
A study suggests that HDACis can enhance the response to
immunotherapy via increasing tumor antigen levels and reactivation
of proapoptotic genes (81).
However, HDACis have side effects, such as lymphopenia, that limit
the efficacy of immunotherapy (82).
Histone methylation is a reversible process on
arginine and lysine residues: Arginine is symmetrically or
asymmetrically methylated, while lysine can be monomethylated,
dimethylated or trimethylated (83). Among these, H3K4, H3K36 and H3K79
are associated with transcriptional activation of genes, whereas
H3K9, H3K27 and H4K20 are associated with the transcriptional
repression of genes (83). For
example, in colorectal cancer, the transcriptional upregulation of
PD-L1 is positively associated with H3K4me3 and negatively
associated with H3K9 tri-methylation (H3K9me3) (84). Inhibitory H3K9me3 and H3K27me3 also
regulate PD-L1 expression in breast cancer tumor-forming cells
(65).
Histone methylation is a complex modification
process regulated by various methyltransferases and demethylases.
Protein arginine methyltransferase 5 (PRMT5) catalyzes the
symmetric dimethylarginine of histone and non-histone proteins and
is closely associated with tumor cell proliferation, invasion and
metastasis (85). In cervical
cancer, PRMT5 promotes the transcription of STAT1, and thus, PD-L1
expression through symmetric dimethylation of histone H3R2
(86). As one of the H3K4
methylation-specific histone methyltransferases, mixed lineage
leukemia 1 catalyzes H3K4me3 to activate the transcription of PD-L1
in pancreatic cancer cells by directly binding to the CD274
promoter (26). Knockdown of WD
repeat domain 5, a key component of the patient SE translocation
1/MLL histone methyltransferase complex, reduces IFN-γ-induced
PD-L1 mRNA and protein levels in prostate cancer (87). EZH2 is a core component of the
polycomb repressive complex 2 and possesses histone
methyltransferase activity (88).
In melanoma, EZH2 inactivation can lead to decreased PD-L1 mRNA
levels (53). Similarly, EZH2 is
also positively associated with PD-L1 levels in lung cancer tissues
and regulates PD-L1 expression through hypoxia-inducible factor 1-α
(89).
Lysine demethylase 4B (KDM4B) is a demethylase that
acts on lysine, and its silencing can reduce PD-L1 expression by
promoting H3K27me3 expression and reducing homeobox C4 (HOXC4)
expression in colorectal cancer cells (90). In addition, lysine-specific histone
demethylase 1 (LSD1) regulates the chromatin landscape and gene
expression by demethylating proteins, such as histone H3 (91). HCI-2509, a noncompetitive highly
potent reversible LSD1 inhibitor, upregulates PD-L1 expression in
breast cancer cells in a dose-dependent manner (92). SP-2577, which is currently
undergoing a phase I clinical trial, is also a potent and
reversible LSD1 inhibitor that can promote PD-L1 expression in
small cell carcinoma of the ovarian hypercalcemic type cells by
inhibiting LSD1 (91). Based on
these developments, LSD1 inhibition may be a promising epigenetic
adjunctive therapy to ICIs. In addition,
5-carboxy-8-hydroxyquinoline (IOX1), a histone demethylase
inhibitor that inhibits Jumonji domain 1A of histone demethylases,
can downregulate PD-L1 expression in a concentration-dependent
manner in various cancer cells, including CT26, HCT116 and MCF-7
cells (93). IOX1 could also
reverse doxorubicin-induced upregulation of PD-L1 expression
(93).
Histone phosphorylation is the most abundant and
dynamic modification during mitosis of tumor cells, and its
occurrence alone or in dense clusters can have a great impact on
the structure and function of modified proteins (94). A study has demonstrated that
histone phosphorylation can dissociate readers of methylated
histones without loss of epigenetic information (95). This allows histone phosphorylation
to serve a functional role in initiating chromatin for reliable
chromosome segregation and preventing genetic instability (96).
Among them, histone H3 phosphorylation is known as a
common epigenetic modification that affects chromatin structure and
gene transcription (97). Pyruvate
kinase isoform M2 (PKM2), a rate-limiting enzyme in glycolysis, is
a transcriptional coactivator of multiple target genes associated
with tumor cell proliferation and metastasis, and can be stimulated
by epidermal growth factor (EGF) to translocate to the nucleus
(98). In hepatocellular
carcinoma, EGF can induce phosphorylation of PKM2 at
Ser37 and translocation of the PKM2 protein to the
nucleus, and then phosphorylation of histone H3 at Thr11
to induce PD-L1 expression (30).
Non-coding RNAs are an abundant component of the
human transcriptome. Since ncRNAs have the ability to regulate gene
expression, protein translation and growth pathways, they can
regulate a variety of cellular processes, such as growth,
differentiation and drug resistance, which are highly related to
the occurrence and development of cancer (99). Furthermore, non-coding RNAs,
particularly microRNAs (miRNAs/miRs), long non-coding RNAs
(lncRNAs) and circular RNAs (circRNAs), can regulate the expression
of immune genes, such as PD-L1, in a variety of tumors, thereby
serving an important role in immunotherapy (100).
miRNAs are highly conserved small non-coding RNAs
comprising 19-22 nucleotides that inhibit gene expression by
binding to complementary nucleotides in the 3′ untranslated region
(3′ UTR) of mRNA targets (101,102). The mechanism of this interaction
occurs under both physiological and pathological conditions, and
thus, serves an important role in a number of biological processes,
including cell proliferation, metastasis, apoptosis and metabolism
(103,104). Aberrant miRNA expression during
tumorigenesis can affect several cancer-related signaling pathways
and transcripts, thereby aberrantly expressed miRNAs are becoming
important diagnostic markers and attractive therapeutic candidates
for multiple cancer types (105).
In addition, a study has indicated that miRNAs can exert profound
regulatory effects on the expression levels of PD-L1 through
complex regulatory mechanisms (106).
In lung cancer, elevated levels of PD-L1 promote
cell proliferation, invasion, migration and immune escape, and
contribute to chemoresistance (107). miR-3127-5p induces PD-L1
expression in lung cancer cells by promoting phosphorylation of
STAT3 (107). PD-L1 serves as a
common downstream target of miR-377-3p and miR-155-5p, and
inhibiting their expression can directly lead to upregulated PD-L1
levels (27). Let-7 miRNA serves a
tumor-suppressive role in multiple cancer types by participating in
the post-transcriptional expression of PD-L1 and has been
implicated in the regulation of tumor immunotherapy (28,108). Hong et al (109) reported that Let-7 miRNA could be
enriched by probes in the 3′ UTR region of PD-L1 mRNA in lung
cancer cells, and overexpression of Let-7 miRNA could inhibit PD-L1
mRNA expression in lung cancer cells (110). Similarly, overexpression of
miR-140 can also suppress PD-L1 expression by directly binding to
its 3′ UTR and participating in the miR-140/PD-L1/cyclin E pathway
in lung cancer to regulate the cell cycle and proliferation
(111).
The miR-200 family, consisting of five members,
miR-200a, miR-200b, miR-200c, miR-429 and miR-141, has also been
implicated in the regulation of PD-L1 and inhibition of tumor cell
proliferation and migration (112). Among them, miR-200b may regulate
PD-L1 expression in lung cancer cells and is negatively associated
with PD-L1 expression in patients with lung cancer (113). miR-200c, which is located on
chromosome 12p13, can inhibit PD-L1 upregulation in ovarian and
breast cancer cells to slow cell proliferation (114,115). In breast cancer, high PD-L1
expression is associated with poor prognosis (116,117), and miR-92 can upregulate PD-L1
expression by promoting YAP1 phosphorylation (118). miR-5119 improved antitumor
immunotherapy efficacy in a mouse breast cancer model, possibly by
downregulating PD-L1 expression (119). Furthermore, miR-570-3p, miR-195
and miR-497 induce apoptosis in breast cancer cells by binding to
the 3′ UTR to regulate CD274 expression (120,121). miR-3609 can also bind to the 3′
UTR of PD-L1 to regulate its expression and reverse the
chemoresistance of breast cancer cells by blocking the PD-L1 immune
checkpoint (122).
Exosomes, subcellular vesicles with a diameter of
30-150 nm, contain numerous miRNAs, mRNAs and functional proteins,
which are released after fusion of multivesicular bodies with the
cell surface (123). Therefore,
the identification of exosome contents may provide more information
about specific tumor biomarkers. As an important part of the tumor
microenvironment, exosomes are one of the most important factors in
promoting tumor metastasis and progression by regulating immune
responses, promoting angiogenesis and blocking EMT (124). As one of the highly enriched
miRNAs found in exosomes of breast cancer cells, miR-27a-3p can
upregulate PD-L1 in macrophages and promote immune evasion of
breast cancer cells by activating the PTEN-AKT/PI3K pathway
(125). PTEN expression is also
inhibited by miR-21 mediated by oral cancer exosomes, which
upregulate PD-L1 expression (126). Exosomal miR-16-5p can
specifically target and downregulate PD-L1 in gastric cancer cells
and block the PD-1/PD-L1 checkpoint to inhibit gastric cancer cell
proliferation, leading to T-cell activation (127). Furthermore, aberrant expression
of PD-L1 in gastric cancer is associated with miR-105-5p and
miR-570 (128,129). In addition, miR-105-5p suppresses
PD-L1 expression by directly targeting important cis-acting
regulatory regions in the PD-L1 3′ UTR to combat immune escape
(128). Furthermore, guanine to
cytosine mutations in the 3′ UTR region can disrupt miR-570
binding, leading to upregulation of PD-L1 expression (129).
In colorectal cancer, PD-L1 expression has been
demonstrated to be regulated by several miRNAs, such as miR-15a,
miR-148a-3p, miR-124 and miR-20b-5p (90,130-132). miR-15a potently represses HOXC4
transcription by targeting KDM4B in colorectal cancer cells,
thereby reducing PD-L1 expression and ultimately inhibiting immune
evasion in colorectal cancer cells (90). miR-148a-3p may directly bind to the
3′ UTR region of PD-L1 to reduce the level of PD-L1 on the surface
of colorectal cancer cells to reduce T-cell apoptosis and restore
its activity (130). It has been
reported that the frequency and activity of regulatory T cells
(Tregs) were increased in human cancer types and that PD-L1 may be
involved in Treg development and enhance their immunosuppressive
capacity (133,134). miR-124 can directly target a
specific region in the PD-L1 3′ UTR to downregulate its expression
and inhibit Treg differentiation, thereby promoting T cell-mediated
anticancer responses in colorectal cancer cells (131). HLA complex group 18 (HCG18)
serves an oncogenic role as a competitive endogenous RNA for
several miRNAs (135). In
colorectal cancer, HCG18 promotes proliferation, inhibits
apoptosis, upregulates PD-L1 by sponging miR-20b-5p, enhances
resistance to cetuximab, and inhibits CD8+ T-cell
activation by targeting the miR-20b-5p/PD-L1 axis (132). In other cancer types of the
digestive tract, several miRNAs exhibit inhibitory effects on the
expression of PD-L1. Bian et al (136) found that miR-493 overexpression
could downregulate PD-L1 expression in esophageal cancer.
Transfection with miR-612 reduces PD-L1 expression in pancreatic
cancer cells (137). In
pancreatic cancer cells, miR-93 and miR-106b can inhibit the
expression of PD-L1 at the mRNA and protein levels (138). A study has demonstrated that
miR-329-3p inhibited PD-L1 expression by targeting and
downregulating lysine demethylase 1A, and it enhanced the response
of hepatocellular carcinoma cells to T cell-induced cytotoxic
effects (139). Furthermore,
miR-22 and miR-24 are negatively associated with plasma PD-L1
levels in renal cancer, suggesting that the miRNA network can
suppress PD-L1 expression (140).
Studies suggest that miRNA-based drugs (miRNA mimics or miRNA
antagonists) are promising and may be a novel strategy for cancer
treatment (141,142).
lncRNAs, RNA transcripts of >200 nucleotides, do
not have protein-coding potential, but appear to be less expressed
than protein-coding genes and have more tissue-specific features
(143,144). lncRNAs can target multiple
mechanisms by affecting different genes, and their abnormal
expression is associated with the occurrence of different diseases,
particularly cancer (145). In
particular, increasing evidence suggests that lncRNAs have
significant potential in immunotherapy by regulating PD-L1
expression in the tumor microenvironment (146,147).
In breast cancer, lncRNA KRT19P3 may inhibit tumor
progression by reducing PD-L1 expression in tumor cells and
activating the tumor-killing potential of CD8+ T cells
(148). However, lncRNA GATA3-AS1
can promote immune evasion of breast cancer cells by regulating
COP9 signalosome subunit 5-mediated PD-L1 deubiquitination
(149). In esophageal cancer and
ovarian cancer, lncRNAs can also mediate immune escape by affecting
PD-L1 expression (29,150,151). After binding to glutathione
peroxidase 4, lncRNA OIP5-AS1 can trigger CD8+ T cell
apoptosis by regulating PD-1/PD-L1, thus promoting immune escape of
esophageal cancer cells (29).
Furthermore, lncRNA HOTTIP upregulates PD-L1 expression in
neutrophils by promoting the secretion of IL-6, thereby inhibiting
T cell activity and antitumor immunity (150). Additionally, lncRNA PVT1 promotes
PD-L1 expression in ovarian cancer by upregulating STAT3
phosphorylation levels (151).
Furthermore, lncRNA small nucleolar RNA host gene 12 promotes
non-small cell lung cancer (NSCLC) cell proliferation and immune
escape by increasing the expression stability of PD-L1 through
binding of the human antigen R gene (152).
A study has demonstrated that lncRNA IFITM4P induced
PD-L1 expression in oral cancer via two mechanisms (153). First, in the nucleus, IFITM4P
decreases PTEN transcription by enhancing lysine demethylase 5A
binding to the PTEN promoter, thereby upregulating PD-L1 expression
(153). Second, in the cytoplasm,
IFITM4P acts as a scaffold, promoting SAM and SH3 domain containing
1 binding and phosphorylating transforming growth factor
β-activated kinase 1, which in turn increases the phosphorylation
of NF-κB, while inducing PD-L1 expression (153). The lncRNA HOTAIR promotes the
immune escape of glioma cells by activating the NF-κB pathway to
abnormally express PD-L1 (154).
It has been reported that lncRNAs could regulate different
biological processes, including gene expression and RNA metabolism,
after binding to protein partners (155). The primary transcript of lncRNA
INCR blocks inhibition of the neighboring gene PD-L1 by binding to
heterogeneous nuclear ribonucleoprotein H1 (156). Notably, in colorectal cancer,
lncRNA MIR17HG can increase PD-L1 expression levels by directly
binding PD-L1 (157).
Furthermore, when lncRNA SNHG29 expression is inhibited, PD-L1
expression is downregulated in colorectal cancer cells to promote
antitumor immunity (147).
circRNAs comprise a large class of endogenous
non-coding RNAs with covalently closed loops that function
independently of linear transcripts transcribed from the same gene
(158). circRNAs are mostly
generated through a process of 'back splicing', in which downstream
splice donor sites are covalently linked to upstream splice
acceptor sites, and are abundant in the cytoplasm (159). On the one hand, circRNAs can act
as transcriptional regulators, miRNA sponges or protein decoys to
serve an important role in tumor development and metastasis
(160,161). On the other hand, circRNAs can
alter drug concentrations in tumor cells by regulating the
expression levels of related genes, such as multidrug
resistance-associated protein-1 and multidrug resistance gene 1,
which affects the drug resistance of tumor cells, such as glioma
and liver cancer cells (162). In
a mouse model of NSCLC, combined anti-PD-L1 and hsa_circ_0003222
inhibitory therapy not only reduced the tumor volume, but
hsa_circ_0003222 inhibition also reduced the anti-PD-L1 resistance
of NSCLC cells in vivo (163).
As the most extensively studied type of epigenetic
modification necessary for the regulation of gene transcription,
DNA methylation is a covalent modification of the nucleotide
cytosine at the 5-position (164). Although it does not alter the DNA
sequence, it has an important effect on gene expression and is
often associated with gene silencing (165). A study has demonstrated that DNA
hypomethylation may lead to the expression of PD-L1 and inhibitory
cytokines, which can be immunosuppressive (166). Therefore, the analysis of the
specific mechanism of DNA methylation in regulating PD-L1 gene
expression may have important clinical and biological
implications.
In gastric cancer, PD-L1 promoter methylation is
associated with PD-L1 protein expression, lymph node stage and the
prognosis of advanced gastric cancer (167). A study has demonstrated that
patients with gastric cancer with a methylated PD-L1 promoter
exhibited shorter PFS and OS times than those without a methylated
PD-L1 promoter (167). DNA
methylation is mainly catalyzed by a family of DNMTs (168). In addition, 5-azacytidine, as a
DNMT inhibitor, can increase PD-L1 expression in gastric cancer
MKN-45 cells, whereas gemcitabine, a DNA demethylation inhibitor,
can inhibit PD-L1 expression in these cells (169). Chondrosarcomas do not typically
express PD-L1 to act as an immune-cold tumor; however, DNMT
inhibitors can induce PD-L1 protein expression (73). In sorafenib-resistant
hepatocellular carcinoma, high DNMT1 expression is positively
associated with upregulation of PD-L1 expression (170). Myocyte enhancer factor 2D (MEF2D)
is a transcription factor involved in a number of tumorigenic
processes, and the reduction of MEF2D methylation increases its
binding to the PD-L1 promoter and elevates PD-L1 expression in
hepatocellular carcinoma (139).
In melanoma, DNMT3A is inversely associated with PD-L1 expression
at both the mRNA and protein levels, and treatment with DNMT
inhibitors strongly increases PD-L1 levels on the surface of
melanoma cells (171). DNMT
inhibitors may also augment the efficacy of PD-L1 blockade therapy
in ovarian cancer (172). Li
et al (173) evaluated the
synergistic effect of DNMT3A and DNMT1 on PD-L1 expression in DU145
prostate cancer cells. Recombinant plasmids containing the
C-terminal domains of DNMT1 and DNMT3A methyltransferases inhibit
PD-L1 expression more potently than those containing DNMT3A alone
(173).
After EMT, tumor cells have increased capacities for
proliferation and metastasis by evading the immune system (174). Asgarova et al (175) found that, during EMT signaling in
NSCLC, TGFβ1 induced PD-L1 promoter demethylation by reducing the
content of DNMT1, leading to the expression of PD-L1. In epidermal
growth factor receptor tyrosine kinase inhibitor-resistant NSCLC,
methylation of the PD-L1 promoter may contribute to the
downregulation of PD-L1 expression (176). In anti-PD-1/PD-L1 therapy,
IFN-γ-induced PD-L1 expression predicts a higher response rate
(175). Lai et al
(177) reported that the
IFN-γ-related genes interferon regulatory factor (IRF)-1 and IRF-7,
which are hypermethylated in lung cancer tissues, were negatively
associated with CD274 expression. The methylation inhibitor
decitabine can demethylate IRF-1 and IRF-7, thereby restoring PD-L1
levels (177). In gliomas,
increased methylation of the PD-L1 promoter downregulates the mRNA
and protein expression levels of PD-L1 (178). Therefore, hypomethylation of the
PD-L1 promoter mediates upregulation of PD-L1 expression (179). A similar relationship has been
demonstrated in patients with breast and colorectal cancer: The
higher the hypomethylation levels were, the higher the PD-L1
expression levels were (180). In
addition, PD-L1 expression in breast cancer cells is also
associated with BRCA1 promoter hypermethylation (181). In patients with colorectal
cancer, PD-L1 expression is more readily observed in microsatellite
unstable cancers caused by mutL homolog 1 promoter methylation
(182). The DNMT inhibitor
5-azacytidine also inhibits the downregulation of PD-L1 mRNA and
protein levels in colorectal cancer cells (183).
This review summarizes the most comprehensive
understanding of epigenetic factors affecting PD-L1 expression in
solid tumors, including histone modifications, noncoding RNAs and
DNA methylation (Table I). In
terms of their potential contribution to PD-L1 expression in solid
tumors, studies of histone modifications have mostly focused on
acetylation, methylation and phosphorylation (54,94).
During this process, multiple chromatin-modifying enzymes, such as
HDACs, HATs, histone methyltransferases and histone demethylases,
regulate PD-L1 expression by affecting modifications that occur on
lysine and arginine residues. Most studies on miRNAs have focused
on their binding to the 3′ UTR of PD-L1 (109-111). As one of the key factors
affecting PD-L1 expression, various miRNAs can inhibit PD-L1
expression by binding to the 3′ UTR of mRNAs. lncRNAs mainly act as
upstream regulators of the PD-1/PD-L1 axis to affect antitumor
immunity. Finally, research on DNA methylation has exclusively
focused on its effect on the PD-L1 promoter, and hypomethylation of
the PD-L1 promoter often leads to upregulation of PD-L1 expression,
thereby exerting immunosuppressive effects (30).
A large number of preclinical studies have revealed
the critical role of epigenetic factors in antitumor immune
responses and reversal of immunosuppression, particularly in
PD-L1/PD-1 blockade (72,91,172,184). The rational application of a
combination of multiple epigenetic targeted drugs, including DNMT
inhibitors and histone-modifying enzyme inhibitors, with anti-PD-L1
immunotherapy, represents an opportunity to improve antitumor
efficacy, enhance response rates to PD-1/PD-L1 blocking antibodies
and reverse drug resistance. However, combinations are still in the
early stages of development and there are still certain problems.
First, the additional toxicity afforded by these epigenetic
molecules cannot be underestimated. Some epigenetic drugs have been
used for a long time with manageable side effects; however, the
side effects associated with ICIs have not been extensively
studied, especially in long-term treatment (16). Second, although exosomes are rich
in miRNAs, mRNAs and functional proteins, and usually serve an
important role in the regulation of PD-L1 expression by epigenetic
factors (125,127), current clinical studies of
exosome-based PD-L1 modification are lacking. Finally, although
studies suggest that upregulated PD-L1 expression may be partly
related to the activity of miRNAs, it is not completely clear
whether tumors with increased PD-L1 expression due to dysregulated
miRNA expression also exhibit higher response rates to ICIs
(27,128,129). The development of large-scale
epigenetic marking studies and the continuous updating of testbed
technologies may open the way to address these issues.
In conclusion, at present, a large amount of work
is still required to explore epigenetic changes in depth. Future
studies may develop more precise and effective drugs and treatment
regimens by identifying more potential therapeutic targets and
mechanisms of action. Epigenetic combination therapies will
ultimately be combined in an optimal manner to enhance the
effectiveness of anti-PD-L1 immunotherapy in solid tumors,
improving the prognosis of patients.
Not applicable.
The research project was designed by XM and CS,
organized by XM, JW and BW, and reviewed and critiqued by CS. The
first draft of the manuscript was written by XM. The content and
grammar of the manuscript was revised by JW, BW, CL, LL and CS. All
authors commented on previous versions of the manuscript. All
authors read and approved the final manuscript. Data authentication
is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81973677 and 82174222) and Shandong
Province Natural Science Foundation (grant nos. ZR2021LZY015 and
ZR202103030292).
|
1
|
Chen J, Jiang CC, Jin L and Zhang XD:
Regulation of PD-L1: A novel role of pro-survival signalling in
cancer. Ann Oncol. 27:409–416. 2016. View Article : Google Scholar
|
|
2
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guan J, Lim KS, Mekhail T and Chang CC:
Programmed death ligand-1 (PD-L1) expression in the programmed
death receptor-1 (PD-1)/PD-L1 blockade: A key player against
various cancers. Arch Pathol Lab Med. 141:851–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang F, Wang JF, Wang Y, Liu B and Molina
JR: Comparative analysis of predictive biomarkers for PD-1/PD-L1
inhibitors in cancers: Developments and challenges. Cancers.
14:1092021. View Article : Google Scholar
|
|
6
|
Liu D, Wang S and Bindeman W: Clinical
applications of PD-L1 bioassays for cancer immunotherapy. J Hematol
Oncol. 10:1102017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Zhang H, Liu C, Wang Z, Wu W,
Zhang N, Zhang L, Hu J, Luo P, Zhang J, et al: Immune checkpoint
modulators in cancer immunotherapy: Recent advances and emerging
concepts. J Hematol Oncol. 15:1112022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim CG, Kim M, Hwang J, Kim ST, Jung M,
Kim KH, Kim KH, Chang JS, Koom WS, Roh MR, et al: First-line
pembrolizumab versus dabrafenib/trametinib treatment for BRAF
V600-mutant advanced melanoma. J Am Acad Dermatol. Sep 3–2022.Epub
ahead of print. View Article : Google Scholar
|
|
9
|
Donne R and Lujambio A: The liver cancer
immune microenvironment: Therapeutic Implications for
hepatocellular carcinoma. Hepatology. Aug 21–2022.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun C, Mezzadra R and Schumacher TN:
Regulation and function of the PD-L1 checkpoint. Immunity.
48:434–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Fu C, Du J, Wang H, He R, Yin X,
Li H, Li X, Wang H, Li K, et al: Enhanced histone H3 acetylation of
the PD-L1 promoter via the COP1/c-Jun/HDAC3 axis is required for
PD-L1 expression in drug-resistant cancer cells. J Exp Clin Cancer
Res. 39:292020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary, adaptive, and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burkitt K and Saloura V: Epigenetic
modifiers as novel therapeutic targets and a systematic review of
clinical studies investigating epigenetic inhibitors in head and
neck cancer. Cancers (Basel). 13:52412021. View Article : Google Scholar
|
|
15
|
Huo M, Zhang J, Huang W and Wang Y:
Interplay among metabolism, epigenetic modifications, and gene
expression in cancer. Front Cell Dev Biol. 9:7934282021. View Article : Google Scholar
|
|
16
|
Perrier A, Didelot A, Laurent-Puig P,
Blons H and Garinet S: Epigenetic mechanisms of resistance to
immune checkpoint inhibitors. Biomolecules. 10:10612020. View Article : Google Scholar :
|
|
17
|
Martínez-Cano J, Campos-Sánchez E and
Cobaleda C: Epigenetic priming in immunodeficiencies. Front Cell
Dev Biol. 7:1252019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuendgen A and Lübbert M: Current status
of epigenetic treatment in myelodysplastic syndromes. Ann Hematol.
87:601–611. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoy SM: Tazemetostat: First approval.
Drugs. 80:513–521. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y and Seto E: HDACs and HDAC inhibitors
in cancer development and therapy. Cold Spring Harb Perspect Med.
6:a0268312016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Y, Liu X, Li Y, Quan C, Zheng L and
Huang K: Lung cancer therapy targeting histone methylation:
Opportunities and challenges. Comput Struct Biotechnol J.
16:211–223. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei Q, Wang D, Sun K, Wang L and Zhang Y:
Resistance mechanisms of anti-PD1/PDL1 therapy in solid tumors.
Front Cell Dev Biol. 8:6722020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Donnell JS, Long GV, Scolyer RA, Teng MW
and Smyth MJ: Resistance to PD1/PDL1 checkpoint inhibition. Cancer
Treat Rev. 52:71–81. 2017. View Article : Google Scholar
|
|
24
|
Shen Y, Liu L, Wang M, Xu B, Lyu R, Shi YG
and Tan L: TET2 inhibits PD-L1 gene expression in breast cancer
cells through histone deacetylation. Cancers (Basel). 13:22072021.
View Article : Google Scholar
|
|
25
|
Fan P, Zhao J, Meng Z, Wu H, Wang B, Wu H
and Jin X: Overexpressed histone acetyltransferase 1 regulates
cancer immunity by increasing programmed death-ligand 1 expression
in pancreatic cancer. J Exp Clin Cancer Res. 38:472019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu C, Paschall AV, Shi H, Savage N, Waller
JL, Sabbatini ME, Oberlies NH, Pearce C and Liu K: The MLL1-H3K4me3
axis-mediated PD-L1 expression and pancreatic cancer immune
evasion. J Natl Cancer Inst. 109:djw2832017. View Article : Google Scholar :
|
|
27
|
Xia R, Geng G, Yu X, Xu Z, Guo J, Liu H,
Li N, Li Z, Li Y, Dai X, et al: LINC01140 promotes the progression
and tumor immune escape in lung cancer by sponging multiple
microRNAs. J Immunother Cancer. 9:e0027462021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gilles ME and Slack FJ: Let-7 microRNA as
a potential therapeutic target with implications for immunotherapy.
Expert Opin Ther Targets. 22:929–939. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou J, Huang Q, Fan Z, Sang H, Wu S, Cheng
S and Li Q: LncRNA OIP5-AS1 knockdown facilitated the ferroptosis
and immune evasion by modulating the GPX4 in oesophageal carcinoma.
Comput Math Methods Med. 2022:81031982022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Liang C, Yao X, Yang RH, Zhang ZS,
Liu FY, Li WQ, Pei SH, Ma J, Xie SQ and Fang D: Corrigendum:
PKM2-induced the phosphorylation of histone H3 contributes to
EGF-Mediated PD-L1 transcription in HCC. Front Pharmacol.
12:7247992021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chung HC, Ros W, Delord JP, Perets R,
Italiano A, Shapira-Frommer R, Manzuk L, Piha-Paul SA, Xu L,
Zeigenfuss S, et al: Efficacy and safety of pembrolizumab in
previously treated advanced cervical cancer: Results from the phase
II KEYNOTE-158 study. J Clin Oncol. 37:1470–1478. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abiko K, Hamanishi J, Matsumura N and
Mandai M: Dynamic host immunity and PD-L1/PD-1 blockade efficacy:
Developments after 'IFN-γ from lymphocytes induces PD-L1 expression
and promotes progression of ovarian cancer'. Br J Cancer. Sep
6–2022.Epub ahead of print. View Article : Google Scholar
|
|
33
|
Mussafi O, Mei J, Mao W and Wan Y: Immune
checkpoint inhibitors for PD-1/PD-L1 axis in combination with other
immunotherapies and targeted therapies for non-small cell lung
cancer. Front Oncol. 12:9484052022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garcia-Diaz A, Shin DS, Moreno BH, Saco J,
Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X,
et al: Interferon receptor signaling pathways regulating PD-L1 and
PD-L2 expression. Cell Rep. 29:37662019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ribas A and Wolchok JD: Cancer
immunotherapy using checkpoint blockade. Science. 359:1350–1355.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Akinleye A and Rasool Z: Immune checkpoint
inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol.
12:922019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Heemskerk B, Kvistborg P and Schumacher
TN: The cancer antigenome. EMBO J. 32:194–203. 2013. View Article : Google Scholar :
|
|
38
|
McLane LM, Abdel-Hakeem MS and Wherry EJ:
CD8 T cell exhaustion during chronic viral infection and cancer.
Annu Rev Immunol. 37:457–495. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Z, Liu S, Zhang B, Qiao L and Zhang
Y and Zhang Y: T cell dysfunction and exhaustion in cancer. Front
Cell Dev Biol. 8:172020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sade-Feldman M, Jiao YJ, Chen JH, Rooney
MS, Barzily-Rokni M, Eliane JP, Bjorgaard SL, Hammond MR, Vitzthum
H, Blackmon SM, et al: Resistance to checkpoint blockade therapy
through inactivation of antigen presentation. Nat Commun.
8:11362017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yeon Yeon S, Jung SH, Jo YS, Choi EJ, Kim
MS, Chung YJ and Lee SH: Immune checkpoint blockade
resistance-related B2M hotspot mutations in microsatellite-unstable
colorectal carcinoma. Pathol Res Pract. 215:209–214. 2019.
View Article : Google Scholar
|
|
42
|
Ngiow SF, Young A, Jacquelot N, Yamazaki
T, Enot D, Zitvogel L and Smyth MJ: A threshold level of intratumor
CD8+ T-cell PD1 expression dictates therapeutic response to
anti-PD1. Cancer Res. 75:3800–3811. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li X, Wenes M, Romero P, Huang SC, Fendt
SM and Ho PC: Navigating metabolic pathways to enhance antitumour
immunity and immunotherapy. Nat Rev Clin Oncol. 16:425–441. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
O'Donnell JS, Teng MWL and Smyth MJ:
Cancer immunoediting and resistance to T cell-based immunotherapy.
Nat Rev Clin Oncol. 16:151–167. 2019. View Article : Google Scholar
|
|
45
|
Pauken KE, Sammons MA, Odorizzi PM, Manne
S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al:
Epigenetic stability of exhausted T cells limits durability of
reinvigoration by PD-1 blockade. Science. 354:1160–1165. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Theivanthiran B, Evans KS, DeVito NC,
Plebanek M, Sturdivant M, Wachsmuth LP, Salama AK, Kang Y, Hsu D,
Balko JM, et al: A tumor-intrinsic PD-L1/NLRP3 inflammasome
signaling pathway drives resistance to anti-PD-1 immunotherapy. J
Clin Invest. 130:2570–2586. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bowman GD and Poirier MG:
Post-translational modifications of histones that influence
nucleosome dynamics. Chem Rev. 115:2274–2295. 2015. View Article : Google Scholar :
|
|
48
|
Bajbouj K, Al-Ali A, Ramakrishnan RK,
Saber-Ayad M and Hamid Q: Histone modification in NSCLC: Molecular
mechanisms and therapeutic targets. Int J Mol Sci. 22:117012021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hu X, Lin Z, Wang Z and Zhou Q: Emerging
role of PD-L1 modification in cancer immunotherapy. Am J Cancer
Res. 11:3832–3840. 2021.PubMed/NCBI
|
|
50
|
Greer EL and Shi Y: Histone methylation: A
dynamic mark in health, disease and inheritance. Nat Rev Genet.
13:343–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li W, Wu H, Sui S, Wang Q, Xu S and Pang
D: Targeting histone modifications in breast cancer: A precise
weapon on the way. Front Cell Dev Biol. 9:7369352021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shi Y, Fu Y, Zhang X, Zhao G, Yao Y, Guo
Y, Ma G, Bai S and Li H: Romidepsin (FK228) regulates the
expression of the immune checkpoint ligand PD-L1 and suppresses
cellular immune functions in colon cancer. Cancer Immunol
Immunother. 70:61–73. 2021. View Article : Google Scholar :
|
|
53
|
Zingg D, Arenas-Ramirez N, Sahin D,
Rosalia RA, Antunes AT, Haeusel J, Sommer L and Boyman O: The
histone methyltransferase Ezh2 controls mechanisms of adaptive
resistance to tumor immunotherapy. Cell Rep. 20:854–867. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gallagher SJ, Tiffen JC and Hersey P:
Histone modifications, modifiers and readers in melanoma resistance
to targeted and immune therapy. Cancers (Basel). 7:1959–1982. 2015.
View Article : Google Scholar
|
|
55
|
Deng S, Hu Q, Zhang H, Yang F, Peng C and
Huang C: HDAC3 inhibition upregulates PD-L1 expression in B-cell
lymphomas and augments the efficacy of anti-PD-L1 therapy. Mol
Cancer Ther. 18:900–908. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mondello P, Tadros S, Teater M, Fontan L,
Chang AY, Jain N, Yang H, Singh S, Ying HY, Chu CS, et al:
Selective inhibition of HDAC3 targets synthetic vulnerabilities and
activates immune surveillance in lymphoma. Cancer Discov.
10:440–459. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hashimoto S, Hashimoto A, Muromoto R,
Kitai Y, Oritani K and Matsuda T: Central roles of STAT3-mediated
signals in onset and development of cancers: Tumorigenesis and
immunosurveillance. Cells. 11:26182022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hu G, He N, Cai C, Cai F, Fan P, Zheng Z
and Jin X: HDAC3 modulates cancer immunity via increasing PD-L1
expression in pancreatic cancer. Pancreatology. 19:383–389. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang YF, Liu F, Sherwin S, Farrelly M, Yan
XG, Croft A, Liu T, Jin L, Zhang XD and Jiang CC: Cooperativity of
HOXA5 and STAT3 is critical for HDAC8 inhibition-mediated
transcriptional activation of PD-L1 in human melanoma cells. J
Invest Dermatol. 138:922–932. 2018. View Article : Google Scholar
|
|
60
|
ML PPV, TK MP, ES JP, KVW CL, FC SD, et
al: Essential role of HDAC6 in the regulation of PD-L1 in melanoma.
Mol Oncol. 10:735–750. 2016. View Article : Google Scholar
|
|
61
|
Keremu A, Aimaiti A, Liang Z and Zou X:
Role of the HDAC6/STAT3 pathway in regulating PD-L1 expression in
osteosarcoma cell lines. Cancer Chemother Pharmacol. 83:255–264.
2019. View Article : Google Scholar
|
|
62
|
Yano M, Katoh T, Miyazawa M, Miyazawa M,
Ogane N, Miwa M, Hasegawa K, Narahara H and Yasuda M:
Clinicopathological correlation of ARID1A status with HDAC6 and its
related factors in ovarian clear cell carcinoma. Sci Rep.
9:23972019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu X, Wang Y, Zhang R, Jin T, Qu L, Jin
Q, Zheng J, Sun J, Wu Z, Wang L, et al: HDAC10 is positively
associated with PD-L1 expression and poor prognosis in patients
with NSCLC. Front Oncol. 10:4852020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xu P, Xiong W, Lin Y, Fan L, Pan H and Li
Y: Histone deacetylase 2 knockout suppresses immune escape of
triple-negative breast cancer cells via downregulating PD-L1
expression. Cell Death Dis. 12:7792021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Darvin P, Sasidharan Nair V and Elkord E:
PD-L1 expression in human breast cancer stem cells is
epigenetically regulated through posttranslational histone
modifications. J Oncol. 2019:39589082019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Makowski AM, Dutnall RN and Annunziato AT:
Effects of acetylation of histone H4 at lysines 8 and 16 on
activity of the Hat1 histone acetyltransferase. J Biol Chem.
276:43499–43502. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jin X, Tian S and Li P: Histone
acetyltransferase 1 promotes cell proliferation and induces
cisplatin resistance in hepatocellular carcinoma. Oncol Res.
25:939–946. 2017. View Article : Google Scholar
|
|
68
|
Halaburková A, Jendželovský R, Kovaľ J,
Herceg Z, Fedoročko P and Ghantous A: Histone deacetylase
inhibitors potentiate photodynamic therapy in colon cancer cells
marked by chromatin-mediated epigenetic regulation of CDKN1A. Clin
Epigenetics. 9:622017. View Article : Google Scholar :
|
|
69
|
Maccallini C, Ammazzalorso A, De Filippis
B, Fantacuzzi M, Giampietro L and Amoroso R: HDAC inhibitors for
the therapy of triple negative breast cancer. Pharmaceuticals
(Basel). 15:6672022. View Article : Google Scholar
|
|
70
|
Knox T, Sahakian E, Banik D, Hadley M,
Palmer E, Noonepalle S, Kim J, Powers J, Gracia-Hernandez M,
Oliveira V, et al: Author correction: Selective HDAC6 inhibitors
improve anti-PD-1 immune checkpoint blockade therapy by decreasing
the anti-inflammatory phenotype of macrophages and down-regulation
of immunosuppressive proteins in tumor cells. Sci Rep. 9:148242019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Briere D, Sudhakar N, Woods DM, Hallin J,
Engstrom LD, Aranda R, Chiang H, Sodré AL, Olson P, Weber JS and
Christensen JG: The class I/IV HDAC inhibitor mocetinostat
increases tumor antigen presentation, decreases immune suppressive
cell types and augments checkpoint inhibitor therapy. Cancer
Immunol Immunother. 67:381–392. 2018. View Article : Google Scholar
|
|
72
|
Que Y, Zhang XL, Liu ZX, Zhao JJ, Pan QZ,
Wen XZ, Xiao W, Xu BS, Hong DC, Guo TH, et al: Frequent
amplification of HDAC genes and efficacy of HDAC inhibitor
chidamide and PD-1 blockade combination in soft tissue sarcoma. J
Immunother Cancer. 9:e0016962021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sheikh TN, Chen X, Xu X, McGuire JT,
Ingham M, Lu C and Schwartz GK: Growth inhibition and induction of
innate immune signaling of chondrosarcomas with epigenetic
inhibitors. Mol Cancer Ther. 20:2362–2371. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Huang R, Zhang X, Min Z, Shadia AS, Yang S
and Liu X: MGCD0103 induces apoptosis and simultaneously increases
the expression of NF-κB and PD-L1 in classical Hodgkin's lymphoma.
Exp Ther Med. 16:3827–3834. 2018.PubMed/NCBI
|
|
75
|
Woods DM, Sodré AL, Villagra A, Sarnaik A,
Sotomayor EM and Weber J: HDAC inhibition upregulates PD-1 ligands
in melanoma and augments immunotherapy with PD-1 blockade. Cancer
Immunol Res. 3:1375–1385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu J, He D, Cheng L, Huang C, Zhang Y,
Rao X, Kong Y, Li C, Zhang Z, Liu J, et al: p300/CBP inhibition
enhances the efficacy of programmed death-ligand 1 blockade
treatment in prostate cancer. Oncogene. 39:3939–3951. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bissonnette RP, Cesario RM, Goodenow B,
Shojaei F and Gillings M: The epigenetic immunomodulator, HBI-8000,
enhances the response and reverses resistance to checkpoint
inhibitors. BMC Cancer. 21:9692021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen MC, Lin YC, Liao YH, Liou JP and Chen
CH: MPT0G612, a novel HDAC6 inhibitor, induces apoptosis and
suppresses IFN-γ-induced programmed death-ligand 1 in human
colorectal carcinoma cells. Cancers (Basel). 11:16172019.
View Article : Google Scholar
|
|
79
|
Shin HS, Choi J, Lee J and Lee SY: Histone
deacetylase as a valuable predictive biomarker and therapeutic
target in immunotherapy for non-small cell lung cancer. Cancer Res
Treat. 54:458–468. 2022. View Article : Google Scholar
|
|
80
|
Kuroki H, Anraku T, Kazama A, Shirono Y,
Bilim V and Tomita Y: Histone deacetylase 6 inhibition in
urothelial cancer as a potential new strategy for cancer treatment.
Oncol Lett. 21:642021. View Article : Google Scholar
|
|
81
|
Hai R, Yang D, Zheng F, Wang W, Han X,
Bode AM and Luo X: The emerging roles of HDACs and their
therapeutic implications in cancer. Eur J Pharmacol.
931:1752162022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Xia C, Leon-Ferre R, Laux D, Deutsch J,
Smith BJ, Frees M and Milhem M: Treatment of resistant metastatic
melanoma using sequential epigenetic therapy (decitabine and
panobinostat) combined with chemotherapy (temozolomide). Cancer
Chemother Pharmacol. 74:691–697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Barski A, Cuddapah S, Cui K, Roh TY,
Schones DE, Wang Z, Wei G, Chepelev I and Zhao K: High-resolution
profiling of histone methylations in the human genome. Cell.
129:823–837. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sasidharan Nair V, Saleh R, Toor SM, Taha
RZ, Ahmed AA, Kurer MA, Murshed K, Abu Nada M and Elkord E:
Epigenetic regulation of immune checkpoints and T cell exhaustion
markers in tumor-infiltrating T cells of colorectal cancer
patients. Epigenomics. 12:1871–1882. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bedford MT and Richard S: Arginine
methylation an emerging regulator of protein function. Mol Cell.
18:263–272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jiang Y, Yuan Y, Chen M, Li S, Bai J,
Zhang Y, Sun Y, Wang G, Xu H, Wang Z, et al: PRMT5 disruption
drives antitumor immunity in cervical cancer by reprogramming T
cell-mediated response and regulating PD-L1 expression.
Theranostics. 11:9162–9176. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhou Q, Chen X, He H, Peng S, Zhang Y,
Zhang J, Cheng L, Liu S, Huang M, Xie R, et al: WD repeat domain 5
promotes chemoresistance and programmed death-ligand 1 expression
in prostate cancer. Theranostics. 11:4809–4824. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kim KH and Roberts CW: Targeting EZH2 in
cancer. Nat Med. 22:128–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhao Y, Wang XX, Wu W, Long H, Huang J,
Wang Z, Li T, Tang S, Zhu B and Chen D: EZH2 regulates PD-L1
expression via HIF-1α in non-small cell lung cancer cells. Biochem
Biophys Res Commun. 517:201–209. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu L, Yu T, Jin Y, Mai W, Zhou J and Zhao
C: MicroRNA-15a carried by mesenchymal stem cell-derived
extracellular vesicles inhibits the immune evasion of colorectal
cancer cells by regulating the KDM4B/HOXC4/PD-L1 axis. Front Cell
Dev Biol. 9:6298932021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Soldi R, Ghosh Halder T, Weston A, Thode
T, Drenner K, Lewis R, Kaadige MR, Srivastava S, Daniel Ampanattu
S, Rodriguez Del Villar R, et al: The novel reversible LSD1
inhibitor SP-2577 promotes anti-tumor immunity in
SWItch/Sucrose-NonFermentable (SWI/SNF) complex mutated ovarian
cancer. PLoS One. 15:e02357052020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Qin Y, Vasilatos SN, Chen L, Wu H, Cao Z,
Fu Y, Huang M, Vlad AM, Lu B, Oesterreich S, et al: Inhibition of
histone lysine-specific demethylase 1 elicits breast tumor immunity
and enhances antitumor efficacy of immune checkpoint blockade.
Oncogene. 38:390–405. 2019. View Article : Google Scholar :
|
|
93
|
Liu J, Zhao Z, Qiu N, Zhou Q, Wang G,
Jiang H, Piao Y, Zhou Z, Tang J and Shen Y: Co-delivery of IOX1 and
doxorubicin for antibody-independent cancer chemo-immunotherapy.
Nat Commun. 12:24252021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Olsen JV, Vermeulen M, Santamaria A, Kumar
C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, et al:
Quantitative phosphoproteomics reveals widespread full
phosphorylation site occupancy during mitosis. Sci Signal.
3:ra32010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Schmitz ML, Higgins JMG and Seibert M:
Priming chromatin for segregation: Functional roles of mitotic
histone modifications. Cell Cycle. 19:625–641. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Santaguida S and Amon A: Short- and
long-term effects of chromosome mis-segregation and aneuploidy. Nat
Rev Mol Cell Biol. 16:473–485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Cerutti H and Casas-Mollano JA: Histone H3
phosphorylation: Universal code or lineage specific dialects?
Epigenetics. 4:71–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chen S, Youhong T, Tan Y, He Y, Ban Y, Cai
J, Li X, Xiong W, Zeng Z, Li G, et al: EGFR-PKM2 signaling promotes
the metastatic potential of nasopharyngeal carcinoma through
induction of FOSL1 and ANTXR2. Carcinogenesis. 41:723–733. 2020.
View Article : Google Scholar :
|
|
99
|
Wang WT, Han C, Sun YM, Chen TQ and Chen
YQ: Noncoding RNAs in cancer therapy resistance and targeted drug
development. J Hematol Oncol. 12:552019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kaur M, Kaur B, Konar M and Sharma S:
Noncoding RNAs as novel immunotherapeutic tools against cancer. Adv
Protein Chem Struct Biol. 129:135–161. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rolfo C, Fanale D, Hong DS, Tsimberidou
AM, Piha-Paul SA, Pauwels P, Van Meerbeeck JP, Caruso S, Bazan V,
Cicero G, et al: Impact of microRNAs in resistance to chemotherapy
and novel targeted agents in non-small cell lung cancer. Curr Pharm
Biotechnol. 15:475–485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Schanza LM, Seles M, Stotz M, Fosselteder
J, Hutterer GC, Pichler M and Stiegelbauer V: MicroRNAs associated
with Von Hippel-Lindau pathway in renal cell carcinoma: A
comprehensive review. Int J Mol Sci. 18:24952017. View Article : Google Scholar
|
|
103
|
Forterre A, Komuro H, Aminova S and Harada
M: A comprehensive review of cancer MicroRNA therapeutic delivery
strategies. Cancers (Basel). 12:18522020. View Article : Google Scholar
|
|
104
|
Anastasiadou E, Faggioni A, Trivedi P and
Slack FJ: The nefarious nexus of noncoding RNAs in cancer. Int J
Mol Sci. 19:20722018. View Article : Google Scholar :
|
|
105
|
Shi C and Zhang Z: The prognostic value of
the miR-200 family in ovarian cancer: A meta-analysis. Acta Obstet
Gynecol Scand. 95:505–512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Cortez MA, Anfossi S, Ramapriyan R, Menon
H, Atalar SC, Aliru M, Welsh J and Calin GA: Role of miRNAs in
immune responses and immunotherapy in cancer. Genes Chromosomes
Cancer. 58:244–253. 2019. View Article : Google Scholar :
|
|
107
|
Tang D, Zhao D, Wu Y, Yao R, Zhou L, Lu L,
Gao W and Sun Y: The miR-3127-5p/p-STAT3 axis up-regulates PD-L1
inducing chemoresistance in non-small-cell lung cancer. J Cell Mol
Med. 22:3847–3856. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Chen Y, Xie C, Zheng X, Nie X, Wang Z, Liu
H and Zhao Y: LIN28/let-7/PD-L1 pathway as a target for cancer
immunotherapy. Cancer Immunol Res. 7:487–497. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hong W, Xue M, Jiang J, Zhang Y and Gao X:
Circular RNA circ-CPA4/let-7 miRNA/PD-L1 axis regulates cell
growth, stemness, drug resistance and immune evasion in non-small
cell lung cancer (NSCLC). J Exp Clin Cancer Res. 39:1492020.
View Article : Google Scholar
|
|
110
|
Zhang Q, Pan J, Xiong D, Wang Y, Miller
MS, Sei S, Shoemaker RH, Izzotti A and You M: Pulmonary aerosol
delivery of Let-7b microRNA confers a striking inhibitory effect on
lung carcinogenesis through targeting the tumor immune
microenvironment. Adv Sci (Weinh). 8:e21006292021. View Article : Google Scholar
|
|
111
|
Xie WB, Liang LH, Wu KG, Wang LX, He X,
Song C, Wang YQ and Li YH: MiR-140 expression regulates cell
proliferation and targets PD-L1 in NSCLC. Cell Physiol Biochem.
46:654–663. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Jo H, Shim K and Jeoung D: Potential of
the miR-200 family as a target for developing anti-cancer
therapeutics. Int J Mol Sci. 23:58812022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Katakura S, Kobayashi N, Hashimoto H,
Kamimaki C, Tanaka K, Kubo S, Nakashima K, Teranishi S, Manabe S,
Watanabe K, et al: MicroRNA-200b is a potential biomarker of the
expression of PD-L1 in patients with lung cancer. Thorac Cancer.
11:2975–2982. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Anastasiadou E, Messina E, Sanavia T,
Mundo L, Farinella F, Lazzi S, Megiorni F, Ceccarelli S, Pontecorvi
P, Marampon F, et al: MiR-200c-3p contrasts PD-L1 induction by
combinatorial therapies and slows proliferation of epithelial
ovarian cancer through downregulation of β-catenin and c-Myc.
Cells. 10:5192021. View Article : Google Scholar
|
|
115
|
Rogers TJ, Christenson JL, Greene LI,
O'Neill KI, Williams MM, Gordon MA, Nemkov T, D'Alessandro A,
Degala GD, Shin J, et al: Reversal of triple-negative breast cancer
EMT by miR-200c decreases tryptophan catabolism and a program of
immunosuppression. Mol Cancer Res. 17:30–41. 2019. View Article : Google Scholar
|
|
116
|
Yao Y, Kong X, Liu R, Xu F, Liu G and Sun
C: Development of a novel immune-related gene prognostic index for
breast cancer. Front Immunol. 13:8450932022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Samanta D, Park Y, Ni X, Li H, Zahnow CA,
Gabrielson E, Pan F and Semenza GL: Chemotherapy induces enrichment
of CD47+/CD73+/PDL1+ immune
evasive triple-negative breast cancer cells. Proc Natl Acad Sci
USA. 115:E1239–E1248. 2018.
|
|
118
|
Dou D, Ren X, Han M, Xu X, Ge X, Gu Y and
Wang X: Cancer-associated fibroblasts-derived exosomes suppress
immune cell function in breast cancer via the miR-92/PD-L1 pathway.
Front Immunol. 11:20262020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhang M, Shi Y, Zhang Y, Wang Y, Alotaibi
F, Qiu L, Wang H, Peng S, Liu Y, Li Q, et al: miRNA-5119 regulates
immune checkpoints in dendritic cells to enhance breast cancer
immunotherapy. Cancer Immunol Immunother. 69:951–967. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wang LL, Huang WW, Huang J, Huang RF, Li
NN, Hong Y, Chen ML, Wu F and Liu J: Protective effect of
hsa-miR-570-3p targeting CD274 on triple negative breast cancer by
blocking PI3K/AKT/mTOR signaling pathway. Kaohsiung J Med Sci.
36:581–591. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yang L, Cai Y, Zhang D, Sun J, Xu C, Zhao
W, Jiang W and Pan C: miR-195/miR-497 regulate CD274 expression of
immune regulatory ligands in triple-negative breast cancer. J
Breast Cancer. 21:371–381. 2018. View Article : Google Scholar
|
|
122
|
Li D, Wang X, Yang M, Kan Q and Duan Z:
miR3609 sensitizes breast cancer cells to adriamycin by blocking
the programmed death-ligand 1 immune checkpoint. Exp Cell Res.
380:20–28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Liu J, Ren L, Li S, Li W, Zheng X, Yang Y,
Fu W, Yi J, Wang J and Du G: The biology, function, and
applications of exosomes in cancer. Acta Pharm Sin B. 11:2783–2797.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: Extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Yao X, Tu Y, Xu Y, Guo Y, Yao F and Zhang
X: Endoplasmic reticulum stress-induced exosomal miR-27a-3p
promotes immune escape in breast cancer via regulating PD-L1
expression in macrophages. J Cell Mol Med. 24:9560–9573. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li L, Cao B, Liang X, Lu S, Luo H, Wang Z,
Wang S, Jiang J, Lang J and Zhu G: Microenvironmental oxygen
pressure orchestrates an anti- and pro-tumoral γδ T cell
equilibrium via tumor-derived exosomes. Oncogene. 38:2830–2843.
2019. View Article : Google Scholar
|
|
127
|
Li Z, Suo B, Long G, Gao Y, Song J, Zhang
M, Feng B, Shang C and Wang D: Exosomal miRNA-16-5p derived from M1
macrophages enhances T cell-dependent immune response by regulating
PD-L1 in gastric cancer. Front Cell Dev Biol. 8:5726892020.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Miliotis C and Slack FJ: miR-105-5p
regulates PD-L1 expression and tumor immunogenicity in gastric
cancer. Cancer Lett. 518:115–126. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wang W, Sun J, Li F, Li R, Gu Y, Liu C,
Yang P, Zhu M, Chen L, Tian W, et al: A frequent somatic mutation
in CD274 3′-UTR leads to protein over-expression in gastric cancer
by disrupting miR-570 binding. Hum Mutat. 33:480–484. 2012.
View Article : Google Scholar
|
|
130
|
Ashizawa M, Okayama H, Ishigame T, Thar
Min AK, Saito K, Ujiie D, Murakami Y, Kikuchi T, Nakayama Y, Noda
M, et al: miRNA-148a-3p regulates immunosuppression in DNA mismatch
repair-deficient colorectal cancer by targeting PD-L1. Mol Cancer
Res. 17:1403–1413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Roshani Asl E, Rasmi Y and Baradaran B:
MicroRNA-124-3p suppresses PD-L1 expression and inhibits
tumorigenesis of colorectal cancer cells via modulating STAT3
signaling. J Cell Physiol. 236:7071–7087. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Xu YJ, Zhao JM, Ni XF, Wang W, Hu WW and
Wu CP: LncRNA HCG18 suppresses CD8+ T cells to confer
resistance to cetuximab in colorectal cancer via miR-20b-5p/PD-L1
axis. Epigenomics. 13:1281–1297. 2021.PubMed/NCBI
|
|
133
|
Whiteside TL: The role of regulatory T
cells in cancer immunology. Immunotargets Ther. 4:159–171. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Cai J, Wang D, Zhang G and Guo X: The role
Of PD-1/PD-L1 axis in treg development and function: Implications
for cancer immunotherapy. Onco Targets Ther. 12:8437–8445. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Li S, Wu T, Zhang D, Sun X and Zhang X:
The long non-coding RNA HCG18 promotes the growth and invasion of
colorectal cancer cells through sponging miR-1271 and upregulating
MTDH/Wnt/β-catenin. Clin Exp Pharmacol Physiol. 47:703–712. 2020.
View Article : Google Scholar
|
|
136
|
Bian W, Li Y, Zhu H, Gao S, Niu R, Wang C,
Zhang H, Qin X and Li S: miR-493 by regulating of c-Jun targets
Wnt5a/PD-L1-inducing esophageal cancer cell development. Thorac
Cancer. 12:1579–1588. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Javadrashid D, Mohammadzadeh R,
Baghbanzadeh A, Safaee S, Amini M, Lotfi Z, Baghbani E, Khaze
Shahgoli V and Baradaran B: Simultaneous microRNA-612 restoration
and 5-FU treatment inhibit the growth and migration of human PANC-1
pancreatic cancer cells. EXCLI J. 20:160–173. 2021.PubMed/NCBI
|
|
138
|
Cioffi M, Trabulo SM, Vallespinos M, Raj
D, Kheir TB, Lin ML, Begum J, Baker AM, Amgheib A, Saif J, et al:
The miR-25-93-106b cluster regulates tumor metastasis and immune
evasion via modulation of CXCL12 and PD-L1. Oncotarget.
8:21609–21625. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Wang Y and Cao K: KDM1A promotes
immunosuppression in hepatocellular carcinoma by regulating PD-L1
through demethylating MEF2D. J Immunol Res. 2021:99650992021.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Incorvaia L, Fanale D, Badalamenti G,
Brando C, Bono M, De Luca I, Algeri L, Bonasera A, Corsini LR,
Scurria S, et al: A 'lymphocyte MicroRNA signature' as predictive
biomarker of immunotherapy response and plasma PD-1/PD-L1
expression levels in patients with metastatic renal cell carcinoma:
Pointing towards epigenetic reprogramming. Cancers (Basel).
12:33962020. View Article : Google Scholar
|
|
141
|
Adil MS, Khulood D and Somanath PR:
Targeting Akt-associated microRNAs for cancer therapeutics. Biochem
Pharmacol. 189:1143842021. View Article : Google Scholar :
|
|
142
|
Xue J, Yang J, Luo M, Cho WC and Liu X:
MicroRNA-targeted therapeutics for lung cancer treatment. Expert
Opin Drug Discov. 12:141–157. 2017. View Article : Google Scholar
|
|
143
|
Pal S, Garg M and Pandey AK: Deciphering
the mounting complexity of the p53 regulatory network in
correlation to long non-coding RNAs (lncRNAs) in ovarian cancer.
Cells. 9:5272020. View Article : Google Scholar :
|
|
144
|
Chen X, Tang FR, Arfuso F, Cai WQ, Ma Z,
Yang J and Sethi G: The emerging role of long non-coding RNAs in
the metastasis of hepatocellular carcinoma. Biomolecules.
10:662019. View Article : Google Scholar
|
|
145
|
Vafadar A, Shabaninejad Z, Movahedpour A,
Mohammadi S, Fathullahzadeh S, Mirzaei HR, Namdar A, Savardashtaki
A and Mirzaei H: Long non-coding RNAs as epigenetic regulators in
cancer. Curr Pharm Des. 25:3563–3577. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Yi K, Cui X, Liu X, Wang Y, Zhao J, Yang
S, Xu C, Yang E, Xiao M, Hong B, et al: PTRF/Cavin-1 as a novel
RNA-binding protein expedites the NF-κB/PD-L1 axis by stabilizing
lncRNA NEAT1, contributing to tumorigenesis and immune evasion in
glioblastoma. Front Immunol. 12:8027952022. View Article : Google Scholar
|
|
147
|
Ni W, Mo H, Liu Y, Xu Y, Qin C, Zhou Y, Li
Y, Li Y, Zhou A, Yao S, et al: Targeting cholesterol biosynthesis
promotes anti-tumor immunity by inhibiting long noncoding RNA
SNHG29-mediated YAP activation. Mol Ther. 29:2995–3010. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Fan Y, Dong X, Li M, Liu P, Zheng J, Li H
and Zhang Y: LncRNA KRT19P3 is involved in breast cancer cell
proliferation, migration and invasion. Front Oncol. 11:7990822022.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zhang M, Wang N, Song P, Fu Y, Ren Y, Li Z
and Wang J: LncRNA GATA3-AS1 facilitates tumour progression and
immune escape in triple-negative breast cancer through
destabilization of GATA3 but stabilization of PD-L1. Cell Prolif.
53:e128552020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Shang A, Wang W, Gu C, Chen C, Zeng B,
Yang Y, Ji P, Sun J, Wu J, Lu W, et al: Long non-coding RNA HOTTIP
enhances IL-6 expression to potentiate immune escape of ovarian
cancer cells by upregulating the expression of PD-L1 in
neutrophils. J Exp Clin Cancer Res. 38:4112019. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Chen Y, Li F, Li D, Liu W and Zhang L:
Atezolizumab and blockade of LncRNA PVT1 attenuate cisplatin
resistant ovarian cancer cells progression synergistically via
JAK2/STAT3/PD-L1 pathway. Clin Immunol. 227:1087282021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Huang Y, Xia L, Tan X, Zhang J, Zeng W,
Tan B, Yu X, Fang W and Yang Z: Molecular mechanism of lncRNA
SNHG12 in immune escape of non-small cell lung cancer through the
HuR/PD-L1/USP8 axis. Cell Mol Biol Lett. 27:432022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Shi L, Yang Y, Li M, Li C, Zhou Z, Tang G,
Wu L, Yao Y, Shen X, Hou Z and Jia H: LncRNA IFITM4P promotes
immune escape by up-regulating PD-L1 via dual mechanism in oral
carcinogenesis. Mol Ther. 30:1564–1577. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Wang Y, Yi K, Liu X, Tan Y, Jin W, Li Y,
Zhou J, Wang H and Kang C: HOTAIR up-regulation activates NF-κB to
induce immunoescape in gliomas. Front Immunol. 12:7854632021.
View Article : Google Scholar
|
|
155
|
Atianand MK, Hu W, Satpathy AT, Shen Y,
Ricci EP, Alvarez-Dominguez JR, Bhatta A, Schattgen SA, McGowan JD,
Blin J, et al: A long noncoding RNA lincRNA-EPS acts as a
transcriptional brake to restrain inflammation. Cell.
165:1672–1685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Mineo M, Lyons SM, Zdioruk M, von
Spreckelsen N, Ferrer-Luna R, Ito H, Alayo QA, Kharel P, Giantini
Larsen A, Fan WY, et al: Tumor interferon signaling is regulated by
a lncRNA INCR1 transcribed from the PD-L1 locus. Mol Cell.
78:1207–1223.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Xu J, Meng Q, Li X, Yang H, Xu J, Gao N,
Sun H, Wu S, Familiari G, Relucenti M, et al: Long noncoding RNA
MIR17HG promotes colorectal cancer progression via miR-17-5p.
Cancer Res. 79:4882–4895. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Yin L, Tang Y and Yuan Y: An overview of
the advances in research on the molecular function and specific
role of circular RNA in cardiovascular diseases. Biomed Res Int.
2022:51541222022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Verduci L, Tarcitano E, Strano S, Yarden Y
and Blandino G: CircRNAs: Role in human diseases and potential use
as biomarkers. Cell Death Dis. 12:4682021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Dong W, Dai ZH, Liu FC, Guo XG, Ge CM,
Ding J, Liu H and Yang F: The RNA-binding protein RBM3 promotes
cell proliferation in hepatocellular carcinoma by regulating
circular RNA SCD-circRNA 2 production. EBioMedicine. 45:155–167.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Wang L, Yi J, Lu LY, Zhang YY, Wang L, Hu
GS, Liu YC, Ding JC, Shen HF, Zhao FQ, et al: Estrogen-induced
circRNA, circPGR, functions as a ceRNA to promote estrogen
receptor-positive breast cancer cell growth by regulating cell
cycle-related genes. Theranostics. 11:1732–1752. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Wang S, Qian L, Cao T, Xu L, Jin Y, Hu H,
Fu Q, Li Q, Wang Y, Wang J, et al: Advances in the study of
CircRNAs in tumor drug resistance. Front Oncol. 12:8683632022.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Li C, Zhang J, Yang X, Hu C, Chu T, Zhong
R, Shen Y, Hu F, Pan F, Xu J, et al: hsa_circ_0003222 accelerates
stemness and progression of non-small cell lung cancer by sponging
miR-527. Cell Death Dis. 12:8072021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Feinberg AP: The key role of epigenetics
in human disease prevention and mitigation. N Engl J Med.
378:1323–1334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Srivastava R and Lodhi N: DNA methylation
malleability and dysregulation in cancer progression: Understanding
the role of PARP1. Biomolecules. 12:4172022. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Emran AA, Chatterjee A, Rodger EJ, Tiffen
JC, Gallagher SJ, Eccles MR and Hersey P: Targeting DNA methylation
and EZH2 activity to overcome melanoma resistance to immunotherapy.
Trends Immunol. 40:328–344. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Lv D, Xing C, Cao L, Zhuo Y, Wu T and Gao
N: PD-L1 gene promoter methylation represents a potential
diagnostic marker in advanced gastric cancer. Oncol Lett.
19:1223–1234. 2020.PubMed/NCBI
|
|
168
|
Del Castillo Falconi VM, Torres-Arciga K,
Matus-Ortega G, Díaz-Chávez J and Herrera LA: DNA
methyltransferases: From evolution to clinical applications. Int J
Mol Sci. 23:89942022. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Lu X, Li Y, Yang W, Tao M, Dai Y, Xu J and
Xu Q: Inhibition of NF-κB is required for oleanolic acid to
downregulate PD-L1 by promoting DNA demethylation in gastric cancer
cells. J Biochem Mol Toxicol. 35:e226212021. View Article : Google Scholar
|
|
170
|
Liu J, Liu Y, Meng L, Liu K and Ji B:
Targeting the PD-L1/DNMT1 axis in acquired resistance to sorafenib
in human hepatocellular carcinoma. Oncol Rep. 38:899–907. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Chatterjee A, Rodger EJ, Ahn A, Stockwell
PA, Parry M, Motwani J, Gallagher SJ, Shklovskaya E, Tiffen J,
Eccles MR and Hersey P: Marked global DNA hypomethylation is
associated with constitutive PD-L1 expression in melanoma.
iScience. 4:312–325. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Peng D, Kryczek I, Nagarsheth N, Zhao L,
Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W, et al: Epigenetic
silencing of TH1-type chemokines shapes tumour immunity and
immunotherapy. Nature. 527:249–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Li X, Wang Z, Huang J, Luo H, Zhu S, Yi H,
Zheng L, Hu B, Yu L, Li L, et al: Specific zinc finger-induced
methylation of PD-L1 promoter inhibits its expression. FEBS Open
Bio. 9:1063–1070. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Garg M: Epithelial-mesenchymal
transition-activating transcription factors-multifunctional
regulators in cancer. World J Stem Cells. 5:188–195. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Asgarova A, Asgarov K, Godet Y, Peixoto P,
Nadaradjane A, Boyer-Guittaut M, Galaine J, Guenat D, Mougey V,
Perrard J, et al: PD-L1 expression is regulated by both DNA
methylation and NF-kB during EMT signaling in non-small cell lung
carcinoma. Oncoimmunology. 7:e14231702018. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Zhang Y, Xiang C, Wang Y, Duan Y, Liu C
and Zhang Y: PD-L1 promoter methylation mediates the resistance
response to anti-PD-1 therapy in NSCLC patients with EGFR-TKI
resistance. Oncotarget. 8:101535–101544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Lai Q, Wang H, Li A, Xu Y, Tang L, Chen Q,
Zhang C, Gao Y, Song J and Du Z: Decitibine improve the efficiency
of anti-PD-1 therapy via activating the response to IFN/PD-L1
signal of lung cancer cells. Oncogene. 37:2302–2312. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Mu L, Long Y, Yang C, Jin L, Tao H, Ge H,
Chang YE, Karachi A, Kubilis PS, De Leon G, et al: The IDH1
mutation-induced oncometabolite, 2-hydroxyglutarate, may affect DNA
methylation and expression of PD-L1 in gliomas. Front Mol Neurosci.
11:822018. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Briand J, Nadaradjane A, Bougras-Cartron
G, Olivier C, Vallette FM and Cartron PF: Diuron exposure and Akt
overexpression promote glioma formation through DNA
hypomethylation. Clin Epigenetics. 11:1592019. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Elashi AA, Sasidharan Nair V, Taha RZ,
Shaath H and Elkord E: DNA methylation of immune checkpoints in the
peripheral blood of breast and colorectal cancer patients.
Oncoimmunology. 8:e15429182018. View Article : Google Scholar
|
|
181
|
Jacot W, Lopez-Crapez E, Mollevi C,
Boissière-Michot F, Simony-Lafontaine J, Ho-Pun-Cheung A, Chartron
E, Theillet C, Lemoine A, Saffroy R, et al: BRCA1 promoter
hypermethylation is associated with good prognosis and
chemosensitivity in triple-negative breast cancer. Cancers (Basel).
12:8282020. View Article : Google Scholar
|
|
182
|
Yamada R, Yamaguchi T, Iijima T, Wakaume
R, Takao M, Koizumi K, Hishima T and Horiguchi SI: Differences in
histological features and PD-L1 expression between sporadic
microsatellite instability and Lynch-syndrome-associated disease in
Japanese patients with colorectal cancer. Int J Clin Oncol.
23:504–513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Hua S, Gu M, Wang Y, Ban D and Ji H:
Oxymatrine reduces expression of programmed death-ligand 1 by
promoting DNA demethylation in colorectal cancer cells. Clin Transl
Oncol. 23:750–756. 2021. View Article : Google Scholar
|
|
184
|
Liu Z, Ren Y, Weng S, Xu H, Li L and Han
X: A new trend in cancer treatment: The combination of epigenetics
and immunotherapy. Front Immunol. 13:8097612022. View Article : Google Scholar : PubMed/NCBI
|