Introduction
Vitiligo is a skin disorder with no fatality but
significant psychosocial consequences. It is characterized by the
progressive depigmentation of sections of skin. While the causes of
this disease remain to be determined, various factors have been
suggested to be involved in the pathogenesis. Cytokines and
reactive oxygen species play important roles in the disappearance
of melanocytes. Further evidence has pointed to the involvement of
cellular immunity (1).
Studies revealing that melanocyte-specific
antibodies (2) and cytotoxic T
cells (3) have been detected in
the peripheral blood of vitiligo patients supports the autoimmune
hypothesis. The cytotoxicity of CD8+ T cells to
melanocytes is considered to make a key contribution to the
pathogenesis of non-segmental vitiligo (4). The autoimmune response is suggested
to trigger the vitiligo but is not the main factor that affects the
extent and duration of the disease (5–7).
It has been reported that T-cell numbers in the
peripheral blood samples of vitiligo patients are normal, while the
ratio of CD4+/CD8+ decreases and the NK cell
number either increases or is unchanged. An immunohistological
study of cutaneous lymphocytes in vitiligo revealed that
infiltration of CD8+ T cells occurs surrounding the
vitiligo lesions (8–12).
In normal skin, similar amounts of CD4+
and CD8+ T cells are found in the dermis (13,14).
T cells on the leading edge of the vitiligo lesion are homing
lymphocytes, which specifically recognize autologous melanocytes
(11,12,15).
Understanding the relationship between CD8+ T-cell
infiltration of the perilesional margin and the loss of melanocytes
in lesional skin should shed light on the possible role of the
autoimmune response in vitiligo pathogenesis.
Given the possibility of the involvement of cellular
immunity in the pathogenesis of vitiligo, we undertook this study
to investigate the potential effects of CD8+ T cells
from vitiligo perilesional margins on the apoptosis of autologous
melanocytes using a co-culture system. Our results support the
theory that CD8+ T cells from the perilesional region of
the vitiligo-affected skin induce autologous melanocyte apoptosis
leading to the disappearance of melanocytes.
Materials and methods
Reagents
RPMI-1640 was purchased from Sijiqing Biological
Engineering Materials Co., Ltd. (Hangzhou, China). Fetal bovine
serum (FBS), recombinant human IL-2 and IL-15,
Dynabeads® Human T-Expander CD3/CD28 and Dead Cell
Apoptosis kit with Annexin V Alexa Fluor® 488 and
propidium iodide (PI) were obtained from Life Technologies
Corporation (Grand Island, NY, USA). PerCP anti-human CD3 antibody,
PE anti-human CD8a antibody and the corresponding anti-human IgG
antibodies were acquired from BioLegend, Inc. (San Diego, CA, USA).
FITC anti-CD69, anti-CD137 and anti-IgG antibodies were obtained
from BD Biosciences (San Jose, CA, USA). The lymphocyte separation
buffer was supplied by Sigma (St. Louis, MO, USA). The
CD8+ T Cell Isolation kit was obtained from Miltenyi
Biotech (Auburn, CA, USA). The RayBio® Human Cytokine
Antibody array was purchased from RayBiotech, Inc. (Norcross, GA,
USA) and data analysis was performed by Shanghai KangCheng Biotech
(Shanghai, China).
Patients
Patients were recruited from the Department of
Dermatology, The Third People’s Hospital of Hangzhou, China. All
participants gave written informed consent. Skin biopsies were
obtained by surgery from patients with stable vitiligo who had
failed to respond to autologous epidermis or melanocyte
transplantation at least twice. Peripheral blood samples were
extracted from the same patients. Systemic application of
glucocorticoid and immunosuppressive drugs was restricted for one
month prior to the surgery.
Isolation and amplification of
CD8+ T cells
A donor skin specimen, 0.5 × 1.0 cm in size, was
obtained from the perilesional margin of a depigmented patch.
Subcutaneous tissue was removed from the skin. The remaining skin
was washed with culture medium (RPMI-1640 with 10% fetal calf
serum, 5 ng/ml IL-15, 2 ng/ml IL-2 and 1% streptomycin-ampicillin)
three times and then cut into small sections (0.1–0.2 cm). The
sections of skin were placed in 12-well plates and cultured in 1 ml
medium together with 1.25 μl CD3/CD28 antibody-coated Dynabeads for
4–5 days. Subsequently, half of the medium was changed every 2–3
days. Sufficient lymphocytes for further experiments were obtained
after 3 weeks.
Characterization of CD8+ T
cells
The primary lymphocyte culture was centrifuged at
1,000 rpm for 10 min. The resulting cell pellet was washed with PBS
twice and re-suspended in 0.1 ml PBS. Either 5 μl PerCP anti-human
CD3 antibody or 20 μl PE anti-human CD8a antibody was added to the
cells which were then incubated at 4°C for 30 min. Expression
levels of CD3 and CD8a were analyzed by flow cytometry. Mouse IgG
was used as a control.
Determination of activation level of
CD8+ T cells
Following the labeling of 0.1 ml CD8+ T
cells (1×106/ml) with 20 μl FITC anti-CD69 or PE
anti-CD137 antibodies, the expression levels of CD69 and CD137 were
examined by flow cytometry.
Isolation of CD8+ T cells from
blood samples
Intravenous blood (5 ml) was extracted from the
vitiligo patients. Peripheral blood mononuclear cells were
separated by the Ficoll/Hypaque density gradient centrifugation
method and washed twice with PBS. The CD8+ T Cell
Isolation kit was then used to purify the CD8+ T
lymphocytes.
Apoptosis analysis
Melanocytes were isolated and cultured by methods
based on those described previously (16,17).
Primary melanocytes (passage 3–5) from vitiligo patients or normal
controls were seeded into 6-well plates at a density of
1×105 cells/well and kept in an incubator for 4 h to
allow them to attach. CD8+ T lymphocytes isolated from
the perilesional margin or peripheral blood of the same patient
were then added to the wells at a ratio of 1:1, 2:1 or 5:1. After
72 h of co-culturing, the supernatant with lymphocytes was removed
and the melanocytes were stained with PI and Annexin V. The
apoptosis rate was then analyzed by flow cytometry.
Cytokine array assay
CD8+ T cells from the perilesional margin
were co-cultured with autologous melanocytes. Monocultures of
CD8+ T cells or melanocytes were prepared in parallel as
controls. Supernatants were collected after 3 days and analyzed for
cytokine level by cytokine array.
Results
Primary culture and characterization of
CD8+ T lymphocytes
To investigate the effect of CD8+ T
lymphocytes on the apoptosis of melanocytes, we first isolated
these cells from the perilesional skin and peripheral blood of the
vitiligo patients. T lymphocytes were isolated from skin
surrounding the depigmented patches. Up to 107 T cells
were prepared after 3 weeks of culture and the success rate was
>95%. It was confirmed by flow cytometry that >90% of this
T-cell population were CD8-positive. CD8+ T cells were
also isolated from peripheral blood of the same donors. A total of
92.05% of prepared cells were confirmed to be CD8-positive.
CD8+ T cells from different
sources have different activation levels
Next we tested the activation levels of the
CD8+ T cells from the two sources. The CD8+ T
cells isolated from peripheral blood or perilesional skin were
examined by flow cytometry to determine the expression levels of
CD69 and CD137, which are indicators of T-cell activation. As shown
in Table I, the CD8+ T
cells from perilesional skin have much higher levels of CD69
(56.13±3.55 versus 29.93±2.35%, p<0.01) and CD137 (41.74±1.06
versus 25.97±1.63%, p<0.01) than CD8+ T cells from
peripheral blood. These data indicate that CD8+ T cells
from the perilesional region are more active (Fig. 1).
 | Table IExpression levels of CD69 and CD137
in CD8+ T cells from 10 vitiligo patients (mean ±
SD). |
Table I
Expression levels of CD69 and CD137
in CD8+ T cells from 10 vitiligo patients (mean ±
SD).
| Marker | Peripheral
blood | Perilesional
margin | t-value | p-value |
|---|
| CD69 | 29.93±2.35 | 56.13±3.55 | 6.154 | <0.0001 |
| CD137 | 25.97±1.63 | 41.74±1.06 | 4.550 | 0.0002 |
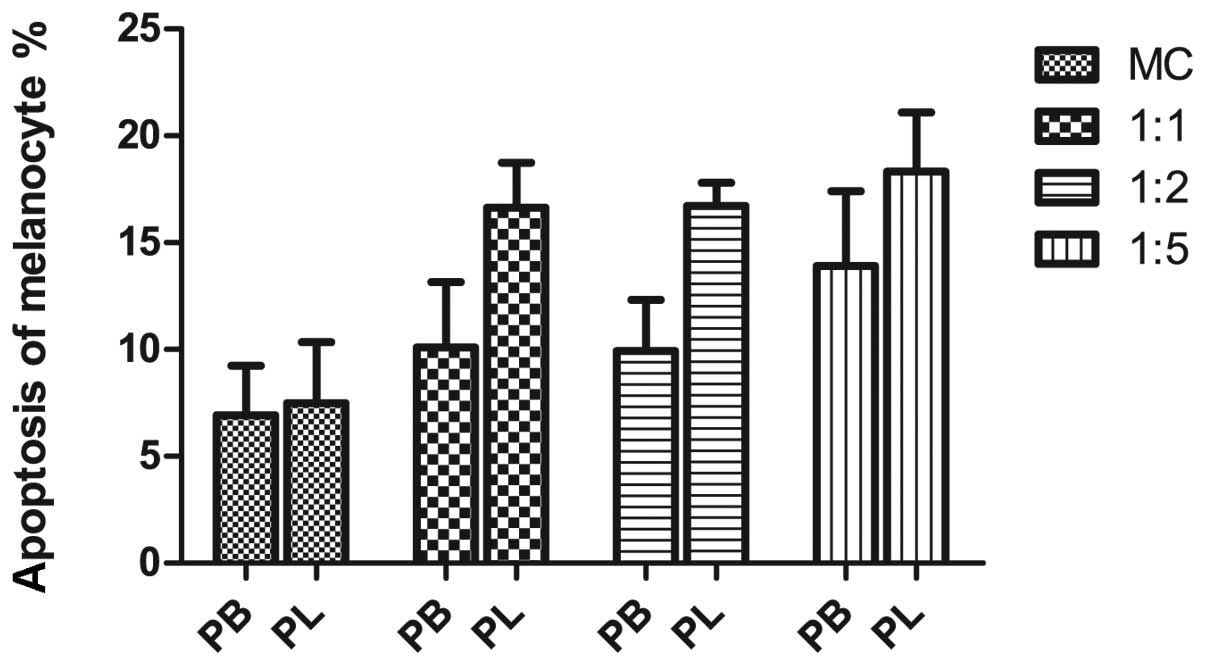
CD8+ T cells from vitiligo
lesions show higher capacity to induce apoptosis in co-cultured
autologous melanocytes
To further investigate the effect of the
CD8+ T cells on autologous melanocytes, we co-cultured
the cells. The results revealed the substantial apoptosis of
melanocytes when purified CD8+ T cells were co-cultured
with autologous melanocytes (Fig.
2). Melanocytes cultured alone had an apoptotic rate of
6.92±1.34%; the percentage of apoptosis increased to 10.10±1.76,
9.93±1.39 and 13.90±2.03% when the melanocytes were co-cultured
with CD8+ T cells from peripheral blood in ratios of
1:1, 1:2 and 1:5, respectively. The apoptotic rate of melanocytes
increased from 7.49±1.65 to 16.63±1.21, 16.71±0.63 and 18.32±1.60%
when co-cultured with CD8+ T cells from the perilesional
margin at ratios of 1:1, 1:2 and 1:5, respectively. While
CD8+ T cells from both sources induced apoptosis in
autologous melanocytes, those from the vitiligo lesion demonstrated
significantly higher cytotoxicity.
CD8+ T cells from perilesional
skin and peripheral blood show different cytokine profiles
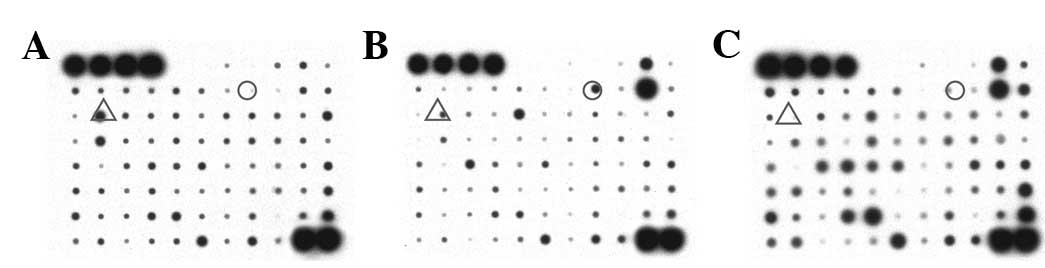
Cytokines are important in cytotoxicity; therefore,
we measured the cytokine profile for the co-culture system.
Cytokine array analysis (Fig. 3)
demonstrated that the level of IL-6 in the supernatant of the
perilesional CD8+ T cell/autologous melanocyte
co-culture was 3.01-fold higher than that in the melanocyte
monoculture (IL-6 standard value: 0.403 versus 0.134) and
17.32-fold higher than that in the peripheral blood CD8+
T cell/melanocyte co-culture (0.403 versus 0.023). IL-13 levels in
the supernatant of the lesional CD8+ T cell/autologous
melanocyte co-culture were also increased 8.02-fold compared with
the melanocyte monoculture (0.321 versus 0.040) but decreased
compared with the blood CD8+ T cell/melanocyte
co-culture (0.321 versus 0.719, 0.56-fold).
Discussion
The in situ activities of perilesional T
cells in the effector phase of depigmentation were analyzed in a
skin explant model by van den Boorn et al who found that T
cells infiltrating the perilesional margin specifically recognize
melanocyte antigen and are reactive to melanocyte antigen-specific
stimulation. These T cells were able to selectively induce the
apoptosis of melanocytes when they infiltrated autologous normally
pigmented skin explants (15).
Our study demonstrated that perilesional
CD8+ T cells express relatively high levels of CD69 and
CD137, the surface markers of activated T cells. The percentage of
CD69 expression in the perilesional CD8+ T-cell
population was determined to be 56.13±3.55%, while that in their
peripheral blood counterparts was 29.93±2.35%. The percentage of
CD137 expression in the perilesional CD8+ T cells was
41.74±1.06%, which is also higher than that in the peripheral blood
CD8+ T cells (25.97±1.63%, p<0.01). These results
suggest that the perilesional CD8+ T cells are more
highly activated than the peripheral blood CD8+ T
cells.
Previous histopathological studies have indicated
that melanocytes in the lesional skin diminish while the
surrounding basal cells remain intact. This phenotype suggests the
occurrence of apoptosis specifically in melanocytes. We co-cultured
the CD8+ T cells from peripheral blood samples or the
perilesional margin of vitiligo skin lesions with autologous
melanocytes at various ratios. The results revealed that
CD8+ T cells from different sources induced apoptosis of
autologous melanocytes. However, the perilesional CD8+ T
cells induced more extensive apoptosis in the autologous
melanocytes, suggesting higher cytotoxicity. The reason may be that
T cells infiltrating the perilesional margin specifically recognize
melanocyte antigen and cause melanocyte-specific killing effects,
while T cells from peripheral blood only cause non-specific
killing. Taken together, our data further support the theory that
CD8+ T-cell infiltration is the mechanism of vitiligo
pathogenesis.
To further investigate the killing mechanism of
CD8+ T cells on autologous melanocytes, we examined the
cytokine levels in the supernatants of CD8+ T cells and
autologous co-culture using cytokine arrays. The results revealed
that IL-6 and IL-13 levels were the highest in the perilesional
CD8+ T cell/melanocyte co-culture. This confirms the
previous observations that IL-6 and IL-8 levels are elevated in the
sera of vitiligo patients while the TNF-α and IFN-γ levels are
decreased (18), and that IL-6 is
highly produced in the vitiligo lesional skin (19–21).
IL-16 is a multi-functional cytokine which is
involved in the regulation of immune response, hematopoiesis, acute
phase reaction and inflammation (22,23).
IL-6 has been reported to significantly upregulate the expression
of ICAM-1, an adhesion molecule among melanocytes which is a potent
growth inhibitor of melanocytes (24–26)
and has been proven to be necessary for lymphocytes to adhere to
and induce immune injury in melanocytes (21). IL-6 itself contributes to the
immune injury of melanocytes by enhancing the migration of effector
cells and the adhesion between effector cells and target cells.
IL-6 downregulates the expression of microphthalmia transcription
factor (MITF) and its downstream genes that cause the shrinkage of
melanocytes and suppress melanin synthesis. The production of IL-6
is significantly increased by IL-17A which is secreted by Th17
cells (27).
Furthermore, IL-6 affects the antibody production of
B cells (28,29), and induces the differentiation of
Th17 (30–32) and cytotoxic T cells (33). Accumulating evidence suggests that
IL-6 is closely related to various autoimmune diseases, including
rheumatoid arthritis, Crohn’s disease, multiple myeloma and
systemic lupus erythematosus. IL-6 levels are significantly
elevated in the blood and the local microenvironment of vitiligo
patients (27). Further study is
required to elucidate how IL-6 is involved in the killing effect of
infiltrating CD8+ T cells on melanocytes.
In our study, we found that IL-13 is produced by
CD8+ T lymphocytes. IL-13, predominantly secreted by
active T cells, is the immune regulator of several cell types.
Anti-CD28 antibody is demonstrated to induce the transcription of
IL-13 mRNA, cause the chemotaxis of monocytes, prolong the survival
time of monocytes ex vivo and inhibit the induction of
monocytes/macrophages or inflammatory factors, including IL-1, IL-6
IL-8 and TNF-α by LPS. IL-13 is one of the main regulators of
allergic inflammation (34) and
the key inducer of several type-2 cytokine-dependent pathologies
(35,36). It functions during the pathogenesis
and prognosis of certain skin disorders. However, its relevance to
the occurrence of vitiligo has not been reported. The role of IL-13
in vitiligo remains to be determined.
In summary, our data demonstrate that
CD8+ T cells from perilesional region of the
vitiligo-affected skin induce autologous melanocyte apoptosis
leading to the disappearance of melanocytes. Our data suggest that
IL-6 and IL-13 are important in the cytotoxicity of CD8+
T lymphocytes to autologous melanocytes.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81071294, A. Xu) and the Natural
Science Foundation of Zhejiang Province, China (Z2100973, A. Xu),
and a grant from NIH (P20 RR016457 from INBRE program of the
National Center for Research Resources, Y. Wan).
References
|
1
|
Yee C, Thompson JA, Roche P, et al:
Melanocyte destruction after antigen-specific immunotherapy of
melanoma: direct evidence of T cell-mediated vitiligo. J Exp Med.
192:1637–44. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kemp EH, Waterman EA, Hawes BE, et al: The
melanin-concentrating hormone receptor 1, a novel target of
autoantibody responses in vitiligo. J Clin Invest. 109:923–930.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Palermo B, Campanelli R, Garbelli S, et
al: Specific cytotoxic T lymphocyte responses against
Melan-A/MART1, tyrosinase and gp100 in vitiligo by the use of major
histocompatibility complex/peptide tetramers: the role of cellular
immunity in the etiopathogenesis of vitiligo. J Invest Dermatol.
117:326–332. 2001. View Article : Google Scholar
|
|
4
|
Glassman SJ: Vitiligo, reactive oxygen
species and T-cells (Review). Clin Sci (Lond). 120:99–120. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Le Poole IC and Luiten RM: Autoimmune
etiology of generalized vitiligo (Review). Curr Dir Autoimmun.
10:227–243. 2008.PubMed/NCBI
|
|
6
|
Le Poole IC, ElMasri WM, Denman CJ, et al:
Langerhans cells and dendritic cells are cytotoxic towards HPV16 E6
and E7 expressing target cells. Cancer Immunol Immunother.
57:789–797. 2008.PubMed/NCBI
|
|
7
|
Ongenae K, Van Geel N and Naeyaert JM:
Evidence for an autoimmune pathogenesis of vitiligo (Review).
Pigment Cell Res. 16:90–100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Badri AM, Todd PM, Garioch JJ, et al: An
immunohistological study of cutaneous lymphocytes in vitiligo. J
Pathol. 170:149–155. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wankowicz-Kalinska A, van den Wijngaard
RM, Tigges BJ, et al: Immunopolarization of CD4+ and
CD8+ T cells to Type-1-like is associated with
melanocyte loss in human vitiligo. Lab Invest. 83:683–695.
2003.
|
|
10
|
Wankowicz-Kalinska A, Le Poole C, van den
Wijngaard R, et al: Melanocyte-specific immune response in melanoma
and vitiligo: two faces of the same coin? Pigment Cell Res.
16:254–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van den Wijngaard RM, Aten J, Scheepmaker
A, et al: Expression and modulation of apoptosis regulatory
molecules in human melanocytes: significance in vitiligo. Br J
Dermatol. 143:573–581. 2000.PubMed/NCBI
|
|
12
|
van den Wijngaard R, Wankowicz-Kalinska A,
Le Poole C, et al: Local immune response in skin of generalized
vitiligo patients. Destruction of melanocytes is associated with
the prominent presence of CLA+ T cells at the
perilesional site. Lab Invest. 80:1299–1309. 2000.PubMed/NCBI
|
|
13
|
Bos JD, Zonneveld I, Das PK, et al: The
skin immune system (SIS): distribution and immunophenotype of
lymphocyte subpopulations in normal human skin. J Invest Dermatol.
88:569–573. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gross AJ, Hunt HH, Cantor AB and Clark BC:
Sample size determination in clinical trials with an emphasis on
exponentially distributed responses. Biometrics. 43:875–883. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van den Boorn JG, Konijnenberg D,
Dellemijn TA, et al: Autoimmune destruction of skin melanocytes by
perilesional T cells from vitiligo patients. J Invest Dermatol.
129:2220–2232. 2009.PubMed/NCBI
|
|
16
|
Hong WS, Hu DN, Qian GP, et al: Treatment
of vitiligo in children and adolescents by autologous cultured pure
melanocytes transplantation with comparison of efficacy to results
in adults. J Eur Acad Dermatol Venereol. 25:538–543. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong WS, Hu DN, Qian GP, et al: Ratio of
size of recipient and donor areas in treatment of vitiligo by
autologous cultured melanocyte transplantation. Br J Dermatol.
165:520–525. 2011.PubMed/NCBI
|
|
18
|
Yu HS, Chang KL, Yu CL, et al: Alterations
in IL-6, IL-8, GM-CSF, TNF-alpha, and IFN-gamma release by
peripheral mononuclear cells in patients with active vitiligo. J
Invest Dermatol. 108:527–529. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moretti S, Spallanzani A, Amato L, et al:
New insights into the pathogenesis of vitiligo: imbalance of
epidermal cytokines at sites of lesions. Pigment Cell Res.
15:87–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moretti S, Spallanzani A, Amato L, et al:
Vitiligo and epidermal microenvironment: possible involvement of
keratinocyte-derived cytokines. Arch Dermatol. 138:273–274. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tu CX, Gu JS and Lin XR: Increased
interleukin-6 and granulocyte-macrophage colony stimulating factor
levels in the sera of patients with non-segmental vitiligo. J
Dermatol Sci. 31:73–78. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Neurath MF and Finotto S: IL-6 signaling
in autoimmunity, chronic inflammation and inflammation-associated
cancer (Review). Cytokine Growth Factor Rev. 22:83–89. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chalaris A, Garbers C, Rabe B, et al: The
soluble Interleukin 6 receptor: generation and role in inflammation
and cancer. Eur J Cell Biol. 90:484–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krasagakis K, Hettmannsperger U, Tebbe B
and Garbe C: Cutaneous metastatic angiosarcoma with a lethal
outcome, following radiotherapy for a cervical carcinoma. Br J
Dermatol. 133:610–614. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krasagakis K, Garbe C, Zouboulis CC and
Orfanos CE: Growth control of melanoma cells and melanocytes by
cytokines. Recent Results Cancer Res. 139:169–182. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krasagakis K, Garbe C, Eberle J and
Orfanos CE: Tumour necrosis factors and several interleukins
inhibit the growth and modulate the antigen expression of normal
human melanocytes in vitro. Arch Dermatol Res. 287:259–265. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kotobuki Y, Tanemura A, Yang L, et al:
Dysregulation of melanocyte function by Th17-related cytokines:
significance of Th17 cell infiltration in autoimmune vitiligo
vulgaris. Pigment Cell Melanoma Res. 25:219–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dienz O and Rincon M: The effects of IL-6
on CD4 T cell responses (Review). Clin Immunol. 130:27–33. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dienz O, Eaton SM, Bond JP, et al: The
induction of antibody production by IL-6 is indirectly mediated by
IL-21 produced by CD4+ T cells. J Exp Med. 206:69–78.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshida H, Hashizume M, Suzuki M and
Mihara M: Anti-IL-6 receptor antibody suppressed T cell activation
by inhibiting IL-2 production and inducing regulatory T cells. Eur
J Pharmacol. 634:178–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshida H, Hashizume M and Mihara M: IL-6
blockade preferentially inhibits Th17 differentiation in
collagen-induced arthritis. Rheumatol Int. 31:127–131. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Romagnani S, Maggi E, Liotta F, et al:
Properties and origin of human Th17 cells (Review). Mol Immunol.
47:3–7. 2009. View Article : Google Scholar
|
|
33
|
Suzuki T, Shoji S, Yamamoto K, et al:
Essential roles of Lyn in fibronectin-mediated filamentous actin
assembly and cell motility in mast cells. J Immunol. 161:3694–3701.
1998.PubMed/NCBI
|
|
34
|
Vercelli D: Genetics of IL-13 and
functional relevance of IL-13 variants (Review). Curr Opin Allergy
Clin Immunol. 2:389–393. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mentink-Kane MM and Wynn TA: Opposing
roles for IL-13 and IL-13 receptor alpha 2 in health and disease
(Review). Immunol Rev. 202:191–202. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mentink-Kane MM, Cheever AW, Thompson RW,
et al: IL-13 receptor alpha 2 down-modulates granulomatous
inflammation and prolongs host survival in schistosomiasis. Proc
Natl Acad Sci USA. 101:586–590. 2004. View Article : Google Scholar : PubMed/NCBI
|