Introduction
Diabetic retinopathy (DR) still remains the leading
cause of blindness worldwide. Consequently, there is a need for
further investigation of the pathogenesis of DR to develop better
more efficient therapeutic techniques. A considerable amount of
evidence supports a causal role for advanced glycation end-products
(AGEs) in the development of diabetic complications. AGEs have
diverse biological properties, which include protein-linking,
cellular activation, growth promotion and induction of vascular
dysfunction (1,2). They represent an integrated measure
of glucose exposure over time (3,4),
increase in diabetic retinas and correlate with the onset and
severity of retinopathy (5).
Müller cells, the predominant glial cells of the
retina, express a diversity of ion channels and are responsive to
numerous growth factors, cytokines and neurotransmitters. Molecules
in the microenvironment regulate Müller cell function, structure,
location, number and intercellular interactions. Müller cells
themselves are sources for molecules that regulate these glia
(6). It has been confirmed that
these factors and cytokines are known to be involved in the
pathogenesis of DR. The molecules include basic fibroblast growth
factor (bFGF) (7), vascular
endothelial growth factor (VEGF) (8,9),
insulin-like growth factor (10),
transforming growth factor-β (11), hepatocyte growth factor (HGF)
(12) and pigment
epithelium-derived factor (PEDF) (13). The angiogenic cytokines contribute
to the regulation of endothelial cell proliferation, migration,
extracellular proteolysis by matrix metalloproteinases (MMPs) and
vessel wall remodeling (14).
VEGF and bFGF (15)
are two of the most important pro-angiogenic cytokines and VEGF may
be associated with the breakdown of this blood-retinal barrier. In
contrast to VEGF, bFGF has broader biological functions in the
development of intraocular neovascularization in DR (16,17).
Although VEGF expression is induced by AGEs (11,18),
it remains unclear whether AGEs initiate the production of bFGF by
Müller cells during the early stages of DR.
In the current study, we performed specific
experiments to examine the correlation between AGEs and the
expression of bFGF in cultured Müller cells in vitro in
order to further explore the mechanisms behind DR.
Materials and methods
Cell cultures
The Müller cell culture was prepared as described
previously (19,20). Briefly, in phosphate-buffered
saline (PBS) with 100 U/ml penicillin, 100 mg/ml streptomycin
solution, the eye of an adult New Zealand white rabbit weighing
2.0–2.5 kg was cut 2 mm away from the limbus circumferentially and
then the anterior segment and vitreous were removed and discarded.
The retina was carefully detached and the avascular non-medullated
fraction was removed in order to prevent contamination by
astrocytes and oligodendrocytes. The residual retina was cut into
0.5×0.5-mm pieces under a biomicroscope and the fragments were
centrifuged at 150 × g for 5 min. The tissue pieces were then
gently dissociated by pipetting up and down and were planted in a
25-cm3 culture flask using Dulbecco’s minimum essential
medium (DMEM) (Gibco-BRL, Grand Island, NY, USA) supplemented with
20% fetal bovine serum (FBS; SAFC, St. Louis, MO, USA), 100 U/ml
penicillin, 100 mg/ml streptomycin and cultured at 37°C in a
humidified incubator saturated with 5% CO2 and 95% air.
Almpost all the explants adhere to the surface of the flask within
7 days. Previous immunocytochemical and electron-microscopic
studies had confirmed the high purity of Müller cell cultures
(19). After reaching 80–100%
confluence, the cells were trypsinized and subcultured onto glass
coverslips at an approximate density of
1×105/cm2 cells. Passage 1 was used for all
experiments.
Preparation of AGEs
Bovine serum albumin (BSA) (fraction V, Sigma
Chemical Co., St. Louis, MO, USA) was glycated by incubation with
0.5 M glucose at 37°C for 6–12 weeks under anaerobic and sterile
conditions, as described previously (21). Control non-glycated BSA (BSA
control) was incubated under the same conditions without glucose.
At the end of the incubation period, samples were extensively
dialyzed against PBS to remove unbound sugars. Dialyzed glycated
protein was characterized based on fluorescence at 446 nm upon
excitation at 360 nm using a fluorescence spectrometer (model
LS-3B, Perkin-Elmer Corp., Norwalk, CT, USA). Endotoxin content in
each sample was determined by the Limulus amebocyte lysate assay
(E-Toxate, Sigma Chemical Co.) and was consistently found to be
below detectable levels (<0.2 ng/ml).
Immunohistochemistry analysis of bFGF
secretion
Müller cells were separately exposed to AGEs (volume
percentage was 0, 4, 8, 16, 32 and 64%) in DMEM supplemented with
10% FBS. The BSA control was used under similar conditions as the
control. Samples were incubated at 37°C in a humidified incubator
saturated with 5% CO2 and 95% air for 1, 3, 6 and 9
days. Conditioned medium without AGEs or BSA control was used as
the blank control.
Unless otherwise stated all washes were for 3×5 min
in PBS at pH 7.4 and were performed at room temperature while
incubations were at 37°C. Sections were brought to room temperature
on days 1, 3, 6 and 9 separately, washed, then fixed by immersion
in acetone (4°C) (Wuhan Boster Biological Technology, Ltd., Wuhan,
China) for 10 min. The slides were air-dried. Endogenous peroxidase
activity in the biopsy specimen cryostat sections was blocked with
3% H2O2 for 30 min and then the slides were
washed and placed in 5% BSA confining liquid for 20 min. The
specimens were incubated at 4°C overnight with a rabbit anti-rabbit
polyclonal antibody against bFGF (Wuhan Boster Biological
Technology, Ltd.) at a dilution of 1:200. The negative controls
were exposed to the secondary antibody only and processed as
described above. The sections were washed and then incubated with
biotinylated goat anti-rabbit secondary antibody (SABC-POD kit,
Wuhan Boster Biological Technology, Ltd.) for 20 min. Subsequently,
a tertiary layer of streptavidin peroxidase was applied according
to the manufacturer’s instructions (SABC-POD kit, Wuhan Boster
Biological Technology, Ltd.). Antigen-antibody complexes were
detected by incubation with diaminobenzidine (DAB) (Wuhan Boster
Biological Technology, Ltd.) at room temperature for 10 to 30 min.
Then slides were lightly counterstained with Mayer’s hematoxylin
(Wuhan Boster Biological Technology Ltd.) for 30 sec. Positive
cells were brown-stained, and non-brown-stained cells were
considered negative. Finally, the sections were washed, dehydrated,
embedded in paraffin and photographed.
Quantitative immunohistochemical
analysis
For immunocytochemical analysis, sections were coded
and counted in a blind fashion by using a light microscope
(Axioscop, Zeiss, Jena, Germany). A total of 6 visual fields from
randomly selected areas in the sample coverslips were examined.
Scopes were chosen as the percentage of angiogenic factor-positive
cells that colocalized with bFGF. Immunohistochemistry staining
gray scale was analyzed by Image-Pro Plus (IPP) software (version
5.0.1, Media Cybernetics Inc., Rockville, MD). The average of the
results was used for statistical analysis and expressed as mean
optical density (MOD).
Statistical analysis
Experiments were repeated in triplicate. All
statistical analyses were performed using the SPSS®
statistical package, version 11.5 (SPSS Inc., Chicago, IL) for
Windows®. Standard deviation and average were
calculated, expressed as the mean ± SD values. The data were
analyzed for significance using one-way analysis of variance
(ANOVA), followed by the Student-Newman-Keuls test for multiple
comparisons. A P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
AGE stimulation upregulates bFGF
expression in Müller cells in a dose-dependent manner
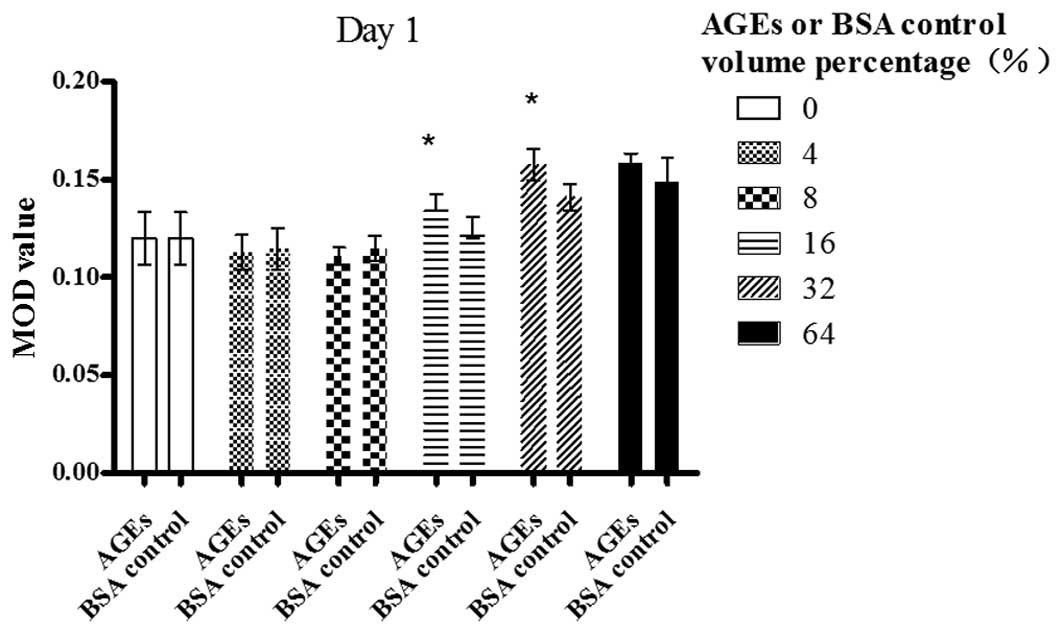
Day 1
A dose-dependent increase in the MOD value of bFGF
was revealed following the exposure of Müller cells to AGE in a
volume percentage ranging from 8 to 32%; however, the value did not
change at 64%, compared with the value at 32% (P>0.05). There
was a statistically significant difference between the cells
treated with AGEs and those treated with the BSA control in a
volume percentage of 16 and 32% (P<0.05) (Fig. 1).
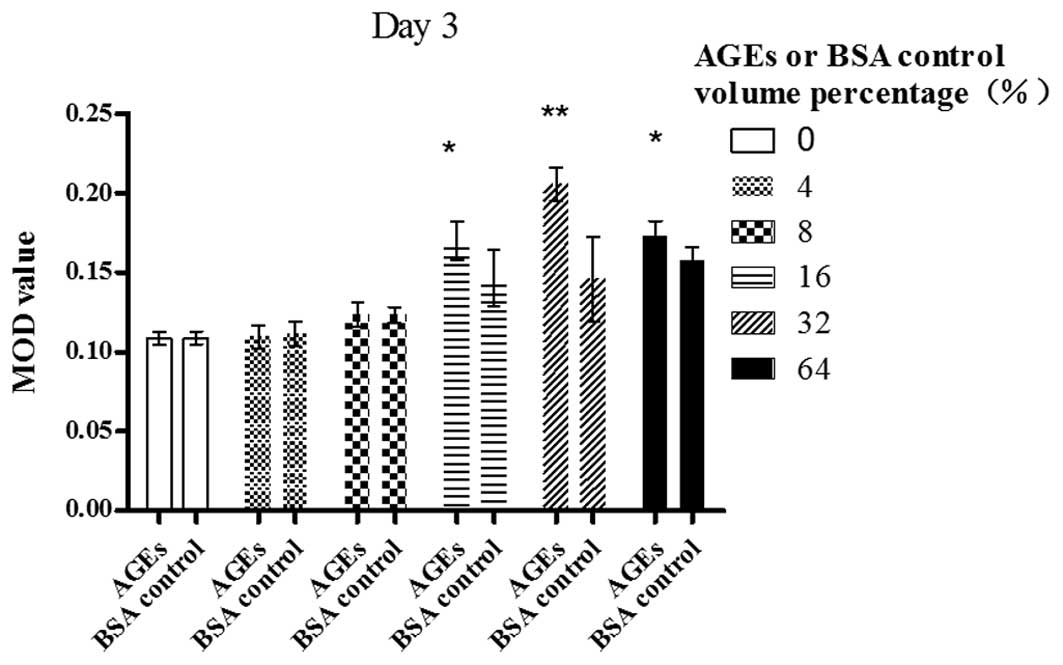
Day 3
A dose-dependent increase in the MOD value of bFGF
was revealed following the exposure of Müller cells to AGE in a
volume percentage ranging from 8 to 32%; however, the value began
to decrease at 64% (P<0.01). AGEs (64% volume percentage) also
significantly enhanced the bFGF expression in contrast with the
blank control (P<0.01). There was a statistically significant
difference between the cells treated with AGEs and those treated
with the BSA control in a volume percentage of 16% (P<0.05), 32%
(P<0.01) and 64% (P<0.05) (Fig.
2).
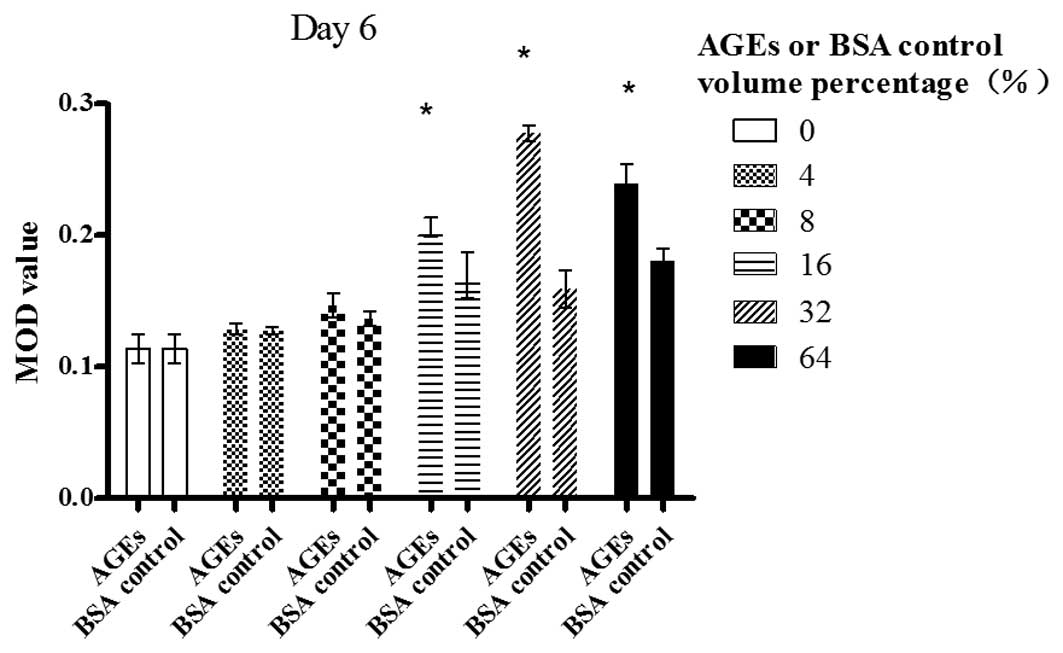
Day 6
A dose-dependent increase in the MOD value bFGF was
revealed following the exposure of Müller cells to AGE in a volume
percentage ranging from 8 to 32%; however, the value began to
decrease at 64% (P<0.01). AGEs (64% volume percentage) also
significantly enhanced bFGF expression in contrast with the blank
control (P<0.01). There was statistically significant difference
between the cells treated with AGEs and those treated with the BSA
control in a volume percentage of 16, 32 and 64% (P<0.05)
(Fig. 3).
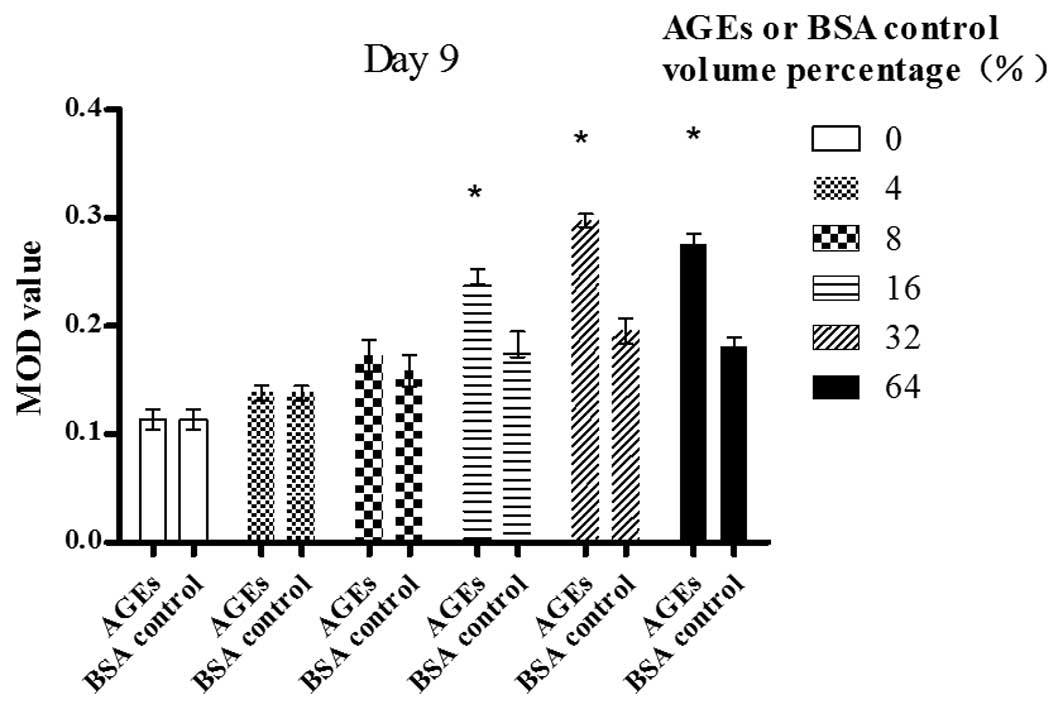
Day 9
A dose-dependent increase in the MOD value of bFGF
was revealed following the exposure of Müller cells to AGE in a
volume percentage ranging from 0 to 32%; however, the value began
to decrease significantly at 64% (P<0.05). AGEs (64% volume
percentage) also significantly enhanced bFGF expression in contrast
with the blank control (P<0.01). There was a statistically
significant difference between the cells treated with AGEs and
those treated with the BSA control in a volume percentage of 16, 32
and 64% (P<0.05) (Fig. 4).
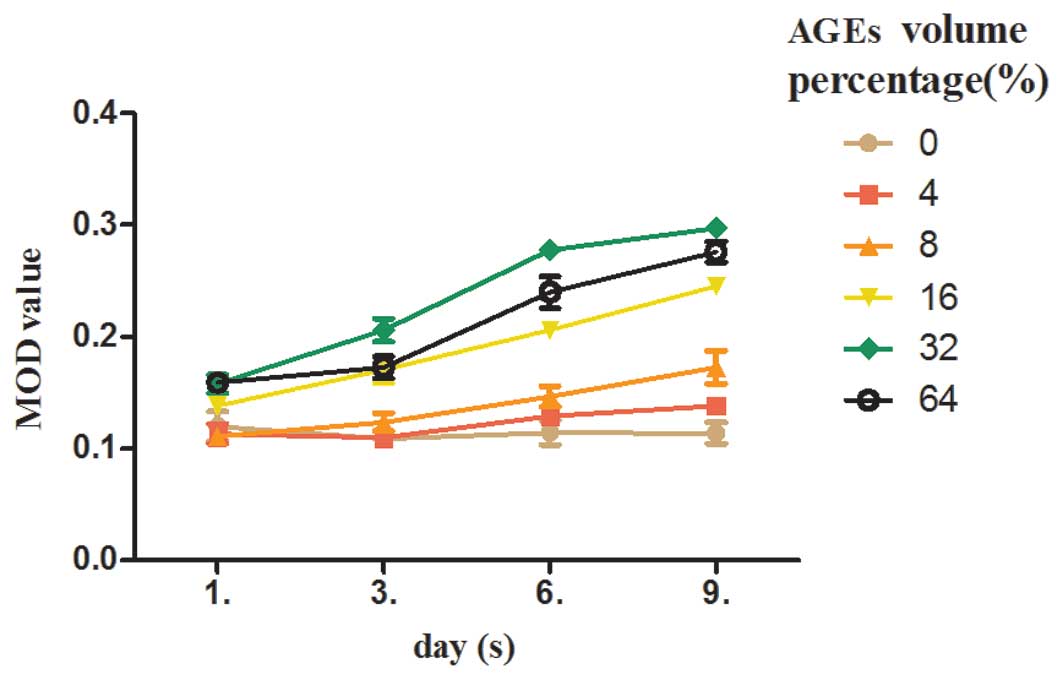
AGE stimulation upregulates bFGF
expression in Müller cells in a time-dependent manner
As shown in Fig. 5,
at a volume percentage of 0% (the blank control), no statistically
significant difference in the expression of bFGF was observed
between the groups (F=1.229, P>0.05). At a volume percentage of
4%, the expression of bFGF increased from day 3 to 6 (P<0.01).
At a volume percentage between 8 and 64%, the expression of bFGF
increased from day 3 to 9 (P<0.05). At a volume percentage
between 16 and 32%, the expression of bFGF increased from day 1 to
9 (P<0.01).
Discussion
The accelerated formation and accumulation of AGEs
has been implicated in the pathogenesis of diabetic vascular
complications. AGEs directly or indirectly induce the production of
various cytokines and growth factors by macrophages, monocytes,
endothelial cells and Müller cells, which leads to the development
of diabetic angiopathy (22–26).
Long-lasting retinal ischemia in DR causes the
outgrowth of new vessels from superficial veins and venules onto
the posterior vitreous cortex. The most severe ocular complications
of diabetes mellitus are associated with proliferative DR (PDR).
Once neovascularization develops, DR is classified as PDR. Various
reactions may be discerned in the development of PDR, including
chemotaxis and cellular migration, cellular proliferation, membrane
formation and contraction (7). In
eyes with PDR and persistent vitreoretinal adhesions, elevated
neovascular fronds may undergo fibrous change and form tight
fibrovascular bands that tug on the retina and exert continued
vitreous contraction. This may cause a progressive traction retinal
detachment.
Müller cells extend from the inner limiting membrane
of the retina to the outer limiting membrane. It has been
demonstrated that Müller cells are directly involved in the
formation of the blood-retinal barrier (6,27)
and grow specifically into the subretinal space and form multiple
layers of cell bodies between the retina and the retinal pigment
epithelium after retinal detachment. Therefore, Müller cells, in
association with blood-derived immune cells and factors within the
vitreous, are suggested to play a crucial role in either
non-proliferative DR (NPDR) or PDR (28,29).
VEGF is deemed to be a part of a pro-survival
response of the hypoxic tissue with actions that include
vasodilatation, endothelial cell survival, inflammation, glial cell
proliferation, neuroprotection, neurogenesis and neovascularization
(30,31). In proliferative vitreous
retinopathy (PVR) and PDR, the vitreal and subretinal concentration
of VEGF is enhanced (32–34). bFGF is involved in many biological
processes in embryonic development, wound healing, hematopoiesis
and angiogenesis (35) and evokes
the release of VEGF and HGF from Müller cells (36). These two cytokines (VEGF and bFGF)
exert a synergistic effect during the several stages of
angiogenesis in the retina (37).
In this study, we demonstrated that AGEs induce bFGF
secretion by Müller cells in a dose- and time-dependent manner
in vitro. However, when the maximum volume percentage was
reached, bFGF expression tended to decrease, since bFGF has a
down-regulatory effect on Müller cells.
As an angiogenic factor in vivo, bFGF is
known to be more potent than VEGF and the formation of
neovascularization is augmented by the accelerated flux through the
bFGF secretion (38). Based on the
present findings, we propose a reciprocal correlation between AGEs
and bFGF expression. AGEs elicit the augmented secretion of bFGF in
Müller cells, which synergistically promotes the development of
diabetic vascular complications with VEGF. AGEs may thus affect the
formation of retinal neovascularization directly, as well as
indirectly, by the induction of bFGF expression; this therefore may
be another relevant mechanism that leads to diabetic vascular
dysfunction.
One limitation of our study is that there is no
standard method of preparing AGE proteins and quantifying their
chemical composition in vitro. A number of widely varying
methods have been used for incubating cells with BSA. The
composition of the final AGE-protein is largely unknown or not
specified in vivo. Therefore, we used volume percentage to
describe the concentrations, after we first measured the
concentrations using a fluorescence spectrometer.
In conclusion, the increased formation of AGEs in
the vitreous may be involved in the initiation and progression of
intraocular neovascularization in diabetes through the production
of bFGF by Müller cells. The results from our study are in accord
with those from other studies suggesting that early vitrectomy in
DR cases by clearing the AGEs and other molecules may contribute to
prevent the progression of DR (7,15,33,39).
Further in vivo studies are required to explore the
importance of AGEs in the initiation and progression of DR and to
develop new therapeutic strategies to prevent DR.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 30901446) and the Programme of
Chinese Medical Science of Zhejiang Province (no. 2009CB040).
References
|
1
|
Daroux M, Prévost G, Maillard-Lefebvre H,
Gaxatte C, D’Agati VD, Schmidt AM and Boulanger E: Advanced
glycation end-products: implications for diabetic and non-diabetic
nephropathies. Diabetes Metab. 36:1–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berrou J, Tostivint I, Verrecchia F,
Berthier C, Boulanger E, Mauviel A, Marti HP, Wautier MP, Wautier
JL, Rondeau E and Hertig A: Advanced glycation end products
regulate extracellular matrix protein and protease expression by
human glomerular mesangial cells. Int J Mol Med. 23:513–520.
2009.
|
|
3
|
Wolffenbuttel BH, Giordano D, Founds HW
and Bucala R: Long-term assessment of glucose control by
haemoglobin-AGE measurement. Lancet. 347:513–515. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meerwaldt R, Links T, Zeebregts C, Tio R,
Hillebrands JL and Smit A: The clinical relevance of assessing
advanced glycation endproducts accumulation in diabetes. Cardiovasc
Diabetol. 7:292008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamagishi S: Role of advanced glycation
end products (AGEs) and receptor for AGEs (RAGE) in vascular damage
in diabetes. Exp Gerontol. 46:217–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bringmann A and Wiedemann P: Involvement
of Müller glial cells in epiretinal membrane formation. Graefes
Arch Clin Exp Ophthalmol. 247:865–883. 2009.
|
|
7
|
Simó R, Carrasco E, García-Ramírez M and
Hernández C: Angiogenic and antiangiogenic factors in proliferative
diabetic retinopathy. Curr Diabetes Rev. 2:71–98. 2006.
|
|
8
|
Aiello LP, Avery RL, Arrigg PG, Keyt BA,
Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et
al: Vascular endothelial growth factor in ocular fluid of patients
with diabetic retinopathy and other retinal disorders. N Engl J
Med. 331:1480–1487. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kakehashi A, Inoda S, Mameuda C, Kuroki M,
Jono T, Nagai R, Horiuchi S, Kawakami M and Kanazawa Y:
Relationship among VEGF, VEGF receptor, AGEs and macrophages in
proliferative diabetic retinopathy. Diabetes Res Clin Pract.
79:438–445. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Payne JF, Tangpricha V, Cleveland J, Lynn
MJ, Ray R and Srivastava SK: Serum insulin-like growth factor-I in
diabetic retinopathy. Mol Vis. 17:2318–2324. 2011.PubMed/NCBI
|
|
11
|
Shimizu F, Sano Y, Haruki H and Kanda T:
Advanced glycation end-products induce basement membrane
hypertrophy in endoneurial microvessels and disrupt the blood-nerve
barrier by stimulating the release of TGF-β and vascular
endothelial growth factor (VEGF) by pericytes. Diabetologia.
54:1517–1526. 2011.PubMed/NCBI
|
|
12
|
Simó R, Vidal MT, García-Arumí J, Carrasco
E, García-Ramírez M, Segura RM and Hernández C: Intravitreous
hepatocyte growth factor in patients with proliferative diabetic
retinopathy: a case-control study. Diabetes Res Clin Pract.
71:36–44. 2006.
|
|
13
|
Yang XM, Yafai Y, Wiedemann P, Kuhrt H,
Wang YS, Reichenbach A and Eichler W: Hypoxia-induced upregulation
of pigment epithelium-derived factor by retinal glial (Müller)
cells. J Neurosci Res. 90:257–266. 2012.PubMed/NCBI
|
|
14
|
He S, Jin ML, Worpel V and Hinton DR: A
role for connective tissue growth factor in the pathogenesis of
choroidal neovascularization. Arch Ophthalmol. 121:1283–1288. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watanabe D, Suzuma K, Suzuma I, Ohashi H,
Ojima T, Kurimoto M, Murakami T, Kimura T and Takagi H: Vitreous
levels of angiopoietin 2 and vascular endothelial growth factor in
patients with proliferative diabetic retinopathy. Am J Ophthalmol.
139:476–481. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao R, Eriksson A, Kubo H, Alitalo K, Cao
Y and Thyberg J: Comparative evaluation of FGF-2-, VEGF-A- and
VEGF-C-induced angiogenesis, lymphangiogenesis, vascular
fenestrations and permeability. Circ Res. 94:664–670. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tokuda H, Adachi S, Matsushima-Nishiwaki
R, Kato K, Natsume H, Otsuka T and Kozawa O: Enhancement of basic
fibroblast growth factor-stimulated VEGF synthesis by Wnt3a in
osteoblasts. Int J Mol Med. 27:859–864. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JJ, Hsiao CC, Yang IH, Chou MH, Wu CL,
Wei YC, Chen CH and Chuang JH: High-mobility group box 1 protein is
implicated in advanced glycation end products-induced vascular
endothelial growth factor A production in the rat retinal ganglion
cell line RGC-5. Mol Vis. 18:838–850. 2012.PubMed/NCBI
|
|
19
|
Wakakura M and Foulds WS:
Immunocytochemical characteristics of Müller cells cultured from
adult rabbit retina. Invest Ophthalmol Vis Sci. 29:892–900.
1988.
|
|
20
|
Liu Y and Wakakura M: P1-/P2-purinergic
receptors on cultured rabbit retinal Müller cells. Jpn J
Ophthalmol. 42:33–40. 1998.
|
|
21
|
Neumann A, Schinzel R, Palm D, Riederer P
and Münch G: High molecular weight hyaluronic acid inhibits
advanced glycation endproduct-induced NF-kappaB activation and
cytokine expression. FEBS Lett. 453:283–287. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vlassara H, Brownlee M, Manogue KR,
Dinarello CA and Pasagian A: Cachectin/TNF and IL-1 induced by
glucose-modified proteins: role in normal tissue remodeling.
Science. 240:1546–1548. 1988. View Article : Google Scholar
|
|
23
|
Walcher D and Marx N: Advanced glycation
end products and C-peptide-modulators in diabetic vasculopathy and
atherogenesis. Semin Immunopathol. 31:103–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamagishi S, Maeda S, Matsui T, Ueda S,
Fukami K and Okuda S: Role of advanced glycation end products
(AGEs) and oxidative stress in vascular complications in diabetes.
Biochim Biophys Acta. 1820:663–671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nam MH, Lee HS, Seomun Y, Lee Y and Lee
KW: Monocyte-endothelium-smooth muscle cell interaction in
co-culture: proliferation and cytokine productions in response to
advanced glycation end products. Biochim Biophys Acta.
1810:907–912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Curtis TM, Hamilton R, Yong PH, McVicar
CM, Berner A, Pringle R, Uchida K, Nagai R, Brockbank S and Stitt
AW: Müller glial dysfunction during diabetic retinopathy in rats is
linked to accumulation of advanced glycation end-products and
advanced lipoxidation end-products. Diabetologia. 54:690–698.
2011.
|
|
27
|
Bhatia B, Jayaram H, Singhal S, Jones MF
and Limb GA: Differences between the neurogenic and proliferative
abilities of Müller glia with stem cell characteristics and the
ciliary epithelium from the adult human eye. Exp Eye Res.
93:852–861. 2011.PubMed/NCBI
|
|
28
|
Guidry C, King JL and Mason JO III:
Fibrocontractive Müller cell phenotypes in proliferative diabetic
retinopathy. Invest Ophthalmol Vis Sci. 50:1929–1939.
2009.PubMed/NCBI
|
|
29
|
King JL, Mason JO III, Cartner SC and
Guidry C: The influence of alloxan-induced diabetes on Müller cell
contraction-promoting activities in vitreous. Invest Ophthalmol Vis
Sci. 52:7485–7491. 2011.
|
|
30
|
Crawford TN, Alfaro DV III, Kerrison JB
and Jablon EP: Diabetic retinopathy and angiogenesis. Curr Diabetes
Rev. 5:8–13. 2009. View Article : Google Scholar
|
|
31
|
Liu W, Xu J, Wang M, Wang Q, Bi Y and Han
M: Tumor-derived vascular endothelial growth factor (VEGF)-a
facilitates tumor metastasis through the VEGF-VEGFR1 signaling
pathway. Int J Oncol. 39:1213–1220. 2011.PubMed/NCBI
|
|
32
|
Dieudonné SC, La Heij EC, Diederen RM,
Kessels AG, Liem AT, Kijlstra A and Hendrikse F: Balance of
vascular endothelial growth factor and pigment epithelial growth
factor prior to development of proliferative vitreoretinopathy.
Ophthalmic Res. 39:148–154. 2007.PubMed/NCBI
|
|
33
|
Funatsu H, Noma H, Mimura T, Eguchi S and
Hori S: Association of vitreous inflammatory factors with diabetic
macular edema. Ophthalmology. 116:73–79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baharivand N, Zarghami N, Panahi F, Dokht
Ghafari MY, Mahdavi Fard A and Mohajeri A: Relationship between
vitreous and serum vascular endothelial growth factor levels,
control of diabetes and microalbuminuria in proliferative diabetic
retinopathy. Clin Ophthalmol. 6:185–191. 2012.
|
|
35
|
Kanda S, Naba A and Miyata Y: Inhibition
of endothelial cell chemotaxis toward FGF-2 by gefitinib associates
with downregulation of Fes activity. Int J Oncol. 35:1305–1312.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hollborn M, Jahn K, Limb GA, Kohen L,
Wiedemann P and Bringmann A: Characterization of the basic
fibroblast growth factor-evoked proliferation of the human Müller
cell line, MIO-M1. Graefes Arch Clin Exp Ophthalmol. 242:414–422.
2004.PubMed/NCBI
|
|
37
|
Zakareia FA, Alderees AA, Al Regaiy KA and
Alrouq FA: Correlation of electroretinography b-wave absolute
latency, plasma levels of human basic fibroblast growth factor,
vascular endothelial growth factor, soluble fatty acid synthase and
adrenomedullin in diabetic retinopathy. J Diabetes Complications.
24:179–185. 2010. View Article : Google Scholar
|
|
38
|
Matsui M and Tabata Y: Enhanced
angiogenesis by multiple release of platelet-rich plasma contents
and basic fibroblast growth factor from gelatin hydrogels. Acta
Biomater. 8:1792–1801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gündüz K and Bakri SJ: Management of
proliferative diabetic retinopathy. Compr Ophthalmol Update.
8:245–256. 2007.
|