Introduction
Adenoid cystic carcinoma (ACC), one of the most
common malignant tumors of the major and minor salivary glands,
displays certain unique characteristics, such as slow but
aggressive growth, strong invasion to peripheral nerves or blood
vessels at the early phase, and a high incidence of recurrence and
distant metastasis (1,2). The incidence of distant metastasis
(mostly in lungs) for patients with salivary gland adenoid cystic
carcinoma (SACC) ranges from 35 to 50%, whereas lymph node
metastases are rare. Although the 5-year survival rate is
approximately 70% for patients with SACC, the survival rates
decrease to 40% at 10 years and 25% at 15 years due to frequent
local recurrence and distant metastasis (3–8). The
current strategy to reduce local recurrence is postoperative
radiotherapy. It has been suggested that neoadjuvant or adjuvant
chemotherapy may reduce the incidence of distant metastasis and
improve the disease-free survival of patients (2,3).
Clinicopathological characteristics are the major factors used to
evaluate the risk of distant metastasis of SACC. For example, a
significantly higher incidence of distant metastasis occurs in
patients with solid histology subtype (6,7). The
size of primary tumors has also been associated with the incidence
of distant metastasis (7).
However, controversial reports have been published in the
literature (3–7). The molecular mechanism involved in
the development and progression of SACC remains uncertain, yet a
strong prognostic indicator is demanded for the appropriate choice
of therapy for SACC patients.
Podoplanin (also termed as T1a-2, aggrus or gp36) is
a small mucin-like protein and its physiological function is
related to tissue development and repair (9,10).
It is highly and specifically expressed in lymphatic endothelial
cells while not found in blood endothelium (9). Therefore, podoplanin is widely used
as a specific marker for lymphatic endothelial cells and
lymphangiogenesis in many species. Recent studies, however, have
shown that podoplanin is also expressed in certain tumor cells,
including many central nervous system, cervix, germinal and
mesothelioma tumors, as well as oral squamous cell carcinoma
(11–15). For cervix and oral squamous cell
carcinoma, podoplanin is associated with migration/invasion, making
it a novel prognostic marker for patients with these tumors
(11,12).
In this study, our team used monoclonal antibody
D2-40, which specifically recognizes podoplanin, to determine the
expression patterns of this protein in patients with primary SACC.
Moreover, we analyzed the associations between the expression
patterns and clinicopathological characteristics, as well as the
clinical outcomes.
Materials and methods
Patient population
All 40 patients analyzed in this study were
diagnosed with SACC at the Department of Oral and Maxillofacial
Surgery, Shanghai Ninth People’s Hospital, between January 1999 and
December 2003, and underwent complete surgical resection with
curative intent. Of the 40 patients, 22 were females and 18 males,
and the median age was 51.9 years (range, 28–76 years). Twenty
tumors (50%) originated from the major salivary glands, and 20
tumors (50%) from the minor salivary glands. The tumor
classification were based on the histological grading system
recommended by Dardick (16):
grade I, tumors with cribriform and/or tubular structures without a
solid component; grade II, tumors composed of cribriform and/or
tubular structures with <30% of solid areas; and grade III,
tumors with >30% of solid areas. The clinical staging of the
patients was determined according to criteria set in the Tumor,
Node, Metastasis (TNM) system of the International Union Against
Cancer, 2000 (17). Tumor
recurrence and metastasis were confirmed by radiographical and
pathological diagnosis. The follow-up period was 6–101 months
(mean, 64.95 months). Of the 40 patients, 3 had enlarged cervical
lymph nodes, 11 had tumor recurrence and 16 had distant metastasis.
Eight (20%) of the 40 patients died of recurrence or metastasis.
All patients signed an informed consent form prior to enrollment in
the study, and permission was obtained from the ethical committee
of the Shanghai Ninth People’s Hospital.
Tumor tissues and immunohistochemistry
(IHC)
Formalin-fixed, paraffin-embedded tissues were cut
into 4-μm sections, mounted on glass slides, and then
deparaffinized in graded alcohol. Endogenous peroxidase activity
was blocked with 3% H2O2 for 20 min. The
slides were incubated overnight at 4°C with the primary antibody
D2-40 (mouse monoclonal, 1:20 dilution; AbD Serotec, MorphoSys UK
Ltd., Oxford, UK). The primary antibody binding was detected using
peroxidase labelled secondary antibody and chromogen,
diaminobenzidine (DAB) Dako EnVision™ Detection Systems (Dako,
Denmark) according to the manufacturer’s recommendations. Tissue
sections were counterstained with hematoxylin. Negative controls
were treated using the same procedures but omitting the use of the
primary antibody. The staining of adjacent lymphatic endothelial
cells within the same sections was used as the positive internal
control. Negative controls were prepared by replacing the primary
antibodies with non-immune mouse serum, and no reactive products
were detected.
Cytoplasmic and/or membrane immunoreactivity were
considered to indicate podoplanin expression positivity. All the
slides were reviewed independently by 2 pathologists (L.Z.W. and
C.Y.Z.) without the knowledge of the clinical information of the
patients. The slides were evaluated using a microscope with a
square grid (10×10 mm) in the ocular lens. Under magnification of
×40 to delineate the area, each case was evaluated in terms of
staining intensity, and the number of positive and negative tumor
cells in at least 4 fields was quantified. Scores were based on
staining extention: no positive tumor cells, 0; <10% positive
tumor cells, 1; 10–30% positive tumors cells, 2; >30% positive
tumors cells, 3. A score of 0 and 1 was considered low reactivity,
while >1 was considered high.
Statistical analysis
Statistical tests were performed using SPSS software
(version 11.0, SPSS Inc, Chicago, USA). The relationship between
podoplanin expression and clinicopathological parameters was
determined using Wilcoxon test amd signed rank sum tests. Fisher’s
exact probability test was used to analyze the statistical
significance. Overall survival time was calculated from the date of
initial diagnosis to death or the last day of follow-up evaluation.
The period from the date of therapy to the date of recurrence or
metastasis was considered as disease-free survival. Survival
analysis was computed by means of the Kaplan-Meier method, and
levels of significance were assessed by means of the log-rank test.
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Podoplanin expression and
clinicopathological features
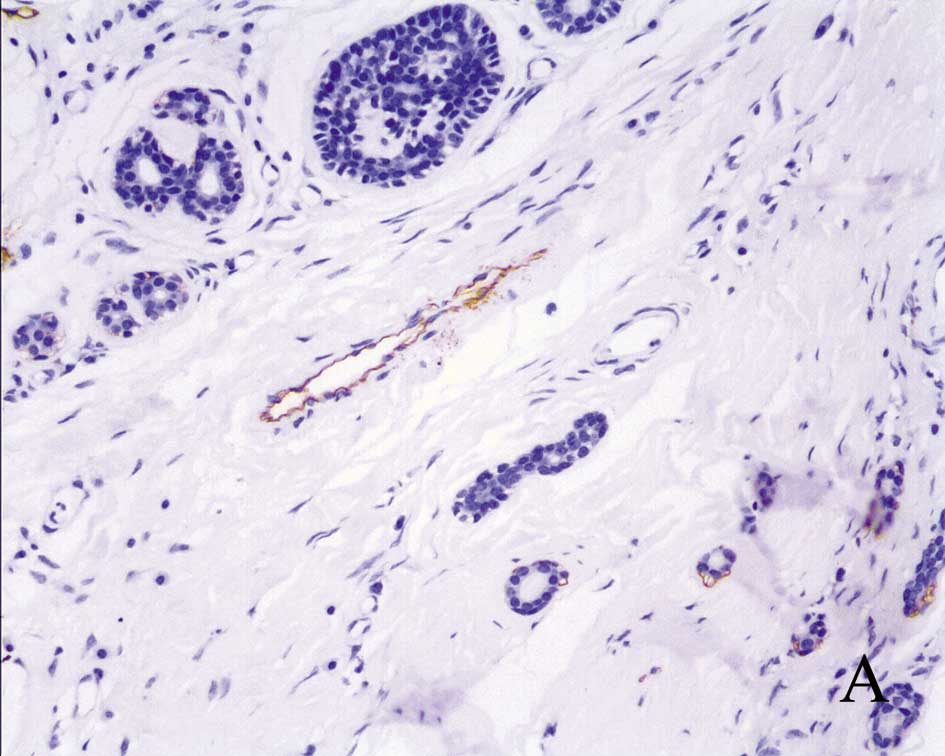
As expected, podoplanin was highly expressed in the
endothelial cells of lymphatic vessels (Fig. 1A). Normal salivary glands around
the tumor revealed a positive cytoplasmic and/or membrane reaction
with the podoplanin antibody; the positive cells were mainly
located in myoepithelial cells and various zones of the secretory
duct system.
Among the 40 tumors analyzed, 9 (22.5%) did not
demonstrate podoplanin expression and 18 (45%) had <10% positive
tumor cells, which were considered to have low expression. The
other 13 samples (32.5%) were considered to have high expression,
of which 8 had 10–30% positive tumor cells, while 5 had >30%
positive tumor cells (Fig. 1B).
The relationship between podoplanin expression and
clinicopathological features is summarized in Table I. Of the 13 specimens with high
positive podoplanin staining, 9 patients developed distant
metastasis. Of the remaining 27 patients with low expression, 7 had
distant metastasis. Statistical analysis indicates that positive
podoplanin staining is significantly correlated with distant
metastasis (P=0.015, n=40). There was no significant difference
between the high and low podoplanin expression group with respect
to age at diagnosis, gender, T-classification tumor histological
grading, regional metastasis and recurrence. However, podoplanin
expression was higher in tumors located in the sublingual gland
than that in tumors located in other glands.
 | Table ICorrelation between podoplanin
expression and patient characteristics. |
Table I
Correlation between podoplanin
expression and patient characteristics.
| Low podoplanin
expression (n=27) | High podoplanin
expression (n=13) | P-value |
|---|
| Age at diagnosis | | | |
| <60 years | 18 | 10 | 0.716 |
| ≥60 years | 9 | 3 | |
| Gender | | | |
| Female | 17 | 5 | 0.314 |
| Male | 10 | 8 | |
| T classification | | | |
| I or II | 15 | 5 | 0.501 |
| III or IV | 12 | 8 | |
| Regional
metastasis | | | |
| With | 3 | 0 | 0.538 |
| Without | 24 | 13 | |
| Distant
metastasis | | | |
| With | 7 | 9 | 0.015 |
| Without | 20 | 4 | |
| Recurrence | | | |
| With | 7 | 4 | 1.000 |
| Without | 20 | 9 | |
| Histological
grading | | | |
| I | 11 | 5 | 0.644 |
| II | 13 | 5 | |
| III | 3 | 3 | |
| SACC tumor location
(gland) | | | |
| Parotid | 4 | 0 | 0.199 |
| Submaxillary | 4 | 1 | |
| Sublingual | 5 | 6 | |
| Small | 14 | 6 | |
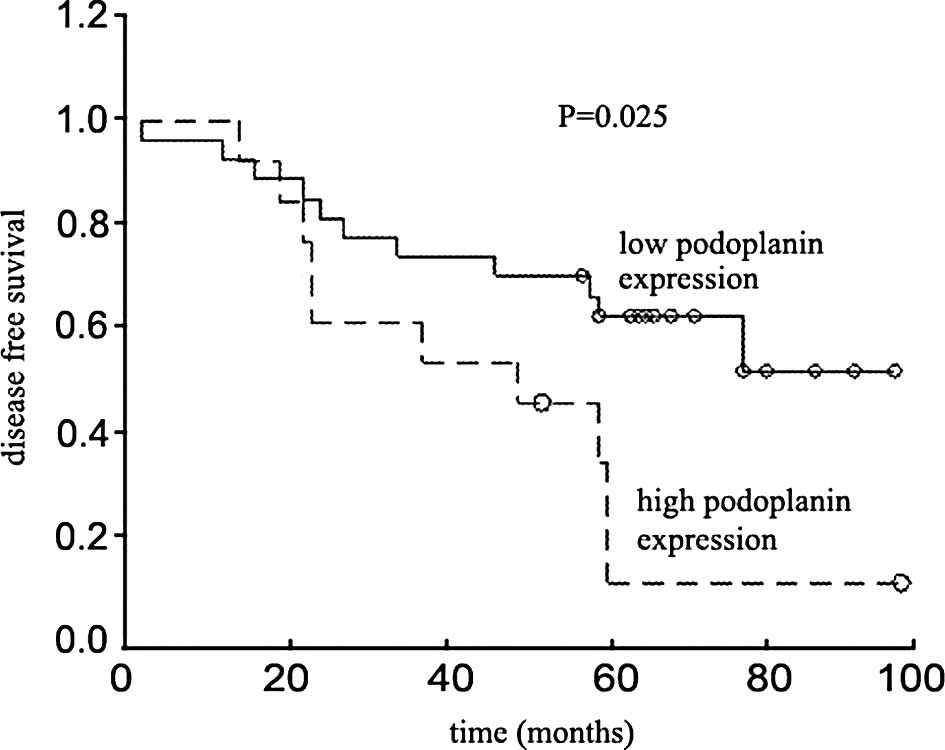
Podoplanin expression and survival
Although patients whose tumors expressed high levels
of podoplanin had a shorter overall survival compared to those
whose tumors expressed low levels of podoplanin, the difference was
not statistically significant (P=0.789, log-rank test). However,
the difference in disease-free survival was statistically
significant (P=0.025, log-rank test) (Fig. 2). Fig.
2 depicts the Kaplan-Meier plot of the survival curves.
Discussion
The monoclonal antibody D2-40 was initially
developed to recognize the M2A antigen, which is an oncofetal
glycoprotein expressed by testicular germ cell neoplasms (11). According to a recent study carried
out using multiple approaches, the D2-40 antibody specifically
recognizes human podoplanin, which has biochemical characteristics
similar to the M2A antigen (11).
In normal human tissue, podoplanin is expressed in kidney
podocytes, skeletal muscle, placenta, lung and heart, myoepithelial
cell of the breast and salivary glands, osteoblasts and mesothelial
cells (10). Although the
biological functions of podoplanin are not fully understood, it may
promote the formation of elongated cell extensions and increase
adhesion, migration, and tube formation of vascular endothelial
cells. ERM-protein phosphorylation may link podoplanin expression
to the observed rearrangement of the actin cytoskeleton (15).
In the mouse salivary gland, podoplanin was found to
be a salivary gland myoepithelial cell antigen, and the detection
level directly reflects myoepithelial cell distribution (18). Strong expression of podoplanin is
also found at the basal portion of the intercalated, striated and
interlobular ducts (19).
Recently, Fujita et al found that various SACC tumor cells
or ‘neoplastic myoepithelial cells’ are podoplanin-positive
(20). In the present research,
the overall expression level of podoplanin in SACC tumor cells was
low (only 13 samples had more than 10% expression). However,
approximately 70% (9 of 13) of patients with podoplanin expression
developed distant metastasis, significantly higher than the
occurrence in the patients with weak or no podoplanin expression.
Importantly, the disease-free survival rate was significantly
lower. These data suggest the potential use of podoplanin as a
molecular maker for distant metastasis in SACC. In mouse salivary
gland, podoplanin expression was rarely found in acini of the
parotid glands, while it was clearly found in the basal portion
surrounding acinar cells of the submandibular and particularly the
sublingual glands (19). Regarding
the high expression in sublingual glands, our results suggest that
podoplanin overexpression may be dependent on the gland involved.
Nevertheless, further studies with larger sample sizes are needed
to validate our findings. Furthermore, the detailed mechanisms
underlying the relationship between high podoplanin expression and
distant metastasis in SACC warrant further investigation.
Nevertheless, of the 16 patients that developed
distant metastasis, 7 did not exhibit high expression of
podoplanin, indicating the limitation of using podoplanin as a
marker of distant metastasis. Additional molecular markers are
desirable to compensate for the limitation of podoplanin.
In summary, our results revealed the differential
expression of podoplanin among SACC cases. There was a close
correlation between podoplanin expression and distant metastasis
and disease-free survival. Further study is needed to elucidate the
main role of podoplanin in tumorigenesis and the biological
behaviors of SACC.
Acknowledgements
This work was supported by the Project of Science
and Technology Commission of Shanghai Municipality (Grant nos.
10410711200, 08140902100 and 11495802000) and the Key Project of
Science and Technology Commission of Shanghai Municipality (Grant
no. 03JC14052). The authors thank Professor Mao L. (Shanghai Key
Laboratory of Stomatology, Department of Oral and Maxillofacial -
Head and Neck Oncology, Ninth People’s Hospital, School of
Medicine, Shanghai Jiao Tong University, Shanghai 200011, P.R.
China) for critical reading of our manuscript.
References
|
1
|
Li J, Wang BY, Nelson M, et al: Salivary
adenocarcinoma, not otherwise specified: a collection of orphans.
Arch Pathol Lab Med. 128:1385–1394. 2004.PubMed/NCBI
|
|
2
|
Bradley PJ: Adenoid cystic carcinoma of
the head and neck: a review. Curr Opin Otolaryngol Head Neck Surg.
12:127–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung MW, Kim KH, Kim JW, et al:
Clinicopathological predictors and impact of distant metastasis
from adenoid cystic carcinoma of the head and neck. Arch
Otolaryngol Head Neck Surg. 129:1193–1197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan AJ, DiGiovanna MP, Ross DA, et al:
Adenoid cystic carcinoma: a retrospective clinical review. Int J
Cancer. 96:149–158. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nascimento AG, Amaral AL, Prado LA,
Kligerman J and Silveira TR: Adenoid cystic carcinoma of salivary
glands. A study of 61 cases with clinicopathological correlation.
Cancer. 57:312–319. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spiro RH, Huvos AG and Strong EW: Adenoid
cystic carcinoma of salivary origin. A clinicopathological study of
242 cases. Am J Surg. 128:512–520. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spiro RH: Distant metastasis in adenoid
cystic carcinoma of salivary origin. Am J Surg. 174:495–498. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
da Cruz Perez DE, de Abreu Alves F, Nobuko
Nishimoto I, de Almeida OP and Kowalski LP: Prognostic factors in
head and neck adenoid cystic carcinoma. Oral Oncol. 42:139–146.
2006.PubMed/NCBI
|
|
9
|
Kahn HJ and Marks A: A new monoclonal
antibody, D2-40, for detection of lymphatic invasion in primary
tumors. Lab Invest. 82:1255–1257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schacht V, Ramirez MI, Hong YK, et al:
T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature
formation and causes lymphedema. EMBO J. 22:3546–3556. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marks A, Sutherland DR, Bailey D, et al:
Characterization and distribution of an oncofetal antigen (M2A
antigen) expressed on testicular germ cell tumours. Br J Cancer.
80:569–578. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan P, Temam S, El-Naggar A, et al:
Overexpression of podoplanin in oral cancer and its association
with poor clinical outcome. Cancer. 107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dumoff KL, Chu C, Xu X, Pasha T, Zhang PJ
and Acs G: Low D2-40 immunoreactivity correlates with lymphatic
invasion and nodal metastasis in early-stage squamous cell
carcinoma of the uterine cervix. Mod Pathol. 18:97–104. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schacht V, Dadras SS, Johnson LA, Jackson
DG, Hong YK and Detmar M: Up-regulation of the lymphatic marker
podoplanin, a mucin-type transmembrane glycoprotein, in human
squamous cell carcinomas and germ cell tumors. Am J Pathol.
166:913–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wicki A and Christofori G: The potential
role of podoplanin in tumour invasion. Br J Cancer. 96:1–5. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dardick I: Adenoid cystic carcinoma. Color
Atlas/Text of Salivary Gland Tumor Pathology. Igaku-Shoin Medical
Publishers; New York, NY: pp. 149–161. 1996
|
|
17
|
Sobin LH and Wittekind C: TNM
Classification of Malignant Tumors. 6th edition. John Wiley &
Sons; New York, NY: 2002
|
|
18
|
Hata M, Amano I, Tsuruga E, Kojima H and
Sawa Y: Immunoelectron microscopic study of podoplanin localization
in mouse salivary gland myoepithelium. Acta Histochem Cytochem.
43:77–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hata M, Ueki T, Sato A, Kojima H and Sawa
Y: Expression of podoplanin in the mouse salivary glands. Arch Oral
Biol. 53:835–841. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujita G, Sato S, Kishino M, Iwai S,
Nakazawa M, Toyosawa S, Yura Y and Ogawa Y: Lymphatic vessels and
related factors in adenoid cystic carcinoma of the salivary gland.
Mod Pathol. 24:885–891. 2011. View Article : Google Scholar : PubMed/NCBI
|