Introduction
The various types of of head and neck cancer
represent ~5% of all the cancer types, and 80–90% of these tumors
constitute squamous cell carcinomas. Despite the rapid progress in
the diagnosis and treatment of this disease, the overall 5-year
survival of this type of cancer is among the lowest of the most
common tumor types (1). Most types
of human oral cancer (63.8%) exhibit either a loss or significant
reduction of P12CDK2AP1 expression. Decreased
P12CDK2AP1 expression has been shown to correlate with
increased tumor invasion, risk of lymph node metastases, and
decreased survival in patients with oral squamous cell carcinoma
(2).
Human cyclin-dependent kinase 2 associated protein 1
(CDK2AP1) is a highly conserved gene. No mutations have been
found in oral and esophageal types of cancer (3,4).
P12CDK2AP1, originally known as deleted in oral cancer-1
(DOC-1), is identified and cloned from the Syrian hamster oral
cancer model (5) and acts as a
growth suppressor by negatively regulating the activity of
cyclin-dependent kinase 2 (CDK2) (6). Human P12CDK2AP1 associates
with DNA polymerase α/primase and mediates the phosphorylation of
the large p180 catalytic subunit, suggesting that it is a potential
regulator of DNA replication in the S phase of the cell cycle
(3). Moreover, the ectopic
expression of P12CDK2AP1 in culture cells leads to
growth suppression, arrests cells in the G1 phase of the cell cycle
and suppresses DNA replication (6,7).
Several functional studies support the role of
P12CDK2AP1 as a growth suppressor (3,5,6,8).
RNA interference (RNAi) is an evolutionarily
conserved mechanism of sequence-specific post-transcriptional gene
silencing mediated by double-stranded RNA molecules that match the
sequence of the target gene (9).
Investigators have focused on HIV-1-derived lentivirus vectors due
to their ability to deliver therapeutic transgenes into a wide
range of difficult-to-transfect target tissues without inducing
significant humoral immune responses (10–12).
In this study, we constructed a lentivirus vector for the
expression of small interfering RNAs (siRNAs) in mammalian cells
and demonstrated its ability to efficiently downregulate the
expression of CDK2AP1 protein. The present study was undertaken to
elucidate the role of P12CDK2AP1 in cell proliferation.
Results demonstrated that P12CDK2AP1 silencing promotes
cell proliferation in vitro.
Materials and methods
Cells, cell culture and materials
HEK293T and normal human skin keratinocytes (HaCaT)
cells were obtained from the China Center for Type Culture
Collection (Wuhan, China). The cells were maintained in Dulbecco’s
modified Eagle’s medium (DMEM) containing 10% fetal bovine serum
(FBS) (Invitrogen, Karlsruhe, Germany) and incubated in a
humidified 37°C, 5% CO2 incubator. The primary antibody
of P12CDK2AP1 was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). GAPDH antibody was
purchased from Millipore (Billerica, MA, USA). SYBR-Green PCR
Master mix for the quantification of CDK2AP1 mRNA was
obtained from Takara (Kyoto, Japan) and oligonucleotides from
Sangon (Shanghai, China).
Construction of plasmid vector for the
expression of siRNA
The sequence of short hairpin RNA (shRNA) targeting
the human CDK2AP1 gene was 5′-GATCTCAGTTCTGCGT TTA-3′,
corresponding to the coding region positions of CDK2AP1
mRNA. The control plasmid was obtained from GenePharma Co., Ltd.
(Shanghai, China). The human U6 promoter was amplified from genomic
DNA and cloned in the AgeI and EcoRI sites of the
pGCSIL-GFP plasmid (GeneChem, Shanghai, China). The
oligonucleotides which were previously shown to induce RNAi against
CDK2AP1 were cloned downstream of the U6 promoter. The
sequence of the oligonucleotide (top strand) was:
5′-CcggtaGATCTCAGT
TCTGCGTTTATTCAAGAGATAAACGCAGAACTGAGATCta-TTTTTg-3′. The
underlined sequence corresponds to nucleotides of human open
reading frame for CDK2AP1. The sequence of the construct was
confirmed by automated fluorescent sequencing.
Construction of lentivirus vector for
siRNA delivery
A self-inactivating lentivirus vector containing a
cytomegalovirus (CMV)-driven enhanced green fluorescent protein
(EGFP) reporter and a U6 promoter upstream of cloning restriction
sites (AgeI and EcoRI) was purchased from GeneChem
Co., Ltd. A control shRNA unrelated to human gene sequences was
used as a negative control. We constructed 3 si-CDK2AP1
lentiviruses, KD-1, KD-2 and KD-3, targeting human CDK2AP1
mRNA. The following custom primers containing AgeI and
EcoRI sites at their 5′ termini were used for PCR
amplification: 5′-CCTATTTCCCATGATTCCT TCATA-3′ and
5′-GTAATACGGTTATCCACGCG-3′. The resulting PCR fragment was digested
with AgeI and EcoRI and cloned into AgeI- and
EcoRI-digested pLenti vector. Oligonucleotides for
constructing the negative control and 3 si-CDK2AP1
lentiviruses were synthesized from Sangon. Correct insertions of
shRNA cassettes were confirmed by restriction mapping and direct
DNA sequencing. KD-3 was identified as the most efficient and was
selected for this study.
Lentiviral vector infection in cultured
HaCaT cells
Cells were replated at 5×103 cells/well
in 96-well plates along with recombinant lentivirus encoding for
shRNA against CDK2AP1 at different multiplicity of
infections (MOIs) in serum-free growth medium containing 5 μg/ml
polybrene at 37°C and 5% CO2. After 4 h,
serum-containing growth medium was added to the cells, and there
was complete replacement of growth medium after 48 h. After 3 days
of post-infection, reporter gene expression was examined using
fluorescent microscopy.
Real-time reverse transcription (RT)-PCR
assay
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). The expression of
CDK2AP1 mRNA was detected using the SYBR-Green miRNA assay
(Takara) (13), and normalized
using the 2−ΔΔCt method (14) relative to human β-actin
(Sangon). Primer sequences and the size of the products are shown
in Table I. To ensure specificity
of the PCR product amplification, the melting curves for standard
and sample products were analyzed. All the real-time RT-PCR assays
were performed three times.
 | Table IPrimer sequences and the length of
products for real-time RT-PCR. |
Table I
Primer sequences and the length of
products for real-time RT-PCR.
| Gene name | Oligonucleotide
sequence | Product size
(bp) |
|---|
| CDK2AP1 | F:
AAGAGCAACCCACCAAACC
R: ATCAACTTACAATAAACGCAGAAC | 92 |
| β-actin | F:
GGCGGCACCACCATGTACCCT
R: AGGGGCCGGACTCGTCATACT | 202 |
Western blot analysis
Cells were seeded in 6-well plates and infected
after 12 h. The cells were harvested 3 days following infection,
washed once in phosphate-buffered saline (PBS) and lysed. Protein
concentration was determined using the bicinchoninic acid (BCA)
assay (Pierce, Rockford, IL, USA). Aliquots (60 μg) were separated
on a 15% SDS-PAGE and transferred to PVDF membrane. The membrane
was incubated with the specific antibody followed by
peroxidase-conjugated secondary antibodies. An enhanced
chemiluminescence detection solution was then applied (Pierce). The
relative protein level in different groups was normalized to the
signal intensity of GAPDH. Quantitative analysis of the blots was
performed using the NIH-ImageJ program.
Measurement of cell growth using the
methyl thiazolyl tetrazolium (MTT) assay
HaCaT cells were seeded at a density of
2×103 cells/well in 96-well plates containing 0.2 ml
DMEM (with 7% FBS) and cultured for 9 days. During this period, the
medium was replaced with fresh complete medium every 3 days. Six
wells from each group were selected randomly for the MTT assay each
day. After 4 h of incubation, the reaction was stopped by adding
150 μl of dimethyl sulfoxide (DMSO; Sigma) to each well, followed
by a 10-min incubation. The percentage of viable cells was
determined by measuring the absorbance at 490 nm on a multiscanner
reader (TECAN-spectra mini; Tecan Austria GmbH, Grödig, Austria).
Cell growth curves were drawn by using average absorbance at 490 nm
from 3 independent experiments.
Flow cytometric analysis of cell
cycle
The cells (72 h) were harvested, washed twice with
ice-cold PBS, fixed with 70% ethanol overnight at 4°C, washed and
resuspended in 100 μl of PBS containing a final concentration of 50
μg/ml RNase A for 30 min at room temperature. The cells were then
stained with 20 μg/ml propidium iodide in a final volume of 300 μl
for 20 min. DNA content and cell cycle were analyzed using a flow
cytometer (FACSCalibur; Becton-Dickinson, Franklin Lakes, NJ, USA),
and ModFit and CellQuest software. The experiments were conducted
three times.
Statistical analysis
The experiments were performed in triplicate and
standard deviations were calculated. The statistical analysis was
performed by a two-tailed paired Student’s t-test or one-way ANOVA
using SPSS 11.5 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Infection efficiency of lenti-shCDK2AP1
in HaCaT cells
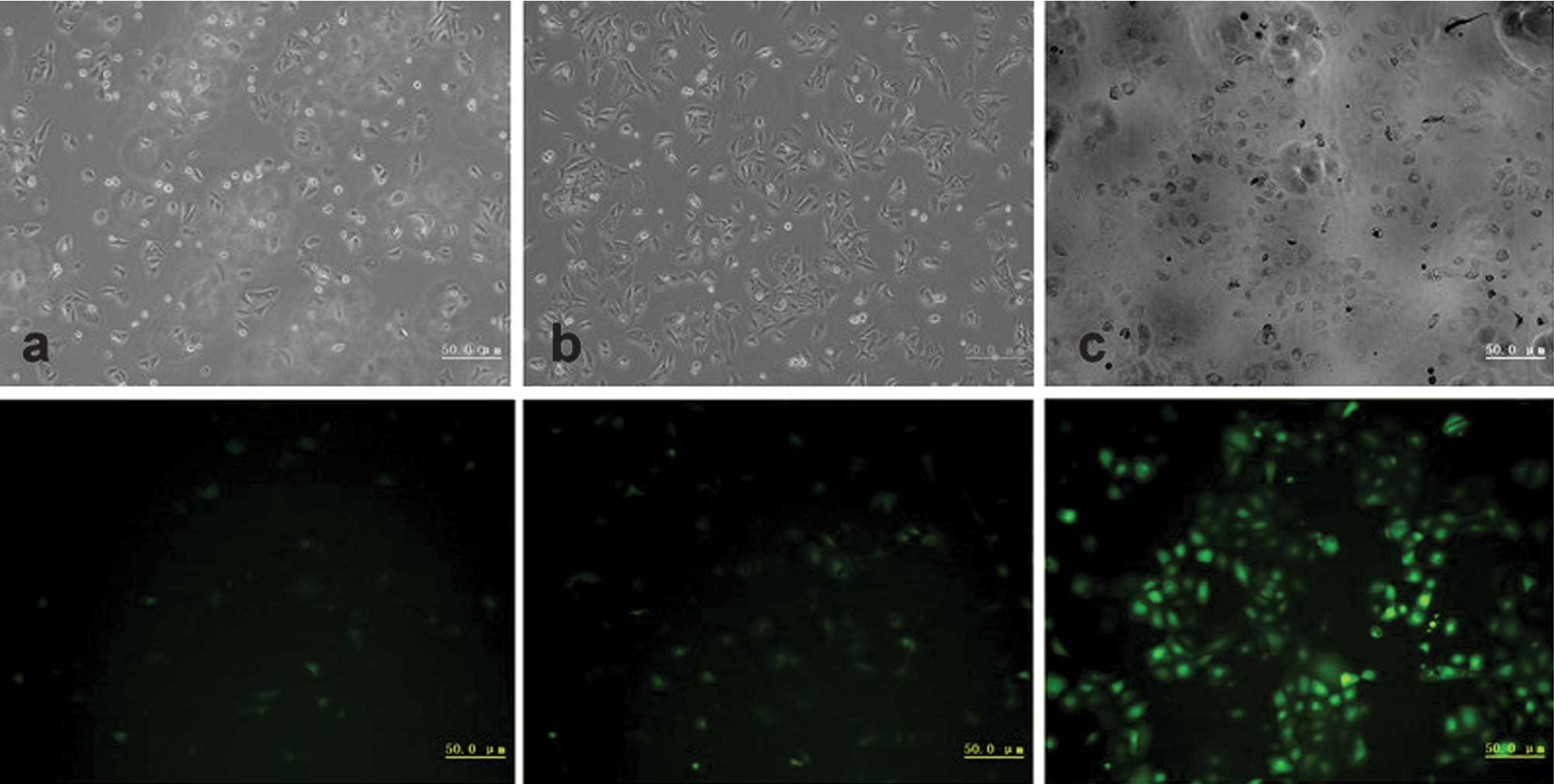
To determine the lentiviral infection efficiency in
HaCaT cells, EGFP expression was examined by microscopy at
different MOIs 3 days after infection. The efficiency of lentiviral
vector infection in HaCaT cells was >90% at an MOI of 40
(Fig. 1). The percentage of
EGFP-expressing cells remained relatively unchanged when MOI was
>40. Based on these results, an MOI of 40 was chosen. Our data
suggested that lentivirus shRNA vector pGCSIL-GFP had high
efficiency for infecting HaCaT cells.
CDK2AP1 mRNA and P12CDK2AP1
expression is efficiently inhibited in lenti-shCDK2AP1-infected
HaCaT cells
The subconfluent cells were infected with either
negative control or shCDK2AP1 lentivirus. Two days
post-infection, the cells were collected and lysed for analysis.
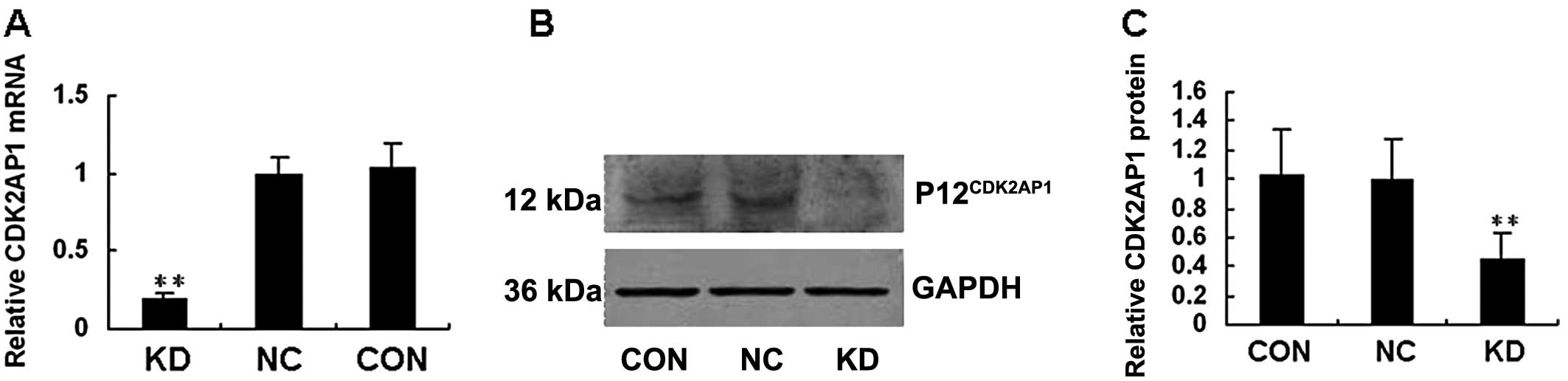
Real-time RT-PCR results showed the mRNA level of CDK2AP1 in
HaCaT cells infected with lenti-shCDK2AP1 to be ~80% lower
compared to that of HaCaT control cells (Fig. 2A). As shown in Fig. 2B and C, the level of
P12CDK2AP1 expression was markedly reduced in HaCaT
cells infected by lenti-shCDK2AP1 compared to that in the
control cells, a fact which was consistent with the downregulation
of CDK2AP1. This study indicated that lentiviral siRNA was
able to provide highly efficient and specific CDK2AP1
knockdown in HaCaT cells.
P12CDK2AP1 knockdown
significantly promotes HaCaT cell growth in vitro
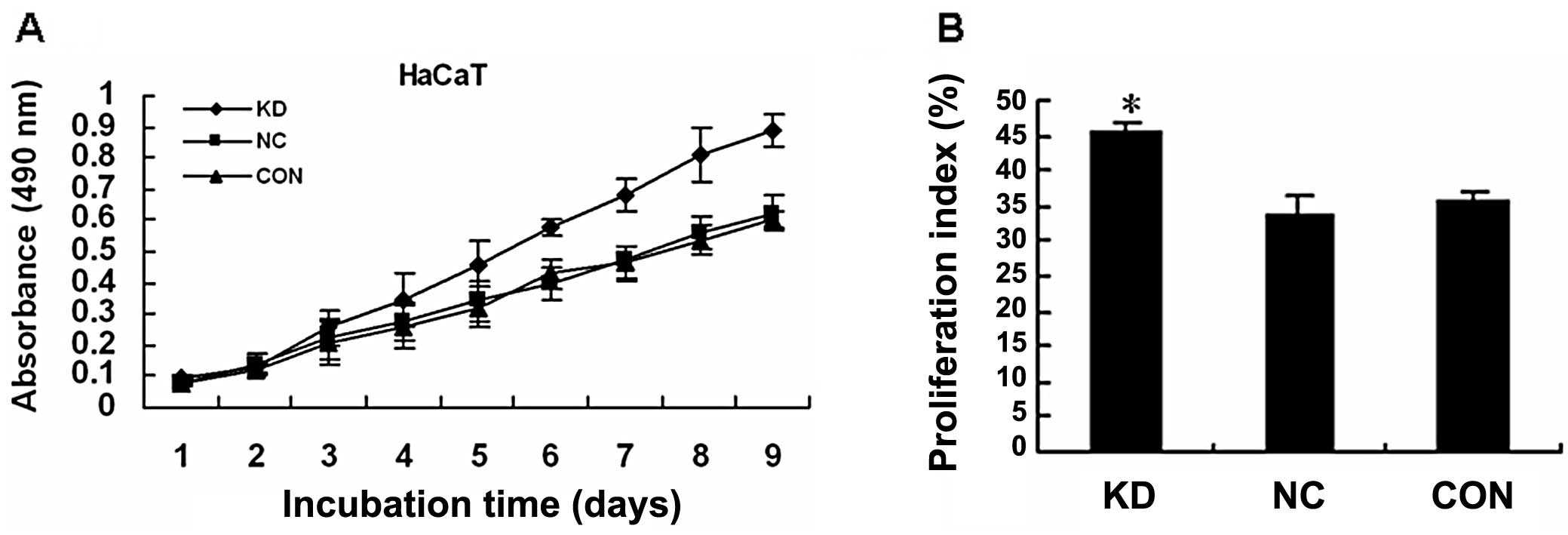
To determine whether silencing P12CDK2AP1
by RNAi had a stimulative effect on HaCaT cell growth,
determination of cell proliferation was performed using the MTT
assay. After 3 days of infection, lenti-shCDK2AP1 did not
positively affect HaCaT cell proliferation. However,
lenti-shCDK2AP1 significantly increased HaCaT cell
proliferation 4–9 days following infection (Fig. 3A). Notably, the suppression of
P12CDK2AP1 led to HaCaT cell growth promotion in a
time-dependent manner and this stimulative effect was not observed
before day 3.
P12CDK2AP1 knockdown affects
HaCaT cell cycle transition
The different proliferation rates of HaCaT cells
infected with lenti-shCDK2AP1 vs. control HaCaT cells may
partly be due to differences in cell cycle regulation. Therefore,
cell cycle distribution was detected using flow cytometry. On day
7, a lower number of HaCaT cells infected by lenti-shCDK2AP1
were arrested in the G0/G1 compared to the S and G2/M phases of the
cell cycle (Fig. 3B).
Discussion
P12CDK2AP1 is crucial in a variety of
responses, including S-phase suppression associated with growth
suppression (6,7), tumor progression (15), malignant transformation (5), apoptosis and oral carcinogenesis
(16). It has also been shown to
be reduced or absent in various types of human oral cancer and it
has been found to be a positive prognostic indicator (2). Several findings have suggested that
P12CDK2AP1 loss or reduced expression contribute to the
multistep nature of oral carcinogenesis, and that
P12CDK2AP1 loss may constitute an event associated with
tumor progression (2,6,7,17).
This may raise the question as to whether blocking
P12CDK2AP1 is beneficial for promoting HaCaT cell
growth, and whether targeting P12CDK2AP1 signaling may
serve as a novel therapeutic strategy for the treatment of HNSCCs
expressing downregulated P12CDK2AP1.
Stable downregulation of gene expression via
retroviral-mediated siRNA delivery has recently been described by
several scientific groups (18–20).
Lentiviral vector is a novel tool for human gene therapy. It has
been shown to be an effective vehicle for stable and long-term gene
expression. The results of this study demonstrated that the rate of
lentiviral vector transducing to HaCaT cells was >90%. The
real-time RT-PCR and western blot analyses indirectly indicated
that the HaCaT cells transduced with the lentiviral shRNA vector
expressed siRNA-targeting CDK2AP1 gene. In this study,
CDK2AP1 siRNA was demonstrated to inhibit, not only
P12CDK2AP1 expression, but also the activity of
P12CDK2AP1 in HaCaT cells.
Previous data have demonstrated that CDK2AP1
is crucial in cancer cell proliferation (2,5,21).
In this study, in order to determine whether CDK2AP1
regulated HaCaT cell proliferation and the cell cycle, transduced
HaCaT cells were analyzed. The results showed that RNAi-targeting
CDK2AP1 promoted the proliferation of HaCaT cells. Flow
cytometric analysis showed that the inhibition of
P12CDK2AP1 expression in HaCaT cells, resulted in a
lower number of cells in the G1 phase of the cell cycle.
Although significant downregulation of
P12CDK2AP1 expression was achieved, complete gene
silencing was not achieved. It is believed that residual
P12CDK2AP1 expression in the mass culture cells is due
to the presence of a few clones that have completely escaped the
effect of siRNA. The incomplete gene silencing of
P12CDK2AP1 observed in this study could be attributed to
the relatively weak expression from Pol III promoters (18–20).
However, it is believed that even incomplete gene silencing would
be of therapeutic utility for a number of human disease conditions.
Studies are underway aiming to investigate the mechanisms
underlying the incomplete gene silencing and a number of strategies
could be established to improve the efficiency of this
procedure.
In this study, a lentiviral vector was constructed
for the delivery of siRNAs and its ability to efficiently
downregulate the expression of a target gene in the infected cells
was demonstrated. It was also shown that P12CDK2AP1
silencing promotes the proliferation of cultured cells. Based on
the well-known ability of lentiviruses to infect dividing and
non-dividing cells as well as to achieve long-term gene expression,
they are an attractive option for the in vitro and in
vivo delivery of siRNAs in cultured cells.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China to Professor M.S. (project nos.
81072230 and 30772428).
References
|
1
|
Moser BA, Subramanian L, Khair L, Chang YT
and Nakamura TM: Fission yeast Tel1(ATM) and Rad3(ATR) promote
telomere protection and telomerase recruitment. PLoS Genet.
5:e10006222009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shintani S, Mihara M, Terakado N, et al:
Reduction of p12DOC-1 expression is a negative prognostic indicator
in patients with surgically resected oral squamous cell carcinoma.
Clin Cancer Res. 7:2776–2782. 2001.PubMed/NCBI
|
|
3
|
Tsuji T, Duh FM, Latif F, et al: Cloning,
mapping, expression, function, and mutation analyses of the human
ortholog of the hamster putative tumor suppressor gene Doc-1. J
Biol Chem. 273:6704–6709. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daigo Y, Suzuki K, Maruyama O, et al:
Isolation, mapping and mutation analysis of a human cDNA homologous
to the doc-1 gene of the Chinese hamster, a candidate tumor
suppressor for oral cancer. Genes Chromosomes Cancer. 20:204–207.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Todd R, McBride J, Tsuji T, et al: Deleted
in oral cancer-1 (doc-1), a novel oral tumor suppressor gene. FASEB
J. 9:1362–1370. 1995.PubMed/NCBI
|
|
6
|
Shintani S, Ohyama H, Zhang X, et al:
p12(DOC-1) is a novel cyclin-dependent kinase 2-associated protein.
Mol Cell Biol. 20:6300–6307. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuo K, Shintani S, Tsuji T, et al:
p12(DOC-1), a growth suppressor, associates with DNA polymerase
alpha/primase. FASEB J. 14:1318–1324. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan Z, Sotsky Kent T and Weber TK:
Differential expression of DOC-1 in microsatellite-unstable human
colorectal cancer. Oncogene. 22:6304–6310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharp PA: RNAi and double-strand RNA.
Genes Dev. 13:139–141. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naldini L and Verma IM: Lentiviral
vectors. Adv Virus Res. 55:599–609. 2000. View Article : Google Scholar
|
|
11
|
Naldini L, Blomer U, Gage FH, Trono D and
Verma IM: Efficient transfer, integration, and sustained long-term
expression of the transgene in adult rat brains injected with a
lentiviral vector. Proc Natl Acad Sci USA. 93:11382–11388. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naldini L, Blomer U, Gallay P, et al: In
vivo gene delivery and stable transduction of nondividing cells by
a lentiviral vector. Science. 272:263–267. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen C, Ridzon DA, Broomer AJ, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mulholland DJ, Xin L, Morim A, Lawson D,
Witte O and Wu H: Lin-Sca-1+CD49fhigh stem/progenitors are
tumor-initiating cells in the Pten-null prostate cancer model.
Cancer Res. 69:8555–8562. 2009.
|
|
15
|
Todd R, Donoff RB and Wong DT: The
molecular biology of oral carcinogenesis: toward a tumor
progression model. J Oral Maxillofac Surg. 55:613–625. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kohno Y, Patel V, Kim Y, et al: Apoptosis,
proliferation and p12(doc-1) profiles in normal, dysplastic and
malignant squamous epithelium of the Syrian hamster cheek pouch
model. Oral Oncol. 38:274–280. 2002. View Article : Google Scholar
|
|
17
|
Shintani S, Mihara M, Nakahara Y, et al:
Expression of cell cycle control proteins in normal epithelium,
premalignant and malignant lesions of oral cavity. Oral Oncol.
38:235–243. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barton GM and Medzhitov R: Retroviral
delivery of small interfering RNA into primary cells. Proc Natl
Acad Sci USA. 99:14943–14945. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brummelkamp TR, Bernards R and Agami R:
Stable suppression of tumorigenicity by virus-mediated RNA
interference. Cancer Cell. 2:243–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Devroe E and Silver PA:
Retrovirus-delivered siRNA. BMC Biotechnol. 2:152002. View Article : Google Scholar
|
|
21
|
Sotsky Kent T, Yuan Z, Miller A and Weber
TK: Deleted in oral cancer-1 expression upregulates proapoptosis
elements in microsatellite-unstable human colorectal cancer. Ann
Surg Oncol. 11:192–196. 2004.PubMed/NCBI
|