Introduction
Rheumatoid arthritis (RA) is characterized by the
proliferation of fibroblast-like synoviocytes (FLSs) in affected
joints. The onset of RA causes dramatic morphological changes in
the synovial membrane, including thickening of the intimal lining.
In addition, the FLSs in RA produce various cytokines and
chemokines that recruit more immune cells into the joint cavities,
and immune cells infiltrate into the proliferating synovial
membranes, forming a pannus that exhibits aggressive, tumor-like
growth and invades and erodes the cartilage and subchondral bone
(1). The FLSs in the pannus
resemble immature, transformed fibroblasts and are, in many ways,
different from normal FLSs (e.g., invasiveness and cartilage
degradation) (2,3). The mechanisms underlying the
tumor-like phenotype of FLSs in RA are poorly understood. Many
attempts have been made to explain the phenotype by
dedifferentiation of normal FLSs, presumably through an
accumulation of genetic and epigenetic abnormalities similar to
cancer (4). A number of
proto-oncogenes, including myc, ras and fos, are overexpressed in
the FLSs of RA (5), but no common
genetic abnormalities have been identified in these genes. Somatic
mutations in the tumor-suppressor gene p53 are associated with RA
(6), but a role for these
mutations in the ‘pseudotransformed’ FLS phenotype is not known.
Slowing of the rapid growth of FLSs after passage in culture is
also against the ‘transformation’ hypothesis (7).
The pannus is comprised of many cell types,
including inflammatory, immune and mesenchymal cells. A fraction of
the heterogeneous FLS population in RA was recently found to have
properties usually associated with mesenchymal stem cells (MSCs).
When appropriately stimulated in culture, a proportion of the FLSs
differentiates into chondrocytes, osteoblasts, adipocytes and
muscle cells (8,9). Animal studies have shown that bone
marrow-derived mesenchymal cells (BM MSCs) appear to contribute to
synovial proliferation (10,11).
By contrast, local inflammatory cytokines suppress the
differentiation of MSCs in RA-affected joints (12,13).
Thus, arthritic FLSs have been suggested to contain a substantial
fraction of BM MSCs, but they are under-differentiated because of
the inflammatory milieu in arthritic joints (14). Inflammatory milieu and hypoxia are
characteristic of arthritic joints. The inflammatory milieu may
induce the dedifferentiation of terminally differentiated cells
(e.g., chondrocytes) in arthritic joints (15). In addition, inflammation-induced
hypoxia may induce the redifferentiation of dedifferentiated
chondrocytes (16).
In this study, to better understand the tumor-like
phenotypes of arthritic FLSs, we investigated whether inflammatory
stimuli and hypoxia in arthritic joints induce the
dedifferentiation of FLSs into mesenchymal stem-like cells, and
whether the dedifferentiated FLSs contribute to the tumor-like
phenotypes of RA FLSs. We compared the expression of MSC surface
markers on fibroblasts from RA and osteoarthritis (OA) patients,
dermal fibroblasts and bone marrow. In addition, we examined
whether synovial or dermal fibroblasts exposed to inflammatory
stimuli and hypoxia have altered expression of MSC surface
markers.
Materials and methods
Cell culture
All in vitro experiments were carried out
with FLSs derived from patients with RA or OA who met the 1987
American College of Rheumatology criteria for diagnosis, had been
treated with non-biological disease-modifying anti-rheumatic drugs
(DMARDs), and underwent therapeutic joint surgery. The synovial
tissues were collected from patients after obtaining informed
consent. The FLSs were isolated and grown in Dulbecco's modified
essential medium (DMEM; low glucose; Invitrogen, Carlsbad, CA,
USA), supplemented with 10% (v/v) fetal bovine serum (FBS;
Invitrogen) and 1X antibiotic-antimycotic (Invitrogen).
Normal dermal fibroblasts were purchased from Modern
Cell and Tissue Technologies (Seoul, Korea) and cultured in DMEM
(high glucose). When the cells had grown to confluence, they were
detached using cell dissociation buffer (Invitrogen) and split at a
1:3 ratio. FLSs from 3 patients at passage 3 were used in all
experiments.
To test the effect of pro-inflammatory cytokines on
dedifferentiation of cells, the cells were cultured for 3 days in
the presence or absence of pro-inflammatory cytokines. For hypoxic
effect, hypoxic conditions were generated by incubating the cells
at 2% O2 in a hypoxic chamber gassed with a combination
of N2 and CO2 (Invivo2 200; Ruskinn
Technology Ltd., Pencoed, UK).
BM MSCs were purchased from Innomedi (Seoul, Korea).
The BM MSCs were expanded for 9–11 days in 100-mm culture dishes,
and then re-plated in 150-mm culture dishes with 0.25% trypsin-EDTA
(Invitrogen) at a density of 5×105 cells. FACS analysis
was performed after culturing for 15 days. This study was approved
by the Institutional Review Board for Human Research of Kyung Hee
University Hospital at Kangdong.
FACS analysis of stem cell markers
The FLSs were treated with cell dissociation buffer
(Invitrogen) for 20 min in a 37˚C incubator and filtered through a
40-μm cell strainer. The dissociated cells (2×105 cells)
were resuspended immediately in PBA (1% bovine serum albumin, 0.02%
NaN3 in PBS, pH 7.4) and incubated with
phycoerythrin-conjugated anti-CD24 (BD Biosciences, Seoul, Korea),
anti-CD44 (BD Biosciences), anti-CD90 (BD Biosciences), anti-CD106
(BD Biosciences), anti-CD146 (BD Biosciences) and anti-STRO-1
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 30 min at 4˚C.
After washing with PBA, the propidium iodide (PI)-negative cells
were analyzed for antibody binding using FACSCalibur (BD
Biosciences) and Cell Quest software (BD Biosciences).
Results
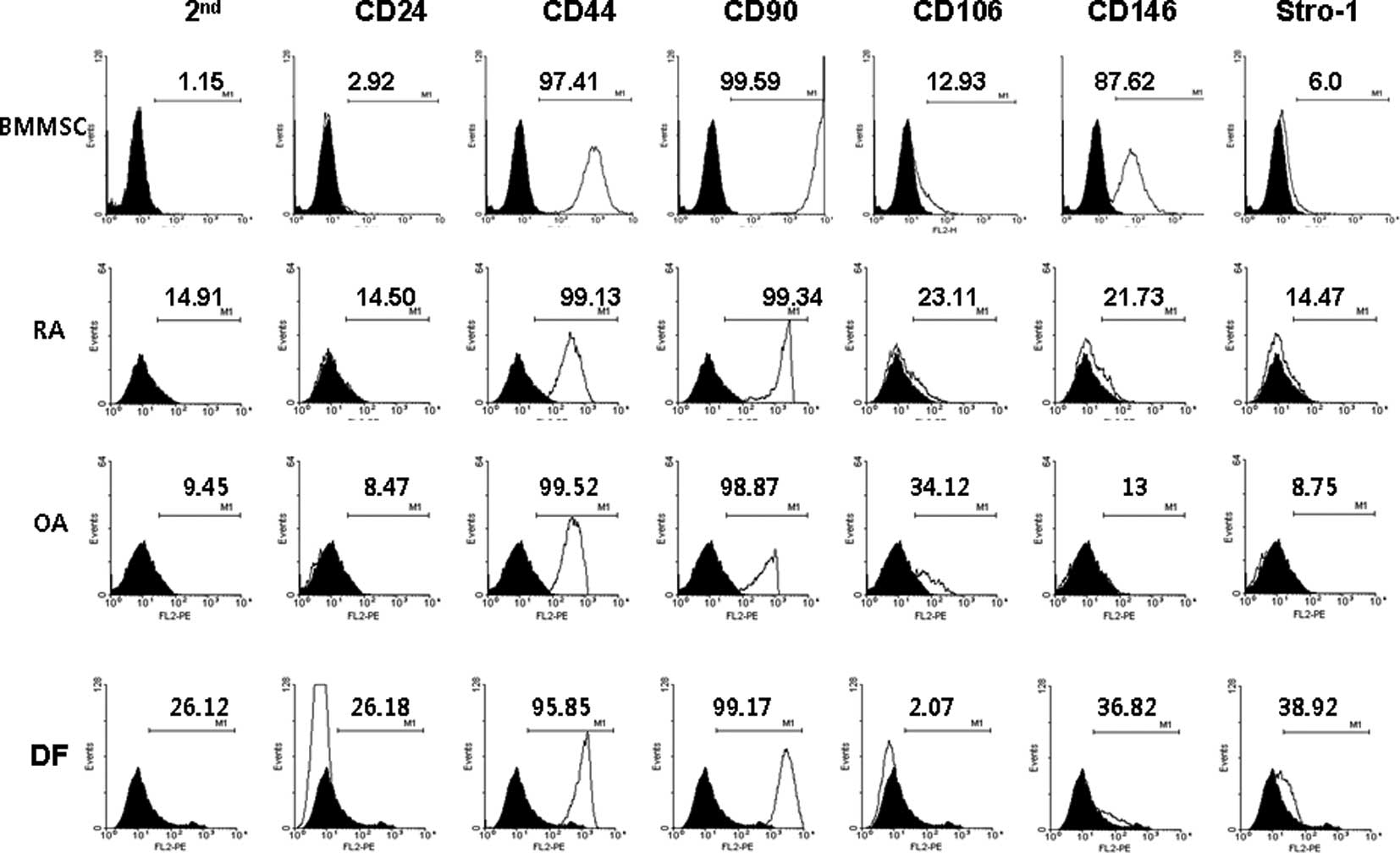
The MSC surface markers CD44 and CD90 were expressed
constitutively in all four cell types (Fig. 1). Only BM MSCs expressed CD146.
None of the cells expressed CD24. The expression in FLSs from RA
patients was distinctly different from that of normal dermal
fibroblasts. Unexpectedly, the expression of stem cell markers in
FLSs from RA patients was similar to that of FLSs from OA patients.
One reason for the lack of difference may be the in vitro
cell culture. The expression of stem cell markers may disappear
during cell culture under conditions lacking inflammation. To
evaluate this limitation, the FLSs from OA patients and normal
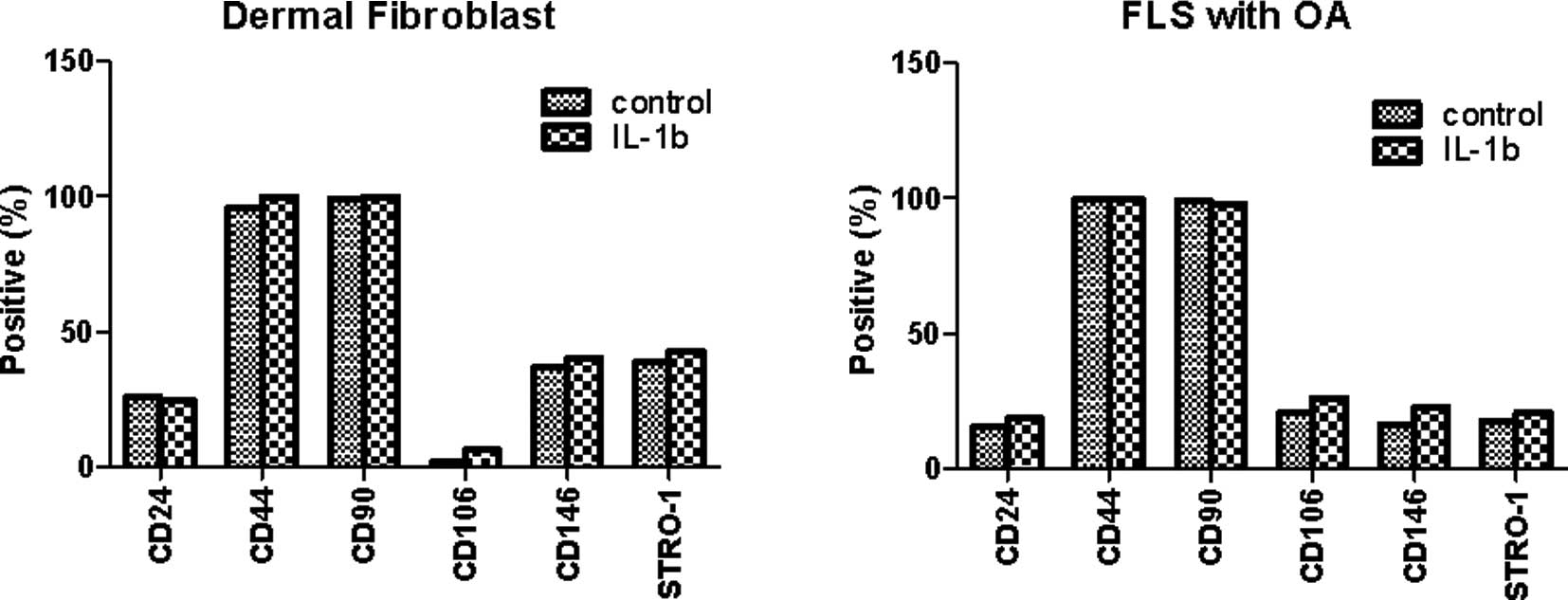
dermal fibroblasts were cultured for 72 h in the presence or
absence of 10 ng/ml IL-1β and the stem cell markers were compared
to those of unstimulated FLSs. As shown in Fig. 2, the expression of CD24, CD44 and
CD90 was similar regardless of IL-1β stimulation. The expression of
CD106, CD146 and Stro-1 was also not significantly different in
response to IL-1β stimulation. Next, we investigated whether the
expression pattern was affected when the cells were cultured under
hypoxic conditions (2% O2 concentration), which is one
of the factors affecting the expression of various genes in
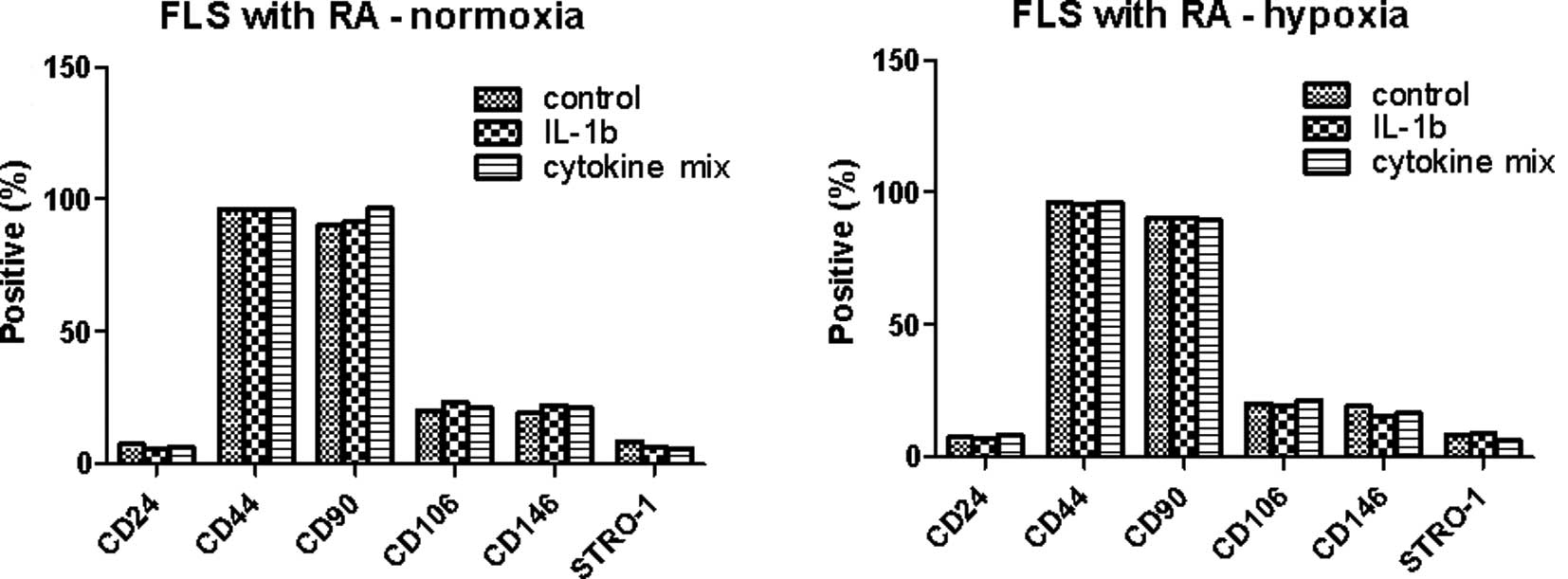
inflammatory arthritic joints. The FLSs from RA patients were
cultured for 72 h with IL-1β (10 ng/ml) or a combination of
cytokines (1 ng/ml each IL-1β, TNF-α, IL-6, IL-17, IL-22 and IL-23)
in the presence or absence of hypoxia (Fig. 3). The MSC markers in the RA FLSs
were not significantly different in the presence of
pro-inflammatory stimulation or under hypoxic conditions.
Discussion
We investigated whether the MSC markers in FLSs from
RA patients indicated dedifferentiation into MSCs in response to
pro-inflammatory cytokines or hypoxic conditions, as this
dedifferentiation may contribute to the tumor-like growth of
synoviocytes in RA during progressive inflammation. Thus, we
compared the expression of MSC surface markers (CD24, CD44, CD90,
CD106, CD146 and Stro-1) on FLSs from RA or OA patients, dermal
fibroblasts and BM MSCs. However, we did not identify any
significant changes in response to pro-inflammatory stimulation or
hypoxic conditions.
Extensive analyses have identified MSC surface
markers in rat, mice and humans (17–20).
Human MSCs have been reported to be positive for CD44, CD90 and
CD105, and negative for CD14, CD34 and CD45 (20). Other cell markers, such as CD106,
CD146 and Stro-1, are also expressed on the surface of MSCs
(21), but their expression
patterns change depending on the culture conditions or specific
types of stimulation (22–24). Surface markers are also
down-regulated with passaging (25). By contrast, the stem cell markers
in chondrocyte dedifferentiation have been employed in autologous
chondrocyte implantation (26).
Chondrocytes isolated from a small biopsy of hyaline cartilage are
expanded ex vivo, and once a sufficient number of cells are
obtained, the chondrocytes are then implanted in the cartilage
defect. However, the proliferating chondrocytes gradually lose
their differentiated phenotype. These phenotypic changes lead to
the production of an extracellular matrix with inferior
biomechanical properties. For tissue, such as cartilage, high
quality biomechanical properties are critical. Therefore,
investigating parameters that favor the redifferentiation of
dedifferentiated chondrocytes before implantation is critical.
Thus, the stem cell markers are investigated to determine
phenotypic changes. However, the stem cell marker changes in FLSs
in RA during FLS dedifferentiation have been rarely studied.
In this study, we attempted to find the parameters
or conditions that favor the dedifferentiation of FLSs, which may
lead to being able to control or inhibit the ‘tumor-like growth’ of
the synovial membrane of patients with RA. However, the in
vitro system in this study has limitations. Thus, we tried to
mimic the in vivo pro-inflammatory conditions, yet it was
still likely not enough to meet the in vivo conditions. In
addition, our in vitro systems may impair the
dedifferentiation of FLSs since the differentiation potential of
cultured cells decreases after every passage (27,28).
Thus, the cell surface markers may not have been significantly
altered by the pro-inflammatory cytokines and/or hypoxia due to
unknown factors in the cell culture. We also examined whether cell
passage affects the expression of stem cell markers. The stem cell
markers of FLSs at the second passage were compared to those of the
sixth passage when the FLSs were prepared at the same time. Cell
passage did not appear to affect the expression pattern of the stem
cell markers in FLSs, even in response to cytokine stimulation
(data not shown). In conclusion, these results indirectly suggest
that the pro-inflammatory milieu may not induce the
dedifferentiation of FLSs in arthritic joints.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (2011-0009061 to KSK; 2011-0002659 and
2011-0027795 to CJR).
References
|
1
|
GS FiresteinEvolving concepts of
rheumatoid
arthritisNature423356361200310.1038/nature0166112748655
|
|
2
|
T PapU Muller-LadnerRE GayS GayFibroblast
biology. Role of synovial fibroblasts in the pathogenesis of
rheumatoid arthritisArthritis
Res2361367200010.1186/ar11311094449
|
|
3
|
JC EdwardsFibroblast biology. Development
and differentiation of synovial fibroblasts in arthritisArthritis
Res2344347200010.1186/ar11011094446
|
|
4
|
GS FiresteinInvasive fibroblast-like
synoviocytes in rheumatoid arthritis. Passive responders or
transformed aggressors?Arthritis
Rheum3917811790199610.1002/art.17803911038912499
|
|
5
|
U Muller-LadnerJ KriegsmannRE GayS
GayOncogenes in rheumatoid arthritisRheum Dis Clin North
Am216756901995
|
|
6
|
Y YamanishiDL BoyleS RosengrenDR GreenNJ
ZvaiflerGS FiresteinRegional analysis of p53 mutations in
rheumatoid arthritis synoviumProc Natl Acad Sci
USA991002510030200210.1073/pnas.15233319912119414
|
|
7
|
R LafyatisEF RemmersAB RobertsDE YocumMB
SpornRL WilderAnchorage-independent growth of synoviocytes from
arthritic and normal joints. Stimulation by exogenous
platelet-derived growth factor and inhibition by transforming
growth factor-beta and retinoidsJ Clin
Invest8312671276198910.1172/JCI1140112784799
|
|
8
|
S YamasakiT NakashimaA KawakamiCytokines
regulate fibroblast-like synovial cell differentiation to
adipocyte-like cellsRheumatology
(Oxford)43448452200410.1093/rheumatology/keh09214734788
|
|
9
|
NJ ZvaiflerV TsaiS AlsalamehJ von KempisGS
FiresteinM LotzPannocytes: distinctive cells found in rheumatoid
arthritis articular cartilage erosionsAm J
Pathol1501125113819979060847
|
|
10
|
S NakagawaY ToritsukaS WakitaniBone marrow
stromal cells contribute to synovial cell proliferation in rats
with collagen induced arthritisJ Rheumatol232098210319968970047
|
|
11
|
L Marinova-MutafchievaRO WilliamsK FunaRN
MainiNJ ZvaiflerInflammation is preceded by tumor necrosis
factor-dependent infiltration of mesenchymal cells in experimental
arthritisArthritis Rheum46507513200210.1002/art.1012611840454
|
|
12
|
S MurakamiV LefebvreB de CrombrugghePotent
inhibition of the master chondrogenic factor Sox9 gene by
interleukin-1 and tumor necrosis factor-alphaJ Biol
Chem27536873692200010.1074/jbc.275.5.368710652367
|
|
13
|
L GilbertX HeP FarmerExpression of the
osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A)
is inhibited by tumor necrosis factor-alphaJ Biol
Chem27726952701200210.1074/jbc.M10633920011723115
|
|
14
|
X LiSS MakarovAn essential role of
NF-kappaB in the ‘tumor-like’ phenotype of arthritic
synoviocytesProc Natl Acad Sci USA10317432174372006
|
|
15
|
N WehlingGD PalmerC
PilapilInterleukin-1beta and tumor necrosis factor alpha inhibit
chondrogenesis by human mesenchymal stem cells through
NF-kappaB-dependent pathwaysArthritis
Rheum60801812200910.1002/art.2435219248089
|
|
16
|
E DuvalS LeclercqJM ElissaldeM DemoorP
GaleraK BoumedieneHypoxia-inducible factor 1alpha inhibits the
fibroblast-like markers type I and type III collagen during
hypoxia-induced chondrocyte redifferentiation: hypoxia not only
induces type II collagen and aggrecan, but it also inhibits type I
and type III collagen in the hypoxia-inducible factor
1alpha-dependent redifferentiation of chondrocytesArthritis
Rheum60303830482009
|
|
17
|
EH JavazonDC ColterEJ SchwarzDJ ProckopRat
marrow stromal cells are more sensitive to plating density and
expand more rapidly from single-cell-derived colonies than human
marrow stromal cellsStem
Cells19219225200110.1634/stemcells.19-3-219
|
|
18
|
MB EslaminejadA NikmahzarL TaghiyarS
NadriM MassumiMurine mesenchymal stem cells isolated by low density
primary culture systemDev Growth
Differ48361370200610.1111/j.1440-169X.2006.00874.x16872449
|
|
19
|
D DochevaC PopovW MutschlerM SchiekerHuman
mesenchymal stem cells in contact with their environment: surface
characteristics and the integrin systemJ Cell Mol
Med112138200710.1111/j.1582-4934.2007.00001.x17367499
|
|
20
|
MF PittengerAM MackaySC BeckMultilineage
potential of adult human mesenchymal stem
cellsScience284143147199910.1126/science.284.5411.14310102814
|
|
21
|
AF SteinertM KunzP PragerMesenchymal stem
cell characteristics of human anterior cruciate ligament outgrowth
cellsTissue Eng Part
A1713751388201110.1089/ten.tea.2010.041321247268
|
|
22
|
JW YangN de IslaC HuselsteinEvaluation of
human MSCs cell cycle, viability and differentiation in micromass
cultureBiorheology43489496200616912420
|
|
23
|
A WiesmannHJ BuhringC MentrupHP
WiesmannDecreased CD90 expression in human mesenchymal stem cells
by applying mechanical stimulationHead Face
Med28200610.1186/1746-160X-2-816573842
|
|
24
|
M HonczarenkoY LeM SwierkowskiI GhiranAM
GlodekLE SilbersteinHuman bone marrow stromal cells express a
distinct set of biologically functional chemokine receptorsStem
Cells2410301041200610.1634/stemcells.2005-031916253981
|
|
25
|
S HalfonN AbramovB GrinblatI GinisMarkers
distinguishing mesenchymal stem cells from fibroblasts are
downregulated with passagingStem Cells
Dev205366201110.1089/scd.2010.004020528146
|
|
26
|
HJ LeeBH ChoiBH MinSR ParkChanges in
surface markers of human mesenchymal stem cells during the
chondrogenic differentiation and dedifferentiation processes in
vitroArthritis Rheum6023252332200910.1002/art.2478619644865
|
|
27
|
CM DigirolamoD StokesD ColterDG PhinneyR
ClassDJ ProckopPropagation and senescence of human marrow stromal
cells in culture: a simple colony-forming assay identifies samples
with the greatest potential to propagate and differentiateBr J
Haematol107275281199910.1046/j.1365-2141.1999.01715.x
|
|
28
|
I SekiyaDC ColterDJ ProckopBMP-6 enhances
chondrogenesis in a subpopulation of human marrow stromal
cellsBiochem Biophys Res
Commun284411418200110.1006/bbrc.2001.489811394894
|