Introduction
Multiple myeloma (MM) is a malignant clone of plasma
cells. The synthesis and secretion of monoclonal immunoglobulin
uniform structure and/or light chains alone, accompanied by a
reduction in normal immunoglobulin levels, is a feature of MM. The
activation of osteoclasts, upsetting the balance of osteoclasts and
osteoblasts, appearance of extensive osteolytic lesions and/or
malignant tumors and osteoporosis are also characteristic of MM
(1). Although high-dose
chemotherapy with autologous stem cell transplation and other
therapies of enhancing response and survival rates have improved,
due to the low proportion of tumor cells (typically less than 1.5%)
(2) and multidrug resistance of
tumor cells, MM treatment response is poor and the disease remains
incurable, ultimately leading to drug resistance and relapse.
Plants of the Strychnos genus have been used
to treat typhoid fever and sore throat, as described in the
‘Compendium of Materia Medica’. The ‘Chinese Medicine chi’ reported
that plants of the Strychnos genus can treat fever,
swelling, ulcers and sores. Deng et al investigated the
effect of Strychnos on HepG2 cells and found that the
Strychnos alkaloid treatment of liver cancer is due to its
direct cytotoxicity (3).
Strychnos alkaloids mostly consist of biologically active
substances, but also contain pharmacological and toxicological
components, of which 80% are strychnine and brucine (4). A previous study (5) found that brucine induces apoptosis
via the death receptor pathway in multiple myeloma. The present
study used reverse transcription polymerase chain reaction (RT-PCR)
and flow cytometry to analyze the apoptotic signaling pathway.
Materials and methods
Cell culture and cell proliferation
assay
U266 cells were maintained in RPMI-1640 culture
medium with 10% fetal calf serum, 100 mg/l penicillin and 100 mg/l
streptomycin, in a 37°C incubator supplied with 5% CO2
at room temperature. The anti-proliferative response of brucine was
assessed by MTT assay. U266 cells were plated at a final
concentration of 5×104 cells/ml in the presence or
absence of brucine (0, 0.05, 0.1, 0.2 and 0.4 mg/ml) in a 96-well
plate. Then 20 μl MTT (5 mg/ml) were added at 24, 48 and 72 h
following treatment. For the MTT assay, the supernatant was
discarded and 200 μl DMSO was added, and the 96-well plate was
agitated on a micro-vibrator for 10 min. The optical density of
each well was measured at λ492 nm by an enzyme-immunoassay
instrument.
Cell cycle analysis
U266 cells (2×106) were treated with or
without brucine (0, 0.05, 0.1, 0.2 and 0.4 mg/ml) for 48 h. The
cells were harvested, washed with PBS at 1,000 rpm for 5 min, fixed
with 70% alcohol, washed again with PBS and stained with propidium
iodide in the presence of 100 μl RNase A for 30 min prior to
analysis by flow cytometry.
RT-PCR
Total RNA was extracted using a TRIzol reagent. cDNA
was amplified from 6 μl of total RNA using ThermoScript RT-PCR
System with 1 μl oligo(dT)18 (0.5 μg/μl), 1 μl TransScript™ RT/RI
enzyme, 10 μl 2× TS Reaction mix, 2 μl RNase-free water, analyzed
on 2% agarose gel and confirmed by nucleotide sequencing. The
primer pairs used for RT-PCR were: caspase-3 (151 bp):
5′-TTTTTCAGAGGGGATCGTTG-3′ and 5′-CGGCCTCCACTGGTATTTTA-3′; c-Jun:
5′-CCCCAA GATCCTGAAACAGA-3′ and 5′-CCGTTGCTGGACT GGATTAT-3′; GADPH:
5′-TGAACGGGAAGCTCACTGG-3′ and 5′-TCCACCACCCTGTTGCTGGA-3′.
Statistical analysis
Data were expressed as the mean ± SD and analyzed
using SPSS software (SPSS Inc., Chicago, IL, USA). The statistical
methods involved two independent sample t-test and analysis of
variance (ANOVA). P<0.05 was considered to indicate a
statistically significant result.
Results
Growth inhibitory effects of brucine on
U266 cells
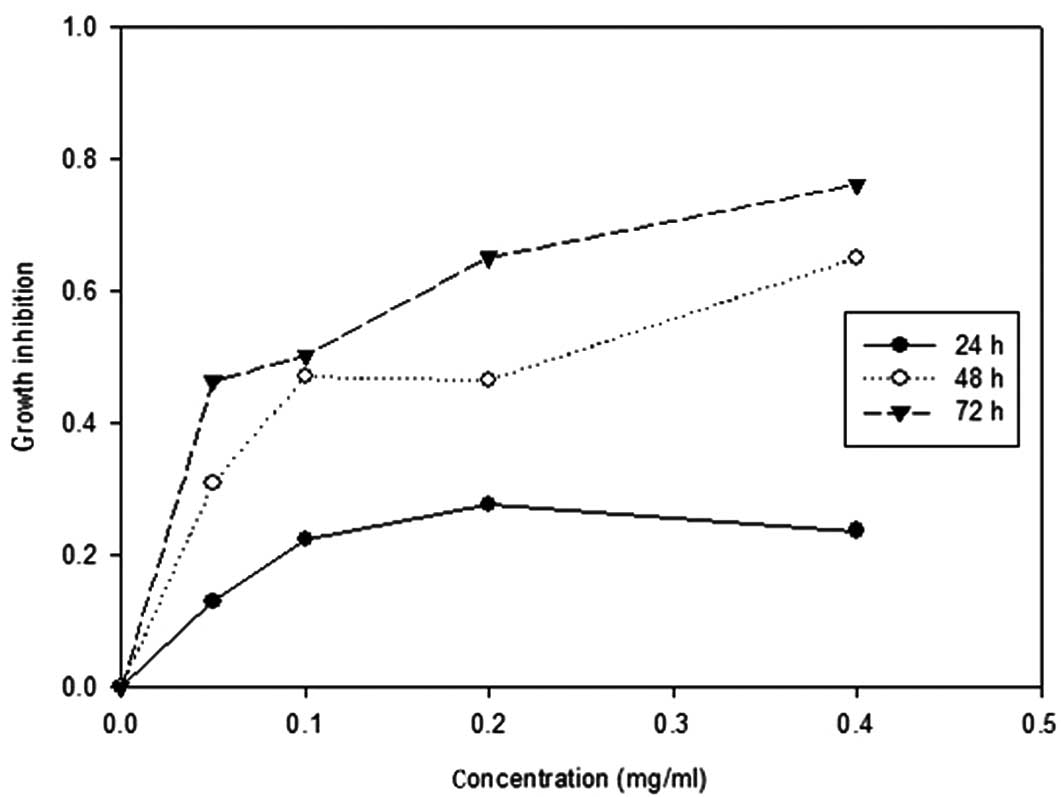
The effect of brucine on the proliferative response
of U266 cells was analyzed by treating cells with different
concentrations of brucine (0, 0.05, 0.1, 0.2 and 0.4 mg/ml) for 24,
48 and 72 h. The growth inhibitory effects of brucine treatment
were assessed by MTT assay. The treated cells showed a significant
decrease in proliferation in a dose- and time-dependent manner
(P<0.05; Fig. 1). The
IC50 value of brucine at 48 h was 0.16 mg/ml.
Cell cycle analysis
The appearance of the
sub-G0/G1 cell is characteristic of
apoptosis. The results of brucine treatment showed that the
sub-G0/G1 phase population significantly
increased following brucine treatment in a dose-dependent manner.
The sub-G0/G1 phase population increased to
4.137, 10.55, 12.31, 27.67 and 29.67% following the exposure of the
cells to 0, 0.05, 0.1, 0.2 and 0.4 mg/ml brucine concentrations,
respectively. This accumulation of cell population at the
sub-G0/G1 phase in a dose-dependent manner
indicated an induction of apoptosis.
Caspase-3 is activated in brucine-induced
U266 cell apoptosis
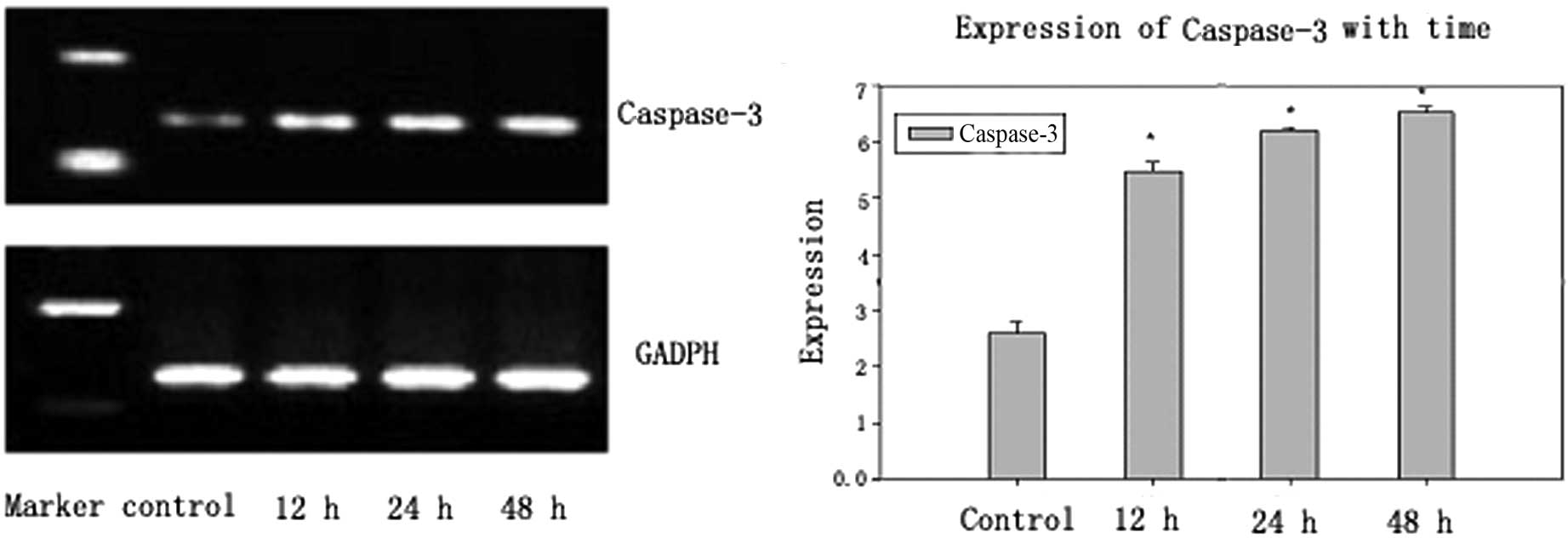
Since caspase-3 is an executor of apoptosis,
caspase-3 expression was measured in brucine-treated U266 cells by
RT-PCR after 12, 24 and 48 h. A brucine-induced time-dependent
increase of caspase-3 was detected (Fig. 2). The gray value of caspase-3 was
0.2597±0.020 in the control group and increased in the
brucine-treated (0.16 mg/ml) group to 0.5488±0.016, 0.6205±0.006
and 0.6533±0.009 on exposure of the cells at 12, 24 and 48 h,
respectively (Table I).
 | Table ImRNA expression of caspase-3 and c-Jun
of U266 cells with or without brucine for 12, 24 and 48 h (mean ±
SD). |
Table I
mRNA expression of caspase-3 and c-Jun
of U266 cells with or without brucine for 12, 24 and 48 h (mean ±
SD).
| Group | Caspase-3a | c-Juna |
|---|
| Control | 0.2597±0.020 | 0.1354±0.0016 |
| 12 h | 0.5488±0.016 | 0.2603±0.0032 |
| 24 h | 0.6205±0.006 | 0.4874±0.0068 |
| 48 h | 0.6533±0.009 | 0.5965±0.0089 |
Effects of brucine on c-Jun
expression
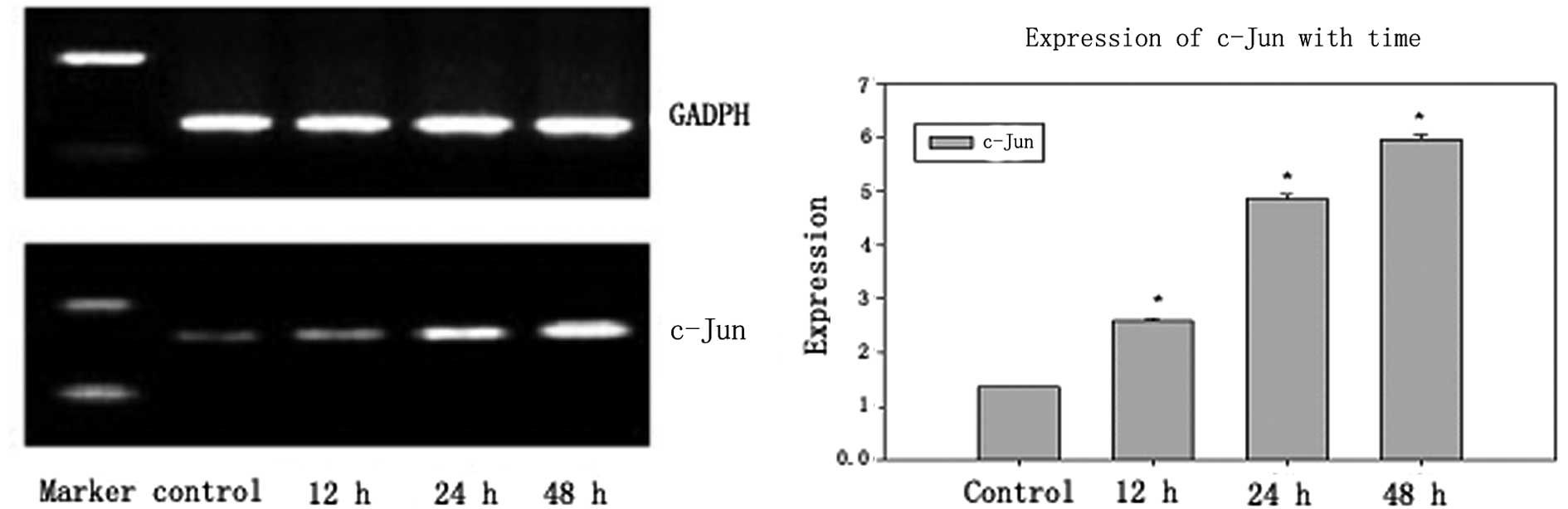
c-Jun is a downstream product of the c-Jun
N-terminal kinase (JNK)/stress-activated protein kinase (SAPK)
signaling pathway. To investigate the effect of c-Jun on the
brucine-induced U266 cell apoptosis, the expression of c-Jun was
measured in brucine-treated U266 cells by RT-PCR at 12, 24 and 48 h
(Fig. 3). The gray value of c-Jun
was 0.1354±0.0016 in the control group and increased in the
brucine-treated (0.16 mg/ml) group up to 0.2603±0.0032,
0.4874±0.0068 and 0.5965±0.0089 upon exposure of the cells at 12,
24 and 48 h, respectively (Table
I).
Caspase-3 and c-Jun expression
To verify that the activation of caspase-3 and c-Jun
is associated to the JNK signaling pathway, the expression of
caspase-3 and c-Jun was measured after the specific inhibitor of
the JNK signaling pathway, SP600125, was added by RT-PCR (Fig. 4). The gray values of caspase-3 and
c-Jun were 0.7683±0.0050 and 0.7961±0.007, respectively, in the
brucine-treated (0.16 mg/ml) group. By contrast, a decrease in the
brucine (0.16 mg/ml) and SP600125 group down to 0.5723±0.0055 and
0.4683±0.003 was detected following treatment for 24 h (Table II).
 | Table IImRNA expression of caspase-3 and c-Jun
of U266 cells with or without SP600125 (mean ± SD). |
Table II
mRNA expression of caspase-3 and c-Jun
of U266 cells with or without SP600125 (mean ± SD).
| Group | Caspase-3a | c-Juna |
|---|
| Brucine | 0.7683±0.0050 | 0.7961±0.007 |
| Brucine+SP600125 | 0.5723±0.0055 | 0.4683±0.003 |
Discussion
The process of programmed cell death (PCD), or
apoptosis, is required to maintain homeostasis (6). Apoptosis is closely correlated with
the occurrence of a variety of diseases (including autoimmune
disease and cancer) (7). Apoptosis
is distinct from necrosis and is a process in which cells are
actively involved in initiating a series of gene activations to
adapt to the environment. MM is an incurable plasma cell neoplasm
characterized by the accumulation of malignant plasma cells in the
bone marrow. The occurrence and development of MM is associated to
abnormal proliferation and the inhibition of apoptosis of tumor
cells.
This study was performed to evaluate the response to
brucine using the human MM line cell, U266. These results confirmed
the anti-proliferative effect of brucine on the U266 cell line,
with an IC50 value of 0.16 mg/ml at 48 h (Fig. 1). Furthermore, the flow cytometric
analysis was performed on brucine-treated U266 cells to analyze the
type of cell death process (apoptosis or necrosis). The results
demonstrated that the sub-G0/G1 cell
population increased in a dose-dependent manner.
Mitogen-actived protein kinase (MAPK) is a
significant method of signal transduction in eukaryotes and is key
in the regulation of gene expression (8). The classic MAPK signaling pathway is
the MAPKKKs → MAPKKs → MAPKs continuous enzymatic reaction
(8,9). The MAPK family includes extracellular
signal regulated kinase (ERK), JNK/SAPK and p38 categories
(10). The dynamic balance of JNK,
p38 and ERK determines cell survival and apoptosis (11). JNK is a serine/threonine protein
kinase located in the cytoplasm with a molecular mass of 54 kDa
(12). Due to its interaction with
the N-terminal activation domain of c-Jun and the phosphorylation
of serine 63 and 73, JNK has been designated as c-Jun N-terminal
kinase (10). c-Jun belongs to the
Jun subfamily (c-Jun, Jun B and Jun D) and forms the center of
transcription factor-activated protein-1 (AP-1) as well as dimers
of basic leucine zippers. c-Jun and Fos (v-Fos, c-Fos, FosB and
FosL1, FosL2) form homo- or heterodimers, which activate
transforming growth factor (ATF-2, LRF-1/ATF-3, B2ATF, JDP1 and
JDP2) or fibrosarcoma tendon membrane protein (c-Maf, MafB, MafA,
MafG/F/K and NRL). Phosphorylation of c-Jun and ATF-2, and
activation of the transcription factor (AP-1), combined with Fas
through the activation of caspase-8 (13). JNK induces apoptosis via the death
receptor pathway. JNK may also act via the phosphorylation of Bcl-2
and Bcl-xL (14). The release of
cytochrome C, and activation of caspase-9 induces apoptosis through
the mitochondrial pathway (15).
In the anti-Fas monoclonal antibody induction apoptosis of MM
cells, JNK/SAPK in the cytoplasm and transcription factor c-Jun
were induced (16,17). The roselle extract is known to
activate JNK/p38MAPK (18). The
phosphorylation of the target proteins of c-Jun activates the
signal transduction of apoptosis-related proteins, including the
Fas-mediated signal, and induces apoptosis (19).
Findings of our previous study (5) have verified that the apoptosis
induced by brucine occurs via the death receptor pathway. To
investigate the signaling pathways of apoptosis, the U266 cells
treated with brucine for 12, 24 and 48 h were collected. c-Jun, the
downstream product of JNK and caspase-3, which is the executor of
apoptosis, was detected (Figs. 2
and 3). The results showed that
the gray value in brucine-treated cells for 12, 12 and 48 h
increased up to 0.5488±0.016, 0.6205±0.006, 0.6533±0.009 for
caspase-3 and 0.2603±0.0032, 0.4874±0.0068, 0.5965±0.0089 for
c-Jun, respectively (Table I;
P<0.01), and demonstrated that caspase-3 and c-Jun were
activated in brucine-treated cells. In addition, caspase-3 and
c-Jun were detected again following the addition of the specific
JNK inhibitor, SP600125 (20). The
results demonstrate that the gray value of caspase-3 and c-Jun were
decreased to 0.5723±0.0055 and 0.4683±0.003, respectively (Fig. 4, Table II), and that the activation of
caspase-3 and c-Jun in brucine-treated cells is capable of
inhibition by the specific JNK inhibitor SP600125. We suggest that
brucine, via phosphorylation of c-Jun by the JNK signaling pathway,
induces apoptosis in the human MM cell line U266.
In the present study, we suggest that the
anti-proliferative activity of brucine on U266 cells is due to
apoptosis. Preliminary analysis of the apoptosis mechanism
demonstrated that the JNK signaling pathway was activated in the
apoptotic U266 cells treated with brucine. The present study
suggests that brucine has possible anti-cancer effects and provides
a theoretical basis for the clinical treatment of MM. However, the
inhibitory growth effect of brucine on other myeloma cell lines and
resistant cell lines as well as other signaling pathways remains to
be determined. Additionally, the overall level of apoptosis and
toxicity caused by brucine requires additional investigation.
References
|
1
|
Wei ZL and Wang XH: The development of
NF-κB in the multiple myeloma. Med Res Nov. 36:98–101. 2007.
|
|
2
|
Spisek R, Charalambous A, Mazumder A, et
al: Bortezomib enhances dendritic cell (DC)-mediated induction of
immunity to human myeloma via exposure of cell surface heat shock
protein 90 on dying tumor cells: therapeutic implications. Blood.
109:4839–4845. 2007. View Article : Google Scholar
|
|
3
|
Rao PS, Ramanadham M and Prasad MN:
Anti-proliferative and cytotoxic effects of Strychnos nux-vomica
root extract on human multiple myeloma cell line - RPMI 8226. Food
Chem Toxicol. 47:283–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deng X, Yin F, Lu X, et al: The apoptotic
effect of brucine from the seed of Strychnos nux-vomica on human
hepatoma cells is mediated via Bcl-2 and Ca2+ involved
mitochondrial pathway. Toxicol Sci. 91:59–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li ZH, Ma YP, Wang YH and Feng J:
Apoptosis of U266 cells induced by brucine through death-receptor
pathway. J Leuk Lymphoma. 19:724–731. 2010.
|
|
6
|
Jiao JX and Gao WJ: The research progress
of the mechanism on the cellular signal transmission. Chin J
Gerontol. 30:853–856. 2010.(In Chinese).
|
|
7
|
Wen ZH and Wang XH: Apoptosis and multiple
myeloma. North China Coal Med Coll. 8:778–781. 2006.
|
|
8
|
Liang XM and Yang KD: Caspase, JNK/SAPK,
P38 MAPK and apoptosis. Foreign Med Sci (section hygiene). 35:5–10.
2008.
|
|
9
|
Wan KF, Chan SL, Sukumaran SK, et al:
Chelerythrine induces apoptosis through a Bax/Bak-independent
mitochondrial mechanism. J Biol Chem. 283:8423–8433. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du L, Wang FY, Zhang L, et al: Advance in
the research of JNK dependent apoptosis. China Trop Med.
18:841–844. 2008.(In Chinese).
|
|
11
|
Xiao Y, Yang FQ, Li SP, et al: Furanodiene
induces G2/M cell cycle arrest and apoptosis through MAPK signaling
and mitochondria-caspase pathway in human hepatocellular carcinoma
cells. Cancer Biol Ther. 6:1044–1050. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Z, Tao YG, Yang LF, et al: Effect of
JIP on the proliferation and apoptosis of nasopharyngeal carcinoma
cells through interaction with JNK mediated pathway. Prog Biochem
Biophys. 30:579–585. 2003.PubMed/NCBI
|
|
13
|
Papa S, Zazzeroni F, Pham CG, et al:
Linking JNK signaling to NF-kappaB: a key to survival. J Cell Sci.
117:5197–5208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murakami Y, Aizu-Yokota E, Sonoda Y, et
al: Suppression of endoplasmic reticulum stress induced caspase
activation and cell death by the over expression of Bcl-xl or
Bcl-2. J Biochem. 141:401–410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng J, Mao XO, Stevenson FF, et al: The
herbicide paraquat induces dopaminergic nigral apoptosis through
sustained activation of the JNK pathway. J Biol Chem.
279:32626–32632. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Largo C, Alvarez S, Saez B, et al:
Identification of overexpressed genes in frequently
gained/amplified chromosome regions in multiple myeloma.
Hematologica. 91:184–191. 2004.PubMed/NCBI
|
|
17
|
Lin HH, Chen JH, Kuo WH, et al:
Chemopreventive properties of Hibiscus sabdariffa L on human
gastric carcinoma cells through apoptosis induction and JNK/p38
MAPK signaling activation. Chem Biol Interac. 165:59–75. 2007.
|
|
18
|
Gong X, Wang M, Tashiro S, et al:
Involvement of JNK-initiated p53 acccumulation and phosphorylation
of p53 in pseudolaric acid B induced cell death. Exp Mol Med.
38:428–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Junttila MR, Li SP and Westermarck J:
Phosphatase-mediated crosstalk between MAPK signaling pathways in
the regulation of cell survival. FASEB J. 22:954–965. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsumoto N, Imamura R and Suda T:
Caspase-8 and JNK dependent AP-1 activation is required for Fas
ligand-induced IL-8 production. FEBS J. 274:2376–2384. 2007.
View Article : Google Scholar : PubMed/NCBI
|