Introduction
Inflammation is a complex defence mechanism in which
leukocytes migrate from the vasculature into damaged tissues to
destroy the agents that potentially cause tissue injury. The
cytokines that are produced during inflammatory processes, and are
involved in them, are stimulators of the production of acute phase
proteins. These inflammation-associated cytokines include
interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α
(1). They are produced by a
variety of cell types, but the most important sources are
macrophages at inflammatory sites. These cytokines activate
macrophages to phagocytose invading pathogens and release toxic
oxygen and nitrogen radicals.
Apoptosis-associated speck-like protein (ASC),
containing a C-terminal caspase recruitment domain, is an adaptor
molecule that mediates inflammatory and apoptotic signals and is
predominantly expressed in macrophages and mucosal epithelial cells
(2). It comprises two
protein-protein interaction domains (PYD, an N-terminal
PYRIN-domain; and CARD, a C-terminal caspase-recruitment domain)
(3). The two domains are members
of the 6-helix bundle death domain-fold superfamily that mediate
the assembly of large signaling complexes in apoptotic and
inflammatory signaling pathways. ASC is mainly known as an integral
component of the inflammasome, which mediates the activation of
caspase-1 (4–6). Within the inflammasome, ASC links
caspase-1 and NOD-like receptors (NLR) (7–10).
It has been reported that ASC functions as a critical component of
the inflammasome by linking microbial and endogenous danger signals
to caspase-1 activation (11).
Caspase-1 is involved in the processing and
secretion of pro-inflammatory molecules and is often referred to as
a pro-inflammatory caspase (12,13).
It is present in the cytosol of macrophage cells as an inactive
zymogen (14,15). Upon stimulation by multiple
microbial and endogenous signals, the dormant pro-caspase-1 zymogen
is self-activated by proteolytic cleavage into an enzymatically
active heterodimer comprising two 10- and 20-kDa subunits (16). Activated caspase-1 is essential for
the processing and release of mature IL-1β, which is the
biologically active form of IL-1β (17,18).
Mature IL-1β is predominantly expressed by activated monocytes,
macrophages and polymorphonuclear phagocytes. It is involved in
numerous immune responses, including the recruitment of
inflammatory cells to sites of infection. Generally speaking, the
biological activities of mature IL-1β are targeted towards
enhancing the host’s inflammatory response.
Given its important role in mediating inflammatory
signals, attention has been focused on the role of ASC. In this
study, we aimed to elucidate the role of ASC on caspase-1
expression and IL-1β, IL-6 and TNF-α secretion in the P388D1
macrophage-like cell line in vitro, which may provide new
insights into the inflammatory responses of the macrophage.
Materials and methods
Materials
The TRIzol reagent (Cat. no. 15596–026; Invitrogen
Life Technologies, Carlsbad, CA, USA) and RT-PCR kit (Cat. no.
DRR024A; Takara Bio, Inc., Shiga, Japan) were obtained from Takara
Biotechnology Co., Ltd. (Dalian, China). Lipopolysaccharide (LPS)
was derived from Escherichia coli serotype O111:B4
(Sigma-Aldrich, St. Louis, MO, USA). ASC antibody (sc-22514-R) and
murine ASC-siRNA (sc-37282) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The plasmid pEGFP-ASC-C2
was a gift from Dr John C. Reed (The Burnham Institute, Scripps
Research Institute, La Jolla, CA, USA).
Cell culture
The P388D1 murine macrophage-like cell line was
purchased from the Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences (Shanghai, China), and maintained in
RPMI-1640 with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 U/ml streptomycin at 37°C in a humidified atmosphere of 5%
CO2 and 95% air.
Immunofluorescence assay
The P388D1 cells were seeded on coverslips at a
density of 5×105 cells/ml and cultured overnight (37°C,
5% CO2, 90% humidity) prior to treatment. The cells were
collected and the coverslips were fixed with freshly prepared 4%
paraformaldehyde for 1 h at room temperature. The coverslips were
then blocked with 5% bovine serum albumin (BSA) at 37°C for 30 min,
washed once with phosphate-buffered saline (PBS), stained with
ASC-goat polyclonal IgG antibody at 37°C for 1 h and washed three
times with PBS, followed by fluorescein isothiocyanate
(FITC)-conjugated rabbit anti-goat IgG at 4°C for 1 h. As a
control, FITC-conjugated rabbit anti-goat IgG was used. Cells were
washed with PBS three times and analyzed by fluorescence microscopy
using an Olympus BX51 microscope (Tokyo, Japan).
RT-PCR analysis
P388D1 cells were treated with 100 ng/ml LPS for
various lengths of time. The total RNA of the cell was extracted
with TRIzol reagent according to the manufacturer’s instructions.
Human β-actin was used as an internal control. Briefly, 2 μg total
RNA was reverse-transcribed into cDNA using a reverse transcription
kit (Takara) under the following conditions: 2 μl 10X reverse
transcriptase buffer, 4 μl MgCl2 (25 mmol/l), 2 μl dNTP
(10 mmol/l), 0.5 μl RNase inhibitor, 1 μl AMV reverse transcriptase
enzyme (5 U/μl) and 1 μl Random 9-mer, made up with DEPC. The
reaction mixture was incubated at 30°C for 10 min and 42°C for 30
min, the reverse transcriptase was then inactivated by heating at
99°C for 5 min, followed by a final incubation at 5°C for 5 min.
cDNA (10 μl) was used as a template for a 50 μl PCR under the
following conditions: 5 μl 10X buffer, 4 μl dNTP, and 0.5 units
Taq DNA polymerase. Primers used were: ASC-specific
5′-TAAGCCCATGTCTCTAAGCAC-3′ (reverse) and
5′-ATGCCATCCTGGACGCTCTT-3′ (forward), caspase-1
5′-CTCCCTCATCTTGTCTTGG-3′ (reverse) and 5′-AGACATGGGCTTACAGGA-3′
(forward), and β-actin 5′-CGTCATACTCCTGCTTGCTGATCCACA TCTGC-3′
(reverse) and 5′-ATCTGGCACCAAACACCTTCT ACAATGAGCTGCG-3′ (forward)
for each 1 μl, made up with water. PCR was performed using a PCR
kit (Takara) under the following conditions: initial denaturation
at 94°C for 2 min, then 35 cycles of 95°C for 30 sec, annealing at
55°C for 45 sec and 72°C for 70 sec and a final extension at 72°C
for 5 min. PCR products were separated by 1.0% agarose gel
electrophoresis and visualized under UV light. β-actin (838 bp) was
used to standardize the amount of cDNA in each sample. To
semiquantitate the PCR products, the intensities of the amplified
bands were analyzed using AlphaImager software. The band
intensities were normalized using the corresponding β-actin signal,
and the correlation ASC/β-actin and caspase-1/β-actin was
determined.
Preparation and expression of the
ASC-overexpressing construct pEGFP-ASC-C2
mASC was inserted into pEGFP- C2 with EcoRI
and SalI sites. The plasmid pEGFP-ASC-C2 was transfected
into P388D1 cells using the Effectene transfection reagent (Cat.
no. 301425, Qiagen, Inc.) according to the manufacturer’s
instructions. In brief, the cells were seeded with 350 μl growth
medium containing serum and antibiotics in a 24-well plate. DNA
(0.2 μg) dissolved in TE buffer was diluted to a total volume of 60
μl. The enhancer was added and mixed by vortexing for 1 sec. The
mixture was incubated at room temperature for 5 min, spun down for
a few seconds to remove drops from the top of the tube, and then 5
μl Effectene reagent and 350 μl growth medium were added to the
tube containing the transfection complexes. After mixing by
pipetting, the transfection complexes were immediately added
drop-wise to the cells in the 24-well plate. Transfected pEGFP-C2
in P388D1 cells was used as a control.
Transfection of ASC-siRNA into P388D1
cells
The P388D1 cells were seeded overnight in 3.5-cm
cell culture dishes, until 40–50% confluence was achieved at the
time of transfection. ASC-siRNA and scrambled siRNA were
transfected using Effectene transfection reagent according to the
manufacturer’s instructions. The effects of the downregulation on
ASC were assessed by flow cytometry, RT-PCR and an
immunofluorescence assay 30 h after the transfection. As a control,
scrambled siRNA was used, a sequence with no significant homology
with target sequence databases.
Cytokine enzyme-linked immunosorbent
assay (ELISA)
Protein levels of IL-1β, IL-6 and TNF-α in the
P388D1 cells were measured using ELISA kits (R&D Systems,
Minneapolis, MN, USA) based on the quantitative immunometric
sandwich enzyme immunoassay technique, according to the
manufacturer’s instructions. Samples were assayed in duplicate and
the results were reported as pg/ml.
Statistical analysis
Data were presented as the mean ± SD. The Student’s
t-test was used for statistical analysis. P<0.05 was considered
to indicate a statistically significant result.
Results
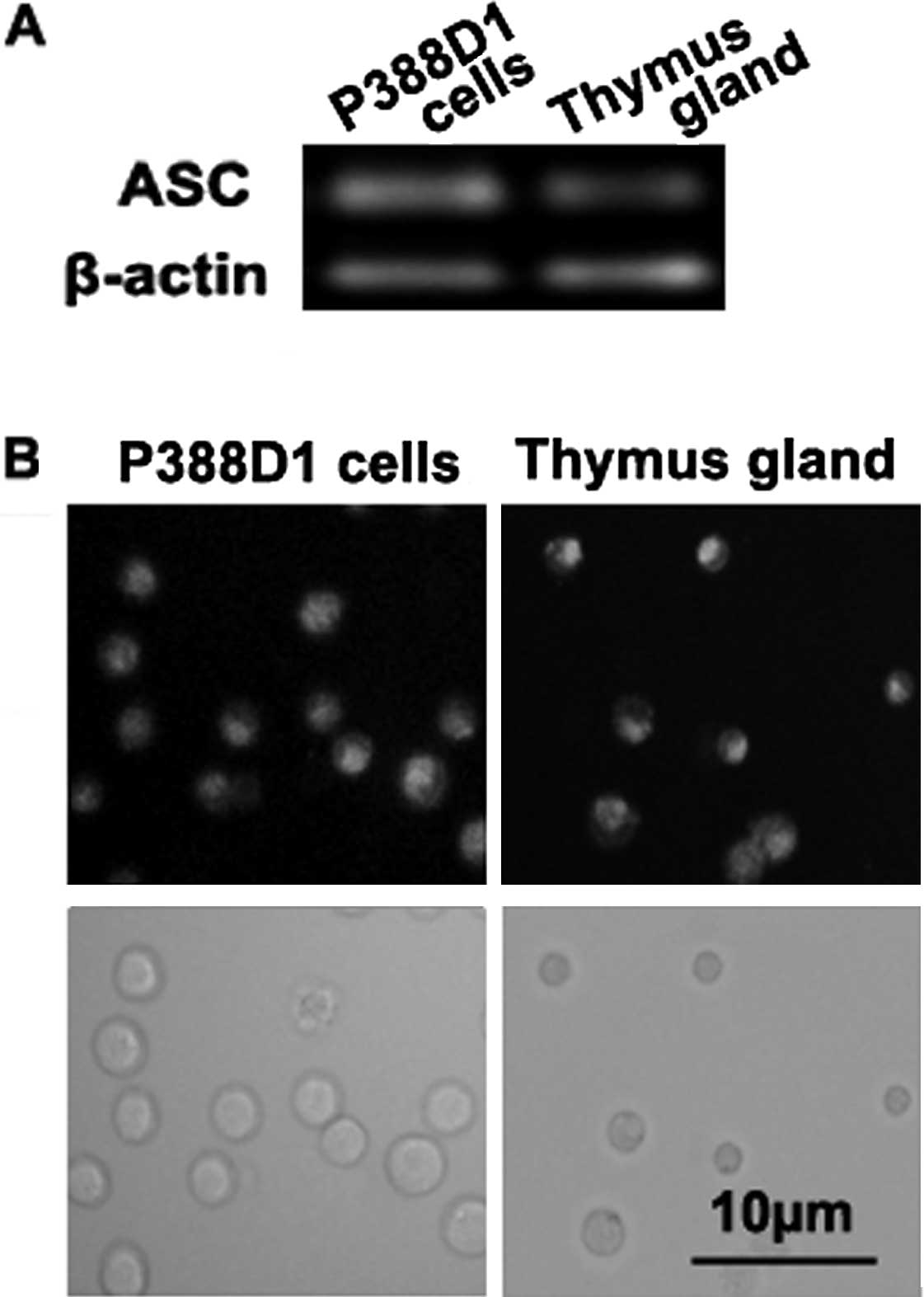
ASC expression in the P388D1
macrophage-like cells
To explore the role of ASC in the P388D1 cells, we
initially detected the expression of ASC by RT-PCR and
immunofluorescence staining assays. RT-PCR demonstrated that the
P388D1 cells expressed ASC (Fig.
1A). The immunofluorescence assay showed that ASC was present
in the cytoplasm of the P388D1 cells (36±3% positive, n=4)
(Fig. 1B), indicating that the
P388D1 cell line expressed ASC. Expression of ASC by C57BL/6 mouse
thymus gland was used as a control.
ASC overexpression promotes caspase-1
activation, IL-1β and IL-6 secretion, but not TNF-α secretion in
P388D1 cells
To verify the possibility that ASC overexpression is
effective in macrophage cells, the plasmids pEGFP-ASC-C2 and
pEGFP-C2 were transfected into P388D1 cells. Following
transfection, the cells expressing high levels of ASC were brightly
stained with green fluorescent protein (GFP). Bright green
fluorescent signals were observed as speck-like aggregates in the
living cells. ASC expression was detected by immunofluorescence
assay 30 h after transfection with pEGFP-ASC-C2 or pEGFP-C2
(Fig. 2A). The ASC protein was
overexpressed in the P388D1 cells following transfection with
pEGFP-ASC-C2. Transfected plasmid pEGFP-C2 was used as a
control.
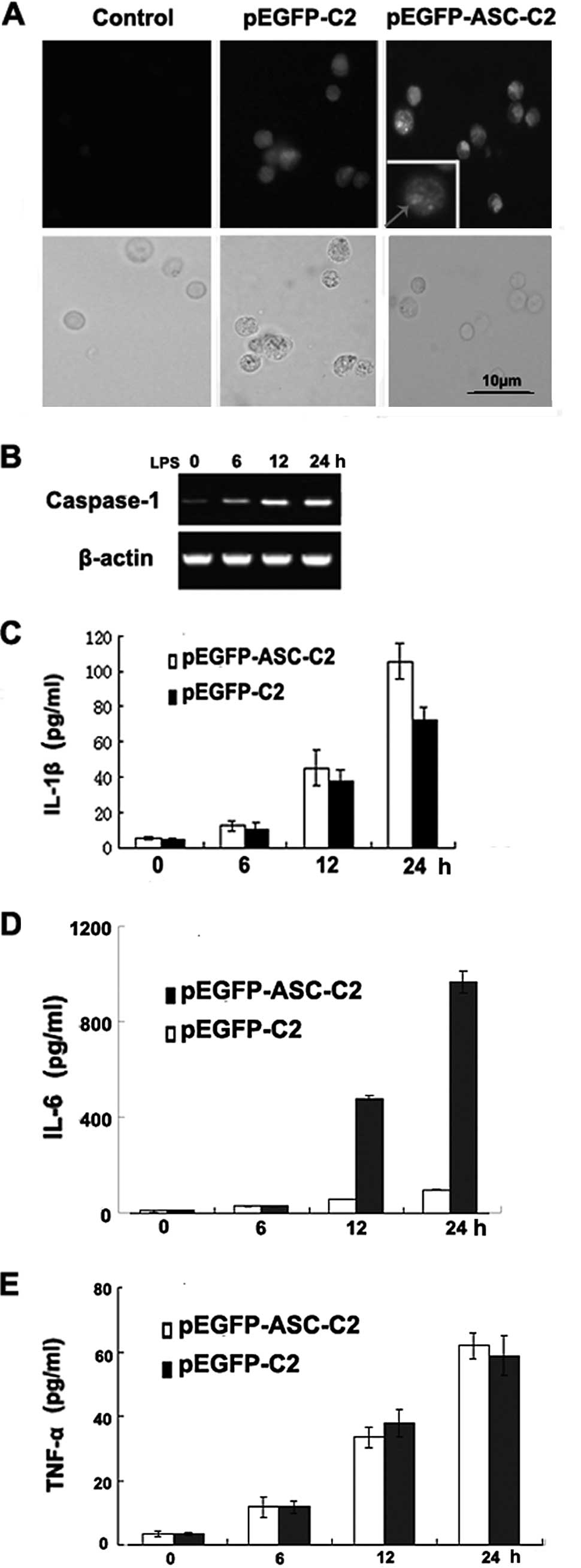 | Figure 2Overexpression of ASC promotes
caspase-1 activation and the secretion of IL-1β and IL-6, but not
that of TNF-α, in the P388D1 macrophage-like cell line. (A) ASC was
overexpressed in P388D1 cells transfected with pEGFP-C2. Signals
were detected by immunofluorescence microscopy following the
staining of ASC with GFP. The right panels show merged images of
the fluorescence and transmitted light images (left: control P388D1
cells; middle: P388D1 cells transfected with pEGFP-C2; right:
P388D1 cells transfected with pEGFP-ASC-C2). (B) RT-PCR analysis of
caspase-1 mRNA. P388D1 cells were transiently transfected with
plasmids expressing ASC and 1 day later the cells were stimulated
with LPS for 6, 12 and 24 h. (C-E) Promotion of IL-1β and IL-6, but
not TNF-α, secretion by ASC in a P388D1 cell culture. Supernatants
were analyzed for IL-1β, IL-6 and TNF-α at 6, 12 and 24 h
post-transfection. Data show the pg/ml of IL-1β, IL-6 and TNF-α
normalized for the cell number (mean ± SD; n=3). ASC,
apoptosis-associated speck-like protein; IL, interleukin; TNF,
tumor necrosis factor; GFP, green fluorescent protein; LPS,
lipopolysaccharide. |
To determine whether ASC is capable of regulating
the production of IL-1β, IL-6 and TNF-α induced by endogenous
caspase-1 in response to a physiologically relevant stimulus,
ASC-overexpressing P388D1 cells were stimulated with LPS for 6, 12
and 24 h to trigger caspase-1 activation and induce IL-1β, IL-6 and
TNF-α secretion. LPS stimulation of the ASC-overexpressing P388D1
cells resulted in an increased caspase-1 mRNA expression (Fig. 2B) and an increased secretion of
IL-1β, IL-6 and TNF-α (Fig. 2C-E).
The enhancing effect of ASC on the caspase-1-induced secretion of
IL-1β and IL-6 was time-dependent and correlated with the amount of
ASC protein produced in the transfected cells. Thus, ASC is capable
of promoting the production of IL-1β and IL-6, but not that of
TNF-α, which results from the activation of endogenous
caspase-1.
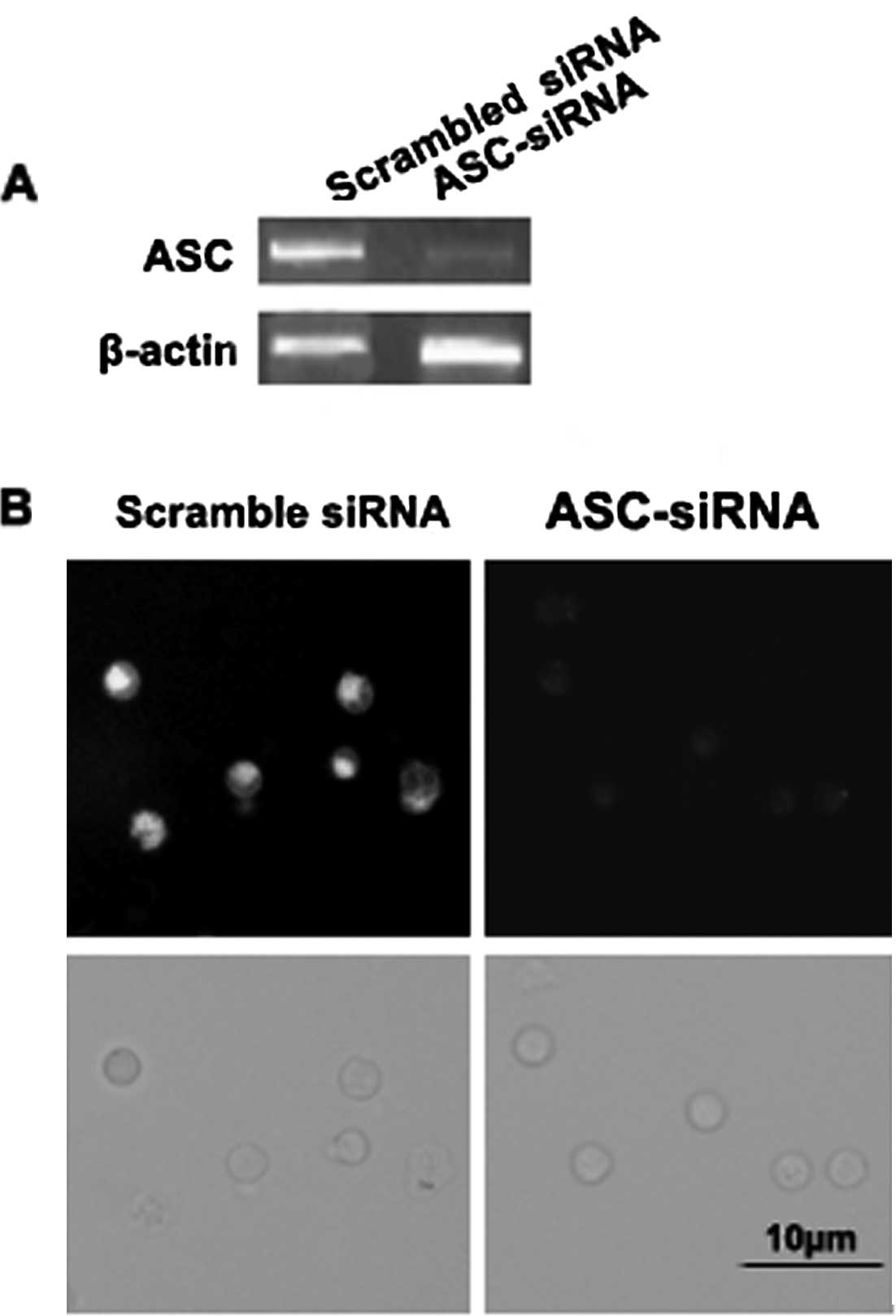
ASC expression is silenced by ASC-siRNA
in the P388D1 macrophage-like cell line
We measured the levels of ASC expression in P388D1
cells transfected with siRNA by RT-PCR and immunofluorescence.
ASC-siRNA was able to silence the expression of ASC in the P388D1
cells (Fig. 3A and B). The
transfected cells exhibited normal morphology and good viability
(Fig. 3B). Silencing of the target
gene was observed after 30 h, whereas a similar effect was not
observed in the cells transfected with the scrambled siRNA
(Fig. 3A and B). The expression of
ASC in the P388D1 cells was found to be silenced by ASC-siRNA.
Silencing ASC expression decreases
caspase-1 activation and IL-1β and IL-6, but not TNF-α, secretion
in the P388D1 macrophage-like cell line
To establish a direct role for ASC-mediated
caspase-1 activation and IL-1β secretion, we used siRNAs to inhibit
the expression of endogenous ASC in the P388D1 cells and measured
caspase-1 activity and IL-1β secretion. Notably, the inhibition of
ASC expression by siRNA decreased the amount of caspase-1 mRNA
(Fig. 4A) and reduced the
secretion of IL-1β and IL-6, but not that of TNF-α (Fig. 4B-D), in the P388D1 cells. By
contrast, the same cells retained their ability to activate
caspase-1 and secrete IL-1β and IL-6 when incubated with scrambled
siRNA. These findings indicate that ASC is required for the
secretion of IL-1β and IL-6 in P388D1 cells.
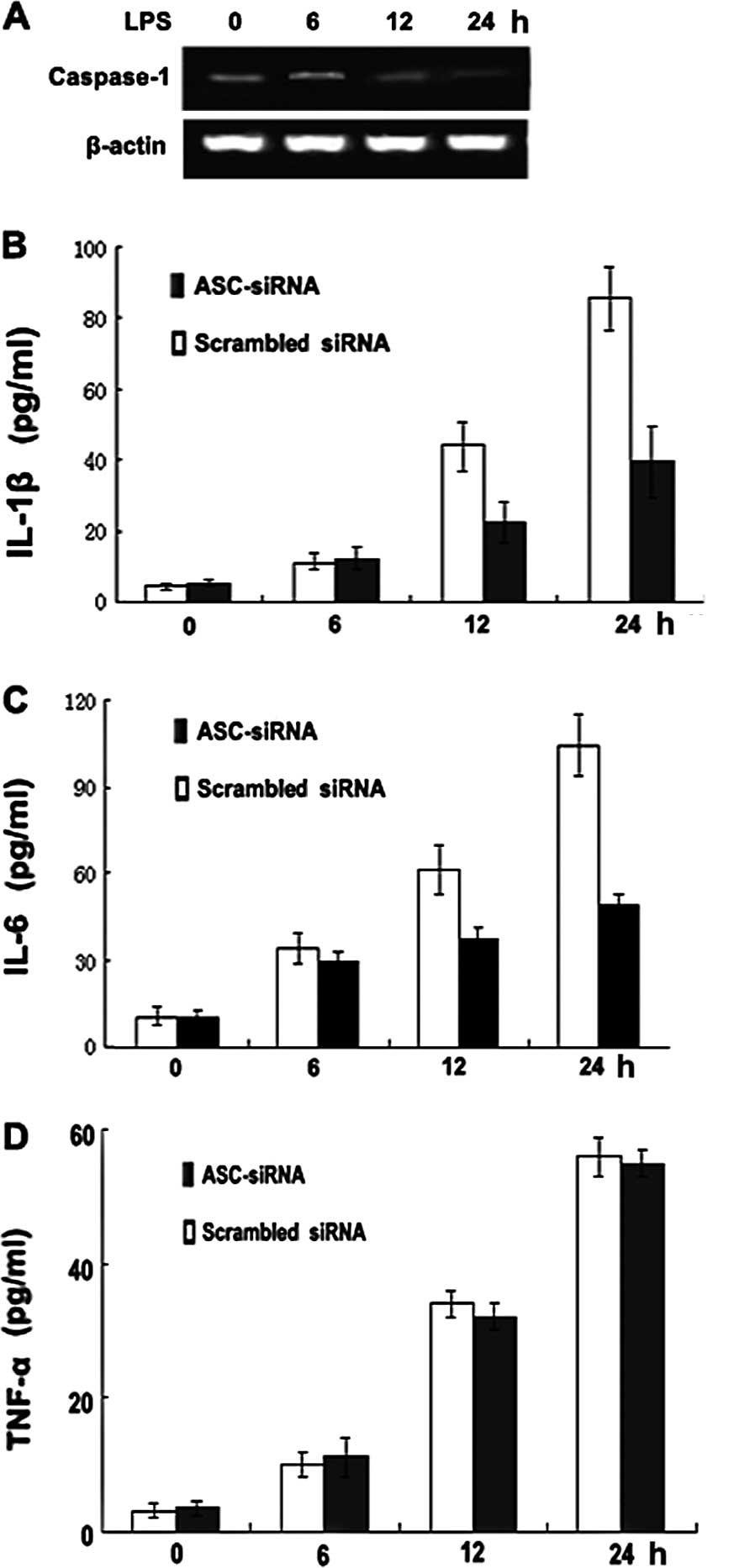 | Figure 4ASC-siRNA decreases caspase-1
activation and IL-1β and IL-6, but not TNF-α, secretion in the
P388D1 macrophage-like cell line. (A) RT-PCR analysis of caspase-1
mRNA. P388D1 cells were transiently transfected with ASC-siRNA and
1 day later were stimulated with LPS for 6, 12 and 24 h. (B-D)
Decrease of IL-1β and IL-6, but not TNF-α, secretion by ASC-siRNA
in a P388D1 cell culture. Supernatants were analyzed for IL-1β,
IL-6 and TNF-α at 6, 12 and 24 h post-transfection. Data show the
pg/ml of IL-1β, IL-6 and TNF-α normalized for the cell number (mean
± SD; n=3). ASC, apoptosis-associated speck-like protein; IL,
interleukin; TNF, tumor necrosis factor; LPS,
lipopolysaccharide. |
Discussion
ASC is one of only two genes in the human genome
that contain both PYD and CARD. Such modular protein-protein
interaction domains are known to play an important role in many
intracellular signal transduction pathways. ASC has been reported
to interact with the CARD of pro-caspase-1 and induce aggregation
of the inflammasome, thereby regulating the activation of caspase-1
and the secretion of IL-1β (19,20).
In this study, we have extended existing data regarding the role of
ASC in caspase-1 activation. We investigated the relationship
between ASC and caspase-1 in P388D1 cells, and our data confirm
that ASC overexpression induced by pEGFP-ASC-C2 transfection
significantly increased caspase-1 expression levels and the
secretion of IL-1β and IL-6, but not TNF-α secretion. We extended
this observation to the opposite situation, in which suppression of
ASC expression, using siRNA, significantly reduced caspase-1
expression and IL-1β and IL-6 secretion.
Caspase-1 exists in two activation states,
unprocessed and fully processed, depending on the composition of
the inflammasome. Inflammasome formation is coordinated by members
of the NLR protein family or the PYHIN protein family that function
as specific sensors for a variety of pathogens and other
inflammatory stimuli (21). ASC
appears to function as a vital adaptor for the recruitment of
caspase-1 to PYRIN-containing receptors. This function indicates
that regulation of the availability of ASC is a potential mechanism
for the modulation of caspase-1-mediated cytokine processing. Other
studies have demonstrated that ASC is crucial for the induction of
caspase-1 processing, which appears to be essential for efficient
cytokine maturation (22). When
activated, the pro-caspase-1 zymogen promotes macrophage cell
death, but requires ASC to be processed to its 10- and 20-kDa
subunits to promote the maturation of cytokines, including IL-1β
and IL-18.
IL-1β increases the activity of nuclear factor κB
(NF-κB), a transcription factor that regulates numerous
pro-inflammatory genes. IL-1β activates NF-κB through a cascade
that involves activating NF-κB-inducing kinase, which then
phosphorylates and activates the inhibitor of NF-κB (IκB) kinase.
Phosphorylation of IκB results in degradation of the IκB inhibitory
subunit, allowing NF-κB to translocate to the nucleus, where it
acts as a transcription factor and regulates its target genes
(23). It has been demonstrated
that the transcription of several inflammatory genes, including
IL-6 and TNF-α, is regulated by NF-κB (24,25).
Consistent with these findings, we found that the upregulation of
ASC induces not only the expression of caspase-1, but also the
secretion of IL-1β and IL-6. Conversely, the downregulation of ASC
inhibits the expression of caspase-1 and the secretion of IL-1β and
IL-6. Notably, the secretion of TNF-α was not significantly
upregulated or downregulated by the changes in the expression of
ASC. One likely reason for the failure of ASC to change the
secretion of TNF-α is that this process also needs the involvement
of additional transcription factors, including STAT3 (26), which may be inadequate.
In conclusion, accumulating evidence suggests that
ASC is important in the regulation of inflammatory responses by
macrophages, and acts primarily by affecting the secretion of
cytokines, such as IL-1β and IL-6. Our results strongly suggest
that ASC is involved in this process.
Acknowledgements
This study was supported by a grant from NSFC
(90709005 and 30973851 and 81173452).
References
|
1
|
Gabay C: Interleukin-6 and chronic
inflammation. Arthritis Res Ther. 8:S32006. View Article : Google Scholar
|
|
2
|
Abdelaziz DH, Gavrilin MA, Akhter A,
Caution K, Kotrange S, Khweek AA, Abdulrahman BA, Grandhi J, Hassan
ZA, Marsh C, et al: Apoptosis-associated speck-like protein (ASC)
controls Legionella pneumophila infection in human
monocytes. J Biol Chem. 286:3203–3208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Masumoto J, Taniguchi S, Ayukawa K,
Sarvotham H, Kishino T, Niikawa N, Hidaka E, Katsuyama T, Higuchi T
and Sagara J: ASC, a novel 22-kDa protein, aggregates during
apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem.
274:33835–33838. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrilli V, Papin S and Tschopp J: The
inflammasome. Curr Biol. 15:R5812005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernandes-Alnemri T, Wu J, Yu JW, Datta P,
Miller B, Jankowski W, Rosenberg S, Zhang J and Alnemri ES: The
pyroptosome: a supramolecular assembly of ASC dimers mediating
inflammatory cell death via caspase-1 activation. Cell Death
Differ. 14:1590–1604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Srinivasula SM, Poyet JL, Razmara M, Datta
P, Zhang Z and Alnemri ES: The PYRIN-CARD protein ASC is an
activating adaptor for Caspase-1. J Biol Chem. 277:21119–21122.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu HB and Finlay BB: The Caspase-1
inflammasome: a pilot of innate immune responses. Cell Host
Microbe. 4:198–208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akhter A, Gavrilin MA, Frantz L,
Washington S, Ditty C, Limoli D, Day C, Sarkar A, Newland C,
Butchar J, et al: Caspase-7 activation by the Nlrc4/Ipaf
inflammasome restricts Legionella pneumophila infection.
PLoS Pathog. 5:e10003612009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lamkanfi M, Kanneganti TD, Van Damme P,
Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, Vandenabeele P,
Gevaert K and Núñez G: Targeted peptidecentric proteomics reveals
Caspase-7 as a substrate of the Caspase-1 inflammasomes. Mol Cell
Proteomics. 7:2350–2363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lamkanfi M, Moreira LO, Makena P,
Spierings DC, Boyd K, Murray PJ, Green DR and Kanneganti TD:
Caspase-7 deficiency protects from endotoxin-induced lymphocyte
apoptosis and improves survival. Blood. 113:2742–2745. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Franchi L, Eigenbrod T, Planillo RM and
Nuñez G: The inflammasome: a caspase-1 activation platform
regulating immune responses and disease pathogenesis. Nat Immunol.
10:241–247. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martinon F and Tschopp J: Inflammatory
caspases and inflammasomes: master switches of inflammation. Cell
Death Differ. 14:10–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nicholson DW: Caspase structure,
proteolytic substrates, and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cerretti DP, Kozlosky CJ, Mosley B, et al:
Molecular cloning of the interleukin-1 beta converting enzyme.
Science. 256:97–100. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thornberry NA, Bull HG, Calaycay JR, et
al: A novel heterodimeric cysteine protease is required for
interleukin-1 beta processing in monocytes. Nature. 356:768–774.
1992. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martinon F and Tschopp J: Inflammatory
caspases: linking an intracellular innate immune system to
autoinflammatory diseases. Cell. 117:561–574. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Netea MG, Nold-Petry CA, Nold MF, et al:
Differential requirement for the activation of the inflammasome for
processing and release of IL-1{beta} in monocytes and macrophages.
Blood. 113:2324–2335. 2009.PubMed/NCBI
|
|
18
|
Kuijk LM, Beekman JM, Koster J, Waterham
HR, Frenkel J and Coffer PJ: HMG-CoA reductase inhibition induces
IL-1beta release through Rac1/PI3K/PKB-dependent caspase-1
activation. Blood. 112:3563–3573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Srinivasula SM, Poyet JL, Razmara M, Datta
P, Zhang Z and Alnemri ES: The PYRIN-CARD protein ASC is an
activating adaptor for caspase-1. J Biol Chem. 277:21119–21122.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stehlik C, Lee SH, Dorfleutner A,
Stassinopoulos A, Sagara J and Reed JC: Apoptosis-associated
Speck-like protein containing a caspase recruitment domain is a
regulator of procaspase-1 activation. J Immunol. 171:6154–6163.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brodsky IE and Monack D: NLR-mediated
control of inflammasome assembly in the host response against
bacterial pathogens. Semin Immunol. 21:199–207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Broz P, Moltke J, Jones JW, Vance R and
Monack DM: Differential requirement for Caspase-1 autoproteolysis
in pathogen-induced cell death and cytokine processing. Cell Host
Microbe. 8:471–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mengshol JA, Vincenti MP, Coon CI,
Barchowsky A and Brinckerhoff CE: Interleukin-1 induction of
collagenase 3 (matrix metalloproteinase 13) gene expression in
chondrocytes requires p38, c-jun N-terminal kinase, and nuclear
factor κB: differential regulation of collagenase 1 and collagenase
3. Arthritis Rheum. 43:801–811. 2000.
|
|
24
|
Hagemann T, Biswas SK, Lawrence T, Sica A
and Lewis CE: Regulation of macrophage function in tumors: the
multifaceted role of NF-κB. Blood. 113:3139–3146. 2009.PubMed/NCBI
|
|
25
|
Karin M and Greten FR: NF-κB: linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005.
|
|
26
|
Biswas S and Lewis CE: NF-κB as a central
regulator of macrophage function in tumors mechanism and repertoire
of ASC-mediated gene expression. JLB. 88:877–884. 2010.
|