Introduction
Chronic obstructive pulmonary disease (COPD) is a
chronic inflammatory disease that has become a significant public
health issue. Airway inflammation, particularly in small airways,
is the basic characteristic of this disease. Pulmonary hypertension
is an important pathophysiological link to the development of a
number of clinical cardiopulmonary diseases. Moreover, COPD is one
of the most common etiologies of pulmonary hypertension. The
majority of previous studies have attributed the pathogenesis of
pulmonary hypertension to advanced hypoxia in COPD (1,2).
However, no effective method of preventing and treating pulmonary
hypertension in COPD has yet been established. Initiation of
pulmonary hypertension in COPD is not considered to be caused by
advanced hypoxia; however, it is closely associated with early
inflammation in COPD (1,2). Pulmonary vascular remodeling occurs
in the early stages of COPD (3)
and correlates with airway and chronic lung inflammation (4). Therefore, early anti-inflammatory
therapy will not only control airway inflammation, but is also
important for the prevention of pulmonary vascular remodeling and
secondary pulmonary hypertension in COPD. Additional studies and
implementation of therapies may improve the recovery rate in COPD
patients.
High-mobility group protein B1 (HMGB1) is an
important non-histone molecule in the eukaryotic nucleus. HMGB1 is
involved in regulation of gene expression and has a number of
ecto-nuclear biological functions. Moreover, it is closely
associated with differentiation, migration, proliferation and
apoptosis of cells, as well as induction of inflammation (5). In addition, HMGB1 functions as a
cytokine (6) and is secreted into
the cytoplasm and outside the cell. HMGB1 and important
inflammatory factors, including interleukin-l (IL-1), IL-6 and
tumor necrosis factor-α (TNF-α), induce one another. In the COPD
process, HMGB1 is involved in airway inflammation and remodeling.
These processes may be mediated by a series of inflammatory
factors, including nuclear factor-κB (NF-κB), vascular endothelial
growth factor (VEGF), TNF-α, monocyte chemotactic protein (MCP)-1,
IL-8 and IL-1β and a final product of receptor for advanced
glycation endproducts (RAGE) (7).
The present study used a COPD rat model to observe the early
effects of the NF-κB inhibitor, pyrrolidine dithiocarbamate (PDTC),
on HMGB1 mRNA and protein expression in rats with COPD and
investigated the mechanisms of signal transduction associated with
this process.
Materials and methods
Animal grouping and modeling
The current study was performed in strict accordance
with the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The animal use protocol was reviewed
and approved by the Institutional Animal Care and Use Committee of
the Affiliated Hospital of Guilin Medical College. A total of 48
male Sprague Dawley rats of specific pathogen-free grade with body
weights ranging between 180 and 220 g were purchased from the
Animal Experimental Center of Guilin Medical College. The test
groups were as follows: group A (normal control); B (COPD); and C
(COPD complicated with hypoxia). The drug intervention groups were
as follows: group A1 (blank control); B1 (COPD intervention); and
C1 (COPD complicated with hypoxia intervention).
Rats were randomly divided into 6 groups, with 6
rats in each group. Groups were set as test or drug intervention.
The test group included groups A, B and C. Rats in group A (normal
control) were bred normally for 6 weeks and then examined. For
group B (COPD), 200 μg LPS (Sigma-Aldrich, St. Louis, MO, USA) was
administered to the airways of the rats on days 1 and 14. On other
days, continuous fresh cigarette smoke (Liuzhou Cigarette Factory,
Guangxi, China) was administered for 1 h/day for 6 weeks.
Cigarettes were lit following placement of rats within the cage and
placed through a small hole on the wall that was connected to the
outside. A smoke exhaust fan was used to control gas flow and
maintain normal pressure. In addition, anhydrous calcium chloride
and calx sodica were placed in the cage. For group C, 200 μg LPS
was administered to the airways of the rats on days 1 and 14. On
other days, smoke fuming was performed for 1 h/day for 6 weeks. In
addition, continuous hypoxia was administered for 8 h/day on weeks
5 and 6 (nitrogen was used to adjust the oxygen concentration to
18% and the oxygen concentration was continuously monitored). The
drug intervention group included groups B1 and C1. The model
preparation methods for groups B1 and C1 corresponded to groups B
and C, respectively. For drug intervention groups, 100 mg/kg/day
(Sigma-Aldrich) PDTC was administered via intraperitoneal injection
every day from day 15. Group Al (blank control) received a dose of
normal saline equivalent to PDTC by intraperitoneal injection every
day from day 15.
Model evaluation
Pathological specimen preparation was performed as
follows: i) middle lobes of the right lungs of the rats were
removed; ii) 10% neutral formalin was injected into the lungs until
the middle lobe of the right lung had swelled completely; iii) the
hilum of the lung was ligated; iv) the middle lobes were soaked in
10% neutral formalin and fixed for 24 h; v) the specimen was sliced
continuously 2–3 mm to the right of the middle lobe; vi)
conventional gradient alcohol dehydration, paraffin embedding and
slicing were performed; and vii) alterations in the airway and
pulmonary alveoli were observed following conventional HE
staining.
Reverse transcription polymerase chain
reaction (RT-PCR)
For the extraction of total RNA, the anterior and
posterior lobes of the right lungs of the rats were homogenized.
Total RNA was extracted using RNAiso PLUS total RNA extraction
reagent according to the manufacturer’s instructions (Takara
Biotechnology Co., Ltd, Dalian, China) and stored at −80°C. RNA (1
μg) was reverse transcribed using PrimeScript RT reagent kit
(Takara Biotechnology) to synthesize cDNA. HMGB1 primer sequences
were as follows: upstream 5′-AGT TCA AGG ACC CCA ATG-3′ and
downstream 5′-TGC TCT TCT CAG CCT TGA CCA-3′. The amplification
fragment size was 285 bp. The β-actin primer sequences were as
follows: upstream 5′-CCC ATC TAT GAG TAC GC-3′ and downstream
5′-TTT AAT GTC ACG CAC GAT TTC-3′. The amplification fragment size
was 150 bp. HMGB1 primers were synthesized by Shanghai Yingweijieji
Biotechnology Co., Ltd. (Shanghai, China). PCR conditions were as
follows: pre-denaturation for 2 min at 94°C; 30 cycles of
denaturation for 30 sec at 94°C, annealing for 30 sec at 58°C and
extension for 30 sec at 72°C; and a final extension for 2 min at
72°C. Subsequently, PCR products were electrophoresed on a 2%
agarose gel. Following electrophoresis, a gel imaging analysis
system was used to determine optical density values of HMGB1 and
the housekeeping gene β-actin. The ratio of the optical density
values of HMGB1 to the optical density value of β-actin was used to
calculate relative HMGB1 mRNA.
Western blot analysis
Western blot analysis was used to determine HMGB1
and NF-κB (p65) protein expression levels in lung tissue. Total
protein in lung tissues was extracted according to the
manufacturer’s instructions (Jiangsu Beyotime Institute of
Biotechnology, China). A BSA kit (Jiangsu Beyotime Institute of
Biotechnology) containing protein standard (5 mg/ml BSA), BCA
reagent A and BCA reagent B was used to determine total protein
concentration. Following this, 40 μg each protein was separated by
electrophoresis on a 15% SDS-PAGE gel. A semi-dry transfer method
was used to transfer proteins onto a membrane, which was then
blocked in 5% dried skimmed milk for 1 h at room temperature.
Primary HMGB, NF-κB antibodies were diluted to 1:1000, and the
blocked membranes were placed in the diluted primary antibodies and
kept at room temperature for 2 h, then removed. The membrane was
then washed three times in TBST for 10 min. Next, the second
antibody (Beijing Zhongshan Company, Beijing, China) was added and
the membrane was incubated for 1 h at room temperature and washed
adequately with TBST. To visualize antibody binding, ECL
luminescence reagent development and X-ray film exposure were
performed in the dark. The Sensi Ansys gel image analysis system
(Shanghai Peiqing, China) was used to capture images of the
membrane and the Sensi Ansys gel imaging analysis software was used
to analyze the films. Ratios of the HMGB1 and NF-κB values to the
β-actin values were used to calculate relative expression levels.
Ratios of the intervention group value to the test group values
were used to conduct comparison and analysis.
Statistical analysis
SPSS 17.0 statistical software was used to analyze
data. One-way ANOVA was used for statistical analysis between
groups. The SNK (homogeneous variance) or Games-Howell method
(inhomogeneous variance) was used to compare two groups with normal
distributions. P<0.05 was considered to indicate a statistically
significant difference.
Results
Pathological changes
Pathological analysis revealed that groups A and A1
exhibited normal airway structures, complete tube walls and no
inflammatory cell infiltration. In group B, inflammatory cell
infiltration was observed in airway tube walls, the airway
epithelium revealed cell proliferation, emphysema was apparent and
lung bullae had formed. These pathological changes were consistent
with COPD. In group C, the airway tube walls had inflammatory cell
infiltration, smooth muscle proliferation and the local muscularis
and lung bullae wall were fractured. These pathological changes
were consistent with COPD complicated with hypoxia. Pathological
results in group Bl were improved compared with B. In group B1,
inflammatory cell infiltration was reduced and an improvement was
identified in terms of emphysema and lung bullae. Inflammatory
infiltration, muscularis fracture and lung bullae wall fracture
were all reduced in group C1 compared with C.
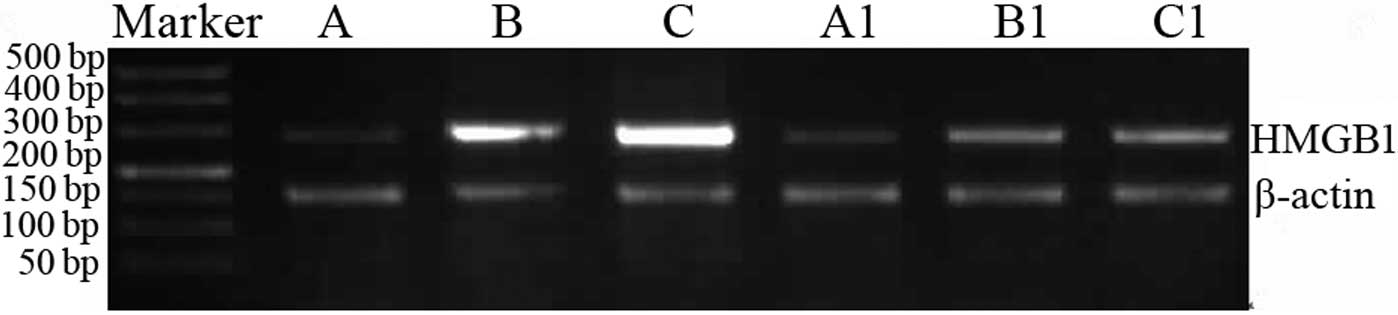
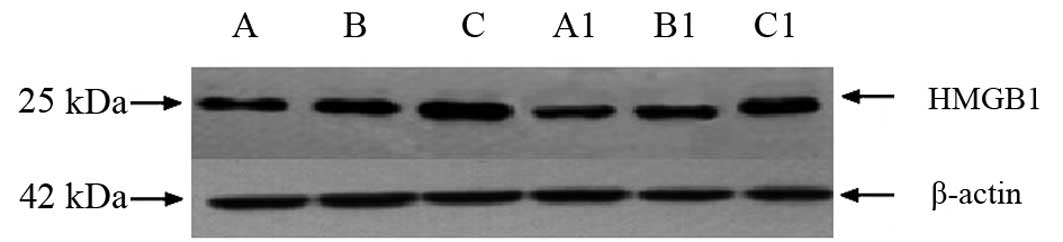
HMGB1 mRNA and protein contents
HMGB1 mRNA and protein expression in groups B and C
was revealed to be significantly increased compared with group A
(P<0.05). In addition, HMGB1 mRNA and protein expression in
groups B1 and C1 was identified to be significantly reduced
compared with groups B and C (Table
I and Figs. 1 and 2).
 | Table IComparison of HMGB1 mRNA, HMGB1
expression in lung tissue between different groups. |
Table I
Comparison of HMGB1 mRNA, HMGB1
expression in lung tissue between different groups.
| Group | Rats | HMGB1
mRNA/β-actin | HMGB1
protein/β-actin | NF-κB(p65)
protein/β-actin |
|---|
| A | 6 | 0.378±0.184 | 0.584±0.198 | 0.368±0.093 |
| B | 6 | 2.551±0.039a | 1.341±0.187a | 1.251±0.088a |
| C | 6 | 4.07±0.420a | 1.563±0.168a | 1.600±0.044a |
| A1 | 6 | 0.437±0.108 | 0.681±0.192 | 0.392±0.123 |
| B1 | 6 | 1.282±0.703b | 0.876±0.455b | 0.935±0.072b |
| C1 | 6 | 1.508±0.231b | 0.910±0.210b | 1.022±0.111b |
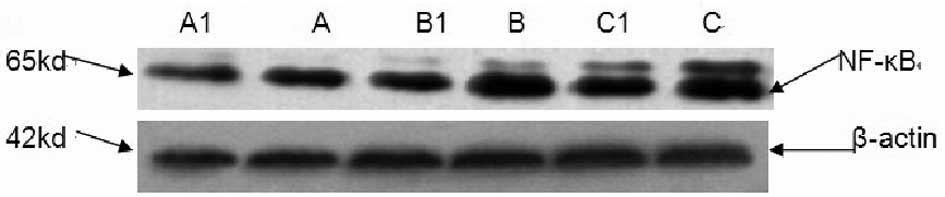
NF-κB protein content
NF-κB levels were low in rat lung tissue under
normal conditions. NF-κB expression in lung tissues in groups B and
C was revealed to be significantly increased compared with group A
(P<0.05). Protein expression in groups B1 and C1 was identified
to be significantly reduced compared with B and C. NF-κB expression
levels are presented in Table I
and Fig. 3.
Correlation analysis
The correlation between NF-κB protein and the HMGB1
mRNA and protein expression levels in rat lung tissue was analyzed
in various rat groups. NF-κB protein expression levels were
positively correlated with HMGB1 mRNA and protein levels in rat
lung tissue (r values were 0.918 and 0.921 for HMGB1 mRNA and
protein, respectively; both P<0.05).
Discussion
COPD is a chronic disease characterized by airflow
restriction. The disease is associated with an abnormal pulmonary
inflammatory response to hazardous gases, including gases generated
by smoking, in smog or hazardous particles. However, the
pathogenesis of COPD remains unclear. COPD is characterized by
chronic inflammation of the central and peripheral airways,
pulmonary parenchyma and vasa publica system (8). The role of chronic inflammation and
the systemic inflammatory reaction in the process of COPD onset is
an important area of research (9,10).
Smoking and infection are major causes of human COPD. Previous
studies have reported that 18% of hypoxia cases develop into
chronic bronchitis. In addition, rats with emphysema have been
identified to tolerate appropriate concentrations of hypoxia. In
the present study, rat models of COPD and COPD complicated with
hypoxia models were developed by combining three pathological
factors, smoking, infection and hypoxia. Pathological changes in
group B were consistent with changes associated with COPD lesions
and changes observed in group C were consistent with COPD
complicated with hypoxia. In addition, airways and pulmonary
alveoli following PDTC treatment were observed to be improved
compared with the corresponding test groups.
HMGB1 is a cytokine associated with a marked
pro-inflammatory effect and has a number of biological functions.
The cytokine is secreted into the cytoplasm or outside the cell to
induce cell differentiation and generate chemotaxis. HMGB1 is an
important inflammatory factor (11), regulating the occurrence,
development and sequelae of the inflammatory reaction due to its
delayed release and long-term functions (12). It is expressed at low levels in
human and rat airways under normal conditions and is involved in
normal maintenance of the respiratory system. In addition, HMGB1
maintains airway inflammation and remodeling in COPD through IL-1β
and RAGE (7). HMGB1 mRNA and
protein expression in the lung tissues of COPD and COPD complicated
with hypoxia groups was increased compared with that of the control
as smoking induces pulmonary inflammation and oxidative stress in
rats, causing an increase in inflammatory factors and cell stress.
These inflammatory factors and cell stress induce inflammatory
cells, including monocytes and macrophages, to secrete HMGB1
(13). Simultaneously, HMGB1
stimulates inflammatory cells, including macrophages and monocytes,
to release multiple inflammatory factors.
Previously, the HMGB1 gene was identified to contain
functional NF-κB binding sites (14). In addition, HMGB1 promotes the
phosphorylation of p38 mitogen-activated protein kinases (MAPK) and
activates NF-κB through RAGE. HMGB1 also increases expression of
inflammatory cytokines (15). The
chemotactic response mechanism of the HMGB1 inflammatory cell
depends on the co-regulation effects of IκB kinase (IKK)-α and
IKK-β on the NF-κB signal (14,16),
indicating that NF-κB indirectly regulates the HMGB1 transcription
process by regulating cytokines (17). PDTC has a specific inhibitory
effect on NF-κB (18–20). In this study NF-κB expression in
rat lung tissue positively correlates with HMGB1 expression. In the
current study, HMGB1 mRNA and protein expression in lung tissues of
various treatment groups was reduced significantly in the presence
of the specific NF-κB inhibitor, PDTC, compared with those of the
test control groups. These observations indicate that HMGB1 gene
expression was downregulated following inhibition of the NF-κB
signaling pathway. HMGB1 is known to be associated with various
inflammatory mediators, including NF-κB. Therefore, inhibition of
the NF-κB signal pathway may block the positive feedback loop of
HMGB1 and early inflammation mediators. Inhibition of this pathway
may relieve pulmonary inflammation and should be investigated
further as a possible therapeutic for the early prevention of
COPD.
In conclusion, the present study demonstrates that
NF-κB may regulate HMGB1 expression. Inhibition of the NF-κB
pathway led to downregulation of the expression of the inflammatory
mediator HMGB1 in early stages of COPD and may relieve tissue
inflammation and delay disease progression. It is currently unclear
whether HMGB1 is involved in the occurrence and development of COPD
through other signal transduction pathways and therefore additional
studies should be performed to develop this hypothesis.
Acknowledgements
The present study was supported by the Foundation of
Health Department (Guangxi no. 200977), Guangxi Science and
Technology Bureau (no. 0679012) and Guilin City (no. [2007]0512).
The authors thank Qian Lv and Xiaoli Liu.
References
|
1
|
Barbera JA, Peinado VI and Santos S:
Pulmonary hypertension in chronic obstructive pulmonary disease.
Eur Respir J. 21:892–905. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dorfmuller P, Perros F, Balabanian K and
Humbert M: Inflammation in pulmonary arterial hypertension. Eur
Respir J. 22:358–363. 2003. View Article : Google Scholar
|
|
3
|
Weitzenblum E, Chaouat A, Canuet M and
Kessler R: Pulmonary hypertension in chronic obstructive pulmonary
disease and interstitial lung diseases. Semin Respir Crit Care Med.
30:458–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barbera JA and Blanco I: Pulmonary
hypertension in patients chronic obstructive pulmonary disease
advances in pathophysiology and management. Drugs. 69:1153–1171.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang H, Yang H and Tracey KJ:
Extracellular role of HMGB1 in inflammation and sepsis. J Intern
Med. 255:320–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grasser M, Lentz A, Lichota J, Merkle T
and Grasser KD: The Arabidopsis genome encodes structurally
and functionally diverse HMGB-type proteins. J Mol Biol.
358:654–664. 2006.
|
|
7
|
Ferhani N, Letuve S, Kozhich A, et al:
Expression of high-mobility group box 1 and of receptor for
advanced glycation end products in chronic obstructive pulmonary
disease. Am J Respir Crit Care Med. 181:917–927. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rabe KF, Hurd S, Anzueto A, et al: Global
strategy for the diagnosis, management and prevention of chronic
obstructive pulmonary disease: GOLD executive summary. Am J Respir
Crit Care Med. 176:532–555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wouters EF, Groenewegen KH, Dentener MA
and Vernooy JH: Systemic inflammation in exacerbation of COPD.
Thorac Soc. 4:626–634. 2007. View Article : Google Scholar
|
|
10
|
Gea J, Barreiro E and Orozco-Levi M:
Systemic inflammation in COPD. Clin Pulm Med. 16:233–242. 2009.
View Article : Google Scholar
|
|
11
|
Yamada S and Maruyama I: HMGB1, a novel
inflammatory cytokine. Clin Chim Acta. 375:36–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Štros M, Bačíková A, Polanská E, Štokrová
J and Strauss F: HMGB1 interacts with human to poisomerase alpha
and stimulates its catalytic activity. Nucleic Acids Res.
35:5001–5013. 2007.
|
|
13
|
Ferhani N, Letuve S, Kozhich A, et al:
Expression of high-mobility group box 1 and of receptor for
advanced glycation end products in chronic obstructive pulmonary
disease. Am J Respir and Crit Care Med. 181:917–927. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Penzo M, Molteni R, Suda T, et al:
Inhibitor of NF-kappa B kinases alpha and beta are both essential
for high mobility group box 1-mediated chemotaxis. J Immunol.
184:4497–4509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palumbo R, Galvez BG, Pusterla T, et al:
Cells migrating to sites of tissue damage in response to the danger
signal HMGB1 require NF-kappa B activation. J Cell Biol. 179:33–40.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qin YH, Dai SM, Tang GS, et al: HMGB1
enhances the proinflammatory activity of lipopolysaccharide by
promoting the phosphorylation of MAPK p38 through receptor for
advanced glycation end products. J Immunol. 183:6244–6250. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kew RR, Penzo M, Habiel DM and Marcu KB:
The IKKα-dependent NF-κB p52/RelB noncanonical pathway is essential
to sustain a CXCL12 autocrine loop in cells migrating in response
to HMGB1. J Immunol. 188:2380–2386. 2012.
|
|
18
|
Frenette PS, Mayadas TN, Rayburn H, Hynes
RO and Wagner DD: Susceptibility to infection and altered
hematopoiesis in mice deficient in both P-and E-selectin. Cell.
84:563–574. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naka Y, Toda K, Kayano K, Oz MC and Pinsky
DJ: Failure to express the P-selectin gene or P-selectin blockade
confers early pulmonary protection after lung ischemia or
transplantation. Proc Natl Acda Sci USA. 94:757–761. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bruck R, Schey R, Aeed H, Hochman A,
Genina O and Pines M: A protective effect of pyrrolidine
dithiocarbamate in a rat model of liver cirrhosis. Liver Int.
24:169–176. 2004. View Article : Google Scholar : PubMed/NCBI
|