Introduction
Tumor metastasis to the lymph nodes is a defining
feature of cancer progression and is associated with poor
prognosis. A number of studies have demonstrated that tumor-induced
lymphangiogenesis plays an important role in the metastatic
progression of tumors (1,2). Notably, studies on the correlation
between tumor-associated macrophages (TAMs) and metastases of
cervical (3), lung (4) and skin cancers (5) have led to the discovery of the role
of TAMs in tumor formation, growth, invasion and metastasis.
Vascular endothelial growth factor-C (VEGF-C) acts predominantly
via VEGF receptor-3 (VEGFR-3), a receptor expressed on the surface
of lymphatic endothelial cells, and plays a critical role in
lymphangiogenesis. Studies have also revealed that VEGF-C is
expressed in more than half of human solid tumors, and its
expression level correlates with tumor-lymph node metastasis
(1,2,6). One
hypothesis is that TAMs secrete VEGF-C to promote lymphangiogenesis
either by inducing hyperplasia via enhanced budding from the
existing lymphatic vessels or by directly transforming the
lymphatic endothelial cells (7). A
recent study demonstrated that VEGF-C expressed by TAMs is closely
related to lymph vessel invasion (LVI) in breast cancer (8). However, the role of TAMs and secreted
VEGF-C in lymphangiogenesis in breast cancer patients remains to be
elucidated. A highly specific and sensitive lymphatic marker, D2-40
(9) may be used to study the
correlation between tumor-induced lymphangiogenesis and tumor
metastasis. In this study, we performed immunohistochemical
double-staining to detect CD68, VEGF-C, D2-40, and Ki-67 expression
in 75 clinical breast tumor tissues. We also examined the
percentage of TAMs expressing VEGF-C and determined the lymphatic
microvessel density (LMVD) and lymphatic endothelial cell
proliferation (LECP) in breast cancer tissues in order to elucidate
the effect of TAMs on lymphangiogenesis and lymphatic metastasis of
breast cancer.
Materials and methods
Patients and samples
We studied paraffin-embedded breast cancer tissue
specimens confirmed by pathological analysis obtained from 75
patients at the Jinhua People’s Hospital from January 2006 to June
2008. Each block of paraffin contained the intratumoral and
peritumoral areas. Peritumoral was defined as the location in
pre-existing mammary stroma at a maximal distance of 2 mm from the
tumor periphery. The patients from whom these specimens were
obtained had not received any chemotherapy or radiotherapy prior to
surgery. The 75 patients consisted of 1 male and 74 females. A
total of 73 had infiltrating ductal carcinoma, while 2 had Paget
disease. A total of 34 cases had lymph node metastasis, while 41
cases did not. The age of the patients ranged from 42 to 63 years,
and the average age at diagnosis was 52.05±9.71 years. According to
the 6th edition of the American Joint Committee on Cancer (AJCC)
tumor-node-metastasis (TNM) staging system, the number of cases in
stages 0, I, II, III, and IV were 3, 23, 39, 8 and 2, respectively.
This study was approved by the institutional review board of the
Jinhua People’s Hospital (Jinhua, China) and written informed
consent was obtained from each participant.
Reagents
The primary antibodies for macrophage- and lymphatic
endothelial cell-specific markers, rat anti-human CD68 monoclonal
antibody (GM081410), and mouse anti-human D2-40 monoclonal antibody
(GM361910), were bought from Gene Technology Co., Ltd. (Shanghai,
China). Rabbit anti-human VEGF-C polyclonal antibody (BA0548) and
rabbit anti-human Ki-67 (proliferation marker) polyclonal antibody
(BA1508) were purchased from Wuhan Boster Biological Engineering
Co., Ltd. (Wuhan, China). An immunohistochemical double-staining
kit (KIT-9999) was purchased from Maxim Biotechnology Development
Co., Ltd. (Fuzhou, China).
Immunohistochemical double-staining
All specimens were serially sectioned into 4-μm
slices. One of the sections was used for hematoxylin and eosin
(H&E) staining, and the remaining sections were used for
immunohistochemical double-staining. For CD68/VEGF-C
double-staining, the protocol was as follows. First, the sections
were sequentially dewaxed, hydrated in graded ethanol, and immersed
in 0.01 M citrate buffer (pH 6.0) and heated in a pressure cooker
for 3 min for antigen retrieval. The sections were treated with
peroxidase-blocking and serum-blocking solutions prior to overnight
incubation with primary anti-CD68 antibody (1:100) at 4°C.
Biotin-labeled secondary antibody, streptavidin-alkaline
phosphatase solution, and 5-bromo-4-chloro-3-indolyl
phosphate/nitroblue tetrazolium (BCIP/NBT) were added, and the
progress of the reaction was monitored under a microscope. A dark
purple color indicated positive staining, and the sections were
washed with distilled water. Next, the sections were treated with
serum-blocking solutions and incubated overnight with primary
anti-VEGF-C antibody (1:100) at 4°C. Biotin-labeled secondary
antibody, streptavidin-peroxidase solution and
3-amino-9-ethylcarbazole (AEC) were added, and the progress of the
reaction was monitored under a microscope. A dark red color
indicated positive staining, and the reaction was terminated with
water. The sections were re-stained with hematoxylin and mounted in
a water-based mounting medium. In the D2-40/Ki-67 double-staining
experiment, the first primary antibody was for D2-40 and the second
primary antibody was for Ki-67. In all experiments, colon tissue
was used as a positive control and phosphate-buffered saline (PBS)
was used instead of a primary antibody as a negative control.
Immunohistochemical evaluation
In the double-stained sections, cells with dark
purple cytoplasmic membranes were positive for CD68 and D2-40
antigens, which indicated that they were macrophages and lymphatic
endothelial cells, respectively. Ki-67 staining was performed to
detect the ability of the tumor to induce lymphangiogenesis. Cells
that expressed VEGF-C and Ki-67 stained dark red in the cytoplasm
and nucleus, respectively. Thus, a dark purple cytoplasm and
membrane with dark red particles in the cytoplasm indicated that
the cell was a VEGF-C-expressing macrophage, and a dark purple
membrane with a dark red nucleus indicated that the cell was a
proliferating lymphatic endothelial cell. The method used to count
the TAMs was based on the study reported by Schoppmann et
al(1). Briefly, we selected 3
zones in the region that demonstrated the most intense staining for
TAMs under low magnification (×40 and ×100), and we then counted
the TAMs in these zones under high magnification (×200, 0.7386
mm2). For analysis, we considered the average of the 3
counts. Next, we counted the number of TAMs that were positive for
VEGF-C expression and determined the VEGF-C positive expression
rate in the TAMs. A cavity with the following features was
identified as a lymph microvessel: D2-40-positive endothelial
cells, intact wall, no muscle or fibrous wall and no red blood
cells. LMVD of the intratumoral and peritumoral areas was
determined according to the method reported by Omachi et
al(10). First, we identified
the region that was the most intensely stained for D2-40 (hot
region) under low magnification. We then selected 3 zones in the
hot region and counted the average number of lymphatic microvessels
under high magnification (×200). LECP was calculated according to
the method reported by Van der Auwera et al(9) and presented as a percentage. Thus,
this method involves counting the number of Ki-67 positive cells
per 100 endothelial cells of the lymphatic microvessels. According
to Arnaout-Alkarain et al(11), LVI was considered positive by Ki-67
staining in the D2-40-positive lymphatic microvessels in the tumor
stoma. All researchers involved in the counting and measuring
processes were unaware of the stage of the clinical specimens.
Statistical analysis
SPSS 14.0 software was used to evaluate the
statistical difference. The data were expressed as the mean ± SD
and compared using Student’s t-test and F-test. The correlation
analysis was performed using the Pearson method. P<0.05 were
considered indicate a statistically significant difference.
Results
VEGF-C expression in TAMs in breast
cancer tissue
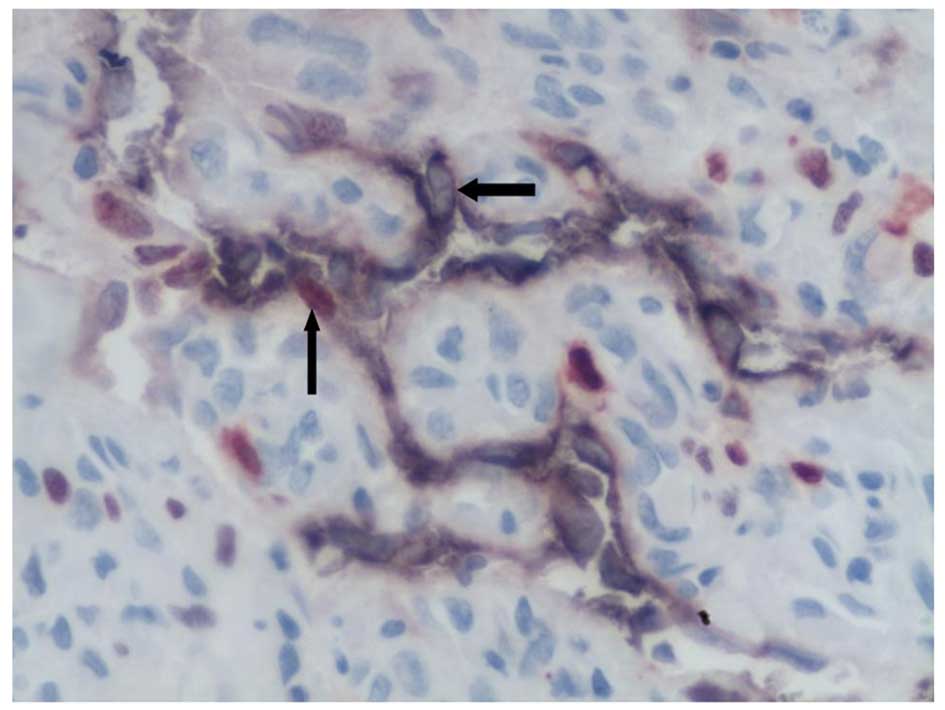
High expression of CD68 was observed in all
specimens obtained from the 75 breast cancer patients. The membrane
and cytoplasm of CD68-expressing cells stained dark purple. Dark
red particles in the dark purple cytoplasm indicated
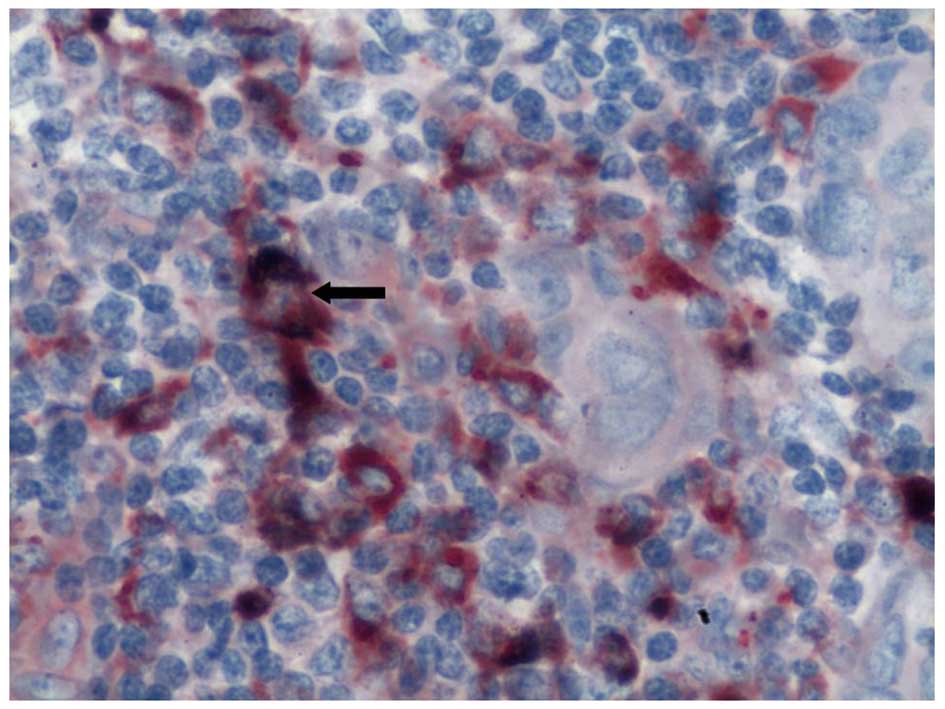
VEGF-C-expressing TAMs (Fig. 1).
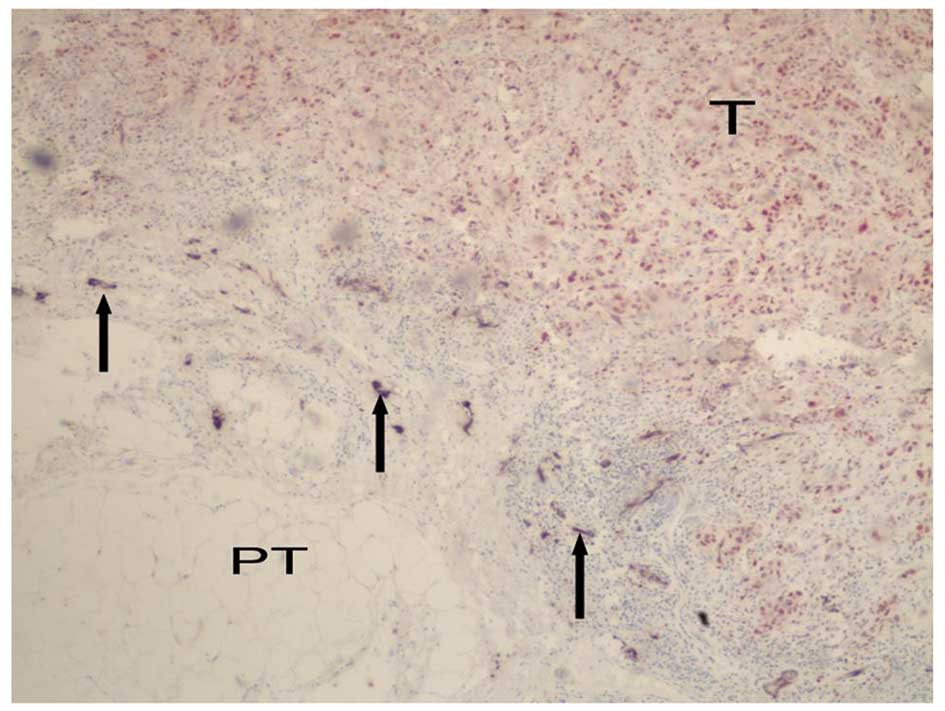
TAMs were morphologically different from other macrophages; they
were generally larger, oval or irregular in shape, and had abundant
cytoplasm. In addition, they were not distributed evenly in the
peritumoral and intratumoral regions; the distribution was dense in
the former and scanty in the latter (Fig. 2). VEGF-C expression was observed in
a variety of cells, but mainly in the breast cancer cells and
inflammatory cells (particularly in the TAMs) in the tumor stroma.
While the tumor cells demonstrated weak expression of VEGF-C, the
stromal cells surrounding the tumor, particularly the TAMs,
demonstrated strong expression. In our specimens, the number of
TAMs was 133.96±46.96. The average abundance of VEGF-C-positive
cells was 29.56±13.93% in the peritumoral areas, while those in the
intratumoral areas it was 60.43±20.23 and 28.02±15.30%,
respectively. The number of TAMs in the peritumoral areas was
significantly greater than that in the intratumoral areas
(P<0.001; Table I), which was
correlated with increased VEGF-C expression.
 | Table IComparison of TAMs, VEGF-C-positive
TAMs, LMVD and LECP in different areas of breast cancer
tissues. |
Table I
Comparison of TAMs, VEGF-C-positive
TAMs, LMVD and LECP in different areas of breast cancer
tissues.
| Peritumoral | Intratumoral |
|---|
| TAMs/×200, 0.7386
mm2 | 133.96±46.96a | 60.43±20.23 |
| VEGF-C expression of
TAMs (%) | 29.56±13.93 | 28.02±15.30 |
| LMVD/×200, 0.7386
mm2 | 12.99±7.97a | 2.06±2.93 |
| LECP (%) | 6.24±4.00a | 2.07±2.19 |
LMVD and LECP in the D2-40-positive
lymphatic microvessel
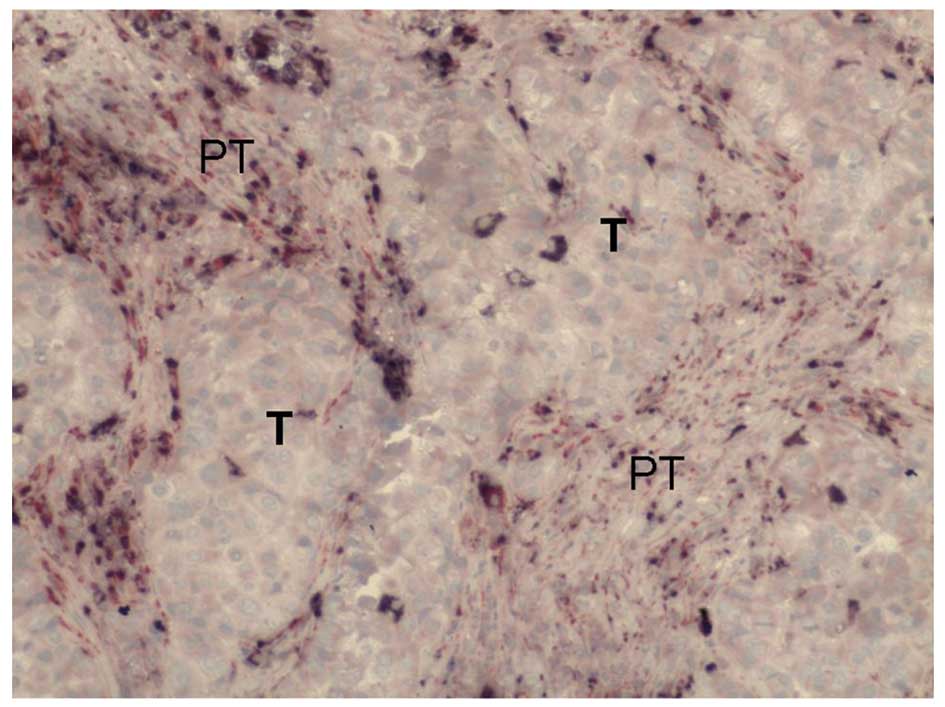
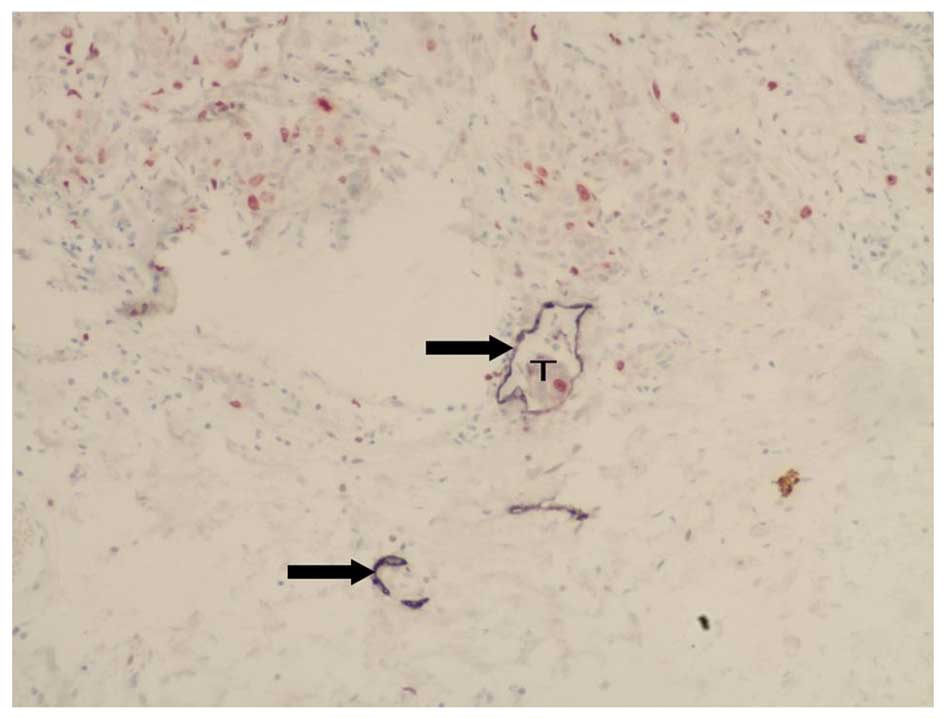
We observed varying degrees of D2-40 expression in
the tissue samples obtained from the 75 breast cancer patients. The
membranes and cytoplasm of cells expressing D2-40 stained dark
purple (Figs. 3 and 4). The D2-40-positive lymphatic
microvessels were irregular, thin-walled, without smooth muscles in
the vessel wall, mostly of expanding shape, variable in size, and
without red blood cells and neutrophils; these features are
consistent with those typical of lymphatic vessels. The lymphatic
microvessels in both the intratumoral and peritumoral areas
contained D2-40-expressing cells, but their morphology and
distribution demonstrated significant heterogeneity.
Morphologically, most of the lymphatic microvessels in the
peritumoral areas were expanded, whereas those in the intratumoral
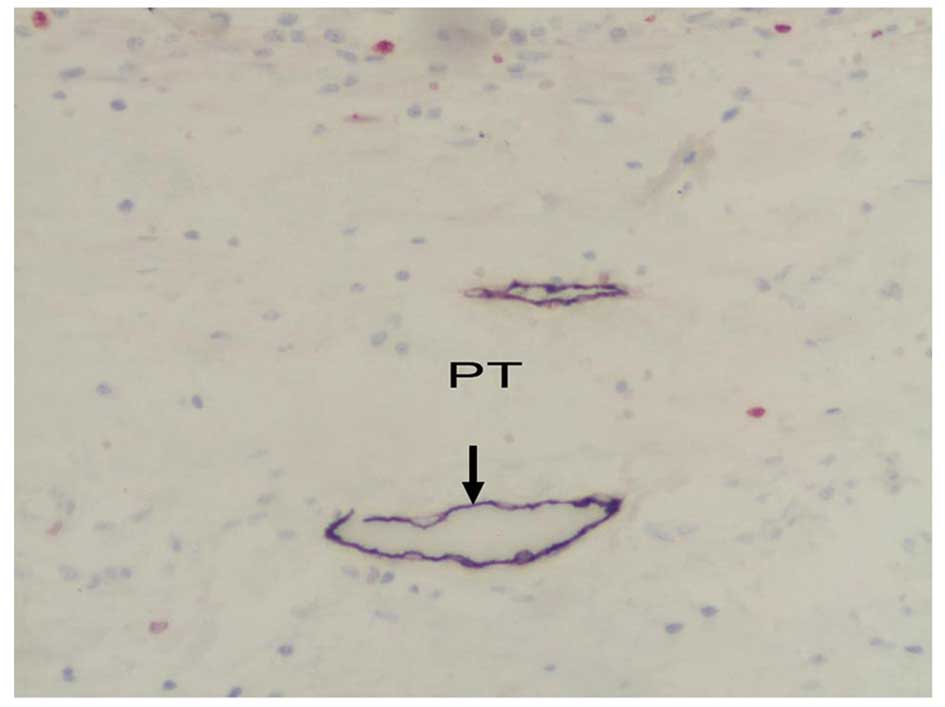
areas had cord-like and crack-like shapes (Figs. 3 and 4). The LMVD in peritumoral areas was
significantly higher (F=54.96, P<0.001; Table I) than that in both intratumoral
areas and normal breast tissue (Fig.
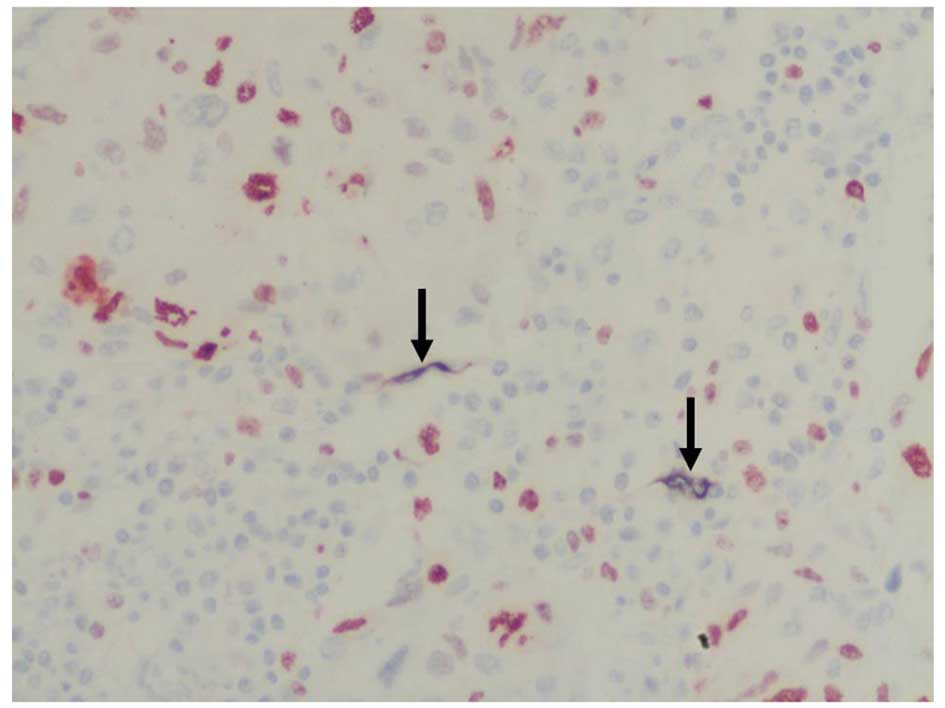
5). In 88% (66 out of 75) of the cases, the nucleus of the
endothelial cells of the D2-40-positive lymphatic microvessels
stained dark red, which indicated varying degrees of Ki-67
expression and suggested LECP (Fig.
6). LECP in the peritumoral areas was significantly higher than
that in the intratumoral areas (F=25.35, P<0.001; Table I).
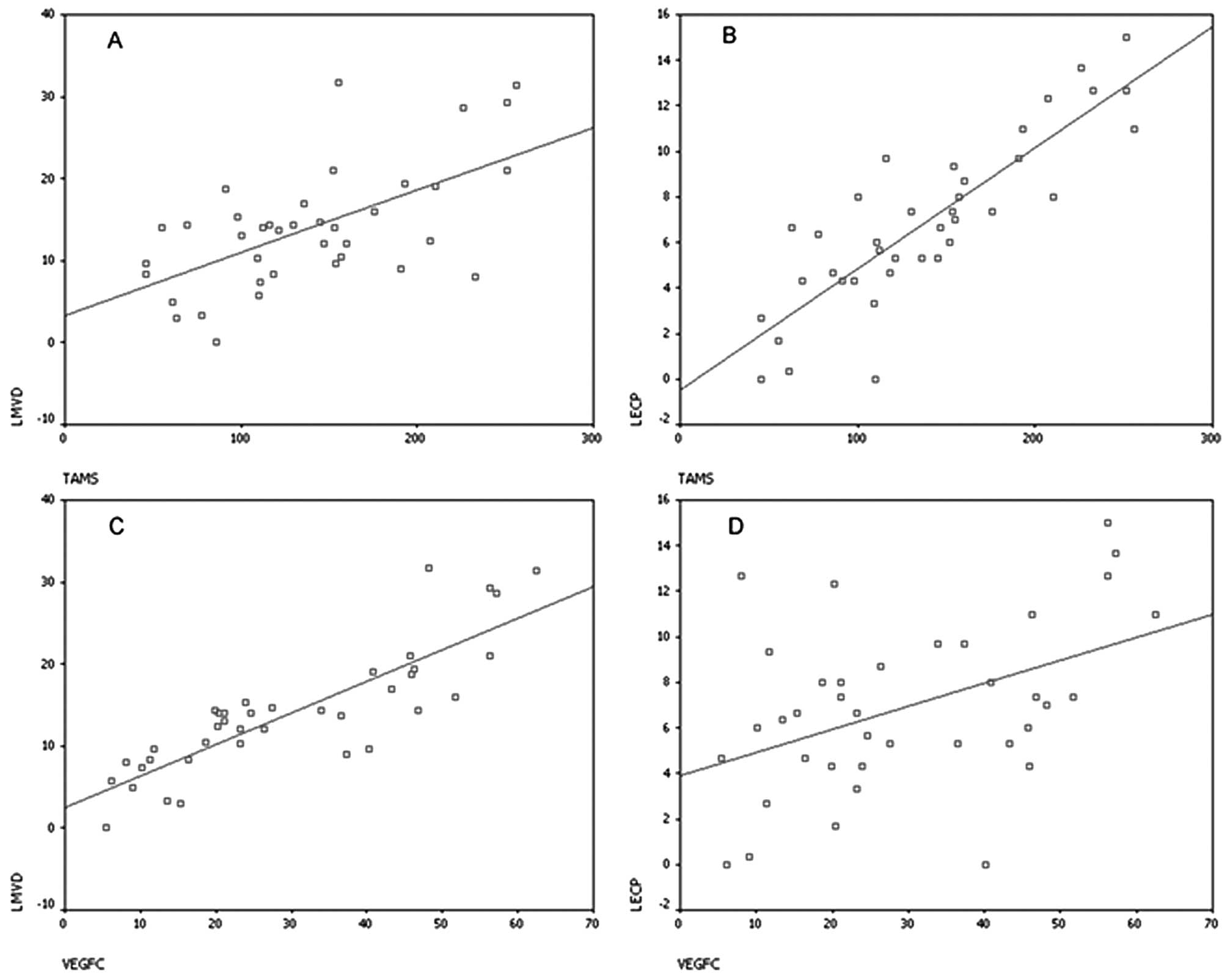
Correlation between the number of TAMs
and VEGF-C positivity in TAMs with LMVD and LECP in the peritumoral
regions
There were positive correlations between the number
of TAMs and LMVD in the peritumoral area (r=0.528, P<0.001) and
between VEGF-C positive expression in the TAMs and the LMVD in the
peritumoral area (r=0.874, P<0.001). Furthermore, LECP in the
peritumoral area was positively correlated with the number of
peritumoral TAMs (r=0.849, P<0.001) and with VEGF-C positivity
in the TAMs (r=0.413, P<0.001; Fig.
7A-D).
Correlation between peritumoral LMVD and
LECP and lymph node metastasis
Lymph node metastasis and LVI were observed in
42.67% (32/75) and 48% (36/75) of the total breast cancer cases,
respectively (Fig. 8, Table II). The LMVD in cases with lymph
node metastasis or LVI (15.36±8.36 or 18.12±9.06, respectively) was
significantly higher than that in cases without lymph node
metastasis or LVI (9.95±6.46 or 11.11±6.76, respectively;
P<0.05). Similarly, the LECP in cases with lymph node metastasis
or LVI (8.98±2.92 or 9.68±2.77%, respectively) was significantly
higher than that in cases without lymph node metastasis or LVI
(3.01±2.32 or 3.59±2.40%, respectively; P<0.001).
 | Table IIComparison of LMVD and LECP in the
presence or absence of lymph node metastasis and LVI. |
Table II
Comparison of LMVD and LECP in the
presence or absence of lymph node metastasis and LVI.
| n | LMVD/×200, 0.7386
mm2 | LECP (%) |
|---|
| Lymph node
metastasis |
| Positive | 32 | 15.36±8.36a | 8.98±2.92b |
| Negative | 43 | 9.95±6.46 | 3.01±2.32 |
| LVI |
| Positive | 36 | 18.12 ± 9.06a | 9.68±2.77b |
| Negative | 39 | 11.11±6.76 | 3.59±2.40 |
Correlation between the total number of
TAMs and VEGF-C-positive TAMs in the peritumoral regions and lymph
node metastasis
The number of TAMs in the peritumoral regions in
cases with lymph node metastasis or LVI (157.14±61.96 or
155.00±55.74, respectively) was significantly higher than the
number of TAMs in the cases without lymph node metastasis or LVI
(108.35±40.25 or 103.63±47.19, respectively; P<0.01).
Furthermore, the percentage of TAMs positively expressing VEGF-C in
the peritumoral regions in cases with lymph node metastasis or LVI
(35.41±16.46 or 26.39±15.09%, respectively) was significantly
higher than that in cases without lymph node metastasis or LVI
(23.13±13.01 or 33.99±16.71%, respectively; P<0.05; Table III).
 | Table IIIComparison of total number of TAMs and
VEGF-C-positive TAMs in the peritumoral regions in the presence or
absence of lymph node metastasis and LVI. |
Table III
Comparison of total number of TAMs and
VEGF-C-positive TAMs in the peritumoral regions in the presence or
absence of lymph node metastasis and LVI.
| n | TAMs/×200, 0.7386
mm2 | VEGF-C expression of
TAMs (%) |
|---|
| Lymph node
metastasis |
| Positive | 32 | 157.14±61.96a | 35.41±16.46b |
| Negative | 43 | 108.35±40.25 | 23.13±13.01 |
| LVI |
| Positive | 36 | 155.00±55.74a | 26.39±15.09b |
| Negative | 39 | 103.63±47.19 | 33.99±16.71 |
Discussion
TAMs account for approximately 30–50% of the
inflammatory cells in the tumor stroma and, thus, represent the
predominant inflammatory cell type in this region (12). Increasing evidence demonstrates
that TAMs play a role in promoting tumorigenesis, growth, invasion
and metastasis (particularly tumor angiogenesis and
lymphangiogenesis). A plausible mechanism to account for these
observations involves autocrine secretion of various growth factors
and inhibitory cytokines that promote the formation of blood
vessels and lymphatic vessels (7,12,13).
The majority of studies on the function of TAMs in lymph node
metastasis involved immunohistochemical analysis. Certain studies
have investigated VEGF-C expression in TAMs. However, in the
majority of the studies, only a single marker was used. These
studies failed to exactly compare the expression and distribution
of VEGF-C in TAMs as VEGF-C is expressed in the TAMs as well as in
a variety of cells, including the tumor cells. Recently, it has
been revraled that both TAMs and various cancer cell types,
including cervical cancer cells and epidermal squamous carcinoma
cells, express VEGF-C and/or VEGF-D. Together, VEGF-C and VEGF-D
bind VEGFR-3 and activate the MEK/ERK and PI3-kinase/Akt pathways
to stimulate lymphatic endothelial cell mitosis and proliferation
and promote lymphangiogenesis in the peritumoral areas. This, in
turn, induces tumor metastasis (3,5). Our
study differs from previous studies in that double-staining was
performed. This technique allowed unambiguous determination of
VEGF-C expression specifically within TAMs. In our study, we
identified that tumor cells weakly express VEGF-C, while TAMs
markedly express VEGF-C. Moreover, the total number of TAMs and
those specifically expressing VEGF-C were both significantly higher
in the peritumoral areas compared to the intratumoral areas. TAMs
are the primary source of VEGF-C in breast cancer tissue. Thus, we
suggest that TAMs may promote lymphangiogenesis in one of two ways
- either by inducing hyperplasia via budding from the existing
lymphatic vessels or by directly transforming the lymphatic
endothelial cells. As a result, LMVD and LECP would also be
expected to be elevated in the peritumoral regions.
As predicted, our data demonstrated that LMVD and
LECP in the peritumoral areas were much higher than in the
intratumoral areas. Our results are consistent with those from
numerous previous studies (14–18),
which have also primarily observed lymphangiogenesis in the
peritumoral areas. Peritumoral lymphatic vessels were considered to
be the main channel for lymph node metastasis. Recently, 107 cases
of breast cancers with lymph node metastasis were analyzed. The
study found that inflammatory infiltration and VEGF-C expression in
macrophages are closely related to LVI (LVI plays an important role
in tumor-induced lymphangiogenesis and lymphatic metastasis)
(8). However, that study differed
from the present study as the former used podoplanin as a marker to
identify lymphatic vessels and failed to detect LECP. Additionally,
in our study, the total number of TAMs and those specifically
expressing VEGF-C were positively correlated with LMVD and LECP.
This suggests that a higher number of TAMs and increased expression
of VEGF-C in TAMs are causal factors resulting in increased LECP
and LMVD in the peritumoral areas. This is notable, particularly
when compared to other studies. Statistics from previous studies
have revealed low correlation between 4 separate data sets. While
it is unclear whether this is simply due to selection bias when
counting, the data is nonetheless intriguing. Significantly, an
additional study limited its focus to the peritumoral area, and no
correlation data was presented for the intratumoral area (19). This further factor that makes our
study presented here unique. One possible explanation for our
results is that cancer cells recruit a large number of TAMs to the
tumor stroma; these TAMs secrete large amounts of VEGF-C that bind
to VEGFR-3 and activate a variety of signaling pathways, including
MEK/ERK and PI3-kinase/Akt. Notably, activation of these signaling
pathways by VEGF-C was revealed to stimulate lymphatic endothelial
cell proliferation, angiogenesis and lymphangiogenesis in a rat
tumor model (20). This study, in
addition to several other preclinical and clinical studies, support
the role of VEGF-C in tumor progression (20–23).
In the early stages of tumorigenesis, the
tumor-associated lymphatic vessel is the primary channel for entry
of the tumor cells into the lymph nodes. There are 2 different
hypotheses regarding the mechanism of metastasis. One hypothesis is
that metastasis occurs through the already existing lymphatic
vessels. The other is that the invasion occurs through newly formed
lymphatic vessels (lymphangiogenesis) (6). In primary solid tumors, VEGF-C and
VEGF-D induce lymphangiogenesis, which provides a direct channel
for tumor cells to invade the lymph nodes (24–26).
For a long time, it was considered that the spreading of tumor
cells through the channel provided by the lymphatic vessels was a
key factor in tumor metastasis. LVI by the tumor cells is the first
step of micrometastasis; however, not all LVIs develop into
metastatic lesions. Studies have revealed that the increased number
of lymphatic vessels in solid tumors is closely related to tumor
LVI (18,27). Therefore, LVI may be used as an
independent predictor of tumor metastasis and prognosis (28,29).
In this study, we further analyzed the correlation between LMVD in
the peritumoral areas of breast cancer and lymph node metastasis.
The results demonstrated that LMVD is positively correlated with
LVI and lymph node metastasis. This suggests that the increase of
LMVD in the peritumoral areas may be related to LVI and lymph node
metastasis. Notably, the LMVD in the peritumoral areas of
metastatic melanoma was much higher than that in non-metastatic
melanoma (14). LMVD in the
peritumoral area is an independent prognostic factor for
disease-free survival and overall survival. It has been revealed
that LMVD in infiltrated breast cancer is significantly associated
with LVI, and the risk of lymphatic metastasis is significantly
higher in LVI-positive cases (19,27).
On the basis of these findings, we consider that, although LVI is
not equivalent to lymph node metastasis, it is an early-stage event
that is closely related to lymph node metastasis. Increased LVI may
induce an increase in lymph node metastasis, which may affect the
treatment and prognosis for cancer patients.
The results from this study reveal that the total
number of TAMs and particularly those expressing VEGF-C are
significantly higher in the peritumoral regions associated with
lymph node metastasis. It is unclear whether this is simply an
association or whether the TAMs in this region support the
metastatic process. Despite this fact, this is an unique
observation that has not been studied in detail in previous
studies. Thus, our findings support a potential role for
VEGF-C-expressing TAMs in peritumoral regions in the metastatic
process and support the need for further investigation.
We have revealed that the formation of new lymphatic
vessels is closely related to metastasis. We provide strong
evidence that TAMs induce lymphangiogenesis in the peritumoral
areas through VEGF-C secretion. This leads to an increase in LMVD
and eventually promotes lymph node metastasis in patients with
breast cancer.
References
|
1
|
Sleeman JP and Thiele W: Tumor metastasis
and the lymphatic vasculature. Int J Cancer. 125:2747–2756.
2009.PubMed/NCBI
|
|
2
|
Achen MG and Stacker SA: Molecular control
of lymphatic metastasis. Ann N Y Acad Sci. 1131:225–234. 2008.
|
|
3
|
Schoppmann SF, Birner P, Stockl J, et al:
Tumor-associated macrophages express lymphatic endothelial growth
factors and are related to peritumoral lymphangiogenesis. Am J
Pathol. 161:947–956. 2002.
|
|
4
|
Zhang B, Wang J, Gao J, et al:
Alternatively activated RAW264.7 macrophages enhance tumor
lymphangiogenesis in mouse lung adenocarcinoma. J Cell Biochem.
107:134–143. 2009.
|
|
5
|
Moussai D, Mitsui H, Pettersen JS, et al:
The human cutaneous squamous cell carcinoma microenvironment is
characterized by increased lymphatic density and enhanced
expression of macrophage-derived VEGF-C. J Invest Dermatol.
131:229–236. 2011.PubMed/NCBI
|
|
6
|
Sundar SS and Ganesan TS: Role of
lymphangiogenesis in cancer. J Clin Oncol. 25:4298–4307. 2007.
View Article : Google Scholar
|
|
7
|
Kerjaschki D: The crucial role of
macrophages in lymphangiogenesis. J Clin Invest. 115:2316–2319.
2005.
|
|
8
|
Schoppmann SF, Fenzl A, Nagy K, et al:
VEGF-C expressing tumor-associated macrophages in lymph node
positive breast cancer: impact on lymphangiogenesis and survival.
Surgery. 139:839–846. 2006.
|
|
9
|
Van der Auwera I, Van den Eynden GG,
Colpaert CG, et al: Tumor lymphangiogenesis in inflammatory breast
carcinoma: a histomorphometric study. Clin Cancer Res.
11:7637–7642. 2005.
|
|
10
|
Omachi T, Kawai Y, Mizuno R, et al:
Immunohistochemical demonstration of proliferating lymphatic
vessels in colorectal carcinoma and its clinicopathological
significance. Cancer Lett. 246:167–172. 2007.
|
|
11
|
Arnaout-Alkarain A, Kahn HJ, Narod SA, Sun
PA and Marks AN: Significance of lymph vessel invasion identified
by the endothelial lymphatic marker D2-40 in node negative breast
cancer. Mod Pathol. 20:183–191. 2007.PubMed/NCBI
|
|
12
|
Solinas G, Germano G, Mantovani A and
Allavena P: Tumor-associated macrophages (TAM) as major players of
the cancer-related inflammation. J Leukoc Biol. 86:1065–1073.
2009.
|
|
13
|
Knowles HJ and Harris AL: Macrophages and
the hypoxic tumour microenvironment. Front Biosci. 12:4298–4314.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Massi D, Puig S, Franchi A, et al: Tumour
lymphangiogenesis is a possible predictor of sentinel lymph node
status in cutaneous melanoma: a case-control study. J Clin Pathol.
59:166–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fernandez MI, Bolenz C, Trojan L, et al:
Prognostic implications of lymphangiogenesis in muscle-invasive
transitional cell carcinoma of the bladder. Eur Urol. 53:571–578.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trojan L, Rensch F, Voss M, et al: The
role of the lymphatic system and its specific growth factor,
vascular endothelial growth factor C, for lymphogenic metastasis in
prostate cancer. BJU Int. 98:903–906. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aishima S, Nishihara Y, Iguchi T, et al:
Lymphatic spread is related to VEGF-C expression and D2-40-positive
myofibroblasts in intrahepatic cholangiocarcinoma. Mod Pathol.
21:256–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schoppmann SF, Bayer G, Aumayr K, et al:
Prognostic value of lymphangiogenesis and lymphovascular invasion
in invasive breast cancer. Ann Surg. 240:306–312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Van den Eynden GG, Van der Auwera I, Van
Laere SJ, et al: Comparison of molecular determinants of
angiogenesis and lymphangiogenesis in lymph node metastases and in
primary tumours of patients with breast cancer. J Pathol.
213:56–64. 2007.PubMed/NCBI
|
|
20
|
Krishnan J, Kirkin V, Steffen A, et al:
Differential in vivo and in vitro expression of vascular
endothelial growth factor (VEGF)-C and VEGF-D in tumors and its
relationship to lymphatic metastasis in immunocompetent rats.
Cancer Res. 63:713–722. 2003.PubMed/NCBI
|
|
21
|
Wissmann C and Detmar M: Pathways
targeting tumor lymphangiogenesis. Clin Cancer Res. 12:6865–6868.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su JL, Yen CJ, Chen PS, et al: The role of
the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer.
96:541–545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sica A, Schioppa T, Mantovani A and
Allavena P: Tumour-associated macrophages are a distinct M2
polarised population promoting tumour progression: potential
targets of anti-cancer therapy. Eur J Cancer. 42:717–727. 2006.
View Article : Google Scholar
|
|
24
|
Royston D and Jackson DG: Mechanisms of
lymphatic metastasis in human colorectal adenocarcinoma. J Pathol.
217:608–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He Y, Karpanen T and Alitalo K: Role of
lymphangiogenic factors in tumor metastasis. Biochim Biophys Acta.
1654:3–12. 2004.PubMed/NCBI
|
|
26
|
He Y, Rajantie I, Pajusola K, et al:
Vascular endothelial cell growth factor receptor 3-mediated
activation of lymphatic endothelium is crucial for tumor cell entry
and spread via lymphatic vessels. Cancer Res. 65:4739–4746. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang XH, Huang DP, Guo GL, et al:
Coexpression of VEGF-C and COX-2 and its association with
lymphangiogenesis in human breast cancer. BMC Cancer. 8:42008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang XL, Fang JP, Tang RY and Chen XM:
Different significance between intratumoral and peritumoral
lymphatic vessel density in gastric cancer: a retrospective study
of 123 cases. BMC Cancer. 10:2992010.PubMed/NCBI
|
|
29
|
Botting SK, Fouad H, Elwell K, et al:
Prognostic significance of peritumoral lymphatic vessel density and
vascular endothelial growth factor receptor 3 in invasive squamous
cell cervical cancer. Transl Oncol. 3:170–175. 2010. View Article : Google Scholar
|