Introduction
Endothelial progenitor cells (EPCs) are a specific
subtype of hematopoietic stem cells present in peripheral blood
that express various combinations of antigens traditionally
associated with hematopoietic stem and endothelial cells, including
kinase insert domain-containing receptor, CD34 and CD133 (1). Previous studies have identified that
EPCs contribute to neovascularization of ischemic tissue and
reversal of endothelial dysfunction (2,3),
indicating a potential application in cell therapy to augment
vascularization in patients with ischemic heart disease (4). However, there are limited numbers of
EPCs in the circulating blood (<0.05% of leukocytes) (5) and the number and functional activity
of EPCs in healthy volunteers and patients with coronary artery
disease is affected by various risk factors, including aging,
diabetes, hypercholesterolemia, hypertension and smoking (6). Allogeneic transfusion of EPCs from
healthy donors leads to immunological incompatibility in specific
individuals; therefore, the functional enhancement of autologous
EPCs, including enhanced mobilization, homing, survival and
secretion of growth factors in a paracrine or autocrine manner,
must be developed for EPC-based therapies for cardiovascular
diseases.
Our previous studies demonstrated that thymosin β4
(Tβ4) increases EPC migration and decreases EPC apoptosis under
serum deprivation via the phosphoinositide 3′-kinase
(PI3K)-Akt-endothelial nitric oxide synthase (eNOS) signal
transduction pathway (7,8). However, the effect of Tβ4 on
senescence of circulating EPCs remains unresolved. Cellular aging
or senescence is characterized by cell cycle arrest and is
triggered by various signaling pathways (9). Understanding of the mechanisms
underlying cellular senescence has developed significantly, and
senescence is currently associated with attrition (i.e., reduced
length) of telomeres (10).
Telomerase, a ribonucleoprotein with reverse transcriptase
activity, extends telomeres of eukaryotic chromosomes and delays
the development of senescence (11). Furthermore, a senescent phenotype
is induced by expression of cyclin-dependent kinase inhibitors
(CDKIs) (12). The
cyclin-dependent kinases (CDKs) are central to the coordination of
the eukaryotic cell cycle and function to integrate diverse growth
regulatory signals. The majority of anti-proliferative signals lead
to induction of CDKIs, including p27Kip1 and
p21Cip1/Waf1 and therefore these cell cycle inhibitors
have been studied as biomarkers of senescence (12).
The aim of the present study was to investigate the
effects of Tβ4 on EPC senescence and telomerase activity and to
define the signal transduction pathway involved in this
process.
Materials and methods
Isolation, cultivation and
characterization of circulating EPCs
EPCs were isolated, cultured and characterized
according to previously described techniques (13,14).
Briefly, peripheral blood mononuclear cells were isolated from
healthy volunteers by density-gradient centrifugation with Ficoll
(Cedarlane Laboratories Ltd., Hornby, ON, Canada) according to the
manufacturer’s instructions. All blood samples were obtained,
processed and analyzed individually in independent experiments.
Following purification with 3 washing steps, 1×107
peripheral blood mononuclear cells were plated on
fibronectin-coated 6-well plates (Chemicon, Temecula, CA, USA).
Cells were cultured in endothelial basal medium-2 (Clonetics Corp.,
Walkersville, MD, USA) with single aliquots of EGM-2MV containing
5% fetal bovine serum, vascular endothelial growth factor,
fibroblast growth factor-2, epidermal growth factor, insulin-like
growth factor and ascorbic acid. Following 4 days in culture,
non-adherent cells were removed by washing with phosphate-buffered
saline (PBS), fresh medium was applied and the culture was
maintained for 7 days. The study was approved by the ethics
committee of Zhejiang University, and performed in accordance with
the 1964 Declaration of Helsinki. Informed consent was obtained
from all volunteers.
Senescence-associated β-galactosidase
(SA-β-gal) staining
Following incubation for 7 days, the culture medium
was replaced with fresh medium and cells were cultured in the
absence or presence of Tβ4 (0, 1, 10, 100 and 1,000 ng/ml)
(ProSpec-Tany TechnoGene Ltd., Rehovot, Israel) for a further 24 h.
EPCs were harvested and SA-β-gal activity was measured with the
Senescence β-gal Staining kit (Cell Signaling Technology Inc.,
Danvers, MA, USA) as described previously (15).
Real-time reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from EPCs with TRIzol
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to a
modification of the method described by Chirgwin et
al(16). RNA (1–2 μg) was
converted into cDNA using murine leukemia virus reverse
transcriptase (Promega Corp., Madison, WI, USA). Transcribed cDNA
was then used as a template in PCR amplification to measure the
expression of hTERT. Primers used for hTERT PCR were as follows:
sense, 5′-AGA GTG TCT GGA GCA AGT TGC-3′; and antisense, 5′-CGT AGT
CCA TGT TCA CAA TCG-3′. Amplification produced a 182-bp hTERT
fragment (nucleotides 1789–1971; GenBank no. AF018167) that was
verified by southern blot analysis and nested PCR as previously
described (17). Primers used for
GAPDH PCR were as follows: sense, 5′-GGG TGT GAA CCA TGA GAA GT-3′;
and antisense, 5′-GAC TGT GGT CAT GAG TCC T-3′. GAPDH was amplified
as a reference and produced a 136-bp product. Real-time PCR was
performed using an ABI 7500 cycler (Applied Biosystems, Foster
City, CA, USA) with SYBR-Green PCR mix (Takara, Shiga, Japan)
according to the manufacturer’s instructions. These experiments
were repeated 3 times for each cell line.
Detection of telomerase activity
EPCs were washed in PBS, lysed with 30 μl lysis
buffer and the telomeric repeat amplification protocol (TRAP) assay
for telomerase activity was performed using the TeloTAGGG
Telomerase PCR ELISA Plus Kit (Roche Diagnostics, Indianapolis, IN,
USA) according to the manufacturer’s instructions, as described
previously (18).
Western blot analysis
Following 7 days of culture, EPCs were deprived of
serum for 12 h to render the cells quiescent. Cells were cultured
for an additional 24 h in the absence or presence of Tβ4 (1,000
ng/ml) and the PI3K-inhibitor wortmannin or the eNOS inhibitor
L-nitroarginine methyl ester hydrochloride (L-NAME; Sigma-Aldrich,
St. Louis, MO, USA). EPCs were collected and lysed with lysis
buffer as previously described (19). Proteins (50 μg/lane) were separated
on SDS-polyacrylamide gels and blotted onto PVDF membranes
(Bio-Rad, Hercules, CA, USA). Western blot analysis was performed
using antibodies against cyclin D1, p21Cip1/Waf1 or
p27Kip1 (Cell Signaling Technology Inc.). Following
reaction with enhanced chemiluminescence reagent (Amersham
Pharmacia Biotech, Haemek, Israel), images were captured using an
image reader LAS 4000 system (Fujifilm, Tokyo, Japan).
Statistical analysis
All experiments were performed at least three times.
Data were analyzed by unpaired Student’s t-test for comparisons
between two groups or one-way ANOVA for multiple comparisons and
are expressed as the mean ± SEM. P<0.05 was considered to
indicate a statistically significant difference.
Results
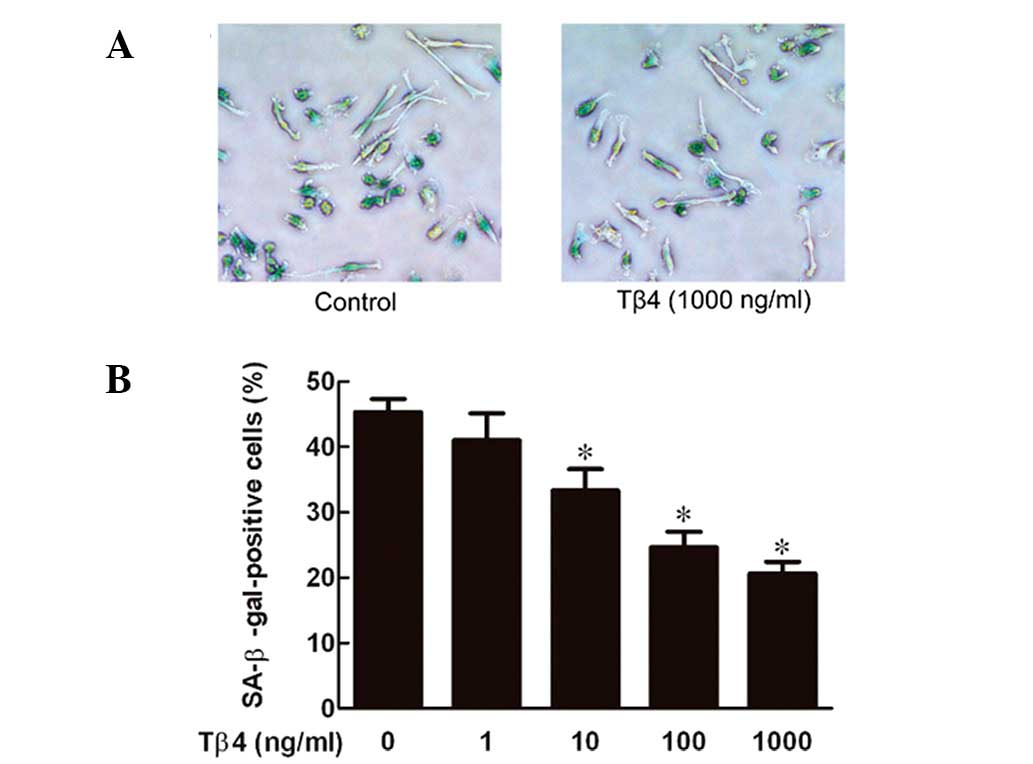
Tβ4 prevents senescence of EPCs in a
dose-dependent manner
EPCs were exposed to Tβ4 at various concentrations
(0, 1, 10, 100 and 1,000 ng/ml) for 24 h and the senescence levels
were measured by SA-β-gal staining, which is widely used as a
biomarker of senescence (15). As
demonstrated in Fig. 1, Tβ4
decreased senescence of EPCs in a dose-dependent manner, with a
maximal effect at 1,000 ng/ml.
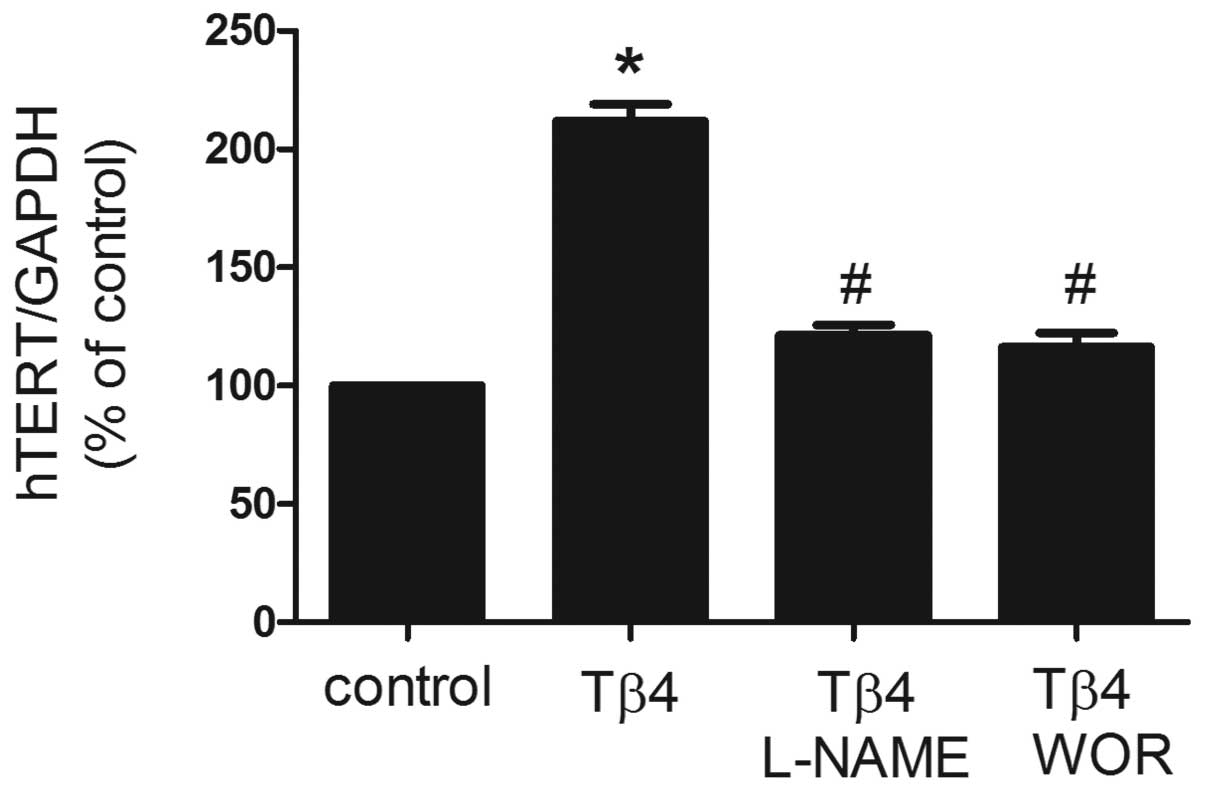
Effects of Tβ4 on hTERT mRNA expression
in EPCs
To investigate the effect of Tβ4 on the expression
of hTERT mRNA, EPCs were treated with Tβ4 (1,000 ng/ml) for 24 h
and real-time RT-PCR analysis was performed. As demonstrated in
Fig. 2, expression of hTERT mRNA
was identified as significantly increased by cotreatment with Tβ4
for 24 h. Previously, Tβ4 treatment of EPCs was revealed to
stimulate time-dependent phosphorylation of Akt and eNOS (8), which is known to regulate the
senescence of mature endothelial cells. In the present study, the
PI3K inhibitor wortmannin and eNOS inhibitor L-NAME were utilized
to assess involvement of the PI3K pathway and eNOS. As revealed in
Fig. 2, pretreatment with
wortmannin (100 nM) or L-NAME (100 μM) for 30 min was identified to
significantly reverse the increase in expression of hTERT mRNA
induced by Tβ4, suggesting that Tβ4 affects EPC senescence via the
PI3K-Akt-eNOS signal transduction pathway (P<0.05).
Tβ4 stimulates telomerase activity in
EPCs
Senescence is hypothesized to be triggered by
shortening of telomere length (18). Telomere shortening during cell
division is counteracted by telomerase activity, therefore, we
analyzed telomerase activity in EPCs. As demonstrated in Fig. 3, incubation of EPCs with Tβ4 was
identified to result in a significant increase in telomerase
activity (P<0.05). The Tβ4-induced increase in telomerase
activity was suppressed by wortmannin (100 nM) or L-NAME (100 μM),
similar to the effect of these inhibitors on hTERT mRNA
expression.
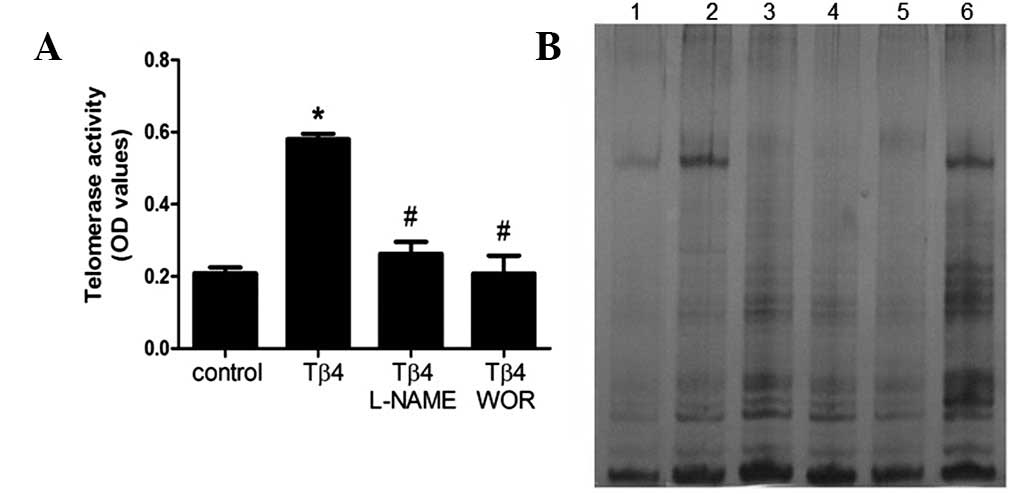 | Figure 3Effect of Tβ4 on telomerase activity.
(A) Following 7 days of cultivation, Tβ4 was added for 24 h with or
without pretreatment with wortmannin (100 nM) or L-NAME (100 μM)
and telomerase activity was measured by the TRAP assay. Data are
presented as the means ± SEM. *P<0.05 compared with
control; #P<0.05 compared with Tβ4 (1,000 ng/ml). (B)
Representative PAGE gel of TRAP-PCR. Lane 1, negative control;
lanes 2 and 3, telomerase activity in the presence of Tβ4 for 24 h
(0 and 1,000 ng/ml); lanes 4 and 5, telomerase activity in the
presence of 1,000 ng/ml Tβ4 for 24 h with pretreatment with
wortmannin (100 nM) or L-NAME (100 μM); lane 6, positive control.
All experiments were repeated at least 3 times and a representative
result is presented. Tβ4, thymosin β4; L-NAME, L-nitroarginine
methyl ester hydrochloride; TRAP, telomeric repeat amplification
protocol. |
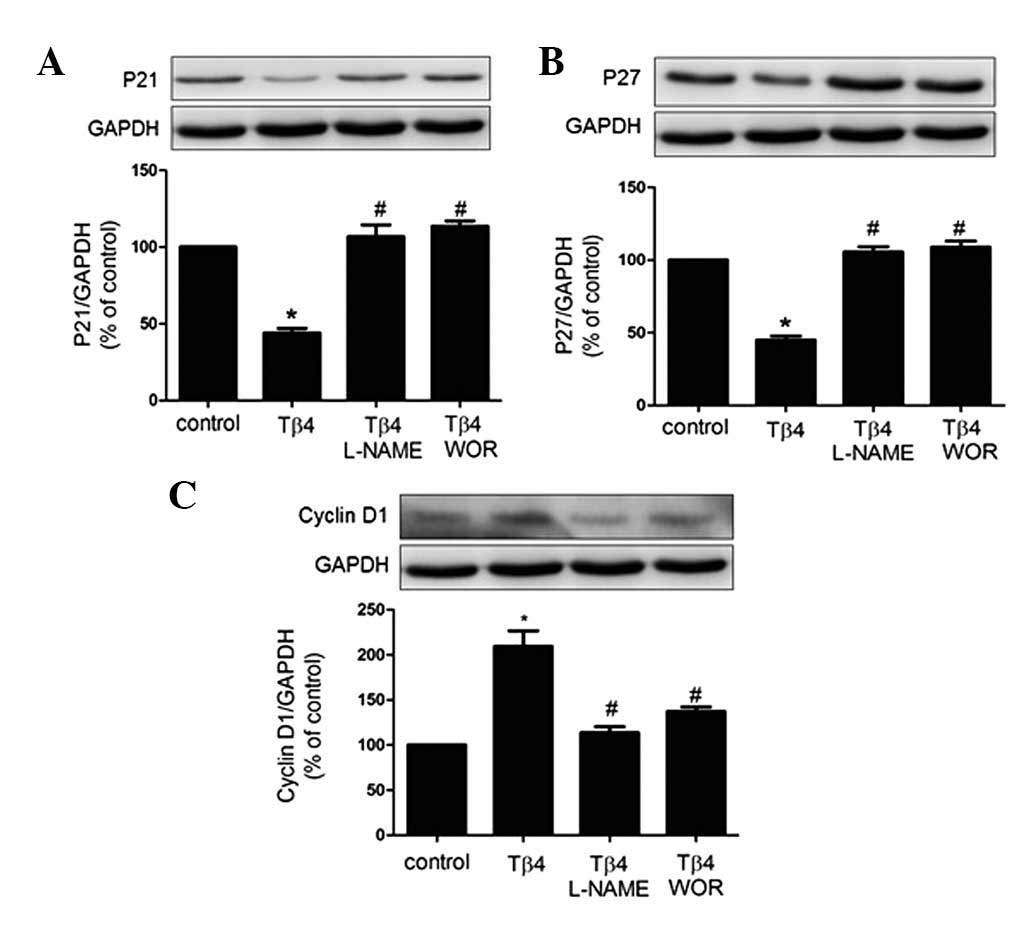
Effects of Tβ4 on cell senescence-related
proteins in EPCs
The senescent phenotype is induced by expression of
CDKIs. To this end, expression patterns of p21, p27 and cyclin D1
have previously been studied as biomarkers of senescence (12). Therefore, investigation of the
expression of the pro-senescent proteins p21 and p27 and the
anti-senescent protein cyclin D1 by western blot analysis was
performed in the present study. As revealed in Fig. 4, p21 and p27 protein expression was
decreased by treatment with Tβ4 for 24 h, whereas cyclin D1 protein
expression was increased. These effects were eliminated by
treatment with wortmannin (100 nM) or L-NAME (100 μM), suggesting
that the PI3K-Akt-eNOS signal transduction pathway is involved in
the process by which Tβ4 attenuates EPC senescence.
Discussion
Results of the present study revealed that exogenous
Tβ4 protected EPCs from senescence in a concentration-dependent
manner and suggested that this may be associated with activation of
telomerase and elongation of telomeres. In addition, the importance
of PI3K-Akt-eNOS activation in this process was demonstrated.
The essential role of vascular endothelium in
cardiovascular disorders is increasingly being recognized. Although
mature endothelial cells contribute to the repair of endothelial
injury, the cells possess limited regenerative capacities (20). Previous studies indicate that
postnatal neovascularization does not rely exclusively on sprouting
of mature endothelial cells in pre-existing vessels (angiogenesis)
but also involves circulating EPCs (1,2).
Importantly, injection of cultivated EPCs enhances
neovascularization (21) and this
process was identified to improve cardiac function (22). These beneficial properties of EPCs
make them attractive candidates for cell therapy that targets the
endothelial regeneration of ischemic tissue. However, ex
vivo cultivation of EPCs leads to rapid onset of EPC senescence
(15), a major risk factor for
coronary and peripheral artery disease. In addition, one of the
consequences of aging is a decline in the ability of the organism
to respond to various stresses, including ischemia. Moreover, the
number and functional activities of EPCs are diminished by aging
(23). Anti-senescence may be a
novel therapeutic strategy for vascular aging and therefore the
prevention of cellular senescence of EPCs may have important
clinical implications, particularly in diseases associated with
increased cellular senescence, including atherosclerosis.
Aging leads to an irreversible state of cell cycle
arrest known as replicative senescence, which is associated with
telomere shortening (11).
Telomerase, a RNA-directed DNA polymerase, extends telomeres of
eukaryotic chromosomes and delays the development of senescence. A
number of previous studies have demonstrated that telomere length
and telomerase activity of circulating EPCs are decreased in
patients with various risk factors for coronary artery disease
(5,24). Similarly, evidence indicates that
EPC subpopulation senescence may be mediated partly by the
telomerase-dependent telomere length mechanism. There is a good
correlation between the expression of hTERT mRNA and the presence
of telomerase activity (25).
Overexpression of hTERT by adenovirus-mediated gene delivery delays
senescence of EPCs (24). In the
present study, telomerase activity in EPCs was increased by
treatment with Tβ4 and this activation was accompanied by increased
expression of hTERT mRNA. Tβ4 delayed EPC senescence through
telomerase activation, which may be associated with Tβ4-induced
upregulation of the expression of hTERT. However, replicative
senescence is also regulated by a telomere-independent mechanism.
In addition, cellular senescence is induced by DNA damage, cellular
stress and oncogenic activation (26). Further studies are required to
elucidate the mechanisms underlying the inhibitory effects of Tβ4
on senescence in EPC.
CDKs regulate and coordinate the eukaryotic cell
cycle. CDKs are controlled through several processes, including
binding of inhibitory Cip subunits (12). For example, p27Cip2
binds to the phosphorylated CDK2-cyclin A complex, interacting with
the CDK and cyclin (27), whereas
p21Cip1 inhibits CDK activity with selectivity for G1/S
phase CDK-cyclin complexes (28).
The majority of anti-proliferative signals lead to induction of CDK
inhibitors. Indeed, senescence augments the expression of
cell-cycle inhibitory proteins, including p27Kip1 or
p21Cip1/Waf1(29,30).
In the present study, Tβ4 treatment upregulated the expression of
cyclin D1 and reduced the expression of p27 and p21. These results
suggest that the effects of Tβ4 on EPC senescence involve
regulation of cyclin D1, p27 and p21, providing a mechanism for the
control of EPC life span by Tβ4.
Possible signal transduction pathways involved in
the effects of Tβ4 on senescence were also investigated. The
PI3K-Akt complex is a critical component of the pathway that
regulates signaling of multiple biological and pathophysiological
processes, including apoptosis, metabolism, cell migration, cell
proliferation and cell growth (31). Previous studies demonstrated that
Tβ4 activates the PI3K-Akt-eNOS pathway (7,8)
which is required to regulate cellular senescence (32). Therefore, we hypothesized that this
pathway may regulate Tβ4-mediated EPC senescence. In the present
study, Tβ4-induced prevention of EPC senescence and upregulation of
telomerase activity and hTERT mRNA were significantly attenuated by
the PI3K inhibitor (wortmannin) and the eNOS inhibitor (L-NAME)
indicating that the PI3K-Akt-eNOS signal transduction pathway is
involved in the effects of Tβ4 on EPC senescence.
In conclusion, the results of the present study
demonstrate that Tβ4 prevents EPC senescence by activation of the
PI3K-Akt-eNOS signaling transduction pathway. These observations
require further investigation of EPC-based cytotherapy in clinical
applications, particularly in diseases associated with increased
cellular senescence, including atherosclerosis.
Acknowledgements
The present study was supported by the Special Major
Projects of Zhejiang Province (2010C13207,N20100746) and the
Research Fund for the Doctoral Program of Higher Education
(J20110122, 20100101120140).
References
|
1
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: characterization and role in vascular biology.
Circ Res. 95:343–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi Q, Rafii S, Wu MH, et al: Evidence for
circulating bone marrow-derived endothelial cells. Blood.
92:362–367. 1998.PubMed/NCBI
|
|
4
|
Assmus B, Schachinger V, Teupe C, et al:
Transplantation of progenitor cells and regeneration enhancement in
acute myocardial infarction (TOPCARE-AMI). Circulation.
106:3009–3017. 2002. View Article : Google Scholar
|
|
5
|
Vasa M, Fichtlscherer S, Aicher A, et al:
Number and migratory activity of circulating endothelial progenitor
cells inversely correlate with risk factors for coronary artery
disease. Circ Res. 89:E1–E7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hill JM, Zalos G, Halcox JP, et al:
Circulating endothelial progenitor cells, vascular function and
cardiovascular risk. N Engl J Med. 348:593–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiu FY, Song XX, Zheng H, Zhao YB and Fu
GS: Thymosin beta4 induces endothelial progenitor cell migration
via PI3K/Akt/eNOS signal transduction pathway. J Cardiovasc
Pharmacol. 53:209–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Y, Qiu F, Xu S, Yu L and Fu G:
Thymosin beta4 activates integrin-linked kinase and decreases
endothelial progenitor cells apoptosis under serum deprivation. J
Cell Physiol. 226:2798–2806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mathon NF and Lloyd AC: Cell senescence
and cancer. Nat Rev Cancer. 1:203–213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Collins K: Mammalian telomeres and
telomerase. Curr Opin Cell Biol. 12:378–383. 2000. View Article : Google Scholar
|
|
12
|
Pavletich NP: Mechanisms of
cyclin-dependent kinase regulation: structures of Cdks, their
cyclin activators and Cip and INK4 inhibitors. J Mol Biol.
287:821–828. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dimmeler S, Aicher A, Vasa M, et al:
HMG-CoA reductase inhibitors (statins) increase endothelial
progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest.
108:391–397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng H, Dai T, Zhou B, et al:
SDF-1alpha/CXCR4 decreases endothelial progenitor cells apoptosis
under serum deprivation by PI3K/Akt/eNOS pathway. Atherosclerosis.
201:36–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Assmus B, Urbich C, Aicher A, et al:
HMG-CoA reductase inhibitors reduce senescence and increase
proliferation of endothelial progenitor cells via regulation of
cell cycle regulatory genes. Circ Res. 92:1049–1055. 2003.
View Article : Google Scholar
|
|
16
|
Chirgwin JM, Przybyla AE, MacDonald RJ and
Rutter WJ: Isolation of biologically active ribonucleic acid from
sources enriched in ribonuclease. Biochemistry. 18:5294–5299.
1979.PubMed/NCBI
|
|
17
|
Fuller RA, Clark J, Kretzner L, et al: Use
of microfluidics chips for the detection of human telomerase RNA.
Anal Biochem. 313:331–334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vasa M, Breitschopf K, Zeiher AM and
Dimmeler S: Nitric oxide activates telomerase and delays
endothelial cell senescence. Circ Res. 87:540–542. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng H, Fu G, Dai T and Huang H:
Migration of endothelial progenitor cells mediated by stromal
cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal
transduction pathway. J Cardiovasc Pharmacol. 50:274–280. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shantsila E, Watson T and Lip GY:
Endothelial progenitor cells in cardiovascular disorders. J Am Coll
Cardiol. 49:741–752. 2007. View Article : Google Scholar
|
|
21
|
Kalka C, Masuda H, Takahashi T, et al:
Transplantation of ex vivo expanded endothelial progenitor cells
for therapeutic neovascularization. Proc Natl Acad Sci USA.
97:3422–3427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawamoto A, Gwon HC, Iwaguro H, et al:
Therapeutic potential of ex vivo expanded endothelial progenitor
cells for myocardial ischemia. Circulation. 103:634–637. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Groleau J, Dussault S, Turgeon J, Haddad P
and Rivard A: Accelerated vascular aging in CuZnSOD-deficient mice:
impact on EPC function and reparative neovascularization. PLoS One.
6:e233082011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murasawa S, Llevadot J, Silver M, Isner
JM, Losordo DW and Asahara T: Constitutive human telomerase reverse
transcriptase expression enhances regenerative properties of
endothelial progenitor cells. Circulation. 106:1133–1139. 2002.
View Article : Google Scholar
|
|
25
|
Greider CW: Telomerase activity, cell
proliferation and cancer. Proc Natl Acad Sci USA. 95:90–92. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Serrano M and Blasco MA: Putting the
stress on senescence. Curr Opin Cell Biol. 13:748–753. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Russo AA, Jeffrey PD, Patten AK, Massague
J and Pavletich NP: Crystal structure of the p27Kip1
cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2
complex. Nature. 382:325–331. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harper JW, Elledge SJ, Keyomarsi K, et al:
Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell.
6:387–400. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Collado M, Medema RH, Garcia-Cao I, et al:
Inhibition of the phosphoinositide 3-kinase pathway induces a
senescence-like arrest mediated by p27Kip1. J Biol Chem.
275:21960–21968. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Macip S, Igarashi M, Fang L, et al:
Inhibition of p21-mediated ROS accumulation can rescue p21-induced
senescence. EMBO J. 21:2180–2188. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oudit GY and Penninger JM: Cardiac
regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res.
82:250–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang SS, Kwon T, Kwon DY and Do SI: Akt
protein kinase enhances human telomerase activity through
phosphorylation of telomerase reverse transcriptase subunit. J Biol
Chem. 274:13085–13090. 1999. View Article : Google Scholar : PubMed/NCBI
|