Introduction
The hepatitis B virus (HBV) has spread worldwide.
Existing data indicate that more than one million patients succumb
to hepatocirrhosis and liver cancer annually. A marked correlation
has been observed between prevalence of HBV carriers and incidence
of hepatocellular carcinoma (1,2).
The HBV genome is a partially double-stranded cyclic
DNA. The X-open reading frame (ORF) that encodes the X protein is
located between 1,376 and 1,837 nt near the cohesive end of the
viral genome and the HBx gene is the smallest gene in the
HBV genome. The encoded X protein is composed of 154 amino acids
with a relative molecular mass of 17 kDa and is mainly located in
the cytoplasm, with a small amount located in the nucleus (3,4). As
a multipurpose viral protein, HBx interacts with a number of
proteins, including cytoplasmic proteins, such as Jak1, PKC binding
protein and Caspase-3; nuclear proteins, such as TFIIB, RXR and
TBP; and proteins shuttling between the cytoplasm and cell nucleus,
such as Smad4 and Tat-binding protein (5). HBx has an extensive gene
transcription regulation function and interacts with numerous
proteins in the host cell to regulate gene expression and cell
protein function, thereby affecting the biological functions of the
virus, including self-duplication, signal transduction of the host
cell, cell multiplication, carcinogenesis and differentiation and
apoptosis (6–10). The HBVX gene and HBx protein
are both involved in HBV infection, duplication, pathogenesis and
possibly carcinogenesis and play an important role in the course of
chronic infection. Clarifying the structure and function of XBP1
and its effect on HBV-induced liver injury facilitates the
investigation of the function and mechanism of action of the
X gene and HBx protein and may contribute to the control and
treatment of chronic HBV infection. The present study lays a
foundation for additional understanding of chronic HBV infection
and the pathogenesis of primary liver cancer.
Materials and methods
XBP1 gene amplification
The study was approved by the ethics committee of
Beijing Ditan Hospital, Beijing, China. Total RNA extraction from
HepG2 cells and cDNA synthesis was performed according to a
previously described method (11).
Sequence-specific primers were designed for XBP1 gene
amplification via PCR according to the full-length XBP1
gene: sense primer 5′-GCCGAATTCATGGCCAAGGACTTTCAAGA-3′ and
antisense primer 5′-TAAGGATCCTCAGGCCACCTCGCCGGTGGC-3′. The
XBP1 gene was amplified via PCR and the target DNA fragments
were subsequently recovered and ligated into the pGEM-T vector
(Promega, Madison, WI, USA), followed by double enzyme digestion
and sequencing.
Recombinant bait vector construction and
self-activation detection
The pGBKT7 yeast plasmid and the XBP1 gene
were digested with EcoRI/SalI, purified and ligated
overnight using T4 DNA ligase at l6°C, transformed into E.
coli DH5α and screened. The plasmid was extracted and
identified via EcoRI and XhoI double enzyme digestion
and sequencing and the recombinant was named pGBKT7-XBP1. The
pGBKT7-XBP1 vector was transformed into the AH109 yeast cells using
the lithium acetate method according to the manufacturer’s
instructions (Clontech Corporation, Mountain View, CA, USA) and
then plated on the synthetic dropout medium-tryptophan (SD/-Trp)
for screening. Colonies (>2 mm in diameter) were identified via
PCR, whereas 100 μl stock solution was directly spread onto the
SD/-Trp/-His/-Ade culture medium with kanamycin for the
self-activation experiment.
Western blot analysis
Protein extract was prepared using the urea/sodium
dodecyl sulfate (SDS) method (12)
and stained using diaminobenzidine tetrachloride with anti-c-Myc
monoclonal antibodies diluted to 1:100 as the primary antibody and
horseradish peroxidase (HRP)-labeled goat anti-mouse IgG diluted to
1:2,500 as the secondary antibody (13).
Liver cDNA library screening
Several AH109 yeast colonies (>2 mm in diameter)
containing the pGBKT7-XBP1 plasmid were selected from the SD/-Trp
culture medium, inoculated into SD/-Trp liquid culture medium,
agitated at 30°C and 250 rpm for 16–24 h and mated with 400 μl of
yeast cells in the liver cell library in 50 ml of 2X yeast peptone
dextrose adenine (YPDA) at 30°C and further agitated at 30–45 rpm
for 18–24 h when the OD600 reached 0.8 and 1.0. Clover
leaf-shaped diploid cells were observed, resuspended in 10 ml 0.5X
YPD culture medium, spread on 25 plates with 150 mm of
SD/-Trp/-Leu/-His and 25 plates with D/-Trp/-Leu/-His/-Ade and
cultured at 30°C until a colony was formed. The monoclonal cells
grown on SD/-Trp/-Leu/-His/-Ade were spread onto QDO with X-α-Gal
for streak culture at 30°C for 4–8 days and the blue colony was the
positive colony. The plasmid from positive yeast was extracted and
transformed into E. coli using electroporation (14), followed by plate cultivation in
ampicillin-containing Luria-Bertani culture medium. The plasmid was
extracted from the obtained colony, digested with BglII,
sequenced and its homology with sequences in the GenBank database
was analyzed.
Subcellular localization of XBP1
The XBP1 primer was designed using the NTI
software package. A ‘G’ was inserted between the EcoRI site
and ATG in this primer via in-frame expression with pEGFP-C1. P1:
5′-GCCGAATTCGATGGCCAAGGACTTTCAAGA-3′ EcoRI; P2:
5′-TAAGGATCCTCAGGCCACCTCGCCGGTGGC-3′ BamHI. The PCR
amplification product was recovered and ligated with the pGEM-T
vector for sequencing. The XBP1 and pEGFP-C1 plasmids were
extracted, digested with EcoRI and BamHI and the
XBP1 and pEGFP-C1 were ligated using ligase and identified
via enzyme digestion. Human hepatocellular carcinoma HepG2 cells
were transfected with the purified plasmid and a blank vector was
used as the blank control. One day prior to transfection, HepG2
cells were trypsinized, counted and inoculated in a small plate
with a diameter of 35 mm and 50% of the cells were cohered on the
day of transfection. At 48 h after transfection, images of the
HepG2 cells were captured using a digital camera under a
fluorescence microscope.
Expression of XBP1 gene in E. coli
The following PCR amplification primers were
designed for the XBP1 gene: sense primer 5′-GCCGAATTCATGGCCAAGGACTTTCAAGA-3′
and antisense strand primer 5′-TAAGTCGACTCAGGCCACCTCGCCGGTGGC-3′;
the underlined sites are EcoRI and SalI enzyme
digestion sites, respectively. The target fragment was recovered
following PCR amplification with the pGEMT-XBP1 plasmid as the
template. The recovered target gene fragment was ligated into the
pGEM-T vector using T4 DNA ligase and those with the correct
sequence were selected for EcoRI/SalI enzyme
digestion, connected to the pET-32a(+) expression plasmid with the
same double enzyme digestion for identification. The pET32a(+)XBP1
plasmid identified to be correct was transformed into E.
coli BL21 for isopropyl-d-thiogalactopyranoside (IPTG) and
SDS-polyacrylamide gel electrophoresis (PAGE) analysis.
Western blot analysis
Anti-His monoclonal antibody diluted to 1:200 and
HRP-IgG diluted to 1:2,500 were used as the primary and secondary
antibodies, respectively. Based on conventional SDS-PAGE and
western blot analysis, membranes were blocked overnight in 5% dried
skimmed milk, incubated with the primary and secondary antibodies,
gently agitated at room temperature following the addition of color
reagent and exposed to X-rays.
Results
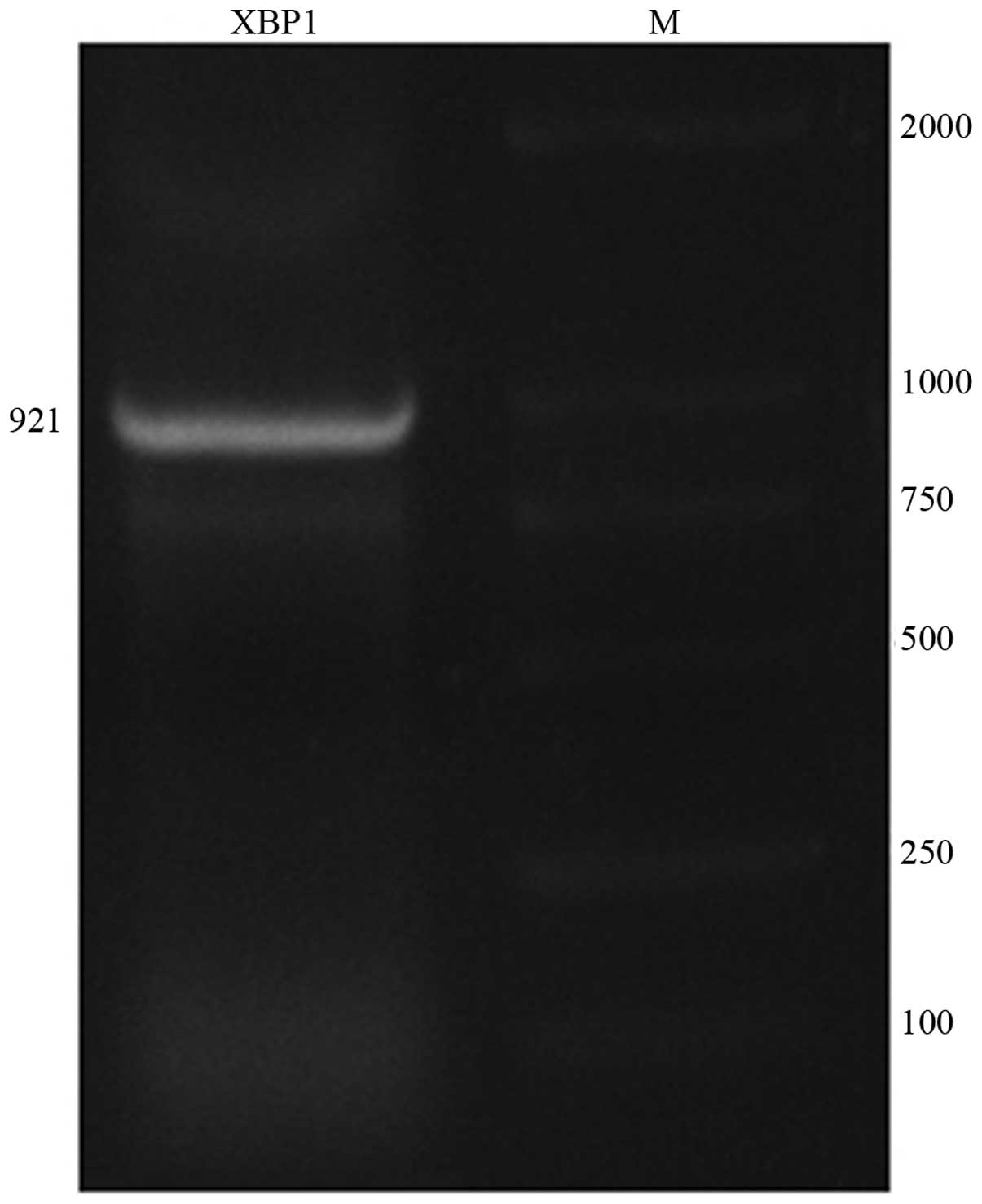
XBP1 gene amplification
The ORF of the XBP1 gene is 921 nt long and
the encoded product is composed of 307 amino acid residues
(Fig. 1). Identification through
enzyme digestion demonstrated that the pGEM-T-XBP1 plasmid digested
with EcoRI and BamHI enzyme had a normal size and was
confirmed as a plasmid.
Recombinant bait vector construction and
self-activation detection
The pGBKT7-XBP1 plasmid construction was analyzed
using the Vector NTI Suite 8.0 software; EcoRV and
SalI restriction enzyme digestion sites present in the
vector and target fragment were selected for enzyme digestion
analysis. Two EcoRV fragments, 7535 and 686 nt, and three
SalI fragments, 5664, 1809 and 748 nt, were obtained. The
XBP1 sequence amplified via PCR from the positive colonies
had a normal size. The colony did not grow on the SD/-Ade-Trp-His
solid culture medium plate, which indicates that this bait strain
had no self-activation phenomenon and subsequent screening by
mating may be performed.
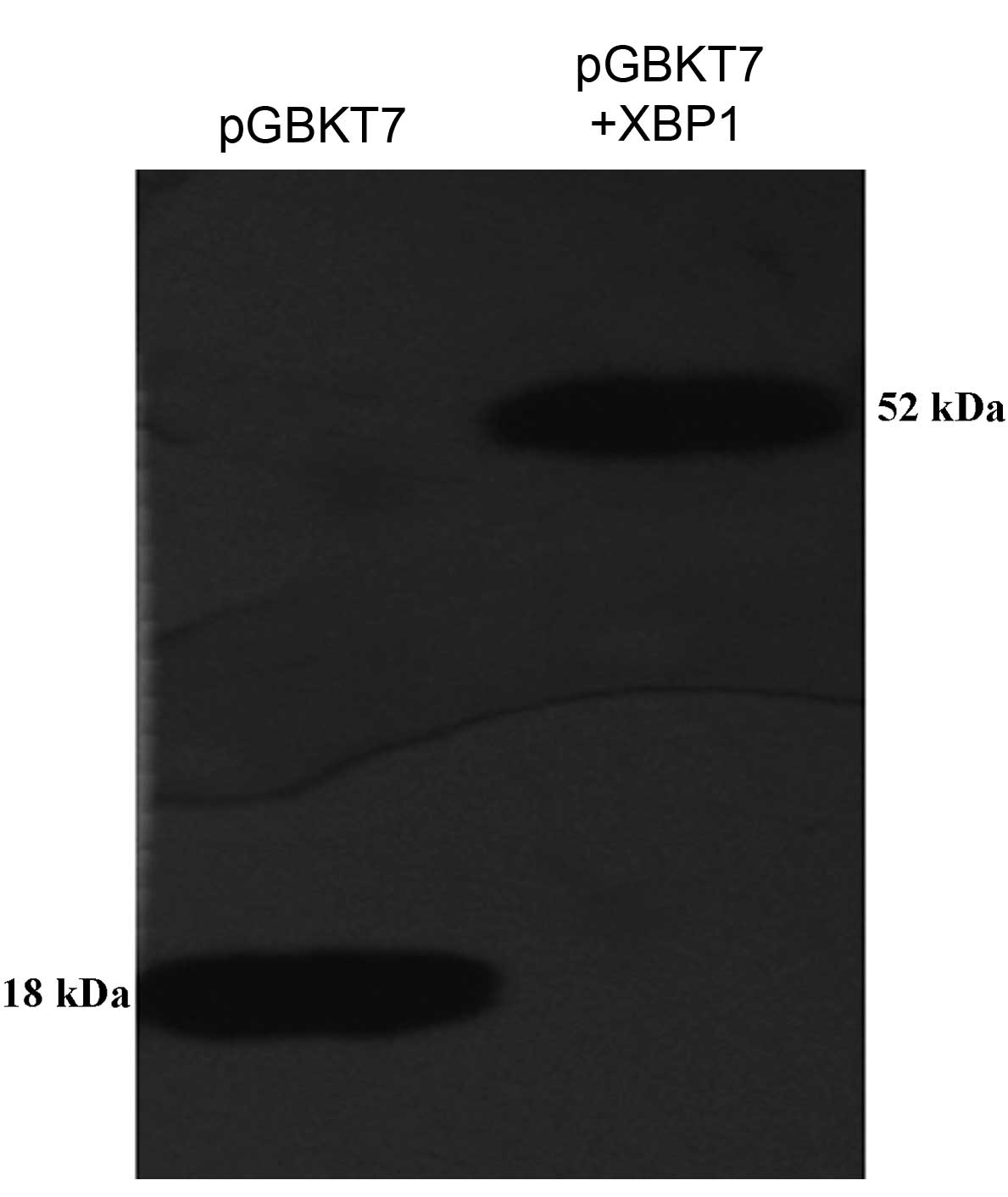
Western blot analysis
Following 12.5% SDS-PAGE of the yeast protein
extract, protein expression was detected by western blot analysis
(Fig. 2). The established ‘bait’
vector pGBKT7-XBP1 was transformed into yeast AH109 and the XBP1
fusion protein was stably expressed. The molecular weight of pGBKT7
in the positive control group was 18,200; XBP1, 33,770; and XBP1
fusion protein in the experimental group, 51,970. Western blot
analysis demonstrated that the yeast extract transforming the
pGBKT7 plasmid and pGBKT7-XBP1 plasmid revealed bands at the
corresponding molecular weight and its molecular weight was
consistent with the theoretical value.
Liver cDNA library screening
The clone numbers of mated product diluted to
1:1,000 and growing on SD/-Trp culture media could not be counted,
and those on SD/-Leu and SD/-Trp-Leu culture media were 187 and 14,
respectively. The survival rate of Y18 was calculated at
1.87×106 cfu/ml (restricted part), whereas that of the
diploid was 1.4×105 cfu/ml and the mating efficiency was
7.5%.
Blue and white screening of the QDO culture medium
containing x-α-gal revealed that blue colony was the positive
colony. On the D/-Trp/-Leu/-His/-Ade/X-α-gal culture medium, 86
positive colonies were screened, 83 yeast plasmids were
successfully extracted and 80 yeast plasmids were successfully
electrotransformed and cloned into E. coli.
The pACT2 library vector includes two BglII
enzyme digestion sites located on both sides of the polyclonal
site. The leukocyte library fragment was released with this enzyme
digestion and 68 plasmids were identified as correct via
BglII enzyme digestion.
The cDNA sequencing and homology analysis results
indicate that 36 positive clones were selected for sequencing and
20 known protein-encoding genes and 1 gene with unknown function
were obtained (Table I).
 | Table IResults of liver cDNA library
screening. |
Table I
Results of liver cDNA library
screening.
| No. | Coding protein with
known homologous sequence | Homologous clone
number | Homology (%) |
|---|
| 1 | Human
asialoglycoprotein receptor 1 | 12 | 98 |
| 2 | Human
betaine-homocysteine methyltransferase | 1 | 100 |
| 3 | Human solute carrier
family 25 | 3 | 100 |
| 4 | Human mitochondrial
DNA | 2 | 100 |
| 5 | Human diazepam
binding inhibitor | 2 | 100 |
| 6 | Transforming growth
factor β1 | 1 | 99 |
| 7 | Human cyclic AMP
response element binding protein 3 | 1 | 99 |
| 8 | Human retinol-binding
protein 4 | 1 | 99 |
| 9 | Human complement
factor B | 1 | 99 |
| 10 | Human CD74 molecule,
major histocompatibility complex, transcript 3 | 1 | 100 |
| 11 | Human metallothionein
2A | 1 | 100 |
| 12 | Human chromosome 10
clone RP11-45D20 | 2 | 98 |
| 13 | Human topoisomerase
IIβ 180-kDa | 1 | 97 |
| 14 | Human serum
albumin | 1 | 99 |
| 15 | Human pyruvic
dehydrogenase kinase 1 | 1 | 99 |
| 17 | Human serine
proteinase inhibitor | 1 | 100 |
| 18 | Smooth muscle
cell-related protein | 1 | 99 |
| 19 | Human complement
C9 | 1 | 100 |
| 20 | Human CD74 molecule,
major histocompatibility complex, transcript 2 | 1 | 100 |
| 21 | Gene 1 with unknown
function | 1 | 97–100 |
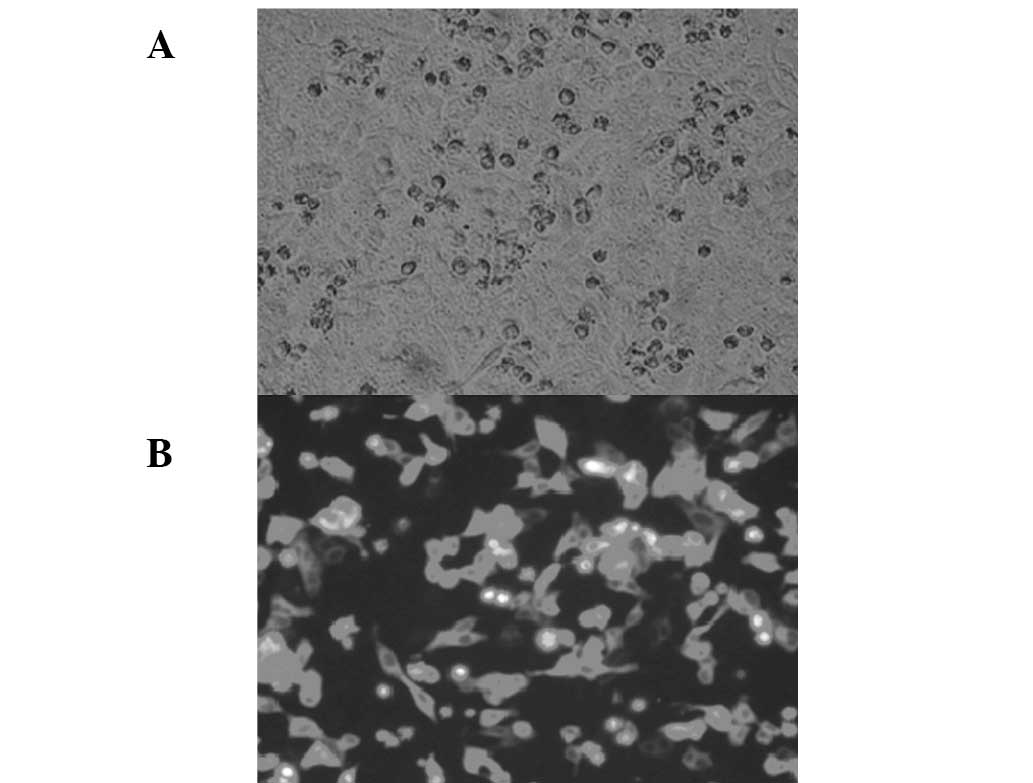
XBP1 subcellular localization
The XBP1 gene was successfully connected to
pEGFP-C1 and was identified as correct via enzyme digestion. As
shown in Fig. 3, the pEGFP-XBP1
expression plasmid was successfully expressed in HepG2 cells and
observation under a fluorescence microscope revealed green
fluorescence diffused in the cytoplasm of the transfected
cells.
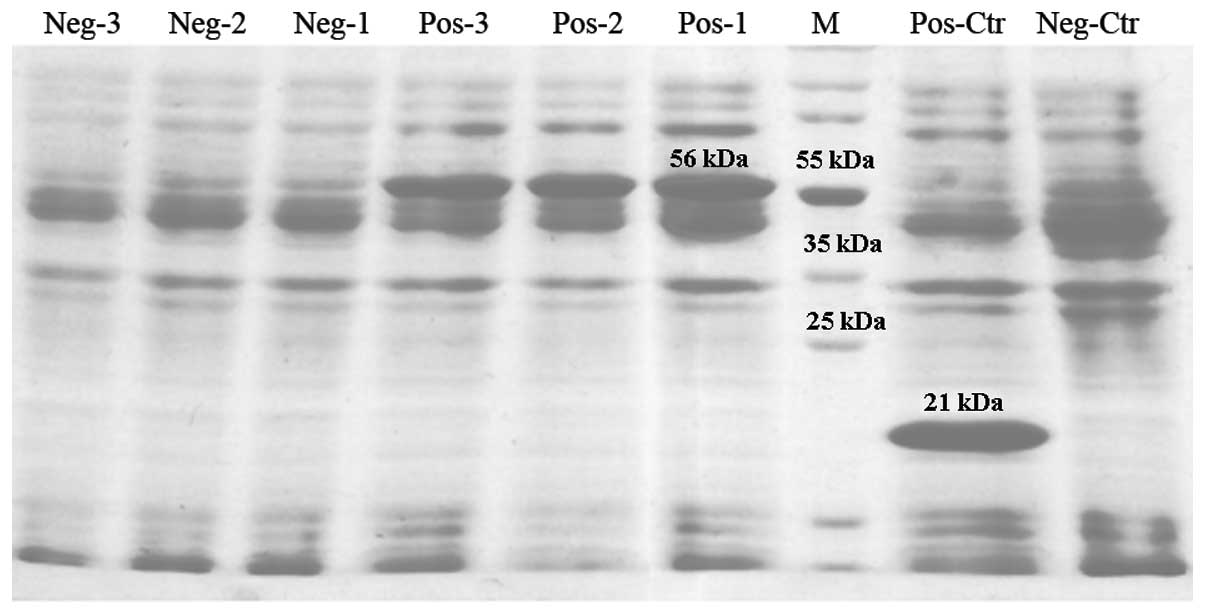
Expression of the XBP1 gene in E.
coli
The pcDNA3.1(−)-XBP1 plasmid was successfully
constructed and verified to be correct via enzyme digestion. The
PET32a(+)-XBP1 plasmid was correct under PCR amplification and
EcoRI and SalI enzyme digestion. The PET-32a-XBP1
recombinant protein in E. coli was detected using 12.5% PAGE
and revealed that the uninduced protein in E. coli BL21 was
expressed with numerous different molecular weights. The
IPTG-induced BL21 expression protein was ~56 kDa, the same as the
predicted molecular weight of the recombinant protein and inhibited
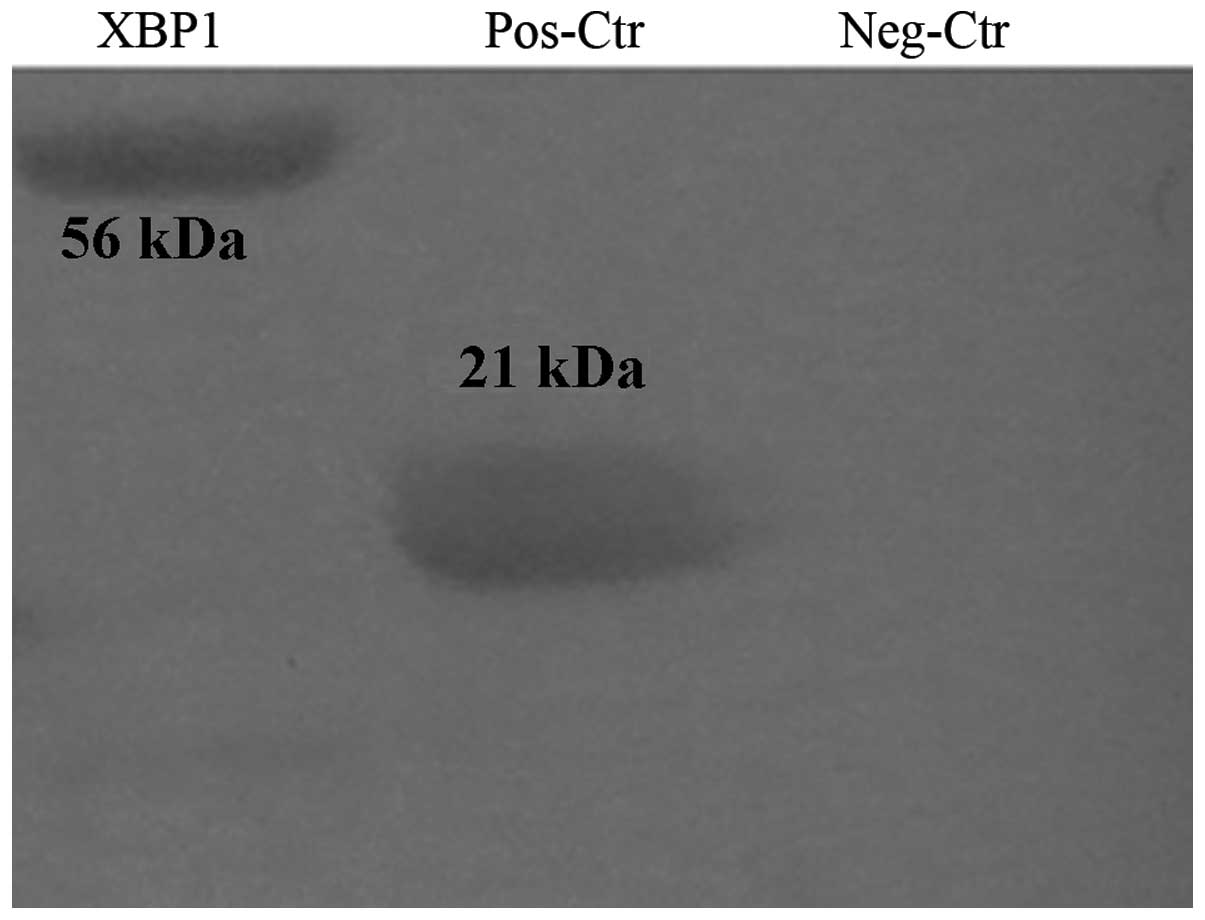
the non-target protein expression (Fig. 4). BL21-pET32a(+) and BL21-pET32a(+)
XBP1 bacterial proteins were detected by western blot analysis and
the results indicate marked banding at 21.8 kDa of the former and
55.6 kDa of the latter (Fig.
5).
Discussion
In the present study, XBP1 was inserted into
the pGBKT7 vector and the fusion protein-expressing recombinant
plasmid was constructed successfully. The plasmid was then
transfected into AH109. Western blot analysis (involving the use of
anti-myc monoclonal antibody prepared using the hybridoma
technique) demonstrated that the fusion protein was expressed,
which laid a basis for further screening, as well as the
application and development of the protein.
XBP1 binding protein genes in the white blood
cell library were screened using yeast two-hybrid assay. Human
asialoglycoprotein receptor 1, human betaine homocysteine
methyltransferase, human cAMP responsive element binding protein 3,
human retinol binding protein 4, human serine proteinase
inhibitors, human CD74 molecules, major histocompatibility complex,
human complement factor B, human complement C9, human pyruvate
dehydrogenase kinase 1, contractile fiber cell associated protein
2, human topoisomerase IIβ 180-kDa, metallothionein 2A and
transforming growth factor β1 were obtained. These molecules
perform important roles, consistent with results of bioinformatics
analysis (15,16).
In the present study, 12 homologous clones were
screened. ASGPR, the endocytic receptor of a heterologous oligomer,
is located on the surface of the cell membrane in the hepatocyte
facing the sinusoid. The functional area wherein ASGPR identifies
and binds with the lactose residue or acetyl galactosamine residue
is called the carbohydrate recognition domain, which includes two
subgroups, H1 and H2. The H1 subgroup is important in identifying
ligands and it mediates endocytosis via ASGPR (17). ASGPR identifies and specifically
binds with sugar chains that contain a galactose or N2-acetyl
galactosamine residue at the end and transports its ligand into the
lysosome for degradation via hepatocellular endocytosis. Therefore,
the main physiological function of ASGPR is the removal of
asialoglycoprotein, apoptotic cells, lipoproteins, etc. (18).
The serine proteinase inhibitor, a serine proteinase
activity regulator, is associated with blood coagulation,
fibrinolysis, complement activation, inflammatory reaction and
tissue reconstruction process and is involved in the inhibition of
tumor invasion and metastasis (19). It performs a transcription
regulation function via autophosphorylation (20). Special attention has been given to
the correlation between cAMP response element-binding (CREB) and
molecular nerve mechanism of learning and memory; CREB promotes the
formation of long-term memory among fruit flies, mice and other
animals (21).
The main function of the smooth muscle cell-related
protein is promoting cell survival, extending cell life, regulating
apoptosis, promoting vascular smooth muscle cell (VSMC)
proliferation and migration and promoting the mammary stromal
differentiation towards myofibroblasts (22). It inhibits cell growth (23) by inhibiting the transcription start
site (24), prevents the entry of
blood cells into the S phase and directly inhibits the action of
pluripotential hematopoietic stem cells.
The indirect enzyme-linked immunosorbent assay for
detecting the anti-HBx antibodies in serum was established using
the recombinant protein HBx to detect and observe changes in levels
of anti-HBx antibodies in the serum of patients with hepatitis B
(25). The XBP1 gene was
expressed using the E. coli system and validated via western
blot analysis. This expression is likely to lay the foundation for
further studies on the effect of X protein on the immunological
function of the host, experiments on further purification of the
immunogenic XBP1 protein and polyclonal antibody and provides the
basis for clinical examination. The XBP1 gene was
successfully connected to pEGFP and identified via enzyme
digestion. The pEGFP-XBP1 expression plasmid was successfully
expressed in HepG2 cells and fluorescence microscopy indicated that
green fluorescence was diffused in the cytoplasm of transfected
cells, but no green fluorescence protein expression was observed in
the cell nucleus. Knowledge of the subcellular localization of XBP1
to the cytoplasm generates additional understanding of gene
function. In future steps, the protein is likely to be purified to
obtain sufficient high-purity XBP1 protein for animal inoculation
and polyclonal and monoclonal antibodies against XBP1 prepared
using the hybridoma technology. Once the antibody is available,
immunohistochemical research may be performed to clarify the
correlation between the protein expression of the gene, its
expression mechanism and clinical disease evolution, thereby
revealing the biological and medicinal significance of the
gene.
Binding proteins screened using yeast two-hybrid
technology were classified as follows: i) proteins related to the
intracellular structure and cell growth; ii) proteins involved in
intracellular metabolism; iii) proteins involved in signal
transduction pathways, immunity and other related proteins; and iv)
proteins involved in DNA duplication, transcription, recombination
and repair. The identification of these binding proteins provides
new insight into the biological function of XBP1, HCV pathogenesis
and the reason for its malignant transformation. Analysis of the
XBP1 indicates that following its intracellular expression, the
expression of genes related to cell growth, differentiation,
material and energy metabolism, signal transduction and
tumorigenesis is increased. The present study indicates that XBP1
may affect numerous systems in vivo, providing new clues as
to the role of XBP1 and HBX in pathogenesis.
References
|
1
|
Wang Y, Liu H, Zhou Q and Li X: Analysis
of point mutation in site 1896 of HBV precore and its detection in
the tissues and serum of HCC patients. World J Gastroenterol.
6:395–397. 2000.PubMed/NCBI
|
|
2
|
Zhuang L, You J, Tang BZ, et al:
Preliminary results of Thymosin-a1 versus interferon-treatment in
patients with HBeA g negative and serum HBV DNA positive chronic
hepatitis B. World J Gastroenterol. 7:407–410. 2001.PubMed/NCBI
|
|
3
|
Henkler F, Hoare J, Waseem N, et al:
Intracellular localization of the hepatitis B virus HBx protein. J
Gen Virol. 82:871–882. 2001.PubMed/NCBI
|
|
4
|
Hoare J, Henkler F, Dowling JJ, et al:
Subcellular localization of the X protein of HBV infected
hepatocytes. J Med Virol. 64:419–246. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang H, Delgermaa L, Huang F, et al: The
transcriptional transactivation function of HBx protein is
important for its augmentation role in hepatitis B virus
replication. J Virol. 79:5548–5556. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang JL, Zhao WG, Wu KL, et al: Human
hepatitis B virus X protein promotes cell proliferation and
inhibits cell apoptosis through interacting with a serine protease
Hepsin. Arch Virol. 150:721–741. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan DW and Ngi O: Knock-down of hepatitis
B virus X protein reduces the tumorigenicity of hepatocellular
carcinoma cells. J Pathol. 208:372–380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park SG, Chung C, Kang H, Kim JY and Jung
G: Up-regulation of cyclin D1 by HBx is mediated by NF-κB2/BCL3
complex through κB site of cyclin D1 promoter. J Biol Chem.
281:31770–31777. 2006.PubMed/NCBI
|
|
9
|
Chen YB, Yan ML, Gong JP, et al:
Establishment of hepatocellular carcinoma multidrug resistant
monoclone cell line HepG2/mdr1. Chin Med J. 120:703–707.
2007.PubMed/NCBI
|
|
10
|
Li H, Cao HF, Wan J, Li Y, Zhu ML and Zhao
P: Growth inhibitory effect of wild-type Kras2 gene on a colonic
adenocarcinoma cell line. World J Gastroenterol. 13:934–938. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sambrook J and Russell DW: Molecular
Cloning: A Laboratory Manual. 3rd edition. Huang P: Science Press;
Beijing: 2002
|
|
12
|
Li K, Wang L, Cheng J, et al: Interaction
between hepatitis C virus core protein and translin protein - a
possible molecular mechanism for hepatocellular carcinoma and
lymphoma caused by hepatitis C virus. World J Gastroenterol.
9:300–303. 2003.
|
|
13
|
Lu YY, Wang L, Liu Y, et al: Interaction
between hepatitis b virus e antigen and the metal sulfur protein.
Jie Fang Jun Yi Xue Za Zhi. 28:451–453. 2003.(In Chinese).
|
|
14
|
Cheng EH, Sheiko TV, Fisher JK, Craigen WJ
and Korsmeyer SJ: VDAC2 inhibits BAK activation and mitochondrial
apoptosis. Science. 301:513–517. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karolchik D, Baertsch R, Diekhans M, et
al; University of California Santa Cruz. The UCSC Genome Browser
Database. Nucleic Acids Res. 31:51–54. 2003. View Article : Google Scholar
|
|
16
|
Rajasekaran S, Thapar V, Dave H and Huang
CH: Randomized and parallel algorithms for distance matrix
calculations in multiple sequence alignment. J Clin Monit Comput.
19:351–359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stocker RJ: The asialoglycoprotein
receptors: relationships between structure, function and
expression. Physiol Rev. 75:591–609. 1995.PubMed/NCBI
|
|
18
|
Hajoui O, Martin S and Alvarez F: Study of
antigenic sites on the asialoglycoprotein receptor recognized by
autoantibodies. Clin Exp Immunol. 113:339–345. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spring P, Nakashima T, Frederick M,
Henderson Y and Clayman G: Identification and cDNA cloning of
headpin, a novel differentially expressed serpin that maps to
chromosome 18q. Biochem Biophys Res Commun. 264:299–304. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Montrminy MR and Bilezikjan LM: Binding of
a nuclear protein to the cyclic-AMP response element of the
somatostatin gene. Nature. 328:175–178. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lundblad M, Andersson M, Winkler C, Kirik
D, Wierup N and Cenci MA: Pharmacological validation of behavioural
measures of akinesia and dyskinesia in a rat model of Parkinson’s
disease. Eur J Neurosci. 15:120–132. 2002.PubMed/NCBI
|
|
22
|
Isoda K, Kamezawa Y, Ayaori M, Kusuhara M,
Tada N and Ohsuzu F: Osteopontin transgenic mice fed a
high-cholesterol diet develop early fatty-streak lesions.
Circulation. 107:679–681. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abba MC, Laguens RM, Dulout FN and Golijow
CD: The c-myc activation in cervical carcinomas and HPV 16
infections. Mutat Res. 557:151–158. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tseng WF, Huang SS and Huang JS:
LRP-1/TbetaR-V mediates TGF-beta1-induced growth inhibition in CHO
cells. FEBS Lett. 562:71–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren F, Jin HY, Guo XH, et al: Erokaryotic
expression and clinical application of X gene of hepatitis B virus.
Wei Chang Bing Xue He Gan Bing Xue Za Zhi. 15:239–241. 2006.(In
Chinese).
|