Introduction
Infections can trigger disease activity in patients
with immune complex glomerulonephritis (ICGN), including GN, IgA
nephropathy (IgAN), renal vasculitis, lupus nephritis and
transplant rejection. However, tubulointerstitial damage has been
demonstrated to be important in progressive renal disease (1). Glomerular injury is thought to
trigger tubular cell activation, leading to tubulointerstitial
inflammation and fibrosis. Activated tubule epithelial cells are
considered to be critical in the pathogenesis of renal injury, due
to their ability to secrete proinflammatory and profibrogenic
substances (2). Therefore, renal
functional damage correlates closely with tubulointerstitial damage
in glomerulonephritis (1).
Toll-like receptors (TLRs) have been found to play a
key role in the recognition of bacterial components during
infection (3). Notably, TLR
interactions with microbial components trigger the expression of
proinflammatory cytokines as well as the functional maturation of
antigen-presenting cells of the innate immune system (3–4). TLR
activation may be a link between mechanical, toxic or ischemic
tubular cell injury and the onset of an inflammatory ‘innate’
immune response in the pathogenesis of acute renal failure
(5). Immune cells and intrinsic
renal cells that respond to TLR activation may become activated by
extracellular matrix molecules, which then promote the secretion of
inflammatory cytokines and chemokines. This process in turn is
followed by additional leukocyte recruitment to the kidney
supporting sustained interstitial inflammation and interstitial
fibrosis (6). Of the 13 known
mammalian TLRs, TLR4 has received particular attention. TLR4 is
involved in the signalling pathway of the lipopolysaccharide (LPS)
receptor complex (7). Virulent
uropathogenic strains (Escherichia coli) express P fimbriae,
which bind to the glycolipid receptors of uroepithelial and kidney
tubular cells, triggering TLR4 activation with subsequent
recruitment of leukocytes and release of proinflammatory cytokines
(8). Cytokines, tumor necrosis
factor α (TNFα) and interleukin-8 (IL-8) homologue are expressed in
response to stimulation by LPS and a synthetic TLR4 agonist in
cultured renal tubular epithelial cells (TECs) (9). Cells lacking the respective TLRs had
a reduced response to this stimulation. The TLR4-mediated response
to stimulation was dependent on nuclear factor κB (NF-κB)
signalling, suggesting a role in the innate immune response to
bacteria during ascending urinary tract infection. It has also been
highlighted that although TLRs have specific ligands, co-operation
may be required between these TLRs and other molecules to achieve a
maximal response (9). Furthermore,
activation of innate immunity through TLR4 in the donor kidney also
contributes to the development of acute rejection after renal
transplantation (10). Therefore,
TLRs of renal cells are important in various kidney disorders and
require further study.
Emodin (1,3,8-trihydroxy-6-methyl-anthraquinone) is
a biologically active natural anthraquinone extracted from the
roots and rhizomes of Rheum palmatum (Chinese name
DaHuang) which is one of the four most well-known crude
drugs in Traditional Chinese Medicine (TCM) history. It was
described in Agriculture God’s Canon of Materia Medica (Chinese
name Shen Nong Ben Cao Jing) (11), in which the use of Rheum can
be traced back to 270 B.C. At present, it is one of the most
effective TCMs for infection and has now been officially listed in
the Chinese Pharmacopoeia (12).
Rheum has been confirmed to possess antimicrobial,
antiviral, anti-inflammatory, antifibrosis, antiulcerogenic,
anti-cancer, immunosuppressive, vasorelaxant and chemopreventive
effects (13–14). Furthermore, the anti-inflammatory
properties of Rheum have been well-established in animal
experiments and clinical studies (14). Emodin also has potential for the
treatment of chronic renal failure (as an immunosuppressant and
vasorelaxant). In a previous study, we found that compounds
produced from the decoction of Centella Asiatica and
Rheum repressed the proliferation and extracellular matrix
production of rat kidney glomerular mesangial cells combined with a
reduction in cyclin D1 and cyclin-dependent kinase 4 expression in
rat (15–16). Although emodin was found to reduce
monocyte/macrophage-chemoattractant protein-1 expression, inhibit
TNFα-induced NF-κB and c-Jun N-terminal kinase, impair cytokine
production (17) and even have an
inhibitory effect against superoxide production (18), the detailed mechanisms underlying
sensitization have not been established. It was recently reported
that amelioration of pancreatic and pulmonary damage by emodin may
partly contribute to the suppression of TLR4 expression (19), however, their relevance to TLR4 in
TECs has not been investigated.
The present study investigated the anti-inflammatory
mechanism of emodin by determining the effects on LPS-induced TLR4
expression in mice TECs cultures and aimed to determine whether
emodin-ameliorated renal damage was mediated by its stimulation of
intrinsic synthesis and release of TNFα and IL-6 homologue in
TECs.
Materials and methods
Antibodies and reagents
Emodin (molecular weight, 270.24; purity, 95%) was
purchased from the National Institute for the Control of
Pharmaceutical and Biological Products (Beijing, China). Dulbecco’s
modified Eagle’s medium (DMEM)-F12 culture medium and fetal calf
serum (FCS) were purchased from Gibco (Carslbad, CA, USA). All
other cell culture reagents were obtained from Sigma (St. Louis,
MO, USA). Mouse anti-rat cytokeratin was purchased from
ABR-Affinity BioReagents (ThermoScientific Pearce, Rockford, IL,
USA). Mouse anti-rat α-smooth actin (α-SMA) and vimentin were
purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China). LPS from E. coli (serotype, 0111:B4; cat. no.,
L-2630) was obtained from Sigma. The CytoTox 96®
Nonradioactive Cytotoxicity Assay kit was obtained from Promega
Corporation (Madison, WI, USA). TRIzol reagent was purchased from
Invitrogen. Reagents for reverse tran-scription-polymerase chain
reaction (RT-PCR) were purchased from Takara Biotechnology (Dalian,
China). The anti-mouse TLR4 antibody was from BD Biosciences
(Franklin Lakes, NJ, USA). Concentrations of TNF-α and IL-6 were
determined in culture supernatants using commercially available
enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems,
Minneapolis, MN, USA).
Cell culture
Male BALB/c mice were purchased from the Shanghai
Laboratory Animal Center Laboratory Co. Ltd. (SLAC, Shanghai,
China). Primary proximal tubular cells (TECs) were obtained from
6–8 week old mice as previously described (20). Briefly, kidneys were bisected and
the outer cortical tissue was separated from the mice. The tissue
was then minced and digested in collagenase II at 37°C for 20 min.
The digest was then passed through various sieves (250, 160, 75 and
40 nm). Cells trapped in the 40 nm sieve were collected by washing
and centrifuging to obtain a pellet that was resuspended in
DMEM-F12 supplemented with 2% FCS, 100 U/ml penicillin, 100 μg/ml
streptomycin, 5 μg/ml insulin transferrin selenite (ITS),
10−12 M triiodothyronine and 40 ng/ml hydrocortisone.
The cell suspension was then seeded onto 1% gelatine-coated culture
plates and incubated at 37°C, at 5% CO2. Cell phenotype
was confirmed by immuno-histochemistry.
Immunohistochemistry
For the measurement of the cytokeratin, α-SMA and
vimentin in cultured primary cells, immunohistochemistry was
performed using mouse anti-rat monoclonal antibody cytokeratin
(dilution, 1:200), mouse anti-rat monoclonal antibody α-SMA and
vimentin (dilution, 1:100), followed by incubation with
biotinylated secondary antibody for 15 min and
streptavidin-conjugated horseradish peroxidase (HRP) complex for a
further 15 min. After washing with phosphate-buffered saline (PBS),
slides were incubated with substrate-chromogen solution
(3,3′-diaminobenzidine, DAB) for 5 min and counterstained with
Mayer’s hematoxylin for 3 min. Images, including almost all of the
cells, were captured using a light microscope.
Cytokine stimulation and emodin treatment
of TECs
TECs were grown until confluent. Culture medium was
changed to serum-free conditions 24 h prior to stimulation with
LPS. Experiments were performed in triplicate using three
consecutive wells of 6-well plates. Each experiment was repeated at
least three times. The cells were incubated with LPS and/or the
indicated concentrations (10, 20 and 40 μM) of emodin for 24 h.
Cell viability (measured by exclusion of trypan blue) was 95–98%
after treatment with the drug concentrations used in the inhibition
studies.
Cytotoxicity assay
The cytotoxicity assay was performed using a CytoTox
96 Nonradioactive Cytotoxicity Assay kit, according to the
manufacturer’s instructions. Briefly, after cells were incubated
with LPS and emodin for 24 h, medium samples were collected and
spun at 4°C at 13,000 rpm for 5 min. To measure lactate
dehydrogenase (LDH) activity, 50 μl of each sample and 50 μl of
substrate were added in each well of 96-well plates. The assay
plate was incubated for 30 min at room temperature. The LDH
activity was recorded after adding stop solution by measuring the
absorbance at 490 nm. Background values were derived from the
median of untreated TECs. Maximum LDH release values were derived
from cell lysates by adding cell lysis solution to the monolayer.
The percentage of cell deaths was determined using the formula:
%Cytotoxicity = [Experimental LDH release (OD490) -
Background values (OD490)]/[Maximum LDH release
(OD490) + Background values (OD490)].
RT-PCR
Cells were treated with 10, 20 and 40 μM emodin for
24 h after stimulation with 102 ng/ml LPS. Total RNA was
extracted using TRIzol reagent. Total RNA (1 μg) was
reverse-transcribed. PCR was performed using a One Step RNA PCR kit
according to the manufacturer’s instructions. The primer sequences
used are shown in Table I. PCR
amplifications were performed as follows: 5 min at 95°C followed by
28–30 cycles consisting of 30 sec at 95°C, annealing (temperatures
shown in Table I) for 30 sec, 30
sec at 72°C, and an additional elongation step for 10 min at 72°C.
The PCR-amplified products were run on a 1.2% agarose gel and
visualized by ethidium bromide staining. The expression intensities
of optimized bands were quantified using a Luminescent Image
Analyzer (Bio-Rad, Hercules, CA, USA).
 | Table ISense and antisense primer sequences
used in reverse-transcription polymerase chain reaction
(RT-PCR). |
Table I
Sense and antisense primer sequences
used in reverse-transcription polymerase chain reaction
(RT-PCR).
| Gene | Primer pair
(forward and reverse) | Tm (°C) | No. of cycles | Size (bp) | Genbank code |
|---|
| TLR4 |
5′-AAATGCCAGGATGATGCCTCCC-3′ | 62 | 28 | 326 | XM-021297 |
|
5′-AGTTTGAGAGGTGGTGTAAGCC-3′ | | | | |
| TNFα |
5′-GCGAGGACAGCAAGGGACTA-3′ | 60 | 30 | 621 | NM-013693 |
|
5′-GTGTGGGTGAGGAGCACGTAG-3′ | | | | |
| IL-6 |
5′-ATTCCAGAAACCGCTATGAA-3′ | 62 | 30 | 653 | XM-031168 |
| 5′-CAC
TAGGTTTGCCGAGTAGAT-3′ | | | | |
| GAPDH |
5′-ACCACAGTCCATGCCATCAC-3′ | 60 | 28 | 453 | XM-033260 |
|
5′-TCCACCACCCTGTTGCTGTA-3′ | | | | |
Cell staining and flow cytometry
Cultured TECs were incubated with 25 mmol/l
ethylenediaminetetraacetic acid (EDTA; pH 7.6) at 37°C for 10 min.
Detached cells were collected and washed in PBS. A total of
5×105 cells were incubated with 0.5 μg of PE-conjugated
anti-mouse TLR4 antibody, followed by incubation with goat
anti-mouse IgG conjugated with fluorescein. The negative control
was prepared by incubating with an isotype-matched control antibody
(IgG2a). The incubation was performed in 50 μl of PBS at 4°C for 30
min, followed by washing three times in 2 ml of PBS with 1% bovine
serum albumin. The cells were then fixed in 400 μl of 2%
paraformaldehyde in PBS and analyzed by FACScan flow cytometry
(Beckman-Coulter, Miami, FL, USA).
ELISA
To quantify the level of TNFα and IL-6 protein
expression under the different experimental conditions, the total
TNFα and IL-6 protein in the culture supernatant was measured using
a commercial sandwich ELISA kit for TNFα and IL-6 according to the
manufacturer’s instructions. Samples were assayed in duplicate.
Statistical analysis
Experiments were performed in triplicate and
repeated three times. The data were shown as the mean ± standard
deviation (SD). Data were analyzed using SPSS 10.0 software (SPSS
Inc., USA). Statistical significance was assessed by ANOVA and the
unpaired t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
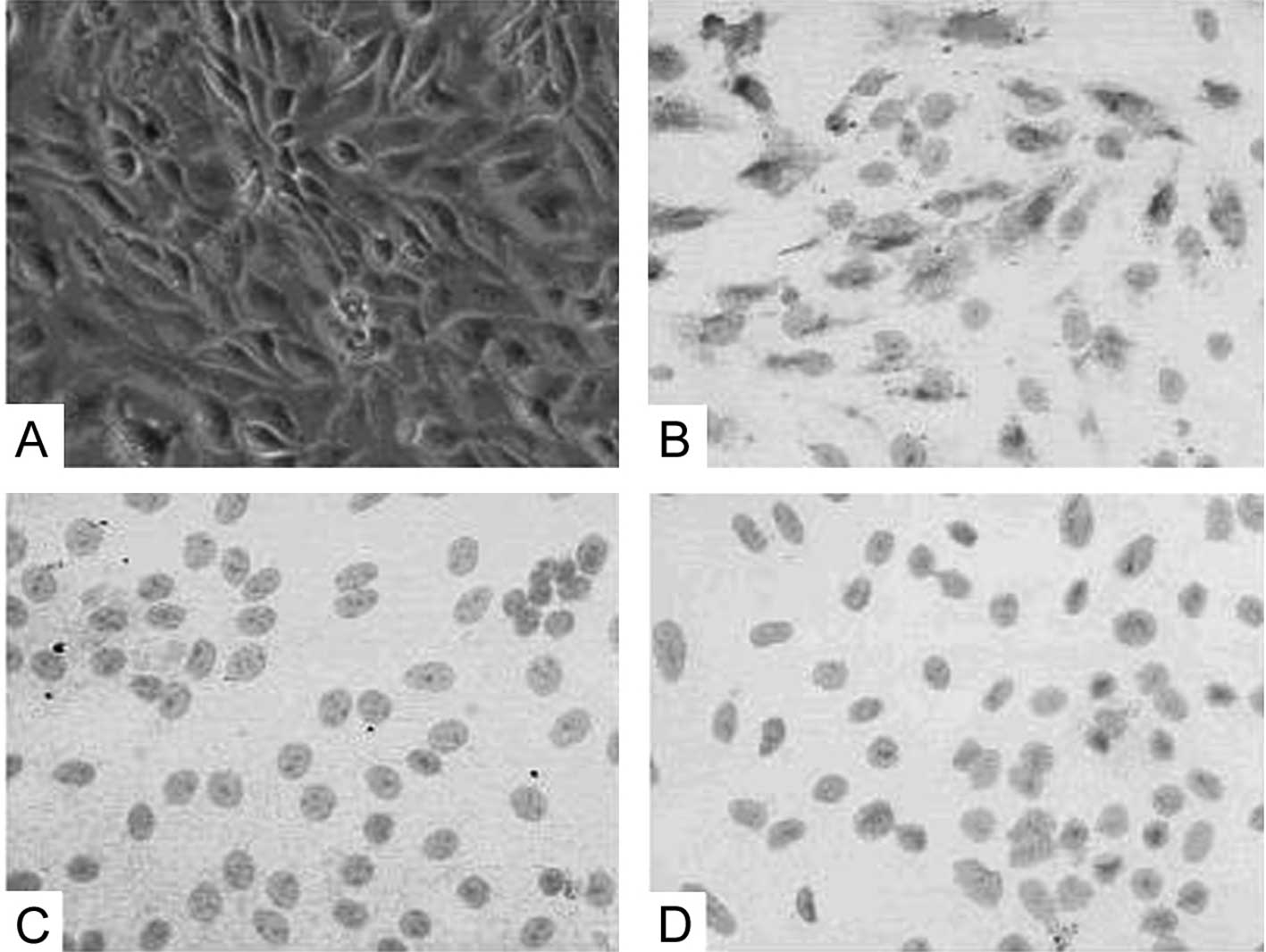
The primary TEC cells were successfully identified
as renal TECs by light microscopy (Fig. 1A). Cell phenotype was confirmed by
positive staining for cytokeratin antibody (Fig. 1B) and negative staining for α-SMA
antibody and vimentin antibody (Fig.
1C and D).
Inhibitory effects of emodin on TLR4 mRNA
and protein expression by LPS-stimulated TECs
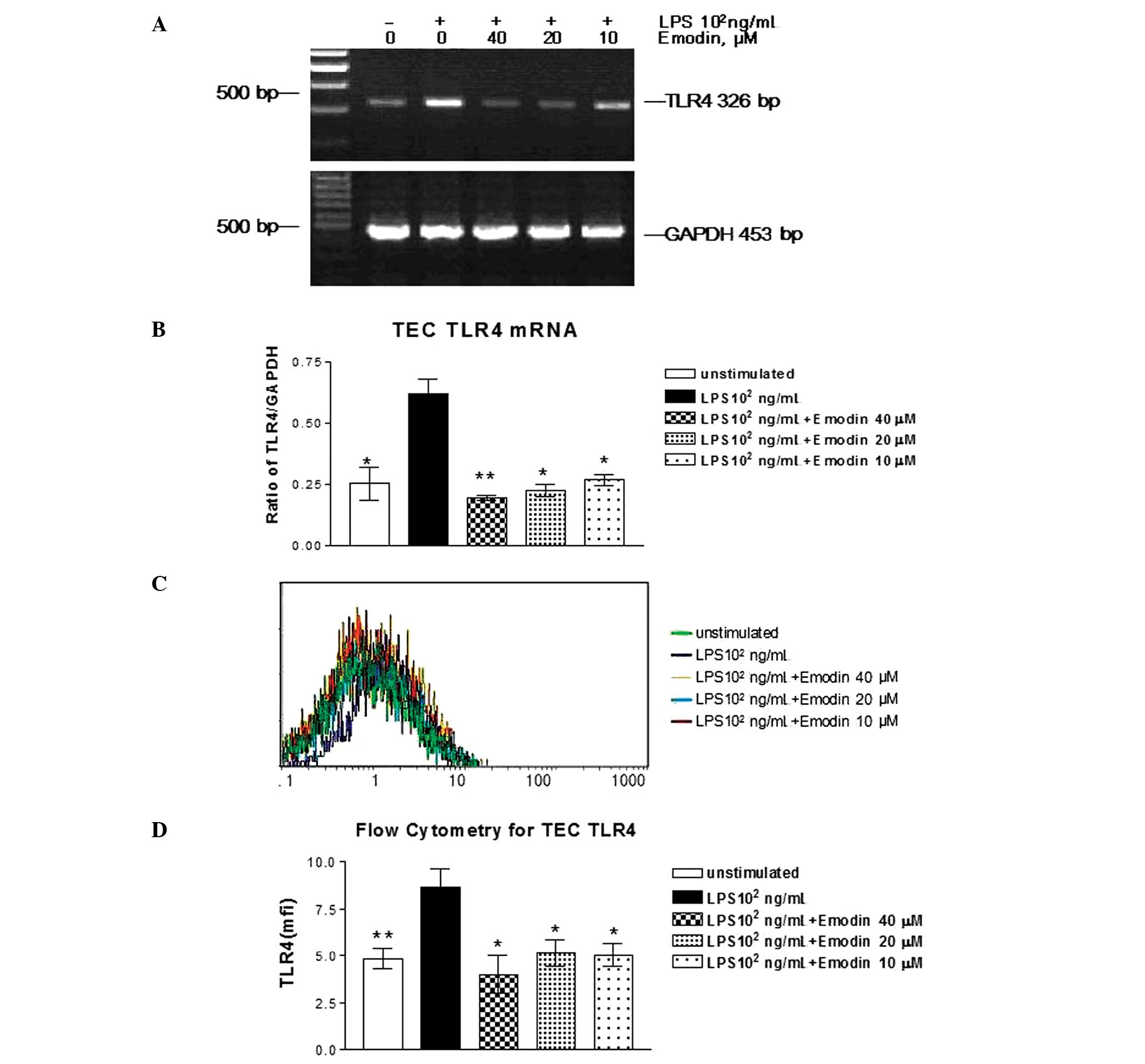
In accordance with previous studies, we found that
TLR4 synthesis by TECs was upregulated following 24 h stimulation
with LPS (7). TLR4 expression was
increased more significantly at a dose of 102 ng/ml than
10 ng/ml and 103 ng/ml (data not shown). Therefore, we
adopted the concentration of 102 ng/ml LPS for our
study.
The concentrations of emodin used in this study were
based on previous experiments (16–17),
and it was verified that these doses were not cytotoxic in cultured
TECs by LDH release assay (Figs.
2–4). To determine the effects
of emodin on TLR4 mRNA expression, TECs stimulated with or without
LPS (102 ng/ml) were incubated with emodin at
concentrations of 10, 20 and 40 μM. Emodin (P<0.05) was able to
inhibit LPS-stimulated TLR4 protein synthesis in cultured TECs, but
with distinct differences in action intensity (Fig. 2A and B). Emodin at a dose of 40 μM
demonstrated maximum suppression of TLR4 mRNA expression in
LPS-stimulated cells. Similar to its effect on TLR4 protein
expression, emodin inhibited the LPS-upregulated synthesis of the
TLR4 surface protein in a dose-dependent manner (Fig. 2C and D).
Inhibitory effects of emodin on TNFα and
IL-6 mRNA and the protein expression by LPS-stimulated TECs
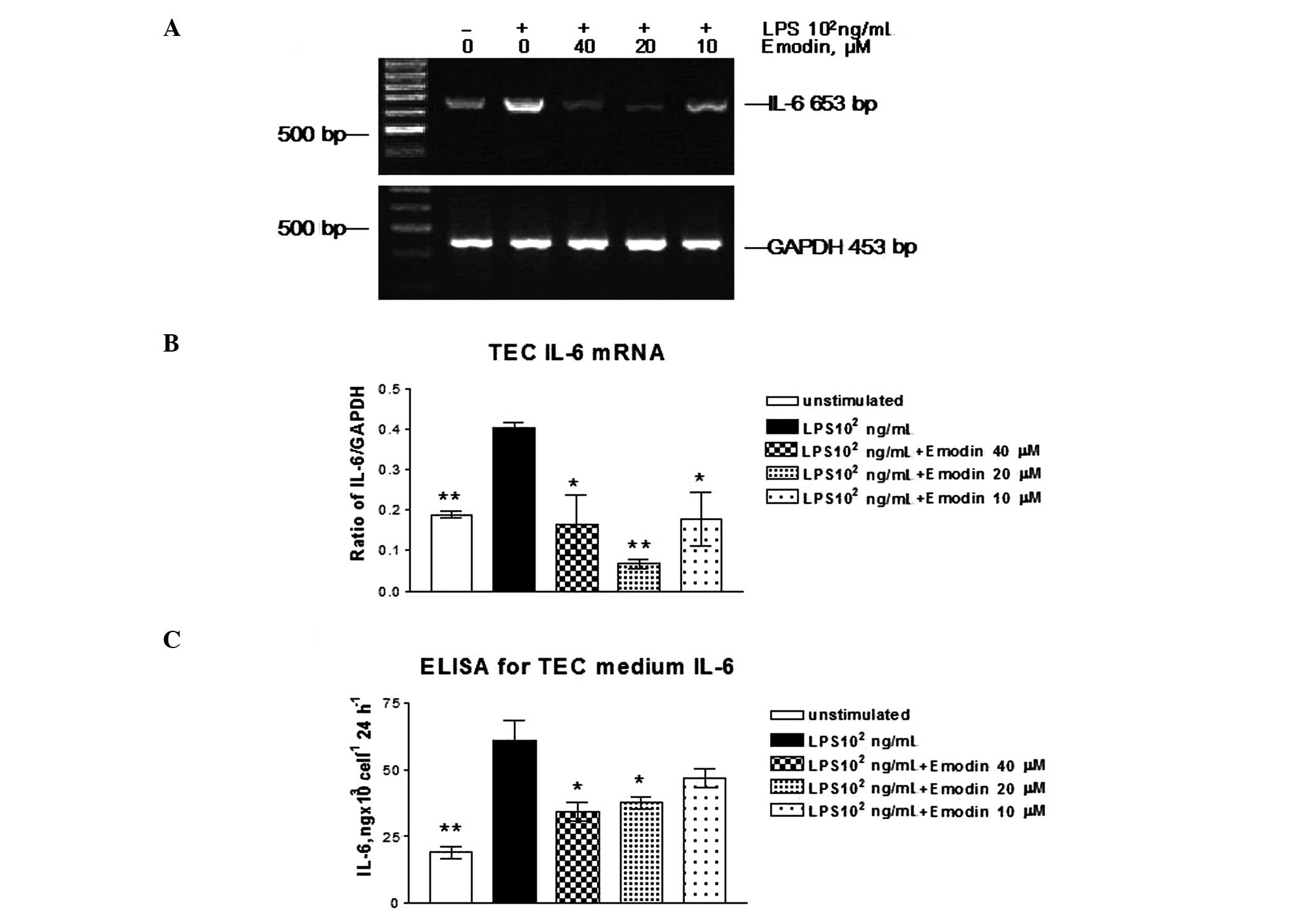
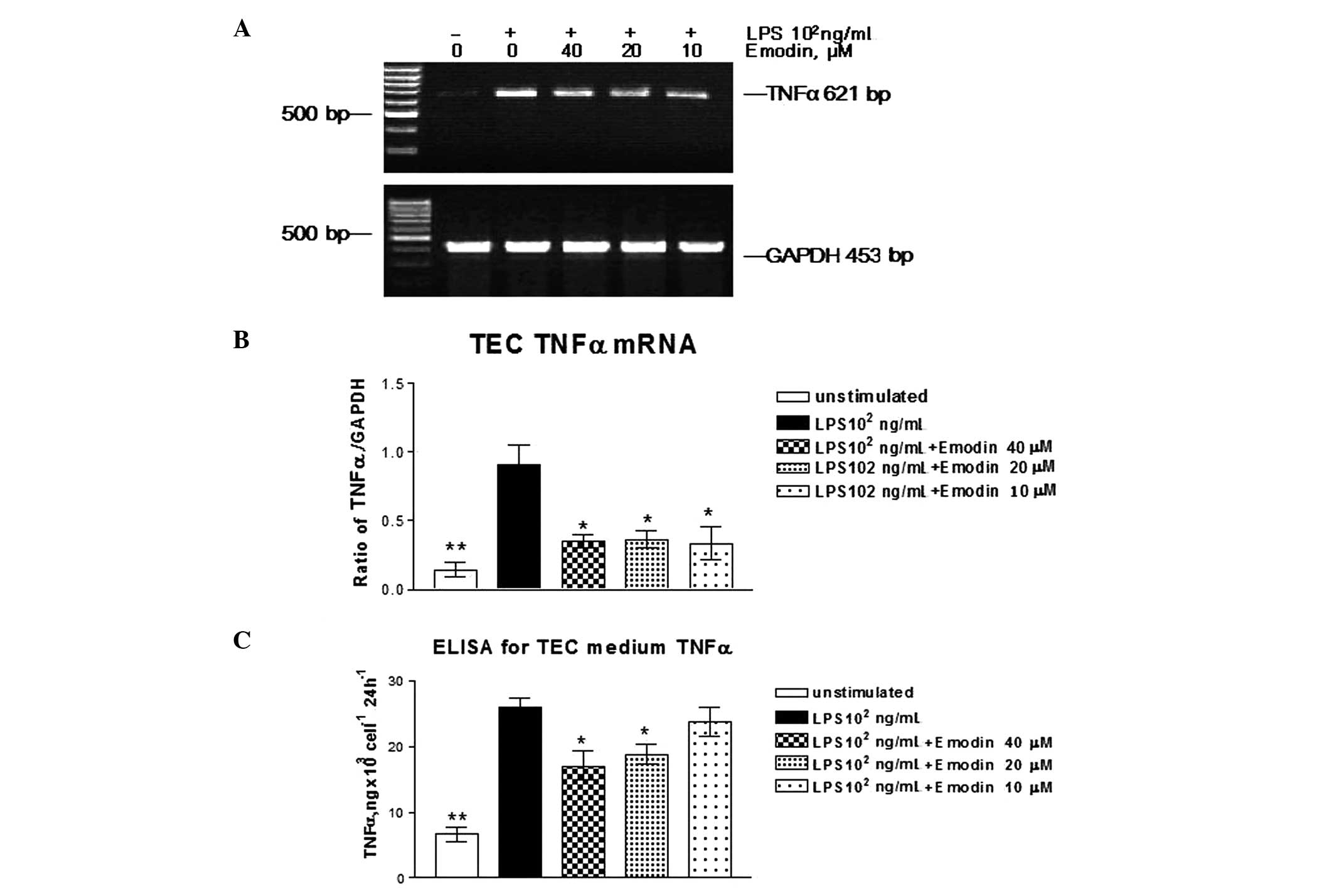
To study the effects of LPS on the expression of
TNFα and IL-6, we evaluated mRNA and protein expression using
RT-PCR and ELISA in LPS-stimulated TECs. TNFα and IL-6 mRNA were
hardly detected in unstimulated TECs. After incubation with LPS
(102 ng/ml) for 24 h, TNFα and IL-6 mRNA expression was
significantly increased (data not shown). TNFα and IL-6 protein
secretions in cell culture medium were increased 2- and 4-fold,
respectively, as demonstrated by using the ELISA method.
Corresponding to TNFα and IL-6 mRNA expression, synthesis of the
TNFα and IL-6 protein was also increased (data not shown).
To examine the effects of emodin on TNFα and IL-6
mRNA expression induced by LPS in TECs, the cells were stimulated
with or without LPS (102 ng/ml) and incubated with
emodin at different doses for 24 h. TNFα and IL-6 mRNA expression
was measured by RT-PCR. Emodin inhibited LPS-induced TNFα and IL-6
mRNA expression (Figs. 3A and
4A). At a dose of 10 μM, the
inhibitory effects of emodin on TNFα and IL-6 protein secretion
were significant, but were lower than the concentrations (Figs. 3B and 4B) of 20 and 40 μM compared with
LPS-stimulated cells (P>0.05). Emodin at concentrations of 40
and 20 μM had an effect on TNFα and IL-6 protein secretion.
Therefore, emodin inhibited TNFα and IL-6 secretion in a
dose-dependent manner.
Discussion
Our results demonstrate for the first time and to
the best of our knowledge that emodin inhibited LPS-induced TLR4
expression in cultured TECs, as well as partly blocked
LPS-stimulated TNFα and IL-6 upregulation. Certain data from our
previous study demonstrated that compounds produced from decoction
of Rheum repressed the proliferation and extracellular
matrix production of glomerular mesangial cells via a decrease in
cyclin D1 and cyclin-dependent kinase 4 levels in rats (15–16).
These studies suggest that emodin is able to inhibit the process of
glomerulosclerosis. In the present study, the effect of emodin in
the TECs is likely to be complex and interrelated to downregulate
TLR4, TNFα and IL-6. It is considered that an increased
understanding of this process is likely to lead to therapies that
are able to effectively prevent or reverse inflammation in
TECs.
LPS is one of the most studied immunostimulatory
components of bacteria that can induce systemic inflammation when
excessive signalling occurs (21)
Thus, it has been widely used as a stimulating factor in research
on TLR4 in infective diseases. We investigated the effects of
emodin on cultured TECs. The results are consistent with previous
findings (9) that TLR4 mRNA and
protein expression were upregulated by LPS, which also paralleled
the severity of increased TNFα and IL-6 mRNA and protein expression
in cultured mouse TECs, supporting the hypothesis that LPS is the
key molecule in the overexpression of TLR4 and cytokines.
Previous reports investigating significant
differences between wild-type and TLR4 knockout mice have
demonstrated that TLR4 is significant in renal pathophysiological
conditions (22). By performing
renal cross-transplantation between wild-type and TLR4 knockout
mice, Cunningham et al revealed that kidneys of TLR4
knockout mice in wild-type hosts remained susceptible to
endotoxin-induced renal failure (23). Furthermore, Tamm-Horsfall
glycoprotein-driven cytokine production was evident in wild-type
mice compared with TLR4-knockout mice. The generation of
Tamm-Horsfall glycoprotein-specific antibodies was consistently
detectable in urinary tract inflammation and was completely blunted
in TLR4-knockout mice (24). TECs
from TLR4-knockout mice failed to respond to LPS and cytokines
released by them were decreased (2). These data suggest that the intrarenal
expression of TLR4 is important for the development of renal
injury. In the present study, we demonstrated that the expression
of TLR4 was significantly upregulated 24 h after stimulation with
102 ng/ml LPS. The detection of TLR4 on TECs also
revealed a potential site for inflammation initiation based on
TLR4. Banas et al detected intense TLR4 immunoreactivity in
mouse models of membranoproliferative glomerulonephritis (25). Zhang et al also provided
compelling evidence that the activation of TLR4 present on renal
parenchymal cells triggers an innate immune response that mediates
cisplatin-induced acute renal failure (26). Thus, TLR4 is critical in the
progression of immunoreactivity. Therefore, the inhibition of TLR4
overexpression has become particularly significant for the
management of renal inflammatory disease. Emodin is the main
effective composition in certain Chinese herbs. Previous studies
have revealed that the laboratory signs of rat renal lesions were
significantly ameliorated by emodin, as demonstrated by decreased
blood creatinine, urea and 24-h urine protein in the renal failure
models after administration with emodin (12,27).
Furthermore, in vitro studies have also revealed that emodin
is able to inhibit proliferation of glomerular mesangial cells and
TECs by decreasing the expression of c-Myc mRNA, increasing the p27
level, delaying the progression from G1 to S phase and promoting
apoptosis of renal fibroblasts, thus intervening in progressive
renal disease (12,28–29).
Previous studies of autoimmunity have demonstrated that emodin
significantly suppressed the expression of pancreatic and pulmonary
TLR4, at the level of mRNA transcription and protein synthesis
(17). The authors speculated that
amelioration of pancreatic and pulmonary damage by emodin may
contribute, at least in part, to the suppression of TLR4
expression. This finding suggested that emodin is a potential drug
target for TLR4 (17). However, no
studies have yet described emodin’s ability to suppress LPS-induced
TLR4 expression in intrinsic renal cells. In the present study, it
was observed that emodin effectively reversed LPS upregulated TLR4
mRNA and protein expression at concentrations of 10, 20 and 40 μM
in cultured mouse TECs. When compared with LPS-stimulated group,
co-culture with emodin significantly reversed LPS-induced
expression of TLR4 in cultured mice TECs, and the effect was
dose-dependent. The results also demonstrated an absence of LDH
release and the appearance of of well-maintained cell viability and
unaltered house keeping gene expression, indicating that reduced
mRNA and protein expression are unlikely to indicate toxicity of
the reagents. The results from the present study raise the
possibility that emodin has good prospects as a treatment for renal
disease by suppression of TLR4.
While we have demonstrated that the LPS-induced
expression of TLR4 was suppressed by emodin in the present study,
the ligation of TLR4-induced production of pro-inflammatory
cytokines cannot be ignored. Consequently, we examined the
inhibitory effects of emodin on the overexpression of TNFα and IL-6
in TECs. Emodin inhibited TNFα and IL-6 activity in a
dose-dependent manner. In the presence of 20 and 40 μM emodin, TNFα
and IL-6 mRNA and protein expression were markedly decreased
compared with the slight inhibition of TNFα and IL-6 protein in the
presence of 10 μM emodin. Thus, TECs respond diffusely to local
infection, with the release of multiple cytokines, chemokines and
other factors that are considered to orchestrate the cellular
constituents of the innate immune response (30). Moreover, the expression of TLR4 may
be involved in regulating immune cells to synthesise cytokines
(31). Other in vivo
studies have also suggested that cytokines released by mesangial
cells may contribute to the pathology and disease progression of
IgA nephropathy (IgAN) (32).
Findings of a previous study showed that the main proinflammatory
cytokines, TNFα and IL-6, were also significantly upregulated in a
rat model of diabetic nephropathy (33). Therefore, it was suggested that
TLR4 is important for the inflammatory response during initiation
and progression of nephropathy. The results from the present study
demonstrate a compelling contribution of emodin in renal
inflammatory disease.
The present study has provided evidence that emodin
not only suppresses LPS-induced TLR4 overexpression, but also
inhibits TNFα and IL-6 activity. Furthermore, the elevated levels
of inflammatory cytokines, as well as an increased expression of
TLR4, were simultaneously inhibited by emodin. Considering that the
TLR4 signalling pathway may stimulate the release of TNFα and IL-6
(34–35), we suggest that the decrease in TNFα
and IL-6 levels by administration of emodin may contribute to the
suppression of TLR4, thus elucidating the mechanism of TLR4
suppression and ICGN alleviation by treatment with emodin.
Acknowledgements
This study was supported by grants from the Project
of National Natural Science Foundation of China (No. 81173426), the
Project of Hangzhou Medical Scientific Technology (No. 2005Z007),
the Project of the Hangzhou Science and Technology Bureau
Foundation (No. 20080333Q17) and the Project of Zhejiang Provincial
Health Department Financed Project (No.2008A135).
References
|
1
|
Theilig F, Kriz W, Jerichow T, et al:
Abrogation of protein uptake through megalin-deficient proximal
tubules does not safeguard against tubulointerstitial injury. J Am
Soc Nephrol. 18:1824–1834. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chassin C, Goujon JM, Darche S, et al:
Renal collecting duct epithelial cells react to
pyelonephritis-associated Escherichia coli by activating
distinct TLR4-dependent and -independent inflammatory pathways. J
Immunol. 177:4773–4784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee HK and Iwasaki A: Innate control of
adaptive immunity: dendritic cells and beyond. Semin Immunol.
19:48–55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zager RA, Johnson AC, Lund S and
Randolph-Habecker J: Toll-like receptor (TLR4) shedding and
depletion: acute proximal tubular cell responses to hypoxic and
toxic injury. Am J Physiol Renal Physiol. 292:304–312. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar
|
|
7
|
Poltorak A, He X, Smirnova I, et al:
Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations
in Tlr4 gene. Science. 282:2085–2088. 1998. View Article : Google Scholar
|
|
8
|
Scherberich JE and Hartinger A: Impact of
Toll-like receptor signalling on urinary tract infection. Int J
Antimicrob Agents. 31:9–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chowdhury P, Sacks SH and Sheerin NS:
Toll-like receptors TLR2 and TLR4 initiate the innate immune
response of the renal tubular epithelium to bacterial products.
Clin Exp Immunol. 145:346–356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palmer SM, Burch LH, Mir S, et al: Donor
polymorphisms in Toll-like receptor-4 influence the development of
rejection after renal transplantation. Clin Transplant. 20:30–36.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li S, Dong X, Wu H, Zhang L and Zhang D:
Progress in studies on pharmacological effect of rhubarb and its
active components. Medical Recapitulate. 1:76–78. 2005.
|
|
12
|
China Pharmacopoeia Committee.
Pharmacopoeia of the People’s Republic of China. (First Division of
2000 ed). China Chemical Industry Press; Beijing: pp. 181999
|
|
13
|
Kwak HJ, Park MJ, Park CM, et al: Emodin
inhibits vascular endothelial growth factor-A-induced angiogenesis
by blocking receptor-2 (KDR/Flk-1) phosphorylation. Int J Cancer.
118:2711–2720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang R, Wan Q, Zhang Y, et al: Emodin
suppresses interleukin-1beta induced mesangial cells proliferation
and extracellular matrix production via inhibiting P38 MAPK. Life
Sci. 80:2481–2488. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu XL, Wang J, Zhou DW, et al:
Experimental studies on prevention and treatment of
glomerulosclerosis with Centella Asiatica compound and its
mechanism. Chin J Nephrol. 17:199–200. 2001.
|
|
16
|
Zhu XL, Wang YJ, Cheng XX, Tong ML, Chen
HY and Zhang MS: Effects of niaodujing II on cyclin D1, CDK4 in
cultured glomerular mesangial cells in human. Chin J Integr Tradit
West Nephrol. 6:316–318. 2004.
|
|
17
|
Kitano A, Saika S, Yamanaka O, et al:
Emodin suppression of ocular surface inflammatory reaction. Invest
Ophthalmol Vis Sci. 48:5013–5022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen RF, Shen YC, Huang HS, et al:
Evaluation of the anti-inflammatory and cytotoxic effects of
anthraquinones and anthracenes derivatives in human leucocytes. J
Pharm Pharmacol. 56:915–919. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Xia X, Zhang S, Zhang A, Bo W and
Zhou R: Up-regulation of Toll-like receptor 4 was suppressed by
emodin and baicalin in the setting of acute pancreatitis. Biomed
Pharmacother. 63:120–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Springall T, Sheerin NS, Abe K, Holers VM,
Wan H and Sacks SH: Epithelial secretion of C3 promotes
colonization of the upper urinary tract by Escherichia coli.
Nat Med. 7:801–806. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beutler B and Rietschel ET: Innate immune
sensing and its roots: the story of endotoxin. Nat Rev Immunol.
3:169–176. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
El-Achkar TM and Dagher PC: Renal
Toll-like receptors: recent advances and implications for disease.
Nat Clin Pract Nephrol. 2:568–581. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cunningham PN, Wang Y, Guo R, He G and
Quigg RJ: Role of Toll-like receptor 4 in endotoxin-induced acute
renal failure. J Immunol. 172:2629–2635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Säemann MD, Weichhart T, Zeyda M, et al:
Tamm-Horsfall glycoprotein links innate immune cell activation with
adaptive immunity via a Toll-like receptor-4-dependent mechanism. J
Clin Invest. 115:468–475. 2005.
|
|
25
|
Banas MC, Banas B, Hudkins KL, et al: TLR4
links podocytes with the innate immune system to mediate glomerular
injury. J Am Soc Nephrol. 19:704–713. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang B, Ramesh G, Uematsu S, Akira S and
Reeves WB: TLR4 signaling mediates inflammation and tissue injury
in nephrotoxicity. J Am Soc Nephrol. 19:923–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Huang H, Liu P, et al: Inhibition
of phosphorylation of p38 MAPK involved in the protection of
nephropathy by emodin in diabetic rats. Eur J Pharmacol.
553:297–303. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu G, Ye R and Tan Z: Effect of emodin on
fibroblasts in lupus nephritis. Zhongguo Zhong Xi Yi Jie He Za Zhi.
20:196–198. 2000.PubMed/NCBI
|
|
29
|
Mei XB, Yuan WJ, Zhan FL, Wu H, Zhang XY
and Cui RL: Role of cyclin kinase inhibitor p27 in inhibition of
emodin on mesangial cell proliferation induced by tumor necrosis
factor-alpha. Zhong Xi Yi Jie He Xue Bao. 2:120–122. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yeboah MM, Xue X, Duan B, Ochani M, Tracey
KJ, Susin M and Metz CN: Cholinergic agonists attenuate renal
ischemia-reperfusion injury in rats. Kidney Int. 74:62–69. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ando M, Shibuya A, Tsuchiya K, Akiba T and
Nitta K: Reduced expression of Toll-like receptor 4 contributes to
impaired cytokine response of monocytes in uremic patients. Kidney
Int. 70:358–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leung JC, Tang SC, Chan LY, Chan WL and
Lai KN: Synthesis of TNF-alpha by mesangial cells cultured with
polymeric anionic IgA - role of MAPK and NF-kappaB. Nephrol Dial
Transplant. 23:72–81. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Navarro JF, Milena FJ, Mora C, León C and
García J: Renal pro-inflammatory cytokine gene expression in
diabetic nephropathy: effect of angiotensin-converting enzyme
inhibition and pentoxifylline administration. Am J Nephrol.
26:562–570. 2006. View Article : Google Scholar
|
|
34
|
Johnson GB, Brunn GJ and Platt JL: Cutting
edge: an endogenous pathway to systemic inflammatory response
syndrome (SIRS)-like reactions through Toll-like receptor-4. J
Immunol. 172:20–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ogawa Y, Tasaka S, Yamada W, Saito F,
Hasegawa N, Miyasho T and Ishizaka A: Role of toll-like receptor 4
in hyperoxia-induced lung inflammation in mice. Inflamm Res.
56:334–338. 2007. View Article : Google Scholar : PubMed/NCBI
|