Introduction
Acute necrotizing pancreatitis (ANP) is a disease
with severe complications and high mortality, despite treatment
(1). Patients with ANP may suffer
from pancreatitis-associated respiratory dysfunction and
gastrointestinal mucosal lesions. Respiratory dysfunction,
presenting as acute lung injury (ALI) or acute respiratory distress
syndrome (ARDS), is frequent in acute pancreatitis and is a major
component of multiple organ dysfunction syndrome (MODS).
Approximately one-third of all mortalities from acute pancreatitis
are reported to occur prior to admission to hospital and, in the
majority of cases, are associated with ALI (2). As a consequence of severe acute
pancreatitis at the early stage, intestinal barrier failure also
contributes to the progression of pancreatitis. It has been
reported that an early increase in intestinal permeability occurs
in patients with severe acute pancreatitis and correlates with the
occurrence of endotoxemia, MODS and mortality (3).
The regenerating gene (Reg) is a multi-gene family
in humans that is involved in tissue regeneration. Okamoto
classified the members of the Reg family and Reg-related genes from
human, rat and mouse into three subclasses, types I, II and III
(4). Regenerating gene I (Reg I)
is mainly expressed in the pancreas and the gastrointestinal tract
and is involved in the pathophysiology of gastritis, pancreatitis,
cancer, inflammatory bowel disease and type 1 diabetes (5–7).
Previous studies have revealed that Reg I is important in acute
pancreatitis due to its involvement in the regeneration and
recovery from the pancreatic injury (8). Viterbo et al revealed a
protective role of Reg I in pancreatitis by the administration of
anti-Reg I antibody to neutralize the pancreatic Reg protein or
siRNA to knockout the Reg gene in animal models (9,10).
However, studies concerning the expression of Reg I in
pancreatitis-induced lung injury and intestinal injury following
ANP are lacking and the correlation between Reg I and the severity
of the disease remains unclear.
Materials and methods
Induction of ANP
A total of 90 male Sprague-Dawley rats, weighing
230–270 g, were randomly divided into 2 groups: an ANP group (n=60)
and a control group (n=30). The animals were anesthetized with 10
mg/ml ketamine at a dose of 10 mg/100 g body weight injected
intraperitoneally. ANP was induced by the retrograde injection of
3% sodium taurocholate into the pancreatic duct. The abdominal
cavity was then entered through a midline incision. After
identifying the duodenum and pancreas, the common bile duct was
ligated at the position of the hepatic hilum. A duodenotomy was
performed ~1 cm distal to the opening of the biliopancreatic duct
into the duodenum and a polyethylene catheter was gently cannulated
into the pancreatic duct via the duodenotomy incision. Sodium
taurocholate solution (3%) was slowly injected at a constant rate
of 0.5 ml/min. The catheter was removed after being kept in place
for 30 min. The rats in the control group underwent sham surgery
(open laparotomy with immediate closure). All animal experiments
were evaluated and approved by the Animal and Ethics Review
Committee of the Southeast University.
Morphological observation and
histopathological assessment
Fresh tissue was collected and fixed in 10%
formaldehyde for 12 h, dehydrated in a conventional manner,
embedded in paraffin, cut into slices and stained with hematoxylin
and eosin (H&E). The morphological observation and histological
assessment were conducted by an experienced pathologist, who was
unaware of the sample identity, using previously described criteria
(11,12).
Harvesting of sample
A total of 20 animals were sacrificed at each time
point (12, 24 and 36 h after the induction of ANP). Ten animals per
group (sham operated) were used as the controls. Samples of the
pancreas, lung and terminal ileum were harvested and a blood sample
was collected from the hepatic portal vein of each animal at the
time of pancreatectomy.
Lung wet/dry weights measurement
To determine the lung wet/dry weight ratio, the
whole left lung, lobes or segments of the peripheral lung were
weighed after initial removal and after drying in an oven at a
constant temperature of 160°C for 48 h.
Intestinal permeability
determination
Prior to the closure of the midline incision, 3.7
MBq technetium-99m diethylenetriaminepentacetic acid
(99mTc-DTPA) was slowly injected into the apical portion
of the jejunum of the rats in the ANP group. Urine was collected
12, 24 and 36 h after the ingestion of the tracer. The
radioactivity of the tracer was detected and the urinary excretion
rate of 99mTc-DTPA was calculated according to the
following equation: urinary excretion rate of 99mTc-DTPA
= [(urinary count-background) × urinary volume/(standard
count-background) × 1000] × 100 (13).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the frozen tissue using
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s instructions and the integrity of the RNA was
confirmed by 1% formaldehyde-agarose gel electrophoresis. The
synthesis of cDNA was conducted using an RT-PCR kit (Takara Bio,
Inc., Shiga, Japan) following the recommendations of the
manufacturer. The primers were designed using Primer premier
software. The primer sequences for the Reg I gene were: upstream:
5′-GCCAGGAGGCTGAAGAAG-3′ and downstream: 5′-CCAGTGTCCCAGGATTTG-3′.
Moreover, the primer sequences for endogenous control GAPDH were:
upstream: 5′-GTTCAACGGCACAGTCAA-3′ and downstream:
5′-CCTCAGTGTAGCCCAGGAT-3′. All primers were synthesized by
Invitrogen Life Technologies. PCR was carried out in a 25-μl
reaction mixture with 3 μl cDNA reaction product as the template
mixed with 22 μl PCR mixture (including 25 mM MgCl2, 5
U/μl Taq polymerase and 1.5 μl of each primer). The thermal
cycling process began with an initial denaturing step at 94°C for 2
min followed by a denaturing step of 30 sec, and annealing at 54°C
for another 30 sec. The synthesis of new DNA was initiated with an
extension step lasting 1 min as the reaction temperature was raised
to 72°C. The next cycle began with a return to 95°C for
denaturation. The process ran for 32 cycles and involved a final
step at 72°C for 7 min. After amplification, the RT-PCR products
were submitted to electrophoresis on 1.5% agarose gel (100 V, 40
min). All PCR assays were conducted in triplicate and the results
were semi-quantitated as the ratio amplicon/GAPDH and presented as
the mean ± SD.
Histological scoring
The histopathological score was evaluated based on
the severity of injury in the pancreas, lung and intestine and used
grading scale criteria. In the pancreas, according to the degree of
edema, acinar necrosis, fat necrosis and perivascular infiltrate,
the score varied from 0 to 4 and then the total score was obtained.
Six parameters including hyaline membranes, microthrombi, edema,
interstitial infiltrates, atelectasis and hemorrhage were taken
into account in the microscopic observation of lung tissue. A score
of 0 represents normal tissue, 1 shows discrete or small foci, 2
indicates moderate or large foci and 3 indicates severe or diffuse
lesions. In the intestine, grade 0 represents normal mucosa and
grades 1 to 5 indicate increasing damage of villi. Grades 6 to 8
represent crypt layer infarction to transmural infarction. A low
score indicates a mild lesion, and a high score depicts serious
damage. The mean was calculated from the sum of the respective
scores.
Statistical analysis
Statistical analysis was achieved using SPSS
software and comparisons between groups were made using an unpaired
Student’s t-test. The correlation between Reg I and each
histological score was assessed using the Pearson correlation test.
Data are expressed as the mean ± SD and P<0.05 was considered to
indicate a statistically significant result.
Results
Serum amylase activity and histological
evaluation of the pancreas
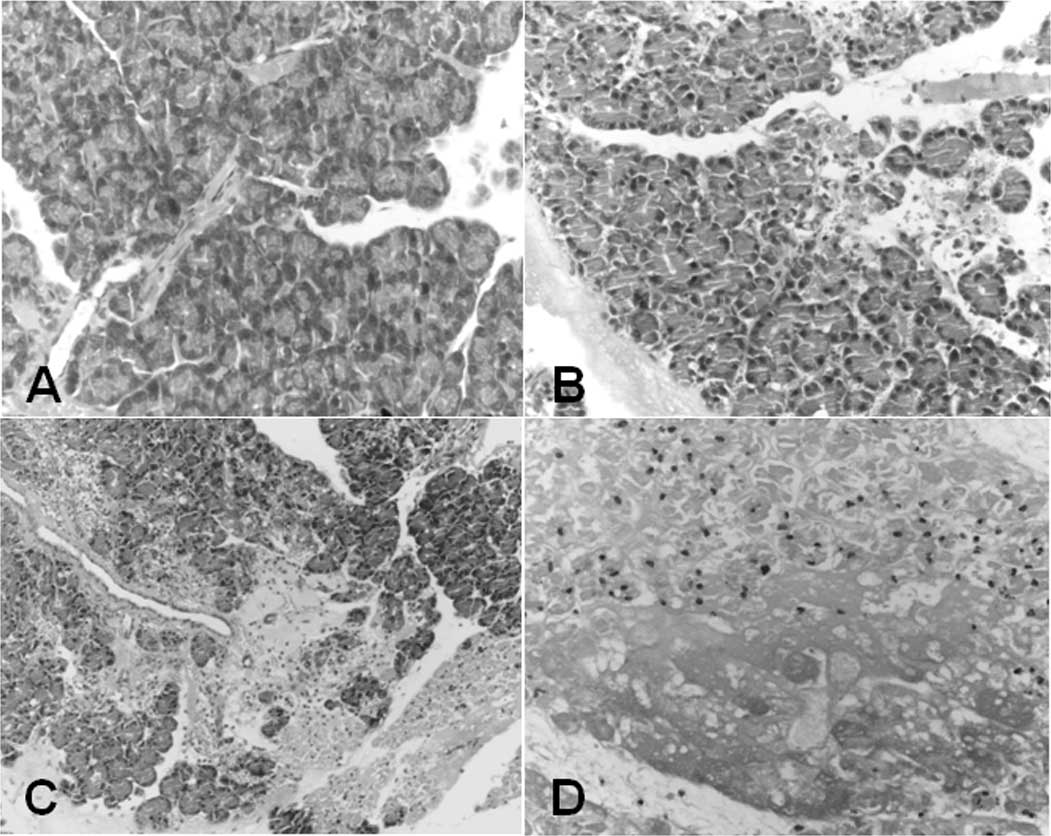
The presence of ANP in the taurocholate-induced
animals was confirmed by serum amylase activity and histology
(Table I and Fig. 1). As demonstrated in Table I, the levels of circulating amylase
in the ANP group were significantly higher than those in the
sham-operated animals 12, 24 and 36 h after surgery (P<0.05).
Bloody ascites in the abdominal cavity, pancreatic bleeding spots
and necrosis were observed when harvesting the samples from the ANP
animals. The histological worsening of the pancreatitis in the
pancreatic parenchyma following sodium taurocholate treatment was
evidenced by hemorrhage (Fig. 1B and
C) and necrosis (Fig. 1D) as
compared with the control (Fig.
1A). The histological scoring of the pancreas was conducted
according to the criteria previously described by Schmidt et
al(14). As shown in Table II, the histological scores for the
pancreas 12, 24 and 36 h after injection in the ANP group were
significantly higher than those in the controls (8.2±1.40,
10.1±1.80, 11.3±1.81 vs. 0.5±0.53, 0.4±0.52, 0.6±0.7, respectively,
P<0.01).
 | Table ISerum amylase activity (U/l). |
Table I
Serum amylase activity (U/l).
| Group | 12 h | 24 h | 36 h |
|---|
| Control | 1,004±94.7 | 1,405±84.5 | 1,134±146.1. |
| ANP | 3,793±185.9a | 5,548±191.5a | 7,071±135.4a |
 | Table IIHistological scores of the
pancreas. |
Table II
Histological scores of the
pancreas.
| Group | 12 h | 24 h | 36 h |
|---|
| Control | 0.5±0.53 | 0.4±0.52 | 0.6±0.7. |
| ANP | 8.2±1.40a | 10.1±1.80a | 11.3±1.81a |
Histological evaluation of pulmonary
injury
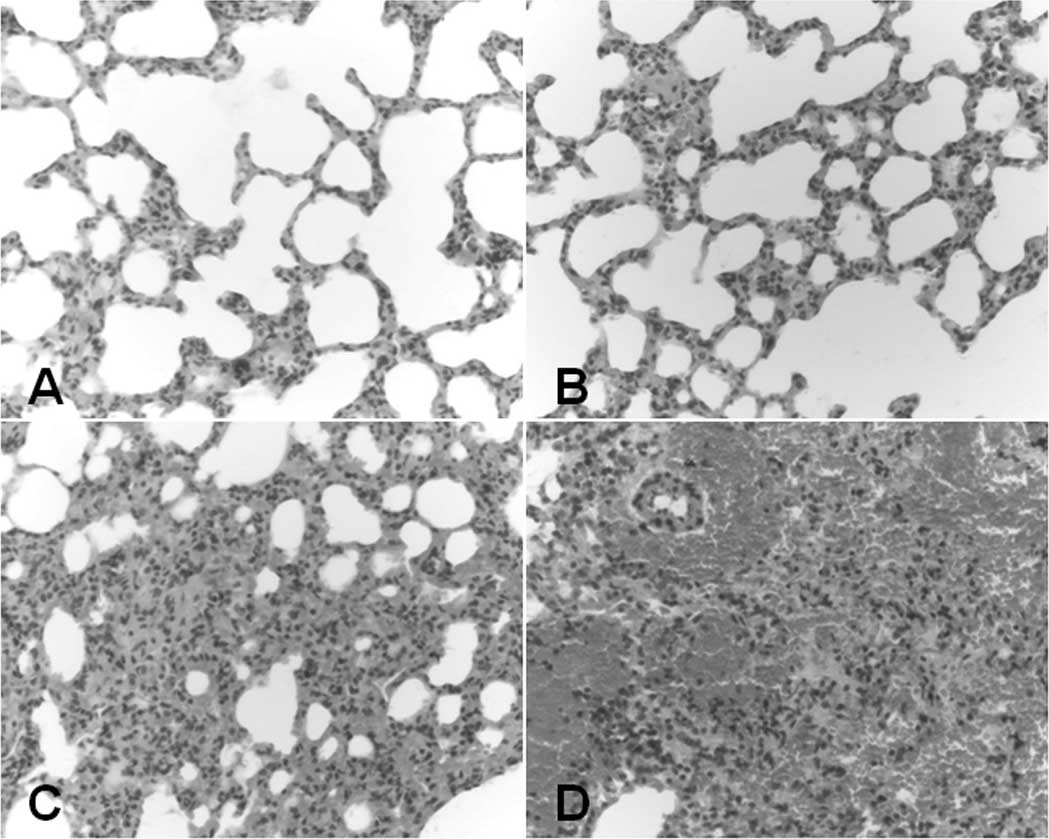
As shown in Fig. 2,
the pulmonary injury induced by ANP was characterized by thickening
of the alveolar wall, pulmonary edema (Fig. 2B), infiltration of neutrophils and
hemorrhage (Fig. 2C and D)
compared with the controls (Fig.
2A). Disruption of the pulmonary alveolus followed by
consolidation and interstitial vasodilation was also observed 36 h
after injection (Fig. 2D). The
histological scores for lung injury were calculated according to
previously described criteria (11). As demonstrated in Table III, the histological scores for
pulmonary injury 12, 24 and 36 h after injection in the ANP group
were significantly higher than those in the controls (8.95±1.88,
12.05±2.11, 13.65±1.81 vs. 0.5±0.71, 0.3±0.48, 0.3±0.67,
respectively, P<0.01). The ratios of rat lung wet/dry weights
were calculated and are shown in Table IV. The ratios increased
significantly in the ANP animals 12, 24 and 36 h after injection
compared with those in the controls (5.28±0.13, 5.39±0.21,
5.47±0.21 vs. 4.47±0.16, 4.48±0.17, 4.47±0.17, respectively,
P<0.05.
 | Table IIIHistological scores of lung
injury. |
Table III
Histological scores of lung
injury.
| Group | 12 h | 24 h | 36 h |
|---|
| Control | 0.5±0.71 | 0.3±0.48 | 0.3±0.67 |
| ANP | 8.95±1.88a | 12.05±2.11a | 13.65±1.81a |
 | Table IVRatio of rat lung wet/dry weights. |
Table IV
Ratio of rat lung wet/dry weights.
| Group | 12 h | 24 h | 36 h |
|---|
| Control | 4.47±0.16. | 4.48±0.17. | 4.47±0.17. |
| ANP | 5.28±0.13a | 5.39±0.21a | 5.47±0.21a |
Histological evaluation of intestinal
injury
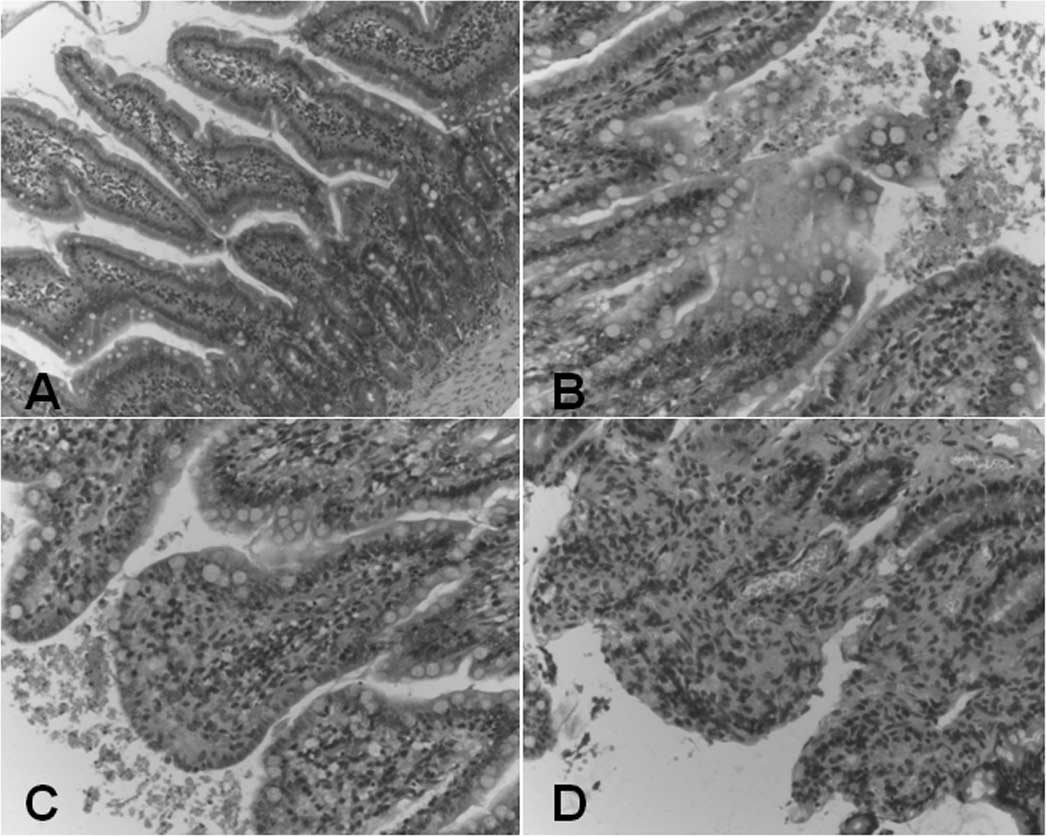
Pancreatitis-induced intestinal injury was mainly
manifested by mild hyperemia and edema in the intestinal mucosa
proper layer 12 h after surgery (Fig.
3B) and accelerated edema as well as focal necrosis were
observed 24 h after surgery (Fig.
3C). Massive inflammatory cell infiltration and exfoliation of
the mucosal epidermis microvilli were found 36 h after surgery
(Fig. 3D) compared with the
controls (Fig. 3A). The
histological scores for intestinal injury were calculated according
to previously described criteria (12). As demonstrated in Table V, the histological scores for
pancreatitis-associated intestinal injury 12, 24 and 36 h after the
induction of ANP were significantly higher than those in the
controls (1.8±0.89, 3.3±1.17, 4.2±0.95 vs. 0.2±0.42, 0.3±0.48,
0.3±0.48, respectively, P<0.01). The intestinal permeability was
assessed by measuring the 12-, 24- and 36-h urinary excretion rates
of ingested 99mTc-DTPA. As demonstrated in Table VI, the urinary excretion rate of
99mTc-DTPA was significantly elevated at each time-point
following the induction of ANP as compared with the controls
(34.70±4.03, 54.63±6.94, 66.83±7.56 vs. 4.62±1.17, 6.14±1.42,
7.48±0.92, respectively, P<0.01). The excretion rate of
99mTc-DTPA tended to increase as the time post-surgery
increased, indicating increased intestinal permeability.
 | Table VHistological scores of intestinal
injury. |
Table V
Histological scores of intestinal
injury.
| Group | 12 h | 24 h | 36 h |
|---|
| Control | 0.2±0.42. | 0.3±0.48. | 0.3±0.48. |
| ANP | 1.8±0.89a | 3.3±1.17a | 4.2±0.95a |
 | Table VI99mTc-DTPA excretion rate
(%). |
Table VI
99mTc-DTPA excretion rate
(%).
| Group | 12 h | 24 h | 36 h |
|---|
| Control | 4.62±1.17 | 6.14±1.42 | 7.48±0.92 |
| ANP | 34.70±4.03a | 54.63±6.94a | 66.83±7.56a |
Overproduction of Reg I in lung and
intestine
To determine whether Reg I mRNA is expressed in rat
lung and intestinal tissue, the total RNA was extracted and RT-PCR
was performed as described in Materials and methods. As shown in
Fig. 4, a PCR product of the
expected size (316 bp) was amplified with specific Reg I primers as
well as the endogenous control GAPDH with an expected size of 671
bp. There was a trend towards an increased expression of Reg I mRNA
in the lung or intestinal tissue as compared with the controls,
indicating that the overproduction of Reg I mRNA correlated with
the severity of the disease, while the expression of endogenous
control GAPDH was basically unchanged. All RT-PCR trials were
performed in triplicate and the intensity ratio of amplicon/GAPDH
was analyzed in a semi-quantitative manner.
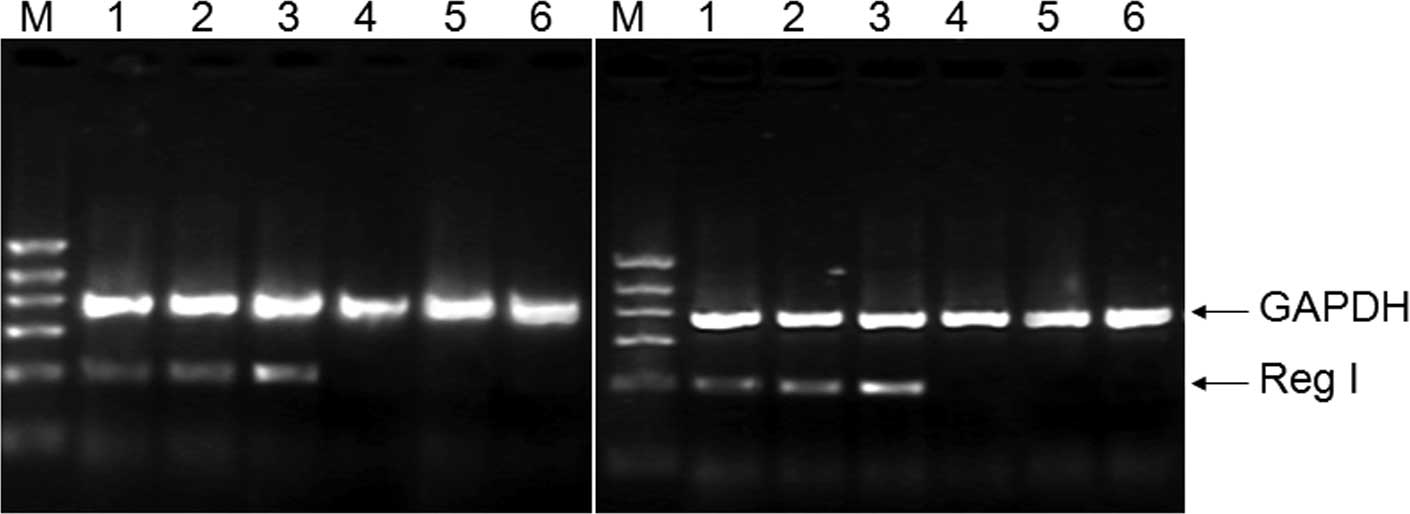 | Figure 4Expression of pancreatic regenerating
gene (Reg I) mRNA in rat lung and intestinal tissue. Lanes 1–3,
acute necrotizing pancreatitis (ANP) 12, 24 and 36 h post-surgery,
respectively; lanes 4–6, control, 12, 24 and 36 h post-surgery,
respectively; M, DNA marker (from top to bottom: 1100, 900, 700,
500, 300 and 100 bp). |
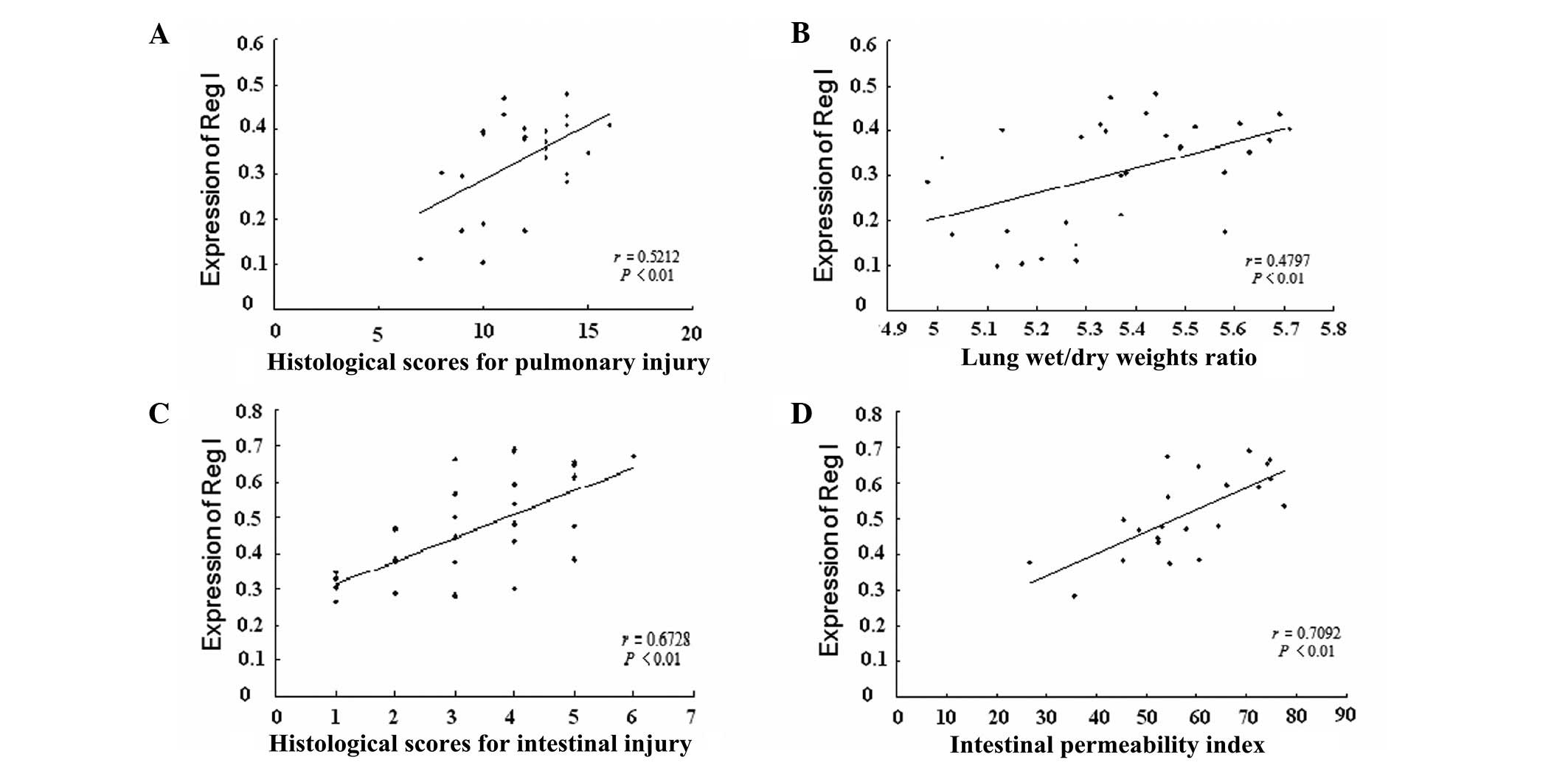
Correlation analysis
To determine whether the expression levels of Reg I
mRNA were correlated with the severity of the pancreatitis-induced
pulmonary and intestinal injury, correlation analysis was performed
between the Reg I mRNA expression levels and histological scores,
ratios of lung wet/dry weights and intestinal permeability indices,
respectively. A strong positive correlation between the expression
levels of Reg I mRNA in the lung tissue and the histological scores
of pulmonary injury was found, with a correlation coefficient of
0.5212 (Fig. 5A, r=0.5212,
P<0.01), implying that the upregulation of Reg I mRNA in lung
tissue is correlated with the severity of the pancreatitis-induced
pulmonary injury. Similarly, a positive correlation between the
expression levels of Reg I mRNA and the ratio of lung wet/dry
weights was also observed (Fig.
5B, r=0.4797, P<0.01). In addition, we also identified a
positive correlation between the Reg I mRNA expression levels in
intestinal tissue and the histological scores of intestinal injury
(Fig. 5C, r=0.6728, P<0.01) as
well as with the intestinal permeability index (Fig. 5D, r=0.7092, P<0.01), implying
that the overproduction of Reg I mRNA in intestinal tissue is
correlated with the severity of the pancreatitis-induced intestinal
injury.
Discussion
Since the discovery of Reg I, considerable attention
has focused on elucidating its role in the pathogenesis of
diseases. Reg I, originally identified as a regenerative growth
factor for rat pancreatic islet cells, has been reported to be
expressed in various organs, including the pancreas, stomach,
kidney and small intestine. It has been shown that Reg I fulfils a
role in cell growth that is required for the regeneration and
maintenance of tissues in these organs. Reg elevation has been
reported in conditions of inflammation and infection, and in
response to surgical stimuli (15). In the present study, we found that
the upregulation of Reg I mRNA in lung tissue has a positive
correlation with the severity of the ALI induced by ANP, which is
in accordance with a study in which PSP/reg was suggested to be a
biomarker related to organ failure and outcome in
ventilator-associated pneumonia (16). Although the mechanism of action of
Reg I in lung tissue is unknown, it is presumed that Reg I
functions as a pro-regenerating mediator in impaired tissue in
response to stress signals, possibly through the induction of the
MAPK p38 pathway due to the expression of Reg I receptors in
impaired lung tissue, based on similar findings in pancreatic
disease (8,17). The upregulation of Reg I in
impaired lung tissue may also be a consequence of stimulation by
inflammatory cytokines, including INF-γ and IL-6, as reported by
Sekikawa et al in early gastric cancer, and its protein
product may protect AGS cells from apoptosis (18). To the best of our knowledge, our
study is the first to show the expression of Reg I in lung tissue.
We also provide evidence of the correlation of Reg I mRNA
expression levels in lung and the severity of the disease, which
indicates that Reg I is a potent biomarker for the severity of
pancreatitis-associated ALI. However, the expression of Reg I in
impaired lung tissue and its trophic role require confirmation by
further study.
Gastrointestinal injury induced by pancreatitis also
contributes to the progress of disease and mortality. Deficiency in
the intestinal mucosal barrier may lead to an increase of
intestinal permeability followed by translocation of bacteria,
endotoxemia and secondary infection of the pancreatic tissue, and
then cause systemic inflammatory reponse syndrome (SIRS) or MODS.
In the present study, a strong positive correlation was found
between the expression of Reg I in intestinal tissue and
histological scores of intestinal injury (r=0.6728, P<0.01).
Since intestinal permeability strongly correlates with endotoxemia,
organ failure and mortality in patients with acute pancreatitis
(3), the correlation between Reg I
expression and intestinal permeability was tested in our study. A
positive correlation was identified (r=0.7092, P<0.01). A recent
study revealed a protective role of Reg I against NSAID-induced
small intestinal injuries in a Reg I-knockout mouse model and
additional recombinant Reg I effectively attenuated such injuries
(19). The results of a further
study support the role of Reg in the healing of gastrointestinal
mucosal lesions (20).
Furthermore, Reg I may also be effective against other small
intestinal injuries, including inflammatory bowel disease, since it
has been demonstrated that Reg I is upregulated and promotes cell
proliferation under other stress conditions (21). Consistent with these studies, we
demonstrated the upregulation of Reg I in injured intestinal tissue
and a strong positive correlation with the severity of the disease.
We postulate that Reg I protected against the intestinal injury
induced by ANP in our rat model.
In summary, we conclude that Reg I may be a potent
biomarker for the severity of pulmonary and intestinal injuries
induced by severe pancreatitis and pancreatitis per se while
its exact mechanism in the pathogenesis of these diseases requires
further investigation.
References
|
1
|
Hartwig W, Werner J, Müller CA, Uhl W and
Büchler MW: Surgical management of severe pancreatitis including
sterile necrosis. J Hepatobiliary Pancreat Surg. 9:429–435. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Appelros S and Borgström A: Incidence,
aetiology and mortality rate of acute pancreatitis over 10 years in
a defined urban population in Sweden. Br J Surg. 86:465–470.
1999.PubMed/NCBI
|
|
3
|
Ammori BJ, Leeder PC, King RF, Barclay GR,
Martin IG, Larvin M and McMahon MJ: Early increase in intestinal
permeability in patients with severe acute pancreatitis:
correlation with endotoxemia, organ failure, and mortality. J
Gastrointest Surg. 3:252–262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okamoto H: The Reg gene family and Reg
proteins: with special attention to the regeneration of pancreatic
beta-cells. J Hepatobiliary Pancreat Surg. 6:254–262. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang YW, Ding LS and Lai MD: Reg gene
family and human diseases. World J Gastroenterol. 9:2635–2641.
2003.PubMed/NCBI
|
|
6
|
Zheng HC, Sugawara A, Okamoto H, Takasawa
S, Takahashi H, Masuda S and Takano Y: Expression profile of the
REG gene family in colorectal carcinoma. J Histochem Cytochem.
59:106–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Planas R, Pujol-Autonell I, Ruiz E,
Montraveta M, Cabre E, Lucas-Martin A, Pujol-Borrell R,
Martinez-Caceres E and Vives-Pi M: Regenerating gene Iα is a
biomarker for diagnosis and monitoring of celiac disease: a
preliminary study. Transl Res. 158:140–145. 2011.
|
|
8
|
Bluth MH, Patel SA, Dieckgraefe BK,
Okamoto H and Zenilman ME: Pancreatic regenerating protein (reg I)
and reg I receptor mRNA are upregulated in rat pancreas after
induction of acute pancreatitis. World J Gastroenterol.
12:4511–4516. 2006.PubMed/NCBI
|
|
9
|
Lin YY, Viterbo D, Mueller CM, Stanek AE,
Smith-Norowitz T, Drew H, Wadgaonkar R, Zenilman ME and Bluth MH:
Small-interference RNA gene knockdown of pancreatitis-associated
proteins in rat acute pancreatitis. Pancreas. 36:402–410. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Viterbo D, Callender GE, DiMaio T, Mueller
CM, Smith-Norowitz T, Zenilman ME and Bluth MH: Administration of
anti-Reg I and anti-PAPII antibodies worsens pancreatitis. JOP.
10:15–23. 2009.PubMed/NCBI
|
|
11
|
Yekebas EF, Strate T, Zolmajd S, et al:
Impact of different modalities of continuous venovenous
hemofiltration on sepsis-induced alterations in experimental
pancreatitis. Kidney Int. 62:1806–1818. 2002. View Article : Google Scholar
|
|
12
|
Park PO, Haglund U, Bulkley GB and Fält K:
The sequence of development of intestinal tissue injury after
strangulation ischemia and reperfusion. Surgery. 107:574–580.
1990.PubMed/NCBI
|
|
13
|
Sun SL, Wu SD and Zhang XB: Oral
(99m)Tc-DTPA simultaneous determination of duodenobiliary reflux
and intestinal permeability in patients after choledocholithotomy
plus T-tube drainage. Hepatobiliary Pancreat Dis Int. 4:593–596.
2005.PubMed/NCBI
|
|
14
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bimmler D, Schiesser M, Perren A, Scheele
G, Angst E, Meili S, Ammann R and Graf R: Coordinate regulation of
PSP/reg and PAP isoforms as a family of secretory stress proteins
in an animal model of chronic pancreatitis. J Surg Res.
118:122–135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boeck L, Graf R, Eggimann P, et al:
Pancreatic stone protein: a marker of organ failure and outcome in
ventilator-associated pneumonia. Chest. 140:925–932. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zenilman ME, Zheng QH, Wu H and
Rengabashyam P: Pancreatic Reg and a conserved bioactive fragment
are mitogenic through the MAPK P38 pathway. Surg Forum. 191(Suppl
1): S292000.PubMed/NCBI
|
|
18
|
Sekikawa A, Fukui H, Fujii S, et al: REG
Ialpha protein may function as a trophic and/or anti-apoptotic
factor in the development of gastric cancer. Gastroenterology.
128:642–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Imaoka H, Ishihara S, Kazumori H, et al:
Exacerbation of indomethacin-induced small intestinal injuries in
Reg I-knockout mice. Am J Physiol Gastrointest Liver Physiol.
299:G311–G319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawanami C, Fukui H, Kinoshita Y, Nakata
H, Asahara M, Matsushima Y, Kishi K and Chiba T: Regenerating gene
expression in normal gastric mucosa and indomethacin-induced
mucosal lesions of the rat. J Gastroenterol. 32:12–18. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dieckgraefe BK, Crimmins DL, Landt V,
Houchen C, Anant S, Porche-Sorbet R and Ladenson JH: Expression of
the regenerating gene family in inflammatory bowel disease mucosa:
Reg Ialpha upregulation, processing, and antiapoptotic activity. J
Investig Med. 50:421–434. 2002. View Article : Google Scholar : PubMed/NCBI
|