Introduction
Cardiac hypertrophy, usually considered as an
effective compensation mechanism, can maintain or even increase
cardiac output. However, in the long term, persistent hypertrophy
can ultimately progress to cardiac dilatation, decreased ejection
fraction finally leading to heart failure (1). Pathological changes in ventricular
hypertrophy often manifest while the morphology of cardiomyocytes
is altered, such as changes in cardiomyocyte hypertrophy,
interstitial fibrosis (2,3) and gene expression (4). Clinically, cardiac hypertrophy caused
by various reasons involves different levels of cardiac matrix
changes, especially in hypertensive heart disease (5,6). It
has been reported in both animal and human studies that in left
ventricular hypertrophy (LVH), gene expression alterations in
connexin 43 (Cx43), as well as gap junction disorganization, are
the basis for the triggering and maintainence of arrhythmias
(7–9). Therefore, the regression of cardiac
hypertrophy is associated with a decreased risk of cardiovascular
diseases.
Hydrogen sulfide (H2S) is well known as a
noxious gas in living organisms (10). However, it is increasingly
recognized that like nitric oxide and carbon monoxide,
H2S is a member of the family of ‘gaseous transmitters’.
Studies have indicated that, although in low amounts,
H2S can be produced in mammalian tissues, and is
controlled by several pyridoxal-5′-phosphate (PLP)-dependent
enzymes, including cystathionine β-synthase (CBS), cystathionine
γ-lyase (CSE) (10,11), and a newly identified enzyme,
3-mercaptopyruvate sulfurtransferase (3-MST) (12,13).
Moreover, increasing evidence has shown that H2S plays
important roles in various systems, particularly in the
cardiovascular system (14),
regulating vascular tone (15) and
protecting heart from ischemic injury (16). These various actions highlight the
potential important functions of H2S for modulation of
the cardiovascular system. While cardiac hypertrophy is a major
risk factor for the development of several cardiovascular diseases,
it is still unknown whether endogenous H2S restrains
cardiac hypertrophy.
In the present study, an in vivo model of
abdominal aortic coarctation was used. This is the most commonly
used method in cardiac hypertrophy research (17). During this study, we investigated
the effects of H2S administration on cardiac hypertrophy
and fibrosis, levels of Ang-II in cardiac tissues and plasma, as
well as the expression levels of Cx43.
Materials and methods
Materials
The animal experiments complied with the guidance
for the Care and Use of Laboratory Animals of Shantou University
Medical College. Adult male Sprague-Dawley rats (170–190 g) were
provided by the Animal Department of Shantou University Medical
College.
Sodium hydrosulfide (NaHS) was purchased from Sigma
(St. Louis, MO, USA). The Ang-II assay ELISA kit for rat was
purchased from Uscnlife Sciences Co., Ltd. (Wuhan, China). The
rabbit monoclonal antibody against Cx43 was purchased from Abcam
PLC (UK).
Abdominal aortic constriction
Abdominal aortic coarctation was carried out as
described by Phrommintikul et al (17) with a few modifications. Rats were
anesthetized with sodium pentobarbital (40 mg/kg). Under sterile
conditions, the skin was cut open along the abdominal midline, 4 cm
away from the xiphoid process. The incision was sutured layer after
layer and the animals were injected with penicillin prophylaxis.
Sham-operated animals serving as controls were subjected to the
same surgical procedure except that the aorta was not
constricted.
Experimental design
Adult male Sprague-Dawley rats, 8-weeks old, were
divided randomly into 3 groups as shown in Table I: i) untreated control (normal,
n=10); ii) sham-operated group (sham, n=10); iii) abdominal aortic
coarctation group (AAC, n=80). One week later, those survival AAC
rats were divided randomly again into three groups: i) abdominal
aortic coarctation only (AAC, n=14); ii) surgery plus enalapril
given by direct gastric gavage (AAC+EN, 5 mg/kg/day, n=14); iii)
surgery plus hydrogen sulfide administered intraperitoneally (i.p.)
(AAC+H2S, 14 μmol/kg/day, n=14). Five weeks after the
surgery, the rats were anesthetized and their hearts were removed
for following analyses.
 | Table ILV weight normalized to body weight
(LVW/BW) was used as an index of cardiac mass for the determination
of cardiac hypertrophy. |
Table I
LV weight normalized to body weight
(LVW/BW) was used as an index of cardiac mass for the determination
of cardiac hypertrophy.
| Initial BW (g) | Final BW (g) | Increased BW
(g) | LVW (mg) | LVW/BW (mg/g) |
|---|
| normal | 180±10 | 276±31 | 96±23 | 582±62 | 2.11±0.14 |
| sham | 175±3 | 276±27 | 101±26 | 570±69 | 2.06±0.07 |
| AAC | 173±2 | 294±25 | 125±24 | 771±126a,b | 2.67±0.23a,b |
| AAC+EN | 179±9 | 234±22 | 54±17 | 590±67a,b,c | 2.50±0.17a,b,c |
|
AAC+H2S | 179±11 | 239±16 | 57±15 | 609±76a,b,c | 2.55±0.16a,b,c |
Determination of cardiac hypertrophy
index
The rats were weighed and then anesthetized with
sodium pentobarbital (40 mg/kg i.p.). The rat heart was removed and
rinsed thoroughly with cold saline, having washed off the blood.
The atria, great vessels, and the right ventricle along its septal
insertion were snipped off. Data were expressed as left ventricular
weight/body weight ratio (mg/g) and were used as an index of
cardiac hypertrophy.
Measurement of endogenous H2S
concentration in plasma
The first 75 μl of plasma was infused into a tube,
with the addition of 250 μl of 1% (w/v) zinc acetate and 425 μl of
distilled water, followed by successive addition of 20 μM
N-dimethyl-p-phenylenediamine sulphate in 7.2 mM HCl (133 μl) and
30 μM FeCl3 in 1.2 mM HCl (133 μl). The test tubes were
kept at room temperature for a 10-min incubation. Then, 250 μl of
10% tricholoacetic acid was added to the reaction mixture, to
remove the protein from the plasma. The OD was measured with a
spectrophotometer at 670 nm.
Histopathological and immunohistochemical
analyses
The ventricles were fixed in 10% formaldehyde and
cut into thin sections (5 μm), followed by staining with
hematoxylin and eosin and picrosirius red in order to determine the
degree of collagen fiber accumulation. The pathological slides were
stained with picrosirius red, and 8 fields were randomly selected
for observation.
Hearts from each group of rats were prepared for
immunohistochemical analysis. A rabbit monoclonal antibody specific
to Cx43 was applied at 4˚C overnight. After being washed with PBS,
the slides were incubated with biotinylated goat anti-rabbit IgG
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 30 min at
room temperature. The immunoreactivity was visualized by the
streptavidin peroxidase staining method.
Determination of hydroxyproline (Hyp)
concentration in cardiac tissue
Hyp was measured by the modified alkaline hydrolysis
method of Reddy and Enwemeka (18)
with modifications. The absorbance was read at 550 nm using a
spectrophotometer. Total collagen content was calculated from the
Hyp concentration assuming that Hyp constitutes 12.5% collagen.
Angiotensin II in plasma and cardiac
concentrations
The concentrations of angiotensin II within the
ventricle and plasma were measured with the method of ELISA.
Absorbance was recorded at 450 nm, and the concentration was
calculated from the generated standard curve of angiotensin II.
Statistical analyses
For comparison among groups, statistical analyses
were performed using one-way analysis of variance followed by post
hoc Tukey's test (SPSS 13.0, Chicago, IL). All values are expressed
as the means ± SD. Prism 4.0 (GraphPad Software, La Jolla, CA) was
used to produce graphs and curve fits. A P-value of <0.05 was
taken to indicate statistical significance.
Results
H2S ameliorates left ventricle
hypertrophy induced by abdominal aorta coarctation
To determine whether H2S affects the left
ventricle hypertrophy induced by aorta coarctation, abdominal
aortic constriction (AAC groups) was conducted. Sham-operated
animals (sham groups) serving as controls were subjected to the
same surgical procedure except that the aorta was not constricted.
The untreated animals (normal groups) were also used as
controls.
Thirty-five days after the surgery of aortic
constriction, the weight of the left ventricle (LVW) normalized to
body weight (BW) increased significantly. When compared to the sham
group, the ventricular mass index of the model group increased by
29.6% (Table I), while the weight
and quality index of the left ventricle of the group treated with
NaHS (14 μmol/kg/day) was reduced by 21.0% (P<0.05) and 4.49%
(P<0.05), respectively. The left ventricle weight and quality
index of the left ventricle of the enalapril-treated (5 mg/kg/day)
group decreased by 23.4% (P<0.05) and 6.38% (P<0.05),
respectively. Therefore, these results suggest that H2S
ameliorates the left ventricle hypertrophy induced by abdominal
aorta coarctation.
Endogenous H2S concentrations
in plasma
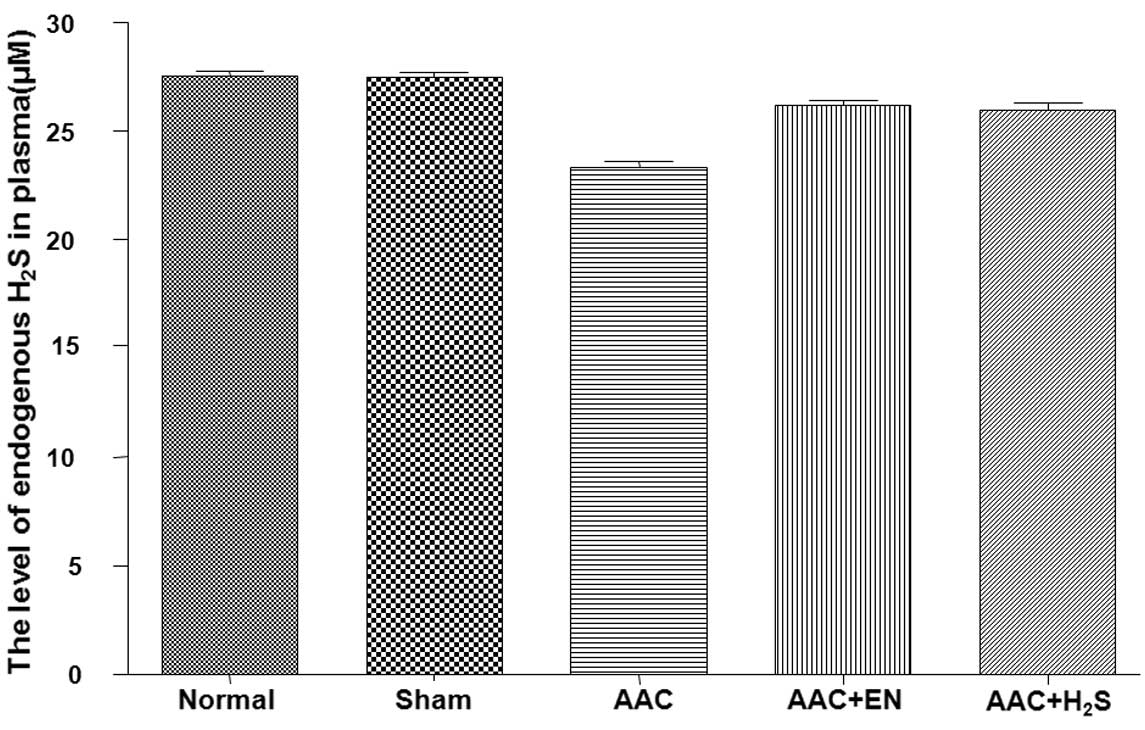
After surgery, the concentration of endogenous
H2S significantly declined (Fig. 1). The contents of endogenous
H2S of the AAC rats, which were treated with enalapril,
significantly increased, while the levels of endogenous
H2S in the rats which received NaHS i.p., obviously
increased.
H2S improves histological
changes in rats treated with abdominal aorta coarctation
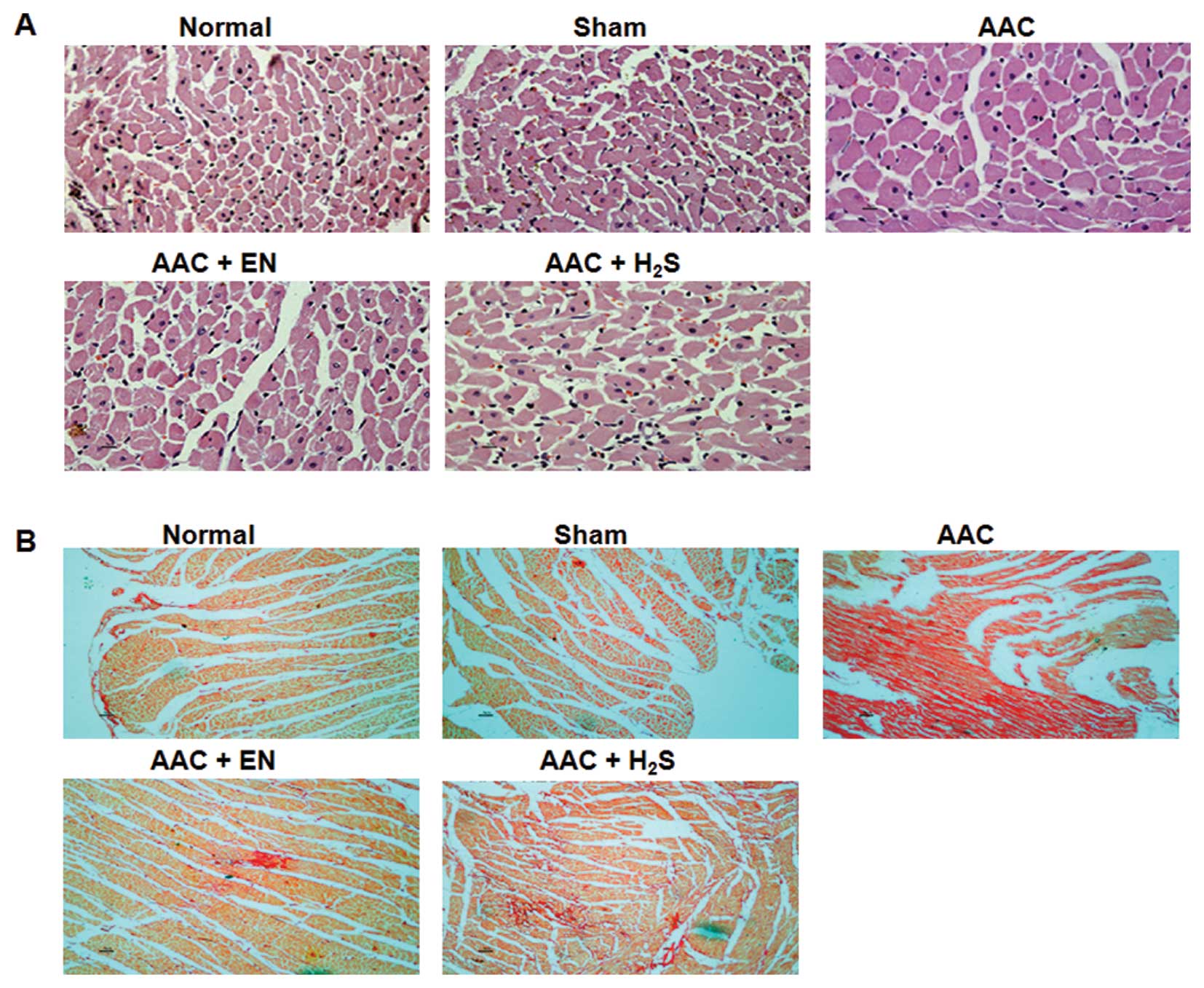
Microscopic examination showed that cardiomyocyte
hypertrophy, as evidenced by the minimum sizes of cells and
cardiomyocyte areas, was significantly increased in AAC rats
(Fig. 2A). The cardiomyocyte
hypertrophy in enalapril rats was lower than that in the model
control, but was still higher than that in the sham control.
Moreover, the administered H2S obviously decreased the
minimum sizes of cells and areas, when compared to AAC rats.
H2S significantly suppresses
development of cardiac fibrosis
The myocardial fibrosis conditions were measured
through Picro-Sirius red staining. The AAC rats showed a
significant increase in cardiac fibrosis levels when compared to
the sham group (Fig. 2B). For rats
in the enalapril or H2S groups, the collagen densities
were significantly lower than those in the AAC group, but were
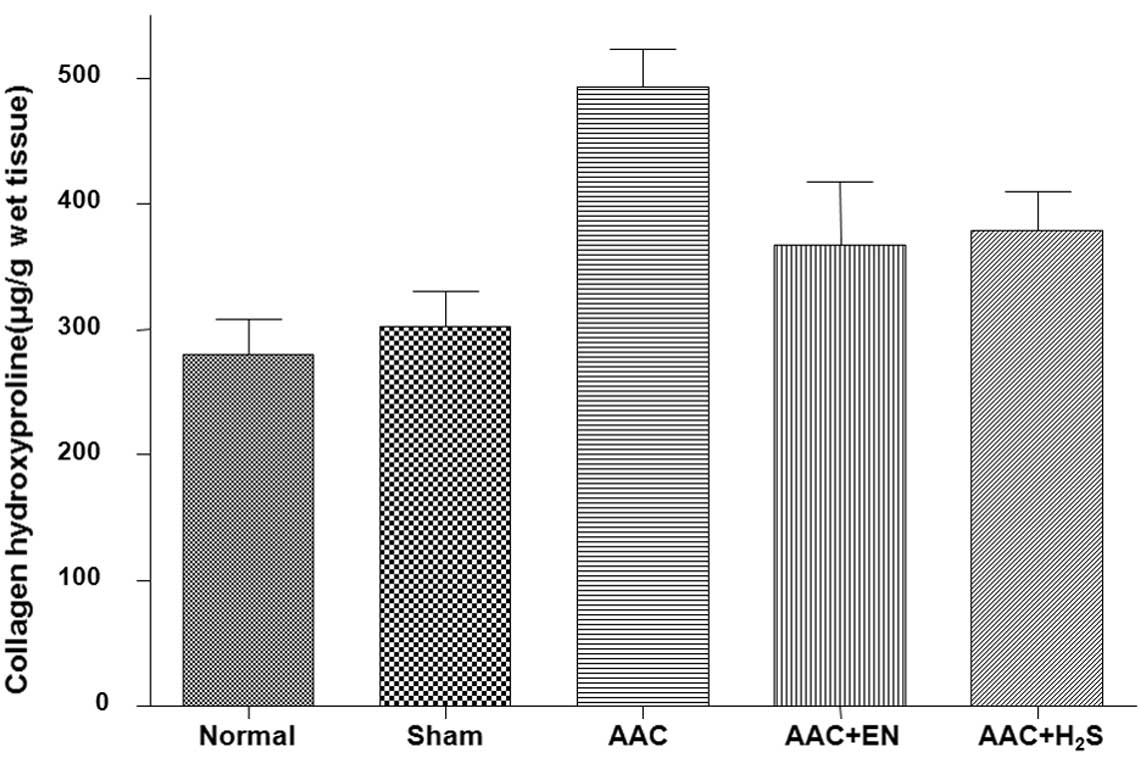
still higher than those in the sham group. The Hyp content was
significantly increased in the AAC group (Fig. 3). Treatments with enalapril or
H2S attenuated increases in the Hyp content.
Angiotensin II content in plasma and
cardiac tissues
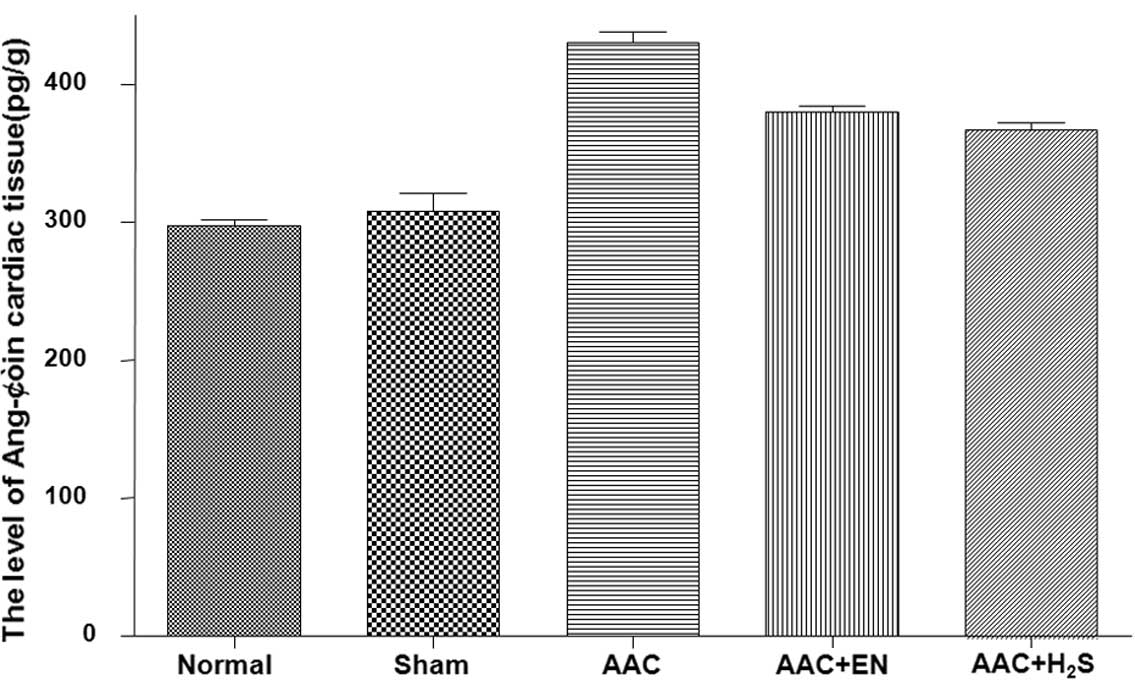
In the cardiac tissues, the Ang-II concentration
markedly increased in the AAC group when compared to the sham
control group (Fig. 4). The
increase in tissues was blocked by enalapril. H2S could
also obviously reduced the high Ang-II contents subjected to AAC.
However, in the plasma, the concentrations of Ang-II were not
significantly different among the groups (data not shown).
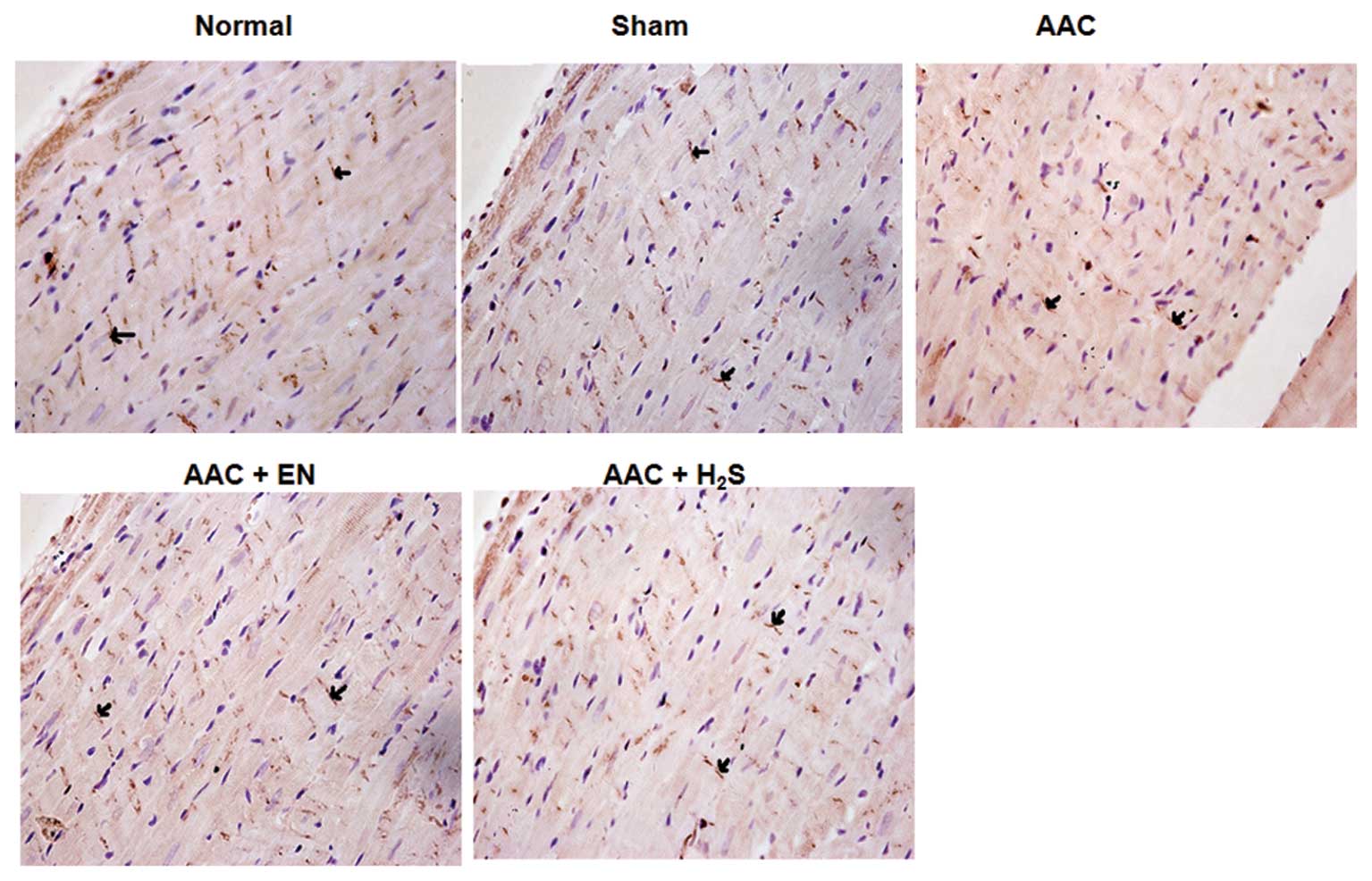
Expression of Cx43 in the myocardial
tissues by immunohistochemical analysis
In the AAC group, a significant decrease in the
density of Cx43 was observed, when compared to the sham group
(Fig. 5). However, enalapril
administration ameliorated expression of Cx43, which was also noted
in the H2S group.
Discussion
This study successfully designed a model of
myocardial hypertrophy through the partial ligation of abdominal
aorta of rats. The presence of myocardial hypertrophy was proven
with typical pathological changes, such as left ventricle
hypertrophy (LVH) (17) and
significant thickness of the left ventricle wall and cardiomyocyte
hypertrophy (19). The study
results showed that the myocardial tissue had increased fibrosis
and collagen deposition in the AAC rats. We also found that the
Ang-II expression was upregulated accompanied by cardiac
hypertrophy in the model group and this is consistent with previous
reports (20,21).
We discovered that compared with those of the sham
group, the levels of endogenous H2S, a newly recognized
gas transmitter (10,11), were greatly decreased in
AAC-induced LVH rats, suggesting that the endogenous H2S
system may be impaired in the process of cardiac hypertrophy
induced by pressure-overload. In addition, the H2S level
was notably elevated in rats administered enalapril, yet the
relevant mechanism is still not clear. The results also revealed
that exogenous administration of H2S via NaHS
significantly suppressed the development of cardiac hypertrophy
induced by pressure-overload, and also greatly downregulated the
Ang-II levels in cardiac tissue. These results suggest that
H2S plays a pivotal role in the development of pressure
overload-induced cardiac hypertrophy. The mechanism may be that
H2S, containing a sulfhydryl-group, interacts with the
zinc ion in the active center of the angiotensin-converting enzyme
(ACE) to modulate enzyme activity (22) suppressing the Ang-II-induced
cardiac hypertrophy. This is supported by the research of Laggner
et al (23), who found that
H2S exerted protection in the vasculature by reducing
the production of Ang-II and inhibiting bradykinin degradation.
However, this experiment found no difference in the expression of
Ang-II in plasma among the groups, proving that Ang-II in
circulation has no correlation with the development of cardiac
hypertrophy. Moreover, H2S has also been described as an
effective antioxidant which increases glutathione production and
suppresses oxidative stress and oxygen species production. Oxygen
species are often increased during cardiac hypertrophy in response
to various stressors (24,25). Therefore, although the effects of
H2S on oxidative stress could not be clarified in the
present study, we could not exclude the possibility that direct or
indirect suppression of oxidative stress might be the relevant
mechanism of H2S to suppress the development of cardiac
hypertrophy.
Myocardial fibrosis is often associated with cardiac
hypertrophy, with the increase in extracellular matrix, such as
collagen (5,6). Progressive myocardial fibrosis
contributes necessarily to an increase in cardiac muscle stiffness,
ultimately leading to impairment of cardiac function. The results
of this study showed that the rats treated with AAC had obvious
cardiac fibrosis, while treatment with enalapril obvious suppressed
the development of myocardial fibrosis, and this is consistent with
a previous report (26). Our
results revealed for the first time that H2S also
markedly prevents the development of cardiac fibrosis, decreasing
the collagen content in the cardiac tissue, yet the detailed
signaling pathway mechanism was not yet elucidated. Li et al
(27) reported that H2S
may reduce collagen accumulation in the pulmonary artery through
increasing its degradation by regulating matrix metalloproteinase
(MMPs) and metalloproteinase (TIMPs) activities. This needs to be
confirmed in future studies on hypertrophied ventricle.
Our study also showed that H2S
ameliorated the expression of Cx43 in cardiac tissue. Cx43 is the
principal connexin in the mammalian ventricle and has been proven
to have a close association with cardiac hypotrophy or arrhythmia
(28). Roell et al
(28) pointed out that engraftment
of Cx43-expressing myocytes has the potential of reducing
life-threatening post-infarct arrhythmias through the augmentation
of intercellular electrical conduction. We also found that the
expression of Cx43 was altered in the LVH rats, representing a
reduction in the number of step-to-step junctions, which was
consistent with previous research (29). Changes in Cx43 within the
hypertrophic ventricles were rather complicated, including the
synthesis, metabolism, redistribution, and mRNA expression of Cx43
(30). Research has demonstrated
that Ang-II, endothelin-I, and transforming growth factor-β
stimulate the expression of Cx43 (31). Our findings indicate that
endogenous H2S may play an important role in regulating
heart function and arrhythmia.
In this study we investigated the chronic effects of
exogenous H2S and proposed novel protective effects of
H2S on cardiac hypertrophy and fibrosis. The limitation
is that we only observed the ameliorating- effects of
H2S on cardiac hypertrophy and fibrosis, but did not
investigated the direct regulating effects of H2S on ACE
activity and mRNA expression. Neither did we observe the oxidative
stress changes in myocytes, which will be the future research
direction of our team.
In summary, our results demonstrated that
H2S has some beneficial effects on deferring or
suppressing the development of left ventricle hypertrophy and
cardiac fibrosis induced by abdominal aorta coarctation in rats.
The mechanisms may be at least partially related to H2S,
regarding its modulation of intracardiac renin-angiotensin system
activity and Cx43.
Acknowledgements
This study was supported by the Foundation for
Natural Science of Guangdong Province, China (no. 6033503).
References
|
1
|
C IndolfiE Di LorenzoC
PerrinoHydroxymethylglutaryl coenzyme A reductase inhibitor
simvastatin prevents cardiac hypertrophy induced by pressure
overload and inhibits p21ras
activationCirculation10621182124200210.1161/01.CIR.0000034047.70205.97
|
|
2
|
K YasunariK MaedaM NakamuraLeft
ventricular hypertrophy and angiotensin II receptor blocking
agentsCurr Med Chem Cardiovasc Hematol
Agents36167200510.2174/156801605277334215638745
|
|
3
|
T NishikimiH MatsuokaCardiac
adrenomedullin: its role in cardiac hypertrophy and heart
failureCurr Med Chem Cardiovasc Hematol
Agents3231242200510.2174/156801605436824115974887
|
|
4
|
I ManabeT ShindoR NagaiGene expression in
fibroblasts and fibrosis: involvement in cardiac hypertrophyCirc
Res9111031113200210.1161/01.RES.0000046452.67724.B812480810
|
|
5
|
CG BrillaRC FunckH RuppLisinopril-mediated
regression of myocardial fibrosis in patients with hypertensive
heart
diseaseCirculation10213881393200010.1161/01.CIR.102.12.138810993857
|
|
6
|
B LópezR QuerejetaN VaroUsefulness of
serum carboxy-terminal propeptide of procollagen type I in
assessment of the cardioreparative ability of antihypertensive
treatment in hypertensive
patientsCirculation104286291200111457746
|
|
7
|
BE TeunissenHJ JongsmaMF
BierhuizenRegulation of myocardial connexins during hypertrophic
remodelingEur Heart
J2519791989200410.1016/j.ehj.2004.08.00715541833
|
|
8
|
S KostinS DammerS HeinConnexin43
expression and distribution in compensated and decompensated
cardiac hypertrophy in patients with aortic stenosisCardiovasc
Res62426436200410.1016/j.cardiores.2003.12.01015094362
|
|
9
|
SB DanikF LiuJ ZhangModulation of cardiac
gap junction expression and arrhythmic susceptibilityCirc
Res9510351041200410.1161/01.RES.0000148664.33695.2a15499029
|
|
10
|
PK MooreM BhatiaS MoochhalaHydrogen
sulfide: from the smell of the past to the mediator of the
future?Trends Pharmacol
Sci24609611200310.1016/j.tips.2003.10.00714654297
|
|
11
|
CS TangXH LiJB DuHydrogen sulfide as a new
endogenous gaseous transmitter in the cardiovascular systemCurr
Vasc Pharmacol41722200610.2174/15701610677520314416472173
|
|
12
|
N ShibuyaM TanakaM
Yoshida3-Mercaptopyruvate sulfurtransferase produces hydrogen
sulfide and bound sulfane sulfur in the brainAntioxid Redox
Signal11703714200910.1089/ars.2008.225318855522
|
|
13
|
N ShibuyaY MikamiY KimuraVascular
endothelium expresses 3-mercaptopyruvate sulfurtransferase and
produces hydrogen sulfideJ
Biochem146623626200910.1093/jb/mvp11119605461
|
|
14
|
JW ElrodJW CalvertJ MorrisonHydrogen
sulfide attenuates myocardial ischemia-reperfusion injury by
preservation of mitochondrial functionProc Natl Acad Sci
USA1041556015565200710.1073/pnas.070589110417878306
|
|
15
|
W ZhaoJ ZhangY LuThe vasorelaxant effect
of H2S as a novel endogenous gaseous K (ATP) channel
openerEMBO J2060086016200111689441
|
|
16
|
JS BianQC YongTT PanRole of hydrogen
sulfide in the cardioprotection caused by ischemic preconditioning
in the rat heart and cardiac myocytesJ Pharmacol Exp
Ther316670678200610.1124/jpet.105.09202316204473
|
|
17
|
A PhrommintikulL TranA KompaEffects of a
Rho kinase inhibitor on pressure overload induced cardiac
hypertrophy and associated diastolic dysfunctionAm J Physiol Heart
Circ
Physiol294H1804H1814200810.1152/ajpheart.01078.200718245565
|
|
18
|
K ReddyCS EnwemekaA simplified method for
the analysis of hydroxyproline in biological tissuesClin
Biochem29225229199610.1016/0009-9120(96)00003-68740508
|
|
19
|
Y IzumiyaS KimY IzumiApoptosis
signal-regulating kinase 1 plays a pivotal role in angiotensin
II-induced cardiac hypertrophy and remodelingCirc
Res93874883200310.1161/01.RES.0000100665.67510.F514551246
|
|
20
|
B DahlöfLeft ventricular hypertrophy and
angiotensin II antagonistsAm J Hypertens141741822001
|
|
21
|
A GonzálezB LópezJ DíezFibrosis in
hypertensive heart disease: role of the
renin-angiotensin-aldosterone systemMed Clin North Am8883972004
|
|
22
|
CM ParkRL NagelWE BlumbergSulfhemoglobin.
Properties of partially sulfurated tetramersJ Biol
Chem2618805881019863722175
|
|
23
|
H LaggnerM HermannH EsterbauerThe novel
gaseous vasorelaxant hydrogen sulfide inhibits
angiotensin-converting enzyme activity of endothelial cellsJ
Hypertens2521002104200710.1097/HJH.0b013e32829b8fd0
|
|
24
|
Y KimuraY GotoH KimuraHydrogen sulfide
increases glutathione production and suppresses oxidative stress in
mitochondriaAntioxid Redox
Signal12113201010.1089/ars.2008.228219852698
|
|
25
|
M SeddonYH LooiAM ShahOxidative stress and
redox signalling in cardiac hypertrophy and heart
failureHeart93903907200710.1136/hrt.2005.06827016670100
|
|
26
|
M PahorR BernabeiA SgadariEnalapril
prevents cardiac fibrosis and arrhythmias in hypertensive
ratsHypertension18148157199110.1161/01.HYP.18.2.1481885222
|
|
27
|
X LiH JinG BinEndogenous hydrogen sulfide
regulates pulmonary artery collagen remodeling in rats with high
pulmonary blood flowExp Biol Med
(Maywood)234504512200910.3181/0807-RM-23019234054
|
|
28
|
W RoellT LewalterP SasseEngraftment of
connexin 43-expressing cells prevents post-infarct
arrhythmiaNature450819824200710.1038/nature0632118064002
|
|
29
|
L BacharovaJ PlandorovaJ KlimasDiscrepancy
between increased left ventricular mass and ‘normal’ QRS voltage is
associated with decreased connexin 43 expression in early stage of
left ventricular hypertrophy in spontaneously hypertensive ratsJ
Electrocardiol417307342008
|
|
30
|
NJ SeversGap junction-mediated
intercellular signaling in health and diseaseNovartis Foundation
Symposium2191882111999
|
|
31
|
AJ van WamelC RuwhofLE van der
Valk-KokshoomThe role of angiotensin II, endothelin-I and
transforming growth factor-beta as autocrine/paracrine mediators of
stretch-induced cardiomyocyte hypertrophyMol Cell
Biochem218113124200111330825
|