Introduction
Endotoxemia presents a serious threat to pediatric
critical care settings, as this illness may rapidly progress to
multiple organ failure and ultimately to death. The mortality rate
of endotoxemia is very high. However, the pathophysiology regarding
the effects of endotoxemia on the central nervous system (CNS)
remains poorly characterized. The enhanced expression of heat shock
protein (HSP) is protective against septic shock in laboratory
models (1). The inducible HSP
isoforms (HSP70 and HSP27) are believed to confer cellular
protection. Previous studies using laboratory models of sepsis,
ischemia-reperfusion or acute lung injury have suggested that these
disorders may be significantly attenuated or prevented by the
enhanced expression of HSPs (2–4).
In vitro and in vivo models have reported that HSP
expression suppresses plasma concentrations of proinflammatory
cytokines (5). HSP70 has been
reported to inhibit the activation of nuclear factor-κB (NF-κB), a
family of transcription factors that activate target genes involved
in inflammation, the immune response and cell apoptosis (6). HSP70 exerts this effect by inducing
IκBα, the inhibitor of NF-κB (7,8).
The nonessential amino acid glutamine (Gln) enhances
the in vitro survival of cells. Platelet-derived growth
factor (PDGF) functions in the development and regulation of the
nervous system (9,10). PDGF is upregulated in the
hypoxic-ischemic neonatal rat brain (11). The expression levels of PDGF and
its receptor following endotoxemic brain damage have not been
examined.
To characterize the protective effect of Gln
following endotoxemic brain damage, particularly in the young
brain, we developed an endotoxemia model by injecting Wistar rats
intraperitoneally with lipopolysaccharide (LPS) 10 days after birth
(12). The expression of NF-κB,
HSP70, platelet-derived growth factor-B (PDGF-B) and PDGF
receptor-β (PDGFR-β) in brain cells was examined by
immunohistochemistry and western blotting. In addition, brain cell
ultrastructure was examined for apoptosis. Comparative analyses
were performed between animals receiving intraperitoneal LPS
injection with or without prior Gln administration. This study
preliminarily supports the use of Gln therapy for treatment of
various infantile brain diseases, particularly ischemic and septic
brain diseases.
Materials and methods
Animals and reagents
Healthy, 10-day-old male and female Wistar rats
(mass 22.3±3.1 g) were provided by the Animal Center of the
Shengjing Hospital of China Medical University. N-(2)-L-alanine-L Gln (20%), containing
13.46% L-Gln, was purchased from Fresenius (Germany). Rabbit
anti-rat HSP70 antibody was obtained from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Rabbit anti-rat NF-κB, PDGF-B, and PDGFR-β
antibodies and ABC kits were purchased from Boster (Wuhan, China).
LPS (E. coli O55:B5) powder,
α-dianisidine, β-naphthyl acid phosphate and hydroxybenzene were
purchased from Sigma (St. Louis, MO, USA). The study was approved
by the Ethics Committee of Shengjing Hospital of China Medical
University.
Rat model of endotoxemia and tissue
preparation
Wistar rats were divided randomly into the control,
LPS and treatment groups. Control rats were intraperitoneally
injected with 1 ml/kg of 0.9% sodium chloride, the same injection
volume as the other groups. LPS group rats were intraperitoneally
injected with a single 5 mg/kg bolus of LPS. Treatment group rats
were injected with single boluses each of Gln and LPS, such that 1
ml/kg solution of Gln (1.346 g/kg) was administered
intraperitoneally 1 h prior to LPS injection. Rats were fed after
injection and were sacrificed at the indicated times. Cerebral
tissues were then harvested. The cutting point was selected as the
junction of the optic chiasm, the middle of premamillaris and the
rear of the cerebrum and cerebellum. Fifteen rats from each group
were used for western blotting. Three rats in each group were
sacrificed 2, 6, 12, 24 and 72 h after the injection and cerebra
were obtained. Remaining brain tissues were preserved at −70°C for
additional protein analyses. For immunohistochemistry and electron
microscopy, 8 rats from each group were terminated 2, 6 and 72 h
post-injection. Brains were divided into 4 sections and immediately
fixed in 40 g/l formaldehyde for 24 h. Samples then were embedded
in paraffin for immunohistochemistry. For electron microscopy, a
coronal block (1 mm3) of the rat brain was excised at
4°C and fixed in 2.5% paraformaldehyde solution for 2 h.
Electron microscopy
Following fixation, blocks were washed with dimethyl
arsenate buffer, fixed in 1% osmium tetroxide for 60 min and
dehydrated in ascending concentrations of ethanol. Samples then
were passed into propylene oxide and embedded in Epon 812 epoxy
resin. After 72 h, 60–80 nm ultra-thin sections were made using a
microtome and were stained with 5-uranyl acetate and lead citrate.
Sections were examined with a Hitachi H-600 transmission electron
microscope.
Immunolocalization of NF-κB, HSP70,
PDGF-B and PDGFR-β
Transverse sections (6 μm) of brain were
immunohistochemically stained for the detection of NF-κB (1:800),
HSP70 (1:100), PDGF-B (1:75) and PDGFR-β (1:150). Indirect
immunoperoxidase (i.e., ABC) methods were used. For ABC reactions,
sections were immersed in 3 ml/l H2O2 for 15
min at 37°C and were washed in PBS. Samples then were incubated for
24 h at 4°C in the primary antibody and in biotinylated anti-rabbit
IgG diluted 1:200 for 1 h. Samples were incubated for 15 min at
37°C in the ABC complex and were visualized in 0.03%
diaminobenzidine containing 0.005% peroxide. The specificity of
immunostaining was confirmed by replacing the primary antibody with
nonimmune normal rabbit serum, which resulted in total abolishment
of immunoreactivity.
Western blotting for HSP70, PDGD-B and
PDGFR-β
Three cerebra from each group were homogenized in
lysis buffer containing 20 mmol/l Tris-HCl (pH 7.5), 10 ml/l
Triton, 0.2 mol/l NaCl, 2 mmol/l EDTA, 2 mmol/l EGTA, 1 mol/l DTT
and 2 mol/l aprotinin. Samples were centrifuged at 12,000 × g for 1
h at 4°C. Equal amounts of brain protein samples (50 μg) were
diluted in SDS incubation medium (100 mmol/l Tris-Cl (pH 6.8), 200
mmol/l DTT, 40 ml/l SDS, 2 ml/l bromophenol blue and 200 ml/l
glycerol) and were applied to 0.8% SDS-PAGE. Samples were
electrophoresed at 100 V for 1 h. Proteins then were transferred
onto nitrocellulose at 50 V and 4°C for 2 h. After blocking with 50
ml/l non-fat dry milk in PBST [PBS (pH 7.4), 0.1% Tween-20] for 1
h, nitrocellulose blots were incubated overnight in primary
antibody [anti-HSP70 (1:200), anti-PDGD-B (1:200), or anti-PDGFR-β
(1:200)] and were then incubated with alkaline
phosphatase-conjugated goat anti-rabbit IgG (1:2000; Chemicon,
Temecula, CA, USA) for 2 h. Immunoreactive proteins were visualized
using alkaline phosphatase. The images were analyzed using imaging
software. A comparison of the LPS and treatment groups was
expressed in terms of the relative protein content (%), which was
determined as the gray value of the protein strip in sample/gray
value of protein strip in the control ×100%.
Statistical analysis
Data were analyzed by Student’s t-test using SPSS
12.0. All data are expressed as the means ± SD for each group.
P<0.05 was considered to indicate a statistically significant
difference.
Results
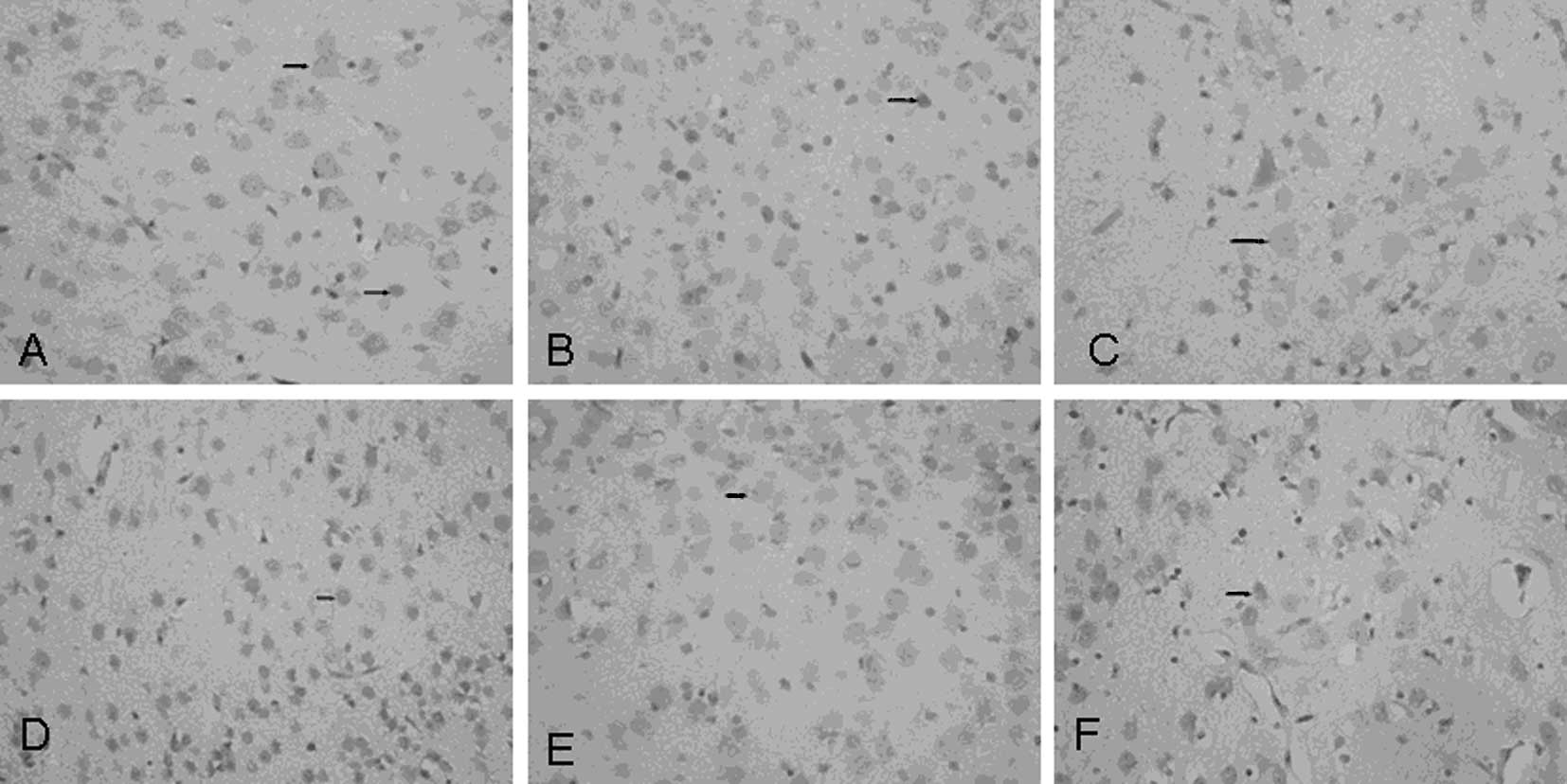
Electron microscopic analysis
The neurocytes of control rats were characterized by
discrete nuclear structures with evenly distributed chromatin,
prominent nucleoli and intact nuclear membranes. Golgi apparatus
and rough endoplasmic reticula were distributed loosely with
ribosomes attached to the surface. Mitochondria were clearly
visible with inner cristae (Fig.
1A). At 2 h post-injection with LPS, nascent apoptosis was
observed with significant swelling of nerve cells, shrunken nuclear
membranes, irregular arrangements of endoplasmic reticula and
degranulated ribosomes (Fig. 1B).
At 6 h post-injection with LPS, the extent of apoptosis was more
pronounced. Nerve cells exhibited chromatin condensation and
nucleolar margination (Fig. 1C).
At 72 h, advanced apoptosis was observed with prominent nuclear
fragmentation and with mitochondria displaying vacuolar
degeneration, defective cristae and decreased matrix density
(Fig. 1D). Near normal neurons
were observed in the Gln treatment group 72 h after LPS injection.
Nuclear membranes, nucleoli and mitochondrial structures were
visible. Endoplasmic reticula were moderately swollen and
irregularly arranged.
 | Figure 1Neurons visualized by electron
microscopy at various magnifications during the process of
lipopolysaccharide (LPS)-induced apoptosis. (A) Normal neuron,
×4,000. (B) Early apoptosis in neuron 2 h after LPS injection,
×10,000. (C) Medium-term apoptosis in neuron 6 h after LPS
injection, ×10,000. (D) Advanced apoptosis in neuron 72 h after LPS
injection, ×10,000. (E) Neuron 72 h after LPS injection in
glutamine (Gln) treatment group, ×5,000. |
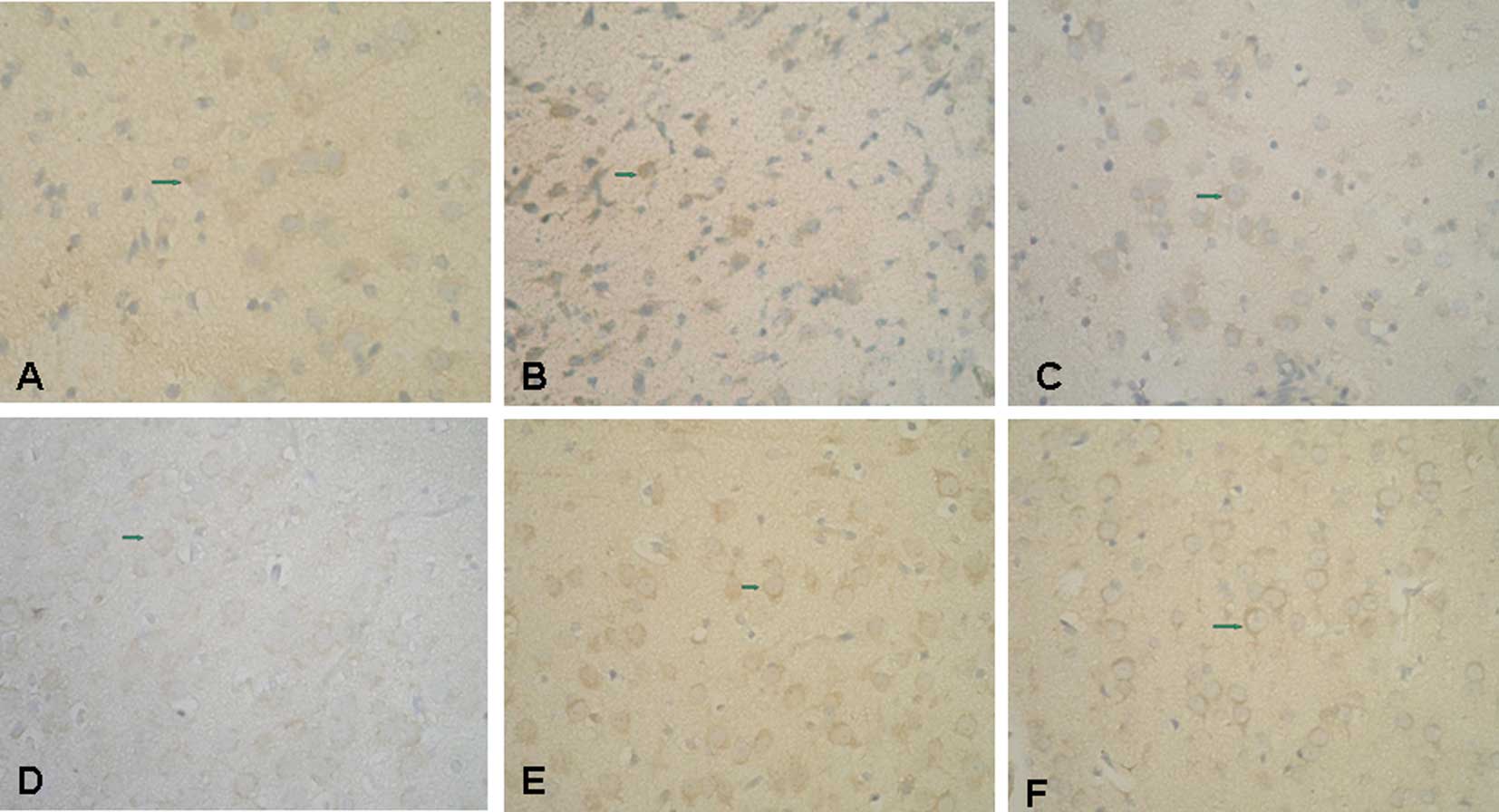
Immunolocalization of NF-κB and HSP70 in
brain tissue
Anti-NF-κB antibody faintly stained the nuclei of
control nerve cells (Fig. 2A). At
2–6 h after LPS injection (LPS group), increased NF-κB
immunoreactivity was observed in the nuclei of several cortical
neurons; immunoreactivity was more distinct at 6 h (Fig. 2B). At 2 h post-injection with LPS,
NF-κB immunoreactivity could barely be observed in the nuclei of
Gln-treated nerve cells (Fig.
2C).
In control brain tissue, HSP70 immunoreactivity was
observed in the nucleoli of cortical neurons (Fig. 2D). Two hours after LPS injection,
the number of positively stained neuronal cells decreased in the
LPS group compared to controls (data not shown). HSP70
immunoreactivity was decreased further in LPS group brain samples 6
h after the injection (Fig. 2E).
In the Gln treatment group, intense HSP70 immunoreactivity was
observed in the nucleoli of cortical neurons 2 h after LPS
injection as compared to the LPS group (Fig. 2F).
Western blot analysis of HSP70 protein
expression following LPS injection
Brain tissues from the LPS group maintained
relatively constant HSP70 levels at 2 and 6 h post-injection
(Fig. 3, Table I). HSP70 expression decreased 12 h
after LPS injection and decreased further at 24 h post-injection.
Gln-treated tissues displayed increasing HSP70 levels at 2 and 6 h
after LPS injection and did not exhibit subsequent decreases in
HSP70. Compared to the LPS group, the expression levels of HSP70
were significantly higher in the Gln treatment group at all
examined time points between 2 and 24 h post-injection.
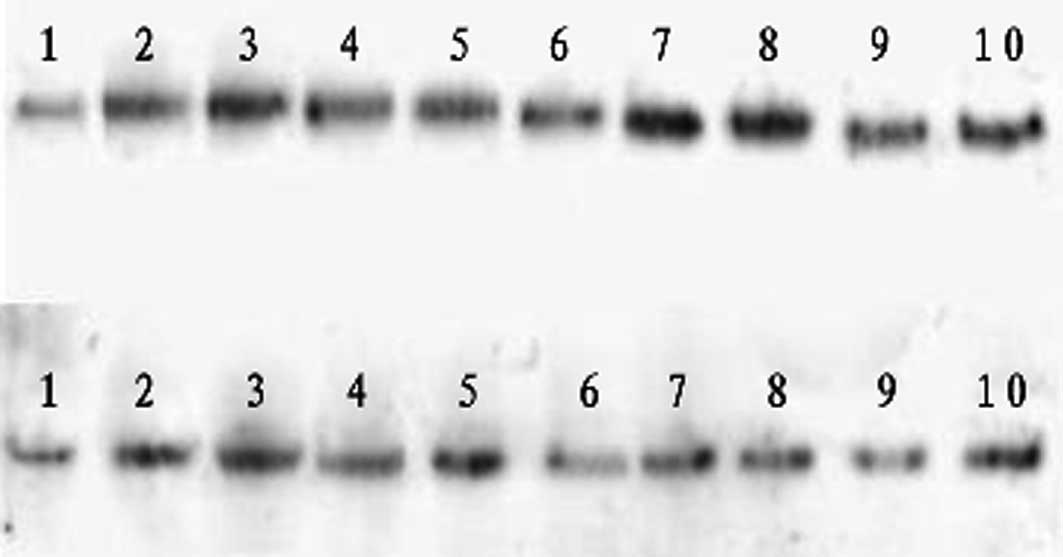 | Figure 3Western blot analysis of heat shock
protein 70 (HSP70) protein expression at various time points
following lipopolysaccharide (LPS) injection. Upper row depicts the
control group from left to right as 1–5 (2, 6, 12, 24 and 72 h) and
the treatment group as 6–10 (2, 6, 12, 24 and 72 h). Lower row
depicts the control group from left to right as 1–5 (2, 6, 12, 24
and 72 h) and the LPS group as 6–10 (2, 6, 12, 24 and 72 h). |
 | Table ISemiquantitative expression of HSP70
in each group (χ̄ ± s, n=3). |
Table I
Semiquantitative expression of HSP70
in each group (χ̄ ± s, n=3).
| Group | 2 h | 6 h | 12 h | 24 h | 72 h |
|---|
| LPS | 1.05±0.04 | 1.04±0.05 | 0.72±0.04 | 0.61±0.03 | 0.92±0.03 |
| Gln | 1.83±0.02 | 1.20±0.03 | 1.03±0.08 | 0.85±0.02 | 1.01±0.07 |
| T | 9.51 | 4.75 | 6.01 | 11.54 | 2.05 |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | >0.05 |
Immunolocalization of PDGF-B and PDGFR-β
in brain tissue
Cytoplasmic PDGF-B immunoreactivity was observed in
the cortical cells of control rats (Fig. 4A). In the LPS group at 2 and 6 h
post-injection, PDGF-B immunoreactivity was lightly positive. At 72
h after injection, LPS group samples displayed markedly positive
PDGF-B immunoreactivity in the nucleoli of cortical neurons
(Fig. 4B). PDGF-B immunoreactivity
was observed at these same time points in the Gln treatment group
(Fig. 4C).
PDGFR-β immunoreactivity was slightly positive in
the membranes of control cortical neurons (Fig. 4D). Conversely, PDGFR-β
immunoreactivity was abolished from 2 to 24 h after injection of
LPS in both the LPS and Gln treatment groups. In the LPS group at
72 h post-injection, PDGFR-β weakly stained neurons (Fig. 4E), whereas PDGFR-β staining was
moderately positive in Gln-treated neurons at the same time points
(Fig. 4F).
Western blot analysis of PDGF-B protein
following LPS injection
PDGF-B protein expression decreased from 2 to 24 h
in the LPS group post-injection and returned to control levels at
72 h post-injection (Fig. 5,
Table II). The decrease in PDGF-B
in the Gln treatment group was less pronounced than that in the LPS
group (Fig. 5, 2 and 12 h), and
PDGF-B significantly increased in the treatment group 72 h
post-injection. Compared to the LPS group, the expression levels of
PDGF-B were significantly increased in the treatment group 2, 12
and 72 h post-injection.
 | Figure 5Western blot analysis of
platelet-derived growth factor-B (PDGF-B) protein at various time
points following injection of lipopolysaccharide (LPS). Upper row
depicts the control group from left to right as 1–5 (2, 6, 12, 24
and 72 h) and the glutamine (Gln) treatment group as 6–10 (2, 6,
12, 24 and 72 h). Lower row depicts the control group from left to
right as 1–5 (2, 6, 12, 24 and 72 h) and the LPS group as 6–10 (2,
6, 12, 24 and 72 h). |
 | Table IISemiquantitative expression of PDGF-B
in LPS and Gln treatment groups (χ̄ ± s, n=3). |
Table II
Semiquantitative expression of PDGF-B
in LPS and Gln treatment groups (χ̄ ± s, n=3).
| Group | 2 h | 6 h | 12 h | 24 h | 72 h |
|---|
| LPS | 0.35±0.02 | 0.42±0.02 | 0.38±0.03 | 0.63±0.03 | 1.08±0.02 |
| Gln | 0.45±0.03 | 0.37±0.03 | 0.62±0.02 | 0.66±0.02 | 1.94±0.05 |
| T | 4.81 | 2.40 | 11.54 | 1.44 | 27.65 |
| P-value | <0.01 | >0.05 | <0.01 | >0.05 | <0.01 |
Western blot analysis of PDGFR-β protein
following LPS injection
PDGFR-β protein expression increased 2 h
post-injection in the LPS group, whereas it progressively decreased
from 12 to 72 h post-injection (Fig.
6, Table III). In the Gln
treatment group, a greater increase in PDGFR-β protein expression
was observed from 2 to 6 h post-injection. In addition, the
late-stage decrease in PDGFR-β was less pronounced than that of the
LPS group. Compared with the LPS group, the expression levels of
PDGFR-β were significantly higher in the Gln treatment group at
each time point.
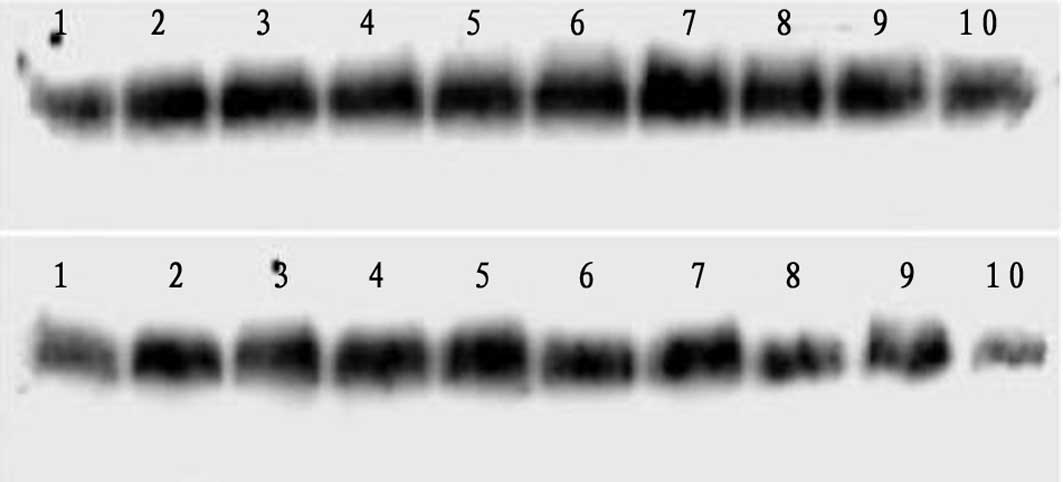 | Figure 6Western blot analysis of PDGF
receptor-β (PDGFR-β) protein at various time points following
injection of lipopolysaccharide (LPS). Upper row depicts the
control group from left to right as 1–5 (2, 6, 12, 24 and 72 h) and
the treatment group as 6–10 (2, 6, 12, 24 and 72 h). Lower row
depicts the control group from left to right as 1–5 (2, 6, 12, 24
and 72 h) and the LPS group as 6–10 (2, 6, 12, 24 and 72 h). |
 | Table IIISemiquantitative expression of PDGFR-β
in LPS and Gln treatment groups (χ̄ ± s, n=3). |
Table III
Semiquantitative expression of PDGFR-β
in LPS and Gln treatment groups (χ̄ ± s, n=3).
| Group | 2 h | 6 h | 12 h | 24 h | 72 h |
|---|
| LPS | 1.30±0.04 | 1.02±0.03 | 0.78±0.02 | 0.80±0.03 | 0.36±0.04 |
| Gln | 1.59±0.03 | 1.20±0.03 | 0.97±0.03 | 0.98±0.02 | 0.77±0.03 |
| T | 10.07 | 7.35 | 9.13 | 8.65 | 10.05 |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Discussion
The present study demonstrates that the apoptotic
process occurs in brain cells subjected to experimental endotoxemia
and that apoptosis is suppressed to a certain extent by
administration of Gln prior to endotoxemia. NF-κB was detected in
brain cells undergoing endotoxemic stress; this transcription
factor was suppressed by administration of Gln. Pretreatment with
Gln prevented endotoxemia-associated suppression of HSP70. Finally,
Gln ameliorated the downregulation in PDGF-B and PDGFR-β proteins
that occurred in untreated brain cells undergoing endotoxemia.
Apoptosis is a process of gene-regulated cell death
that involves special biochemical and morphological manifestations.
Morphologically, apoptosis is characterized by pyknosis with
chromatin condensation, such that chromatin forms crescent bodies
within intact nuclear membranes. The mechanism of apoptosis induced
by endotoxin injection has been postulated as Akt pathway
activation (13), Bcl-2 family
induction (14) or release of
inflammatory cytokines (15). In
concordance with a previous study that reported brain cell
apoptosis upon systemic induction of inflammation (16), our study observed enhanced
apoptosis in response to LPS injection. Gln pre-administration
protected against brain cell apoptosis, supporting the theory that
Gln may be effective in reducing endotoxemic brain damage.
NF-κB is one of the most important mediators of
stress and inflammatory gene expression. Activation of NF-κB plays
an important role in the CNS, particularly in the neopallium
neocortex, olfactory bulb, amygdaloid nucleus and hippocampus. Chen
and other researchers discovered that NF-κB is activated
immediately upon injury, peaking at 12 h. A separate study reported
that 2–6 h after intraperitoneal injection with endotoxin, NF-κB is
activated in neurons of the pallium; after 12 h, NF-κB is activated
in astrocyte nuclei surrounding denatured neurons. In premature
rats subjected to endotoxemia, the activation of NF-κB is strongly
associated with neuronal survival in the early stages of brain
injury and inflammation (17,18).
HSP72 may play a major role in attenuating the inflammatory
response following Gln administration in sepsis (19). The present study demonstrated that
the expression of HSP70 is suppressed during endotoxemia and that
this effect could be avoided by Gln pretreatment.
The expression of PDGF-B is capable of relieving
hypoxemic brain injury after shock. In macaques with simian
immunodeficiency virus encephalitis, the expression of PDGF-B mRNA
increased in the brains (20).
Previous studies demonstrated that endogenous PDGF-B plays
important roles in healing after trauma, synapse regeneration and
functional recovery (21). We
detected PDGF-B expression in the cytoplasm of cortical neurons and
PDGFR-β expression in the cell membranes of cortical neurons.
Post-injection with LPS, PDGF-B expression decreased significantly
at 2, 12 and 72 h. At 72 h, PDGF-B expression recovered, indicating
that PDGF-B is capable of protecting nerves by regulating neuroglia
cell differentiation following brain injury. PDGFR-β expression is
increased early following experimental endotoxemia, indicating that
organisms may sustain and nourish cells by expressing PDGFR-β in
the early stages of infectious stress.
The mechanism by which PDGF and its β receptor
protect nerve cells may involve the following: (i) Prior PDGF
exposure protects neurons against excitotoxicity (22); (ii) PDGF-B may increase neuron
viability by inhibiting NMDA receptor activation, thereby
inhibiting excessive influx of Ca2+; (iii) PDGF may
promote the activity of superoxide dismutase and glutathione
reductase and protect the nerves (23); (iv) activation of PI-3 may inhibit
the apoptosis of nerve cells (24); (v) PDGF-B is necessary for
survival, and induction of PDGF-B can increase cell migration and
neural differentiation by basic fibroblast growth factor as well as
decrease other open channels (25), causing prolonged suppression of the
NMDA receptor (26). In the
present study, expression of PDGF-B decreased during experimental
sepsis for 2–24 h. By 72 h, PDGF-B expression recovered, but did
not reach the same levels as in the Gln group. The expression of
PDGF-B and PDGF in neurons of the pallium in the early and late
stages of sepsis in premature rats, suggest that the mechanism of
protecting brain injury may be related to these factors.
In conclusion, the present study demonstrates that
Gln administration modifies the process of endotoxemic brain damage
in an experimental model of sepsis in young rats. Although the
exact mechanism of Gln mediation in this process has not been
elucidated, the enhanced expression of HSP70 and the preservation
of the PDGF signal transduction system are likely to be involved in
the protective effect of Gln.
References
|
1
|
Wischmeyer PE, Kahana M, Wolfson R, Ren H,
Musch MM and Chang EB: Glutamine induces heat shock protein and
protects against endotoxin shock in the rat. J Appl Physiol.
90:2403–2410. 2001.PubMed/NCBI
|
|
2
|
Opie LH: Reperfusion injury and its
pharmacologic modification. Circulation. 80:1049–1062. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ribeiro SP, Villar J and Slutsky AS:
Induction of the stress response to prevent organ injury. New
Horiz. 3:301–311. 1995.PubMed/NCBI
|
|
4
|
De Maio A: Heat shock proteins: facts,
thoughts, and dreams. Shock. 11:1–12. 1999.
|
|
5
|
Chu EK, Ribeiro SP and Slutsky AS: Heat
stress increases survival rates in lipopolysaccharide-stimulated
rats. Crit Care Med. 25:1727–1732. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stice JP and Knowlton AA: Estrogen,
NFkappaB, and the heat shock response. Mol Med. 14:517–527. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Voegeli TS, Wintink AJ, Chen Y and Currie
RW: Heat shock proteins 27 and 70 regulating angiotensin II-induced
NF-kappaB: a possible connection to blood pressure control? Appl
Physiol Nutr Metab. 33:1042–1049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoo CG, Lee S, Lee CT, Kim YW, Han SK and
Shim YS: Anti-inflammatory effect of heat shock protein induction
is related to stabilization of I kappa B alpha through preventing I
kappa B kinase activation in respiratory epithelial cells. J
Immunol. 164:5416–5423. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rogister B, Ben-Hur T and Dubois-Dalcq M:
From neural stem cells to myelinating oligodendrocytes. Mol Cell
Neurosci. 14:287–300. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dubois-Dalcq M and Murray K: Why are
growth factors important in oligodendrocyte physiology? Pathol Biol
(Paris). 48:80–86. 2000.PubMed/NCBI
|
|
11
|
Morioka I, Tsuneishi S, Takada S and
Matsuo M: PDGF-alpha receptor expression following hypoxic-ischemic
injury in the neonatal rat brain. Kobe J Med Sci. 50:21–30.
2004.PubMed/NCBI
|
|
12
|
Liu JC, Wang HT and Wang W: Protective
effects of alanyl-glutamine on acute lung injury induced by
lipopolysaccharide in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
33:1095–1100. 2008.PubMed/NCBI
|
|
13
|
Jacob A, Hensley LK, Safratowich BD, Quigg
RJ and Alexander JJ: The role of the complement cascade in
endotoxin-induced septic encephalopathy. Lab Invest. 87:1186–1194.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caruso C, Durand D, Schiöth HB, Rey R,
Seilicovich A and Lasaga M: Activation of melanocortin 4 receptors
reduces the inflammatory response and prevents apoptosis induced by
lipopolysaccharide and interferon-gamma in astrocytes.
Endocrinology. 148:4918–4926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Islam Z, Amuzie CJ, Harkema JR and Pestka
JJ: Neurotoxicity and inflammation in the nasal airways of mice
exposed to the macrocyclic trichothecene mycotoxin roridin a:
kinetics and potentiation by bacterial lipopolysaccharide
coexposure. Toxicol Sci. 98:526–541. 2007. View Article : Google Scholar
|
|
16
|
Semmler A, Okulla T, Sastre M,
Dumitrescu-Ozimek L and Heneka MT: Systemic inflammation induces
apoptosis with variable vulnerability of different brain regions. J
Chem Neuroanat. 30:144–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen G, Shi J, Hu Z and Hang C: Inhibitory
effect on cerebral inflammatory response following traumatic brain
injury in rats: a potential neuroprotective mechanism of
N-acetylcysteine. Mediators Inflamm. 2008:7164582008. View Article : Google Scholar
|
|
18
|
Chen NJ, Chio II, Lin WJ, et al: Beyond
tumor necrosis factor receptor: TRADD signaling in toll-like
receptors. Proc Natl Acad Sci USA. 105:12429–12434. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang XM, Liang MF, Yuan Y and Jiang W: The
different effects of glutamine on macrophage cytokines release in
vivo and in vitro. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue.
20:456–460. 2008.(In Chinese).
|
|
20
|
Potula R, Dhillion N, Sui Y, Zien CA, Funa
K, Pinson D, Mayo MS, Singh DK, Narayan O and Buch S: Association
of platelet-derived growth factor-B chain with simian human
immunodeficiency virus encephalitis. Am J Pathol. 165:815–824.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiyang YB, Liu S, Liu J, Hao CG, Wang ZJ,
NI W, Wang XY and Wang TH: Roles of platelet-derived growth
factor-B expression in the ventral horn and motor cortex in the
spinal cord-hemisected rhesus monkey. J Neurotrauma. 26:275–287.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tseng HC and Dichter MA: Platelet-derived
growth factor-BB pretreatment attenuates excitotoxic death in
cultured hippocampal neurons. Neurobiol Dis. 19:77–83. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iantomasi T, Favilli F, Catarzi S and
Vincenzini MT: GSH role on platelet-derived growth factor receptor
tyrosine phosphorylation induced by H2O2.
Biochem Biophys Res Commun. 280:1279–1285. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng F, Dhillon N, Callen S, Yao H,
Bokhari S, Zhu X, Baydoun HH and Buch S: Platelet-derived growth
factor protects neurons against gp120-mediated toxicity. J
Neurovirol. 14:62–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishii Y, Matsumoto Y, Watanabe R, Elmi M,
Fujimori T, Nissen J, Cao Y, Nabeshima Y, Sasahara M and Funa K:
Characterization of neuroprogenitor cells expressing the PDGF
beta-receptor within the subventricular zone of postnatal mice. Mol
Cell Neurosci. 37:507–518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beazely MA, Weerapura M and Macdonald JF:
Abelson tyrosine kinase links PDGFbeta receptor activation to
cytoskeletal regulation of NMDA receptors in CA1 hippocampal
neurons. Mol Brain. 1:202008. View Article : Google Scholar : PubMed/NCBI
|