Introduction
Cyclin-dependent kinases (CDKs) play a pivotal role
in the control of aberrant cell cycle and/or the proliferation
mechanism in malignant cells (1).
Aberrations in the function of CDKs have also been found in
Alzheimer's and Parkinson's diseases, illnesses caused by viral
infections, ischemia or other proliferative disorders (2–4).
Therefore, small-molecule inhibitors have been designed to explore
the molecular functions of CDKs within a thereapeutic
framework.
Roscovitine
(2-(1-ethyl-2-hydroxyethylamino)-6-benzylamino-9-isopro-pylpurine)
is a new generation reversible inhibitor of CDKs, such as Cdc2,
Cdk2, Cdk5 and Cdk7, by behaving as a competitor for ATP binding
(5–8). Roscovitine triggers cell cycle arrest
at the G1 and G2/M phases (6) and
leads to apoptotic induction which can be determined in each phase
of the cell cycle (9). It has also
been shown that following roscovitine treatment, DNA synthesis is
inhibited and induces apoptosis by causing nucleolar fragmentation
in various cancer cells. Due to these cell growth-inhibiting
activities, roscovitine is being considered as a potential
anticancer agent and a suitable candidate for combination treatment
models (10–13). In vitro studies have
revealed that roscovitine is a promising therapeutic agent by
inducing apoptosis in prostate cancer (14), breast cancer (10,15)
and leukemia cells (16).
Roscovitine has also been presented as a sensitizing drug in
combination with other conventional therapeutic options in the
treatment of cancer (11,13).
Purvalanol was recently designed as a protein kinase
inhibitor with high selectivity for Cdc2 and CDK2 through
competitive inhibition of ATP binding resulting in G2/M cell cycle
arrest (17). Recent studies have
shown that purvalanol may increase drug-induced apoptosis by
inhibiting formation of CDK2/cyclin B and CDK2/cyclin A, and
CDK2/cyclin E and CDK5/p35 complexes (18–20).
Purvalanol treatment also led to significant downregulation of
anti-apoptotic molecules, such as survivin, Bcl-XL and
Bcl-2 by globally inhibiting RNA synthesis (21,22).
Purvalanol also induced the activation of caspase-dependent
apoptosis by altering mitochondrial membrane functions in various
cancer cell lines (23,24).
The natural polyamines (PAs) putrescine (Put),
spermidine (Spd) and spermine (Spm) are ubiquitous polycationic
amine derivatives found in all eukaryotic cells (25,26).
PAs are considered essential elements for cell proliferation,
differentiation and growth in normal and cancer cells (27–29).
Their cellular levels are referred to as critical regulators of
cell cycle, survival and death mechanisms (30). PA metabolic regulation is
characterized by several enzyme activities. Ornithine decarboxylase
(ODC) is a rate limiting enzyme which induces synthesis of Put from
L-arginine (31). High
accumulation of PAs in cells are regulated by PA catabolic pathway
players, spermidine/spermine N1-acetyltransferase (SSAT), spermine
oxidase (SMO) and polyamine oxidase (PAO). These enzymes induce
excretion of acetylated PA derivatives or provide a back-conversion
pathway by oxidizing several compounds in the cells (32). Previous reports have shown that PA
depletion by specific inhibitor DL-α-difluoromethylornithine (DFMO)
treatment may increase the apoptotic efficiency of drugs (33). Therefore, PA metabolic
pathway-targeted therapies are gaining importance in the increase
of combination therapy efficiency in clinics (34,35).
However, the molecular mechanism involved in drug-induced apoptosis
related to PA biosynthetic regulation has yet to be fully
understood.
In the present study, we aimed to determine the
potential role of CDK inhibitors, roscovitine and purvalanol, on
the apoptotic cell death mechanism related to the PA catabolic
pathway in Caco-2 colon carcinoma cells.
Materials and methods
Chemicals, antibodies and primers
Roscovitine (Sigma, St. Louis, MO, USA) and
purvalanol (Tocris Bioscience, Bristol, UK) were dissolved in DMSO
to make a 10-mM stock solution and stored at −20˚C. Put, Spd and
Spm standards were purchased from Sigma. 3,3-Dihexyloxacarbocyanine
iodide (DiOC6) was purchased from Calbiochem (La Jolla,
CA, USA). Caspase inhibitors (each 10-mM stock solution),
z-DEVD-FMK (caspase-3), z-LEHD-FMK (caspase-9), z-VAD-FMK (general
caspase) and Z-FA-FMK (negative caspase) were purchased from BD
Biosciences (San Jose, CA, USA).
β-actin (1:2,000), Bcl-XL (1:1,000), Bax
(1:1,000), PUMA (1:1,000), Bim (1:1,000), PARP (1:1,000), cleaved
PARP (1:1,000), cleaved caspase-3 (1:1,000) and pro-caspase-3
(1:1,000) anti-rabbit antibodies were purchased from Cell Signaling
Technology (CST; Danvers, MA, USA). ODC, SSAT and PAO anti-rabbit
antibodies (1:2,000) were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Horseradish peroxidase (HRP)-conjugated
secondary anti-rabbit and anti-mouse antibodies (1:5,000) were from
CST.
Cell culture
Caco-2 colon carcinoma cells (HTB-37) (ATCC) were
maintained in minimal essential medium (PAN Biotech, Aidenbach,
Germany) with 2 mM L-glutamine, 20% fetal calf serum (PAN Biotech),
1% non-essential amino acids (Biological Industries) and 100
units/100 mg/ml penicillin/streptomycin (Biological Industries,
Kibbutz Beit-Haemek, Israel) and grown in the presence of 5%
CO2 in humidified air at 37˚C.
Cell viability assay
Cells were seeded in 96-well plates and co-treated
with various concentrations of roscovitine or purvalanol (0–50 μM)
for 24 h. The cytotoxic effect of CDK inhibitors on Caco-2 cells
was determined by colorimetric
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT;
Roche, Indianapolis, IN, USA) cell proliferation assay, which is
based on the conversion of MTT to MTT-formazan by mitochondrial
enzymes. Absorbance was determined with a microplate reader at 570
nm (Bio-Rad, Hercules, CA, USA).
Apoptosis determination
Caco-2 cells (1×104) were seeded in
96-well plates, co-treated with 0–50 μM roscovitine and purvalanol
for 24 h. Cytoplasmic histone-associated-DNA fragments (mono- and
oligonucleosomes) were determined using Cell Death Detection
ELISAPlus assay, according to the manufacturer's instructions
(Roche). Briefly, cell lysates were placed in a streptavidin-coated
microplate. A mixture of anti-histone-biotin and anti-DNA-POD was
added and incubated for 2 h at 15–25˚C. Following the removal of
unbound antibodies by a washing procedure, POD was determined
photometrically at 405 nm with ABTS as substrate. In order to
determine the DNA fragments following drug treatment in colon
carcinoma cells, total DNA content was isolated.
Mitochondrial membrane potential
assay
Caco-2 cells (1×105) were seeded in
12-well plates, allowed to attach overnight and treated with
desired concentrations of roscovitine for 24 h. Cells were washed
once with 1X PBS and then stained with DiOC6
(Calbiochem) fluorescent probe. Mitochondrial membrane potential
(MMP) loss was measured by Fluoroskan Ascent fluorometer (Thermo
Labsystems, Beverly, MA, USA) (excitation/emission = 488 nm/525
nm).
Polyamine determination
PA content of the cells was determined by the
benzoylation procedure using HPLC. Cells (0.6×106) were
seeded in 6-well plates and allowed to attach overnight. Caco-2
cells were treated with roscovitine or purvalanol for 24 h and were
then washed with 1X PBS. The scraped cell lysates were transferred
into a new microfuge tube. Trichloroacetic acid (TCA; 50%) was
added to each sample (1:10, v:v). All samples were kept at −20˚C
until the benzoylation process. Following benzoylation, samples
were immediately run on HPLC using UV detector on 226 mV. The data
obtained were evaluated according to internal standards
1,6-diaminoheptane and the standard curves of Put, Spd and Spm.
Preparation of siRNA transfection
Caco-2 colon carcinoma cells were placed in a 6-well
plate 24 h prior to transfection. Cells were transfected with
different SSAT siRNA complexes that target SSAT-encoding genes SAT1
or SAT2 at two different mRNA binding sites (each 20 nM; Gene
Globe, Qiagen, Heidelberg, Germany). The transfection ratio was 1:6
and transfection was performed (RNAifect; Qiagen) according to the
manufacturer's protocol. After 48 h of incubation, the silencing
effect was analyzed with western blot analysis.
Immunoblot analysis
Caco-2 colon carcinoma cells were treated with the
appropriate concentrations of drugs. The samples were initially
washed with ice-cold 1X PBS and lysed on ice in a solution
containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, Nonidet P-40 0.5%
(v/v), 1 mM EDTA, 0.5 mM PMSF, 1 mM DTT, protease inhibitor
cocktail (Complete; Roche). Following cell lysis, cell debris was
removed by centrifugation for 15 min at 13,200 × g, and protein
concentrations were determined with a Bradford protein assay. Total
protein lysates (30 μg) were separated on a 12% SDS-PAGE and
transferred onto PVDF membranes (Amersham Pharmacia Biotech, Little
Chalfont, UK). The membranes were then blocked with 5% milk
blocking solution in Tris buffer saline (TBS) Tween-20 (Sigma) and
incubated with appropriate primary and HRP-conjugated secondary
antibodies (CST) in antibody buffer containing 5% (v/v) milk
blocking solution. After washing with TBS Tween-20, proteins were
analyzed using an enhanced chemiluminescence detection system (ECL
or ECL-Advance; Amersham Pharmacia Biotech) and exposed to
Hyperfilm-ECL (Amersham Pharmacia Biotech).
Statistical analysis
All samples were evaluated statistically using an
Excel calculation file. The MTT and cell death ELISA assay results
are shown as the means ± standard deviation, and the Student's
t-test was applied to evaluate the probability efficiency.
Differences were regarded as statistically significant at
p<0.05.
Results
Roscovitine and purvalanol are potent
apoptotic inducers
We used Caco-2 cells to investigate whether
roscovitine or purvalanol decreases cell viability through the
induction of apoptosis. To assess the cytotoxic effects of both CDK
inhibitors, Caco-2 cells were treated with roscovitine or
purvalanol at various concentrations (0–50 μM) for 24 h and MTT
cell viability assay was assessed. CDK inhibitors decreased cell
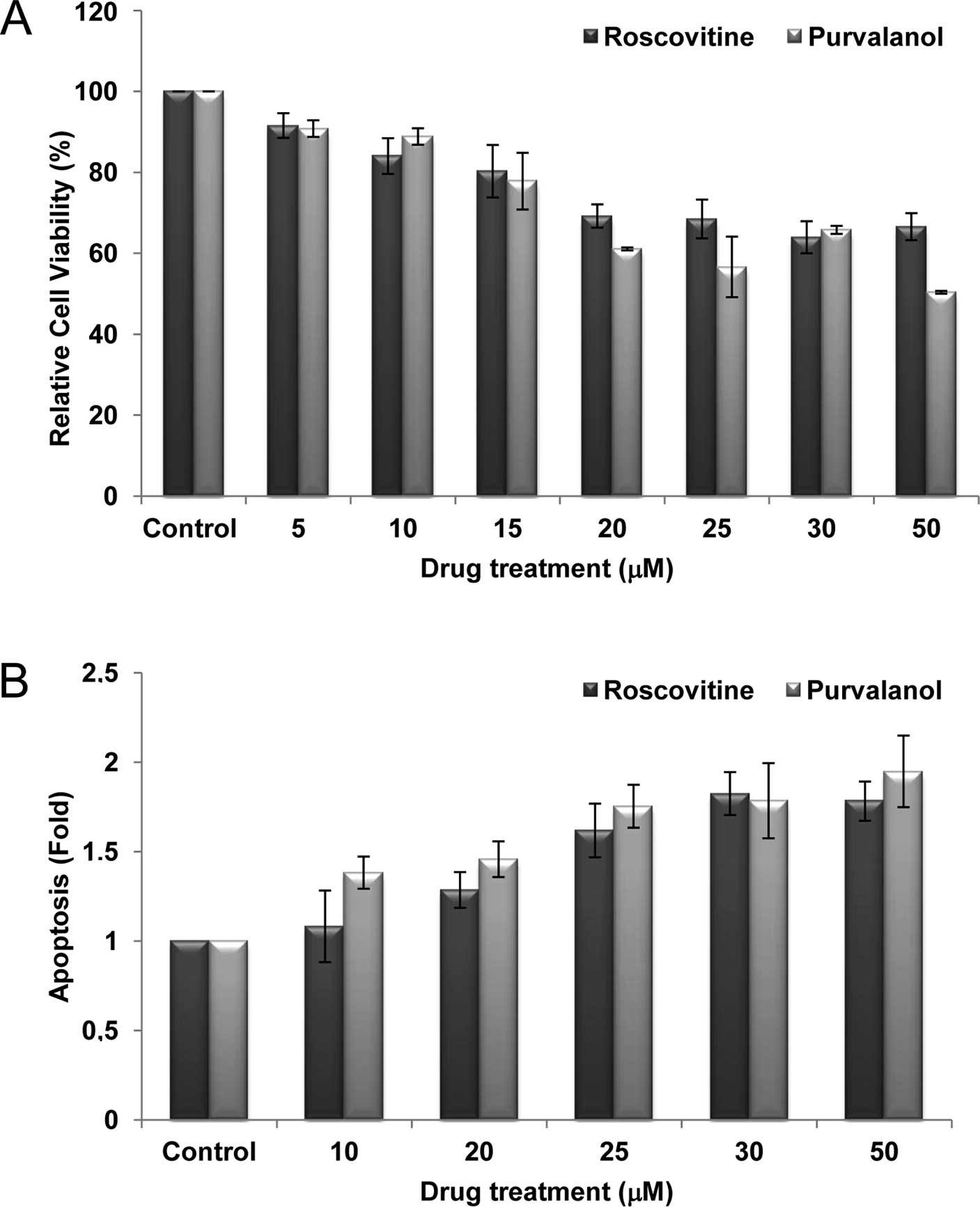
viability in a dose-dependent manner. According to the MTT,
roscovitine and purvalanol (each 20 μM) decreased cell viability by
~30% in Caco-2 cells (Fig. 1A). To
confirm that the decrease in cell viability was indeed due to
apoptosis, we performed the Cell Death ELISA Plus assay (Fig. 1B). Roscovitine or purvalanol
treatment led to a significant 2-fold increase in apoptotic cell
death in Caco-2 cells compared to the untreated samples.
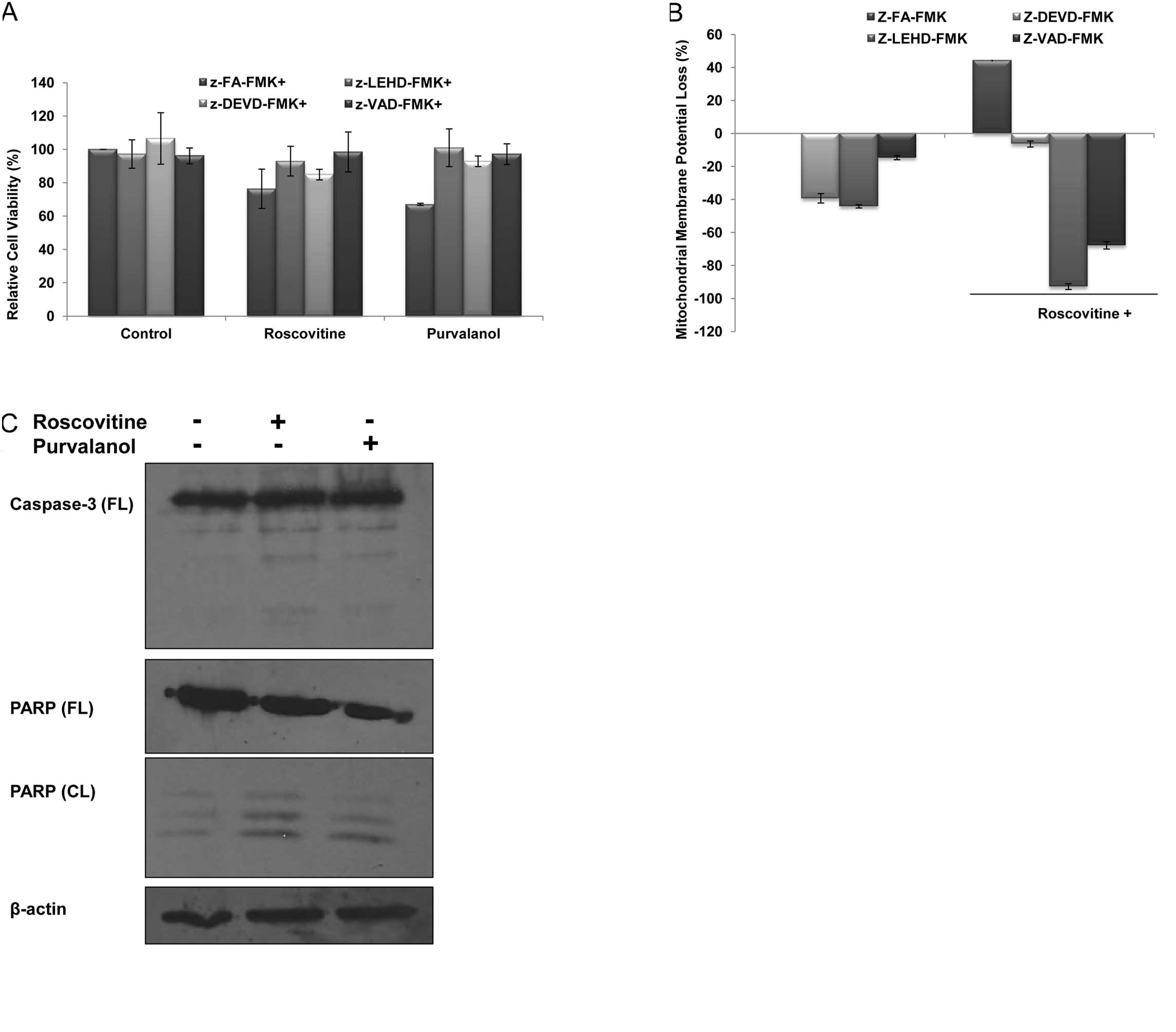
In order to understand CDK inhibitor-induced
apoptosis due to activation of caspases, cells were co-treated with
several caspase inhibitors (each 2.5 μM) and purvalanol or
roscovitine for 24 h; the MTT cell viability assay was then
performed. Co-treatment with caspase inhibitors, z-VAD-FMK
(pan-caspase inhibitor), z-LEHD-FMK (caspase-9 inhibitor),
z-DEVD-FMK (caspase-3 inhibitor) significantly prevented
roscovitine- or purvalanol-induced apoptosis, respectively
(Fig. 2A). These observations were
also confirmed by the determination of MMP loss by DiOC6
staining. As shown in Fig. 2B,
when the cells were treated with roscovitine (20 μM) in the
presence of each caspase inhibitor for 24 h, roscovitine-induced
MMP loss was prevented. To assess whether drug-induced apoptosis is
mediated by caspases, the proteolytic activation of caspase-3 was
also examined in Caco-2 cells. Both CDK inhibitors induced cleavage
of pro-caspase-3 to the active form (p19/17) (Fig. 2C). Involvement of apoptosis was
further confirmed by the detection of PARP degradation in Caco-2
cells compared to untreated samples. Furthermore, CDK inhibitors
were able to alter Bcl-2 family members (Fig. 2D) which are critical in the
apoptotic decision in the cells. Although roscovitine did not alter
Bcl-XL and Puma expression profiles, the exposure of
cells to roscovitine led to upregulation of Bax expression in
Caco-2 cells. Purvalanol upregulated both anti-apoptotic
Bcl-XL and pro-apopotic Bax and Puma protein expression
profiles.
CDK inhibitors modulate polyamine
metabolism in the drug-induced apoptotic mechanism
Since PAs are critical in cellular homeostasis and
in conducting several signalling networks, including the apoptotic
mechanism, we investigated whether modulation of the PA metabolic
pathway impacts the drug-induced apoptotic mechanism. Initially, we
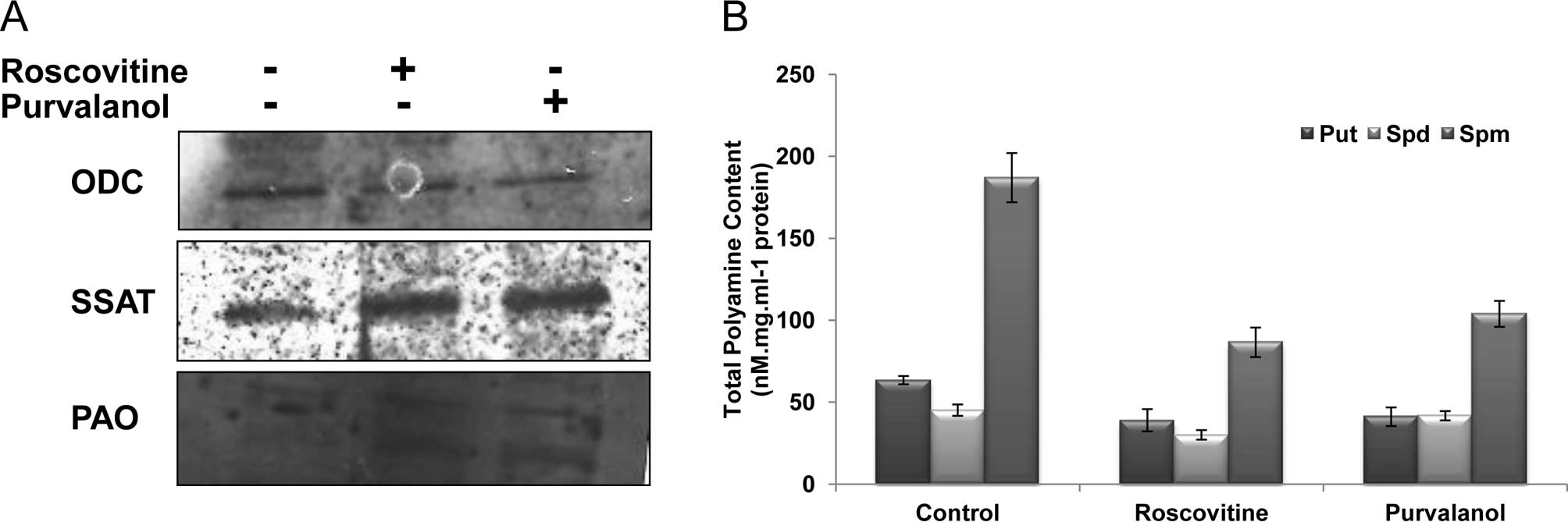
determined the expression of the PA biosynthesis enzyme ODC and
catabolic enzymes SSAT and PAO in Caco-2 cells following CDK
inhibitor treatment for 24 h. As shown in Fig. 3A, while both CDK inhibitors
downregulated ODC gene expression, roscovitine and purvalanol
upregulated SSAT and PAO protein expression in Caco-2 cells.
Following drug treatments, total PA content of the
Caco-2 cells was decreased. The sharp decrease was observed in Spm
levels following roscovitine or purvalanol treatments. Although
roscovitine led to a significant decrease in Put and Spd levels in
Caco-2 cells, purvalanol did not exert any significant effect
compared to untreated samples (Fig.
3B).
SSAT silencing attenuates the apoptotic
effect of CDK inhibitors
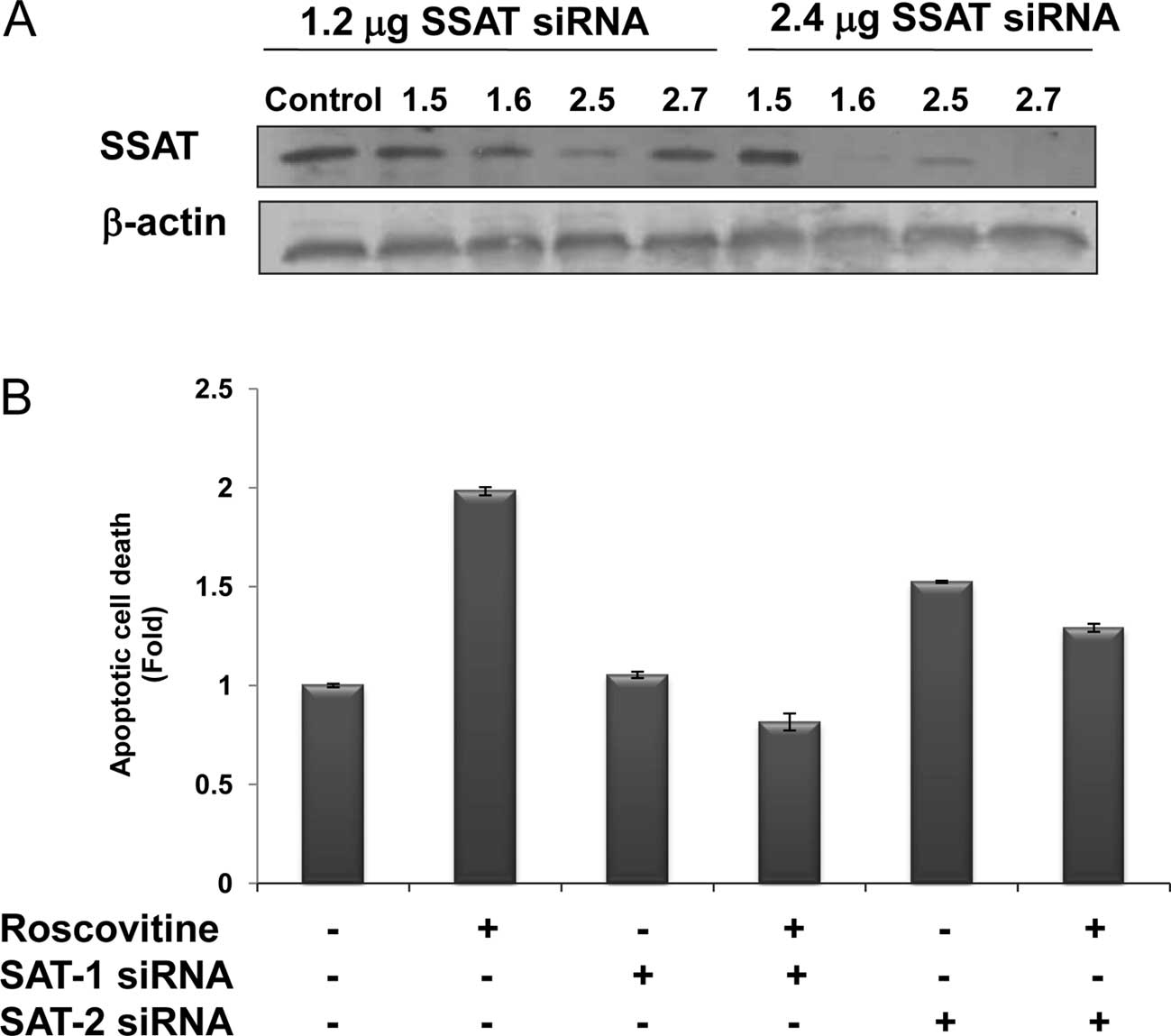
The efficiency of SSAT siRNAs on SSAT levels was
assessed at 48 h by the treatment of four different siRNA duplexes
targeting SAT1 and SAT2 mRNAs, which expressed the SSAT in the
human genome. According to SSAT immunoblotting results, the
transfection efficiency of SAT2 (2.6) was the most marked, compared
to the other siRNA duplexes. SAT1 mRNA product was downregulated by
SAT1.6 siRNA duplex (2.4 μg) and SAT2 mRNA was degraded by the
treatment of SAT2.7 (2.4 μg) following 48 h of treatment (Fig. 4A). However, the treatment of SAT1.5
siRNA duplex targeting SAT1 mRNA was not efficient.
The potential role of SSAT in the drug-induced
apoptosis mechanism was modelled by the treatment of roscovitine in
SSAT-silenced Caco-2 cells (Fig.
4B). Although roscovitine treatment caused a significant 2-fold
apoptosis induction compared to untreated samples, roscovitine did
not induce apoptosis in SAT1.6 siRNA-transfected cells. According
to the immunoblotting results, SAT2.7 siRNA treatment caused a
significant downregulation of SSAT expression, and also had a
smaller impact on roscovitine-induced apoptotic mechanism in Caco-2
cells.
Discussion
Genetic alterations in the regulation of the natural
PAs Put, Spd and Spm which are essential for cell growth and
differentiation may induce cell cycle arrest and apoptosis in
various cancer cell lines (36).
Recent studies have indicated that chemotherapeutic agents, such as
cisplatin, paclitaxel, doxorubicin, 5-FU and oxaliplatin, may
induce SSAT expression and decrease total PA content in cells
(37,38). It has been shown that 5-FU and
oxaliplatin upregulated SSAT expression in a panel of colon cancer
cells; H630, HT-29, LoVo, HCT175 and HCT248 colorectal cancer cell
lines. Only treatment with oxaliplatin exerted a significant effect
on SSAT expression in RKO colorectal cancer cells (39). Moreover, aspirin, a non-steroidal
anti-inflammatory drug, decreases the intracellular PA content by
inducing SSAT expression in Caco-2 cells (40). In the present study, we tested the
potential role of SSAT, a PA catabolic pathway key enzyme, in the
drug-induced apoptotic mechanism in Caco-2 colon cancer cells. To
achieve this, cells were treated with CDK inhibitors roscovitine
and purvalanol, which are strong apoptotic inducers (Fig. 1A and B). Although both CDK
inhibitors lead to apoptotic cell death in various cancer cell
types, their targets for apoptotic induction differs. Thus, in
order to determine the chemotherapeutic potential of these CDK
inhibitors, it is essential to investigate the molecular targets of
these CDK inhibitors during apoptotic cell death. According to our
previous results, roscovitine induced apoptotic cell death
modulated by the PA biosynthetic pathway, and depletion of PAs by a
specific inhibitor of ODC increased the roscovitine-induced
apoptosis in HCT116 colon carcinoma cells (41). Similar to previous results, we
established that roscovitine and purvalanol caspase-dependently
induced apoptosis and altered the mitochondrial pathway in Caco-2
cells (Fig. 2). The co-treatment
of caspase inhibitors prevented drug-induced cytotoxic effects
within 24 h. Consistent with these findings, roscovitine was also
shown as a caspase-3 activator in A4573, TC-71 Ewing's sarcoma
family of tumour cells, and SK-N-SH neuroblastoma cells and the
presence of Ac-DEVD-CHO (a specific caspase-3/-7 inhibitor)
prevented roscovitine-induced apoptosis (42). Purvalanol and paclitaxel combined
treatment leads to mitochondrial-dependent apoptotic cell death by
activating caspase-3 and -9 in HeLa cells (22).
To understand the potential effects of these CDK
inhibitors on the PA metabolism, we determined the ODC, SSAT and
PAO expression levels following drug treatment for 24 h. As shown
in Fig. 3A, while both CDK
inhibitors downregulated ODC expression, they upregulated SSAT and
PAO gene expression. Moreover, these alterations caused a
significant decrease in Put and Spm levels, but no alteration was
observed in the Spd content (Fig.
3B). In accordance with these results, PA biosynthesis was
inhibited by ODC downregulation and catabolic enzymes were induced
by the following treatment of cancer cells with PA analogs, such as
PG11047 (37) and classical
chemotherapeutic agents (43).
We also tested whether enhanced PA metabolism is
critical in the apoptotic decision by transiently transfecting
cells with SSAT-targeting siRNA complexes for 48 h. As shown in
Fig. 4B, when the induction of
SSAT is prevented, Caco-2 cells become more resistant to the
apoptotic effects of roscovitine than parental controls. Similar to
this finding, the silencing of both SMO and SSAT prevented
BENSpm-induced growth inhibitory effect on MDA-MB-231 cells
(44).
In summary, these findings show that SSAT is a
significant target in the regulation of apoptosis induced by
roscovitine. Therefore, the modulation of cellular targets related
to PA metabolism may be critical in evaluating the efficiency of
drugs in cancer cells. This study also underlines the potential
therapeutic efficacy of new generation CDK inhibitors, roscovitine
and purvalanol, in colon carcinoma. Although a number of studies
have shown the molecular targets of roscovitine, purvalanol has yet
to be thoroughly investigated. Therefore, this study is the first
to show the therapeutic potential of purvalanol in colon carcinoma
cells. Further studies are required to clarify the apoptotic cell
death mechanism of purvalanol in colon cancer.
Acknowledgements
The authors thank Pelin Ozfiliz for the technical
assistance during the study. This study was supported by the
Istanbul Kultur University Research Fund.
References
|
1
|
S BlagdenJ de BonoDrugging cell cycle
kinases in cancer therapyCurr Drug
Targets6325335200510.2174/138945005376582415857291
|
|
2
|
KH ChangY de PabloHP LeeHG LeeMA SmithK
ShahCdk5 is a major regulator of p38 cascade: relevance to
neurotoxicity in Alzheimer's diseaseJ
Neurochem11312211229201020345761
|
|
3
|
LM SchangAdvances on cyclin-dependent
kinases (CDKs) as novel targets for antiviral drugsCurr Drug
Targets Infect Disord52937200510.2174/156800505317460915777196
|
|
4
|
J CicenasM ValiusThe CDK inhibitors in
cancer research and therapyJ Cancer Res Clin
Oncol13714091418201110.1007/s00432-011-1039-421877198
|
|
5
|
H IsekiTC KoXY XueA SeapanMR HellmichCM
Townsend JrCyclin-dependent kinase inhibitors block proliferation
of human gastric cancer
cellsSurgery122187195199710.1016/S0039-6060(97)90008-89288122
|
|
6
|
L MeijerA BorgneO MulnerBiochemical and
cellular effects of roscovitine, a potent and selective inhibitor
of the cyclin-dependent kinases cdc2, cdk2 and cdk5Eur J
Biochem243527536199710.1111/j.1432-1033.1997.t01-2-00527.x
|
|
7
|
B SchutteL NielandM van EngelandME
HenflingL MeijerFC RamaekersThe effect of the cyclin-dependent
kinase inhibitor olomoucine on cell cycle kineticsExp Cell
Res236415199710.1006/excr.1997.37009344580
|
|
8
|
J Wesierska-GadekSB HajekB SargS WandlE
WalziH LindnerPleiotropic effects of selective CDK inhibitors on
human normal and cancer cellsBiochem
Pharmacol7615031514200810.1016/j.bcp.2008.07.04018761330
|
|
9
|
T David-PfeutyPotent inhibitors of
cyclin-dependent kinase 2 induce nuclear accumulation of wild-type
p53 and nucleolar fragmentation in human untransformed and
tumor-derived cellsOncogene1874097422199910.1038/sj.onc.1203103
|
|
10
|
MV AppleyardMA O'NeillKE MurraySeliciclib
(CYC202, R-roscovitine) enhances the antitumor effect of
doxorubicin in vivo in a breast cancer xenograft modelInt J
Cancer124465472200910.1002/ijc.2393819003963
|
|
11
|
G Ortiz-FerronR YerbesA EramoAI
Lopez-PerezR de MariaA Lopez-RivasRoscovitine sensitizes breast
cancer cells to TRAIL-induced apoptosis through a pleiotropic
mechanismCell Res18664676200810.1038/cr.2008.54
|
|
12
|
J Wesierska-GadekMP KramerM
MaurerResveratrol modulates roscovitine-mediated cell cycle arrest
of human MCF-7 breast cancer cellsFood Chem
Toxicol4613271333200810.1016/j.fct.2007.09.00417933449
|
|
13
|
F ZhangT ZhangZP GuEnhancement of
radiosensitivity by roscovitine pretreatment in human non-small
cell lung cancer A549 cellsJ Radiat Res
(Tokyo)49541548200810.1269/jrr.0802418728343
|
|
14
|
S MohapatraB ChuX ZhaoJ DjeuJQ ChengWJ
PledgerApoptosis of metastatic prostate cancer cells by a
combination of cyclin-dependent kinase and AKT inhibitorsInt J
Biochem Cell
Biol41595602200910.1016/j.biocel.2008.07.01318708158
|
|
15
|
M MaurerO KominaJ
Wesierska-GadekRoscovitine differentially affects asynchronously
growing and synchronized human MCF-7 breast cancer cellsAnn NY Acad
Sci1171250256200910.1111/j.1749-6632.2009.04717.x19723062
|
|
16
|
JD ZolnierczykJZ BlonskiT RobakZM
KilianskaJ Wesierska-GadekRoscovitine triggers apoptosis in B-cell
chronic lymphocytic leukemia cells with similar efficiency as
combinations of conventional purine analogs with
cyclophosphamideAnn NY Acad
Sci1171124131200910.1111/j.1749-6632.2009.04903.x
|
|
17
|
D IizukaO InanamiI KashiwakuraM
KuwabaraPurvalanol A enhances cell killing by inhibiting
up-regulation of CDC2 kinase activity in tumor cells irradiated
with high doses of X raysRadiat
Res167563571200710.1667/RR0622.117474786
|
|
18
|
L CerquettiC SampaoliD AmendolaMitotane
sensitizes adrenocortical cancer cells to ionizing radiations by
involvement of the cyclin B1/CDK complex in G2 arrest and mismatch
repair enzymes modulationInt J Oncol374935012010
|
|
19
|
T HikitaC OneyamaM OkadaPurvalanol A, a
CDK inhibitor, effectively suppresses Src-mediated transformation
by inhibiting both CDKs and c-SrcGenes
Cells1510511062201010.1111/j.1365-2443.2010.01439.x20825494
|
|
20
|
PM PriceF YuP KaldisDependence of
cisplatin-induced cell death in vitro and in vivo on
cyclin-dependent kinase 2J Am Soc
Nephrol1724342442200610.1681/ASN.200602016216914540
|
|
21
|
D IizukaA OguraM KuwabaraO
InanamiPurvalanol A induces apoptosis and downregulation of
antiapoptotic proteins through abrogation of phosphorylation of
JAK2/STAT3 and RNA polymerase IIAnticancer
Drugs19565572200810.1097/CAD.0b013e3282fe330e18525315
|
|
22
|
M PennatiAJ CampbellM CurtoPotentiation of
paclitaxel-induced apoptosis by the novel cyclin-dependent kinase
inhibitor NU6140: a possible role for survivin down-regulationMol
Cancer Ther413281337200510.1158/1535-7163.MCT-05-002216170024
|
|
23
|
VM YenugondaTB DebSC GrindrodFluorescent
cyclin-dependent kinase inhibitors block the proliferation of human
breast cancer cellsBioorg Med
Chem1927142725201110.1016/j.bmc.2011.02.05221440449
|
|
24
|
L RingerP SirajuddinVM YenugondaVMY-1–103,
a dansylated analog of purvalanol B, induces caspase-3-dependent
apoptosis in LNCaP prostate cancer cellsCancer Biol
Ther103203252010
|
|
25
|
LJ MartonAE PeggPolyamines as targets for
therapeutic interventionAnnu Rev Pharmacol
Toxicol355591199510.1146/annurev.pa.35.040195.0004157598507
|
|
26
|
LJ MartonAE PeggDR MorrisDirections for
polyamine researchJ Cell
Biochem4578199110.1002/jcb.2404501052005186
|
|
27
|
G AnderssonO HebyKinetics of cell
proliferation and polyamine synthesis during Ehrlich ascites tumor
growthCancer Res37436143661977922727
|
|
28
|
O HebyG AnderssonJW GrayInterference with
S and G2 phase progression by polyamine synthesis inhibitorsExp
Cell Res111461464197810.1016/0014-4827(78)90192-1627248
|
|
29
|
U BachrachPolyamines as chemical markers
of malignancyItal J Biochem2577931976773897
|
|
30
|
MG MontiL PerneccoR ManfrediniInhibition
of cell growth by accumulated spermine is associated with a
transient alteration of cell cycle progressionLife
Sci5820652072199610.1016/0024-3205(96)00200-78649191
|
|
31
|
Y WangRA Casero JrMammalian polyamine
catabolism: a therapeutic target, a pathological problem, or both?J
Biochem1391725200610.1093/jb/mvj02116428315
|
|
32
|
RA CaseroAE PeggPolyamine catabolism and
diseaseBiochem J421323338200910.1042/BJ2009059819589128
|
|
33
|
Y HuangA PledgieRA Casero JrNE
DavidsonMolecular mechanisms of polyamine analogs in cancer
cellsAnticancer
Drugs16229241200510.1097/00001813-200503000-0000215711175
|
|
34
|
Y ChenRS WeeksMR BurnsDW BoormanA
Klein-SzantoTG O'BrienCombination therapy with
2-difluoromethylornithine and a polyamine transport inhibitor
against murine squamous cell carcinomaInt J
Cancer11823442349200610.1002/ijc.2162116331620
|
|
35
|
MR BurnsGF GraminskiRS WeeksY ChenTG
O'BrienLipophilic lysine-spermine conjugates are potent polyamine
transport inhibitors for use in combination with a polyamine
biosynthesis inhibitorJ Med Chem5219831993200910.1021/jm801580w
|
|
36
|
L MyhreK AlmC HegardtDifferent cell cycle
kinetic effects of N1,N11-diethylnorspermine-induced polyamine
depletion in four human breast cancer cell linesAnticancer
Drugs19359368200810.1097/CAD.0b013e3282f7f51818454046
|
|
37
|
K DredgeJA KinkRM JohnsonI BythewayLJ
MartonThe polyamine analog PG11047 potentiates the antitumor
activity of cisplatin and bevacizumab in preclinical models of lung
and prostate cancerCancer Chemother
Pharmacol65191195200910.1007/s00280-009-1105-719685053
|
|
38
|
S HectorR TummalaND KisielPolyamine
catabolism in colorectal cancer cells following treatment with
oxaliplatin, 5-fluorouracil and N1, N11 diethylnorspermineCancer
Chemother
Pharmacol62517527200810.1007/s00280-007-0633-217987291
|
|
39
|
WL AllenEG McLeanJ BoyerThe role of
spermidine/spermine N1-acetyltransferase in determining response to
chemotherapeutic agents in colorectal cancer cellsMol Cancer
Ther6128137200710.1158/1535-7163.MCT-06-030317237273
|
|
40
|
N BabbarEW GernerRA Casero JrInduction of
spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in
Caco-2 colon cancer cellsBiochem
J394317324200610.1042/BJ2005129816262603
|
|
41
|
ED ArisanA CokerN Palavan-UnsalPolyamine
depletion enhances the roscovitine-induced apoptosis through the
activation of mitochondria in HCT116 colon carcinoma cellsAmino
AcidsAug22011(E-pub ahead of print).
|
|
42
|
OM TiradoS Mateo-LozanoV
NotarioRoscovitine is an effective inducer of apoptosis of Ewing's
sarcoma family tumor cells in vitro and in vivoCancer
Res6593209327200510.1158/0008-5472.CAN-05-127616230394
|
|
43
|
R VarmaS HectorWR GrecoPlatinum drug
effects on the expression of genes in the polyamine pathway:
time-course and concentration-effect analysis based on Affymetrix
gene expression profiling of A2780 ovarian carcinoma cellsCancer
Chemother
Pharmacol59711723200710.1007/s00280-006-0325-317021820
|
|
44
|
A PledgieY HuangA HackerSpermine oxidase
SMO(PAOh1), not N1-acetylpolyamine oxidase PAO, is the primary
source of cytotoxic H2O2 in polyamine
analogue-treated human breast cancer cell linesJ Biol
Chem2803984339851200516207710
|